Abstract
BACKGROUND AND PURPOSE:
Mild traumatic brain injury results in a heterogeneous constellation of deficits and symptoms that persist in a subset of patients. This prospective longitudinal study identifies early diffusion tensor imaging biomarkers of mild traumatic brain injury that significantly relate to outcomes at 1 year following injury.
MATERIALS AND METHODS:
DTI was performed on 39 subjects with mild traumatic brain injury within 16 days of injury and 40 controls; 26 subjects with mild traumatic brain injury returned for follow-up at 1 year. We identified subject-specific regions of abnormally high and low fractional anisotropy and calculated mean fractional anisotropy, axial diffusivity, radial diffusivity, and mean diffusivity across all white matter voxels brain-wide and each of several white matter regions. Assessment of cognitive performance and symptom burden was performed at 1 year.
RESULTS:
Significant associations of brain-wide DTI measures and outcomes included the following: mean radial diffusivity and mean diffusivity with memory; and mean fractional anisotropy, radial diffusivity, and mean diffusivity with health-related quality of life. Significant differences in outcomes were found between subjects with and without abnormally high fractional anisotropy for the following white matter regions and outcome measures: left frontal lobe and left temporal lobe with attention at 1 year, left and right cerebelli with somatic postconcussion symptoms at 1 year, and right thalamus with emotional postconcussion symptoms at 1 year.
CONCLUSIONS:
Individualized assessment of DTI abnormalities significantly relates to long-term outcomes in mild traumatic brain injury. Abnormally high fractional anisotropy is significantly associated with better outcomes and might represent an imaging correlate of postinjury compensatory processes.
Mild traumatic brain injury (mTBI) is associated with a heterogeneous constellation of deficits and symptoms that persist for the long term in 20% of patients who experience concussion.1 The syndrome can entail cognitive impairment, most prominently in memory, attention, and executive function, and postconcussion symptoms (PCS) and limitations in daily functioning.
Notwithstanding earlier constructs that frame PCS as largely factitious or psychogenic and without a biologic basis, it is now widely understood that even uncomplicated mTBI-related dysfunction results from structural pathology such as traumatic axonal injury.2,3 The inability of imaging techniques such as CT and MR imaging to detect traumatic axonal injury has led to delayed understanding of the clinical mTBI syndrome, despite human (eg, Bigler, 20042) and animal (eg, Mac Donald et al, 20074) studies delineating trauma-related histopathology following even mild head trauma.
More recently, DTI has become an established means for the detection of human traumatic axonal injury pathology in vivo; the overwhelming consensus of >120 published studies indicates that despite methodologic heterogeneity, abnormally low fractional anisotropy (FA) derived from DTI is characteristic of patients with mTBI.5 Cross-sectional associations of DTI abnormalities and functional outcomes support the clinical significance of these imaging findings.6–8
Despite strong evidence supporting the ability of DTI to detect clinically salient traumatic axonal injury pathology and its potential to identify patients at risk for poor long-term outcomes, to date DTI has not yet yielded a validated prognostic biomarker for several reasons: First, only a small number of longitudinal studies have assessed the relationship between early imaging and later outcomes.9,10 Second, most studies define outcomes as performance on tests of cognitive function.7,8,11,12 Only a few have examined the relationship between early imaging and subjective measures, which may more closely approximate real-world functioning, such as PCS10,11 and health-related quality of life (HRQoL).13 The morbidity of mTBI in real-world settings may reflect deficits not captured by standard formal cognitive testing, such as multimodal processing and divided attention.14 Third, image-analysis approaches have largely (though not exclusively) used group-level delineation of DTI measures. However, delineation of ROIs at the group level, whether on an a priori basis or by using a voxelwise analysis, is insensitive to the unique spatial distribution of traumatic axonal injury, which is likely present in each patient. Finally, most studies report the association of low FA with poor mTBI outcomes7,8 but do not report abnormally high FA. The few studies assessing the functional significance of abnormally high FA do so at a relatively short follow-up.9,15 The objective of this study was to address current gaps in knowledge by characterizing the relationship between acute diffusion abnormalities in patients with uncomplicated mTBI and their 1-year functional outcomes, including cognition, PCS, and HRQoL. To address the importance of spatial heterogeneity of traumatic axonal injury across subjects, we used an individualized approach to identification of abnormality in each patient with mTBI, Enhanced Z Score Microstructural Assessment of Pathology (EZ-MAP),16,17 and we considered both regional and brain-wide measures and their relationship outcome. If identified prospectively, those with worse prognosis could be targeted for studies of interventions designed to improve outcomes following mTBI.
Materials and Methods
Subject Enrollment and Study Design
This study was approved by the Einstein Institutional Review Board and was conducted in accordance with the Health Insurance Portability and Accountability Act. All subjects provided written, informed consent for participation in the study.
Thirty-nine subjects with mTBI were prospectively enrolled from 2 urban emergency departments.
Inclusion and exclusion criteria for subjects are detailed in Table 1. Subjects were evaluated in the emergency department within 48 hours of injury, and diagnosis of mTBI/concussion was made by an emergency department physician. If CT was performed for clinical care of the current head injury, then an American Board of Radiology Certificate of Added Qualification–certified neuroradiologist reviewed the CT images. Subjects were excluded if skull fracture or any acute or chronic posttraumatic abnormality such as gliosis, localized encephalomalacia, or remote hemorrhage was identified.
Table 1:
Subject inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age 18–70 years | Focal neurologic deficits |
| Availability for testing within 2 weeks of concussion | History of head injury (based on history and medical record) |
| Glasgow Coma Scale = 13–15 | Chronic posttraumatic abnormal findings on CT/MRI |
| Loss of consciousness < 20 minutes | Hospitalization due to the current head injury |
| Posttraumatic amnesia < 24 hours | History of a neurodevelopmental or neurologic disorder |
| English or Spanish proficiency | Major psychiatric disorder |
| Illicit drug use within 30 days |
Forty healthy volunteers were recruited from the community via printed advertisements. The inclusion criterion for controls was 18–70 years of age. Exclusion criteria were the same as those for subject enrollment. This study used a prospective, within-subjects design: The role of the group of 40 controls was to provide imaging for comparison, to allow identification of abnormal FA.
In this longitudinal study, imaging was performed within 16 days of injury (median, 7.5 days; range, 1–16 days), and cognitive function, PCS, and HRQoL were assessed at 1 year following injury.
Data Acquisition
Imaging.
Imaging was performed by using a 3T MR imaging scanner (Achieva TX; Philips Healthcare, Best, the Netherlands) with a 5-channel head coil (SENSE Head Coil; Philips Heathcare). T1-weighted whole-head structural imaging was performed by using sagittal 3D magnetization-prepared rapid acquisition of gradient echo imaging (TR/TE, 9/4.6 ms; FOV, 240 mm2; matrix, 240 × 240; section thickness, 1 mm). T2-weighted whole-head imaging was performed by using axial 2D turbo spin-echo imaging (TR/TE, 4000/100 ms; FOV, 240 mm2; matrix, 384 × 512; section thickness, 4.5 mm) and axial 2D fluid-attenuated inversion recovery turbo spin-echo (TR/TE, 1100/120 ms; TI, 2800 ms; FOV, 240 mm2; matrix, 384 × 512; section thickness, 4.5 mm; number of signals acquired, 1). DTI was performed by using single-shot spin-echo echo-planar imaging (TR/TE, 3800/88 ms; FOV, 240 mm2; matrix, 112 × 89; section thickness, 4.5 mm; independent diffusion-sensitizing directions, 32; b=800 s/mm2 images).
Outcome Measures
Cognition.
Tests of cognitive function were administered to all subjects, by using IntegNeuro (Brain Resource Company, Sydney, Australia), a computerized battery of cognitive tasks.18 A summary z score was computed for each of 3 cognitive domains (executive function, episodic memory, and attention), selected for study because of their known associations with the mTBI syndrome, by using an international database of >5000 age-, sex-, and education-matched healthy individuals, as detailed in Table 2.
Table 2:
Cognitive domains and component cognitive tasks
| Cognitive Domain | Constituent Cognitive Tasks |
|---|---|
| Executive Function | Digit Span Backward |
| Switching of attention (digits/letters) | |
| Verbal interference | |
| Executive maze task | |
| Episodic Memory | Verbal list learning task (immediate recall, delayed recall, and recognition) |
| Attention | Digit Span Forward |
| Continuous Performance Task | |
| Switching of attention (digits) | |
| Visual memory |
Premorbid intelligence was estimated by using the Spot-the-Word test, which is a measure of word recognition ability and lexical decision-making, with good reliability (0.88) and convergent validity (0.60–0.86).19
Postconcussion Symptoms and Health-Related Quality of Life Outcome Measures
At 1 year postinjury, the Rivermead Post Concussion Symptoms Questionnaire20 was administered to assess PCS, and the Sickness Impact Profile21 was administered to assess HRQoL. Patients were classified as having postconcussion syndrome on the basis of outcomes at 1 year rather than 3 or 6 months; thus, those with postconcussion syndrome that lasted <1 year were not classified in this manner. This classification restricts the postconcussion syndrome patient group to those with more persistent and chronic symptoms.
The Rivermead Post Concussion Symptoms Questionnaire consists of a series of 16 symptoms; subjects are asked to rate the severity for each item, relative to preinjury experience, on a scale from 0 to 4. Symptoms are categorized into cognitive, somatic, or emotional factors; and each factor is used as an independent outcome measure.22
The Sickness Impact Profile includes 68 questions pertaining to daily functioning and is graded on a dichotomous scale (0 or 1) yielding 5 subscores. The physical dimension is assessed as “somatic autonomy” and “mobility control”; the psychological dimension, as “psychological autonomy and communication”; and the social dimension, as “mobility range” and “social behavior.”23
Data Analysis
Neuroradiology Assessment.
An American Board of Radiology Certificate of Added Qualification–certified neuroradiologist reviewed structural MR images for posttraumatic pathology, including hemorrhage, extra-axial collection, contusion, or traumatic axonal injury.
Calculation of Diffusion Parameter Images.
The 32 diffusion-weighted image sets (32 b=800 s/mm2 images) were corrected for head motion and eddy current effects by using an affine registration algorithm, with the b=0 s/mm2 image as the target, and tensor fitting was performed at each voxel by using the FMRIB Diffusion Toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT).24
Image Registration.
All analyses were performed after transformation of diffusion parameter images to match a high-resolution T1-weighted template (Montreal Neurological Institute).25 The registration process includes correction for EPI distortions and linear within-subject and nonlinear subject-to-template registration steps as previously reported.17 This spatial normalization procedure has been shown to be robust across subjects.26 Nonetheless, the results of each registration are critically assessed by viewing each stage of the registration output, with particular assessment of the alignment of brain surface; deep structures including the brain stem, corpus callosum, and fornix; and gray/white margins in both the deep gray matter structures and at the cortical margin. These landmarks must align within 2 voxel dimensions for the registration to be accepted, though alignment is typically nearly exact.
White Matter Segmentation.
The FMRIB Automated Segmentation Tool (FAST; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FAST)27 was used to generate a white matter mask for the 3D T1-weighted template brain images. This mask was eroded by 3 voxels to eliminate locations most at risk of misregistration and was used to restrict subsequent statistical analysis of FA to white matter voxels.
Subregion Segmentation of White Matter.
While the analysis was performed in the Montreal Neurological Institute template space, the Johns Hopkins University white matter atlas28 was adapted and used for segmentation of white matter subregions. This segmentation procedure eliminates potential observer bias introduced by intra- and interrater variance inherent in manually delineated ROIs. With the FMRIB Linear Image Registration Tool (FLIRT; http://www.fmrib.ox.ac.uk/),27 the T1-weighted template for the Johns Hopkins University white matter atlas was registered to the T1-weighted Montreal Neurological Institute template used for DTI analysis. The resulting transformation matrix was applied to the white matter segmentation volume of the Johns Hopkins University white matter atlas to bring it into registration with the Montreal Neurological Institute template. Individual Johns Hopkins University regions were combined to generate larger white matter regions used for analysis, as detailed below.
Adjustment for Demographic Covariates.
Before lesion detection, multiple linear regression analysis was performed to adjust for the effects of age, sex, and education as detailed in Kim et al.17 Regression coefficients were determined from control subjects only, to avoid potential interaction effects of mTBI on the aforementioned putative risk factors, and were applied to the voxels within each subject's FA image, where covariate effects on individual voxels were significant at P < .05 and >100 significant voxels formed a contiguous cluster.
EZ-MAP Analysis for FA Lesion Detection.
The Enhanced Z Score Microstructural Assessment of Pathology17 is a method for delineating abnormal regions in individual patients with mTBI. The EZ-MAP is based on a whole-brain voxelwise z score of a subject , calculated with the mean (x̄) and SD [sd (x)] from healthy controls at each voxel, where we denote SD from healthy controls as sd(x). We here omit the voxel index for notation convenience. The EZ-MAP is more robust than standard z score analysis because it incorporates estimated sampling variance of individual z scores by using a bootstrap procedure, which is finally calculated as . σ̂B(x) is >1 due to sample-to-sample variation of z scores.17 Abnormal clusters were further delineated by applying a cluster size threshold determined on the basis of the Gaussian random field theory. This technique has been optimized and validated previously for assessment of individual subjects, and the EZ-MAP showed greater robustness in varying control samples compared with the z score and the 1-versus-many t test, approaches that have been adopted in other single-subject analyses of FA imaging data in mTBI.16,17 The EZ-MAP thus generated was thresholded with 2 criteria: |EZ| > 1.96 for each voxel and cluster size P value 1% (corrected for multiple comparisons by the Gaussian random field theory).16,17 A subject FA value from an abnormal FA lesion detected by EZ-MAP is in the range of magnitude: y < x̄ ± 1.96 × K(x), where K(x) = sd(x) × σ̂B(x). Because σ̂B(x) is >1 as aforementioned, the value of SD (K) from the control mean in the EZ-MAP is >1.96. Validation of the EZ-MAP method in Kim et al17 serves as the premise for its application in the current study.
Calculation of Imaging Variables
The procedure for calculating brain-wide and regional DTI measures for subsequent analysis is summarized in On-line Figs 1 and 2.
Brain-Wide Imaging Measures.
Each subject-specific EZ-MAP of abnormal FA regions was segregated into 2 separate maps: 1) all voxels showing abnormally high FA, indicated below as hFA, and 2) all voxels showing abnormally low FA, referred to below as lFA. These maps were then used as masks and were applied to each subject's DTI parameter images: FA, axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD). Mean FA, AD, RD, and MD were thus calculated separately across all hFA voxels and across all lFA voxels, yielding 2 measures for each diffusion parameter in each subject. Additionally, the total volume (number of 1-mm3 voxels) of hFA and lFA was also computed for each subject (On-line Fig 1).
Regional Imaging Measures.
We selected 9 white matter regions known to be susceptible to mTBI and/or to serve functions associated with mTBI morbidity, as follows: the left frontal lobe, right frontal lobe,8 left temporal lobe, right temporal lobe,10 left thalamus, right thalamus,12 left cerebellum and right cerebellum,29 and corpus callosum (On-line Fig 2).7
Two new class variables were generated, representing the following: a) the presence (1) or absence (0) of hFA in each region, and b) the presence (1) or absence (0) of lFA for each region (On-line Fig 2). The 2 class variables were independently generated for each region. As a result, it is possible that hFA and lFA lesions will coexist within a single brain region. The 2 subgroups of subjects classified by using each class variable (a or b) were tested for significant differences in long-term outcomes.
Statistical Analyses: Relationship between Early Imaging and Long-Term Outcomes
All statistical analyses were conducted in SPSS (Version 22.0, Released 2013; IBM, Armonk, New York). Both brain-wide and regional imaging measures (as defined above) were used in distinct analyses.
The Spearman rank correlation analysis is robust to outliers and was used to evaluate the monotonic association of brain-wide early imaging variables and long-term (1 year) outcomes.
In addition, a regional analysis of abnormal FA was performed to facilitate investigation of structure-function associations, despite the fact—which is an expected feature of traumatic brain injury (TBI) pathology—that not all subjects exhibited abnormalities in the same brain location. Student 2-sample t tests were used to compare long-term outcomes between groups of subjects who did-versus-did not exhibit hFA or lFA within a given anatomic region (listed above).
False Discovery Rate Control for Statistical Analyses
The total number of hypotheses tested for association between imaging measures and long-term outcomes are as follows: In the brain-wide assessment of abnormal diffusion metrics across all areas of hFA and lFA in each subject, 5 imaging parameters (FA lesion volume, mean FA, mean AD, mean RD, and mean MD) were correlated with 12 outcomes for each total hFA and total lFA, totalling 120 (5 × 2 ×12) tests. For regional analyses, we examined the difference between the 2 groups in 12 outcome measures for each of 9 brain regions, in which subjects were classified by the presence or absence of hFA and lFA within each brain region; 216 (2 × 9 × 12) tests were required for these analyses. Thus, in total, 336 comparisons were performed across the entire study. We grouped the 336 comparisons into 10 subgroups by 5 outcome categories (memory, attention, executive, PCS, and HRQoL) and analysis approaches, brain-wide and regional. The significance of individual tests was determined by using the Benjamini Hochberg method at a false discovery rate = 0.15 for each subgroup.30,31
Results
Study Subjects: Sample Size, Demographics, Mechanisms of Injury, and Baseline Functional Status
Thirty-nine subjects with mTBI and 40 control subjects met the inclusion criteria. Subjects with mTBI underwent DTI within 16 days of injury. Twenty-six subjects returned for 1-year follow-up and were included in the analysis. During the time elapsed between enrollment and follow-up, 6 of the 26 subjects moved to locations too far from the testing center to allow on-site, computerized cognitive assessment; they were, however, available to complete the Rivermead Post Concussion Symptoms Questionnaire and Sickness Impact Profile by phone interview.
Table 3 details demographic and injury characteristics of the 26 subjects and 40 controls used in the analysis. We found no significant difference in age (t = −0.134, P = .894), sex (χ2 = 1.247, P = .264), or years of education (t = −1.919, P = .059) between the 26 subjects and 40 controls. Nevertheless, potential effects of age, sex, and education were addressed by voxelwise regression adjustment, with application of regression coefficients to all FA voxels in which demographic covariate effects were significant at P < .05 across >100 contiguous voxels.16 All subjects were diagnosed with mTBI in the emergency department, and no abnormalities were identified on conventional CT or MR imaging. In this relatively young sample, with a mean age of 38.5 years, changes of microvascular ischemia and stroke were not observed.
Table 3:
Demographic and injury features of subjects and controls
| Subjects | Controls | |
|---|---|---|
| Mean age (yr) (range) | 38.5 (24–64) | 38.85 (20–60) |
| Sex | 10 Men (38.5%) | 21 Men (52.5%) |
| 16 Women (61.5%) | 19 Women (47.5%) | |
| Mean years of education (range) | 14.3 (8–24) | 16.4 (2–26) |
| Mechanism of injury | Motor vehicle crash: 3 | NA |
| Sports accident: 2 | ||
| Fall: 7 | ||
| Assault: 7 | ||
| Falling object: 7 | ||
| Posttraumatic amnesia | 2/26 | |
| Loss of consciousness | 10/26 |
Note:—NA indicates not applicable.
Preinjury cognitive ability was estimated through administration of the Spot-the-Word test, a measure of reading achievement, a “hold” ability that is resistant to the effects of brain injury.19 We found no difference between the 26 subjects and a subset of 18 controls (those for whom data were available) on the Spot-the-Word test (t = −1.152, P = .256), indicating that the subjects' preinjury intellectual functioning was not significantly different from that of controls. There were no differences in age (t = 1.353, P = .184), sex (χ2 = 0.834, P = .361), years of education (t = 1.227, P = .228), Spot-the-Word scores (t = −0.019, P = .985), and loss of consciousness at the time of injury (χ2 = 1.805, P = .179) between the 26 subjects included in the analysis and the 13 subjects lost to follow-up (note that loss of consciousness data were available for only 12/13 lost to follow-up).
Functional Outcomes at 1 Year: Cognitive Function, HRQoL, and PCS
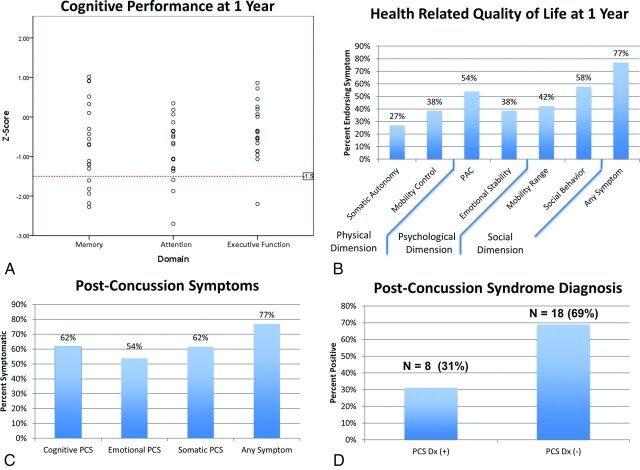
Figure 1A depicts a range of z scores for each of the 3 cognitive domains, with impairment defined as z score values of >1.5 SDs below the mean for the given domain. Figure 1B shows the prevalence of symptom endorsement related to HRQoL according to dimension (social, psychological, and physical). Figure 1C, -D demonstrate the prevalence of symptom endorsement related to PCS and the percentage of those meeting the criteria for the diagnosis of postconcussion syndrome based on the Rivermead Post Concussion Symptoms Questionnaire cutoff criterion.32
Fig 1.
Outcomes in mTBI. A, Cognitive outcomes. The red dotted line indicates impairment in the given domain, defined as a z score less than −1.5. B, Health-related quality of life (Sickness Impact Profile), grouped according to dimension. C, Postconcussion symptoms (Rivermead Post Concussion Symptoms Questionnaire). D, Postconcussion syndrome diagnosis. PAC indicates psychological autonomy and communication.
Microstructural Abnormalities at the Time of mTBI
Using the procedures described above, we detected regions of hFA in 24/26 subjects (mean total volume = 9398 μL; maximum = 27,660 μL) and lFA in 25/26 subjects (mean total volume = 6923 μL, maximum = 39,686 μL). All subjects showed at least 1 region of hFA or lFA, though not all showed both. The mean hFA averaged across all subjects (0.641) was significantly higher than the mean lFA (0.278, P < .001). MD and RD were significantly higher in lFA (0.625, 0.567) regions than in hFA regions (0.517, 0.357) (P < .001, P < .001). AD was not significantly different between hFA (0.805) and lFA (0.740) regions.
Loss of Consciousness and Microstructural Abnormalities
Ten of 26 of subjects experienced loss of consciousness at the time of injury. Mean hFA and mean lFA values within 16 days of injury were not significantly different between those with and without loss of consciousness at the time of injury (t = −0.904, P = .375; t = 0.923, P = .365, respectively).
Relationship between Microstructural Abnormalities and Functional Outcomes
Whole White Matter Assessment.
Brain-Wide Imaging Measures and Cognitive Performance. Imaging measures were associated with 1-year memory performance, but not with attention or executive function. The associations with memory were significant for higher RD from areas of hFA at baseline (ρ = −0.562, P = .015) and higher MD from areas of hFA at baseline (ρ = −0.488, P = .040) (On-line Fig 3).
Brain-Wide Imaging Measures and PCS. Neither hFA nor lFA were associated with PCS when averaged across the whole brain.
Brain-Wide Imaging Measures and HRQoL. Higher MD and higher RD from areas of lFA (ρ = 0.513, P = .009; ρ = 0.514, P = .009) were significantly associated with worse somatic autonomy. Lower mean FA from regions of lFA were significantly associated with worse psychologic autonomy and communication (ρ = −0.596, P = .002) and worse emotional stability (ρ = −0.581, P = .002) at 1 year postinjury (On-line Fig 4). FA, AD, RD, and MD from regions of hFA were not significantly associated with HRQoL.
Regional White Matter Assessment.
Regional Imaging Measures and Cognitive Performance. Subjects with hFA in the left frontal and left temporal white matter performed better than those without hFA in these regions on tasks of attention at 1 year postinjury (t = 2.985, P = .008 and t = 3.322, P = .004, respectively) (Fig 2).
Fig 2.
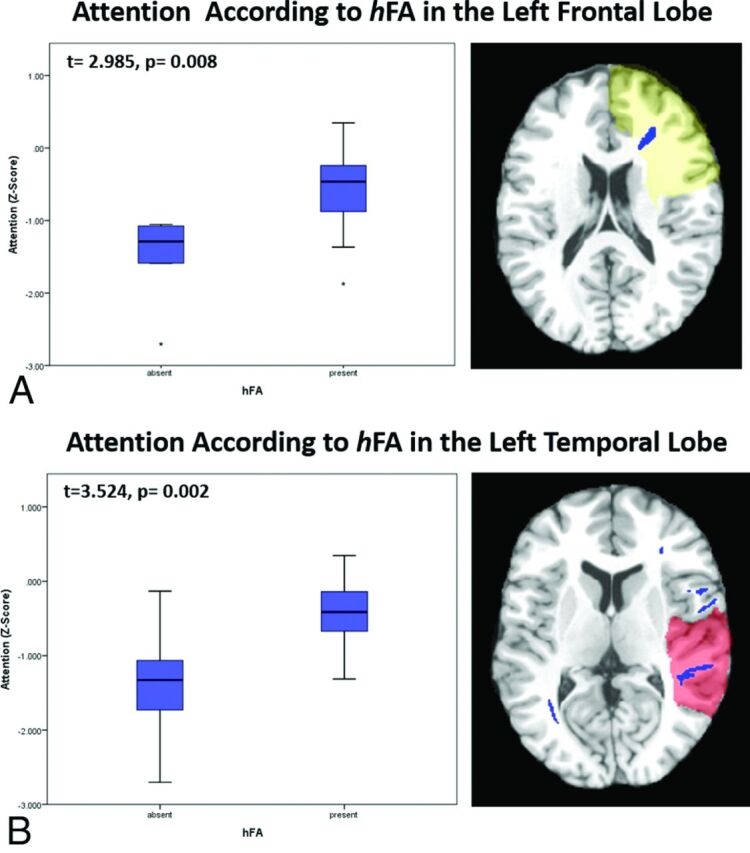
Associations between regional imaging measures and long-term cognitive outcomes. A, Subjects with high FA in the left frontal lobe perform significantly better on tasks of attention at 1 year postinjury than do those without it (t = 2.985, P = .008). B, Subjects with high FA in left temporal lobe perform significantly better on tasks of attention at 1 year postinjury than do those without it (t = 3.524, P = .002).
Regional Imaging Measures and PCS. Subjects with hFA in the right thalamus white matter experienced fewer emotional postconcussion symptoms than did those without hFA in this region (t = −0.398, P = .003). Subjects with hFA in the left or right cerebellar hemisphere experienced fewer somatic postconcussion symptoms than did those without abnormality (t = −3.365, P = .003; t = −3.38, P = .003, respectively) (Fig 3A, -C).
Fig 3.
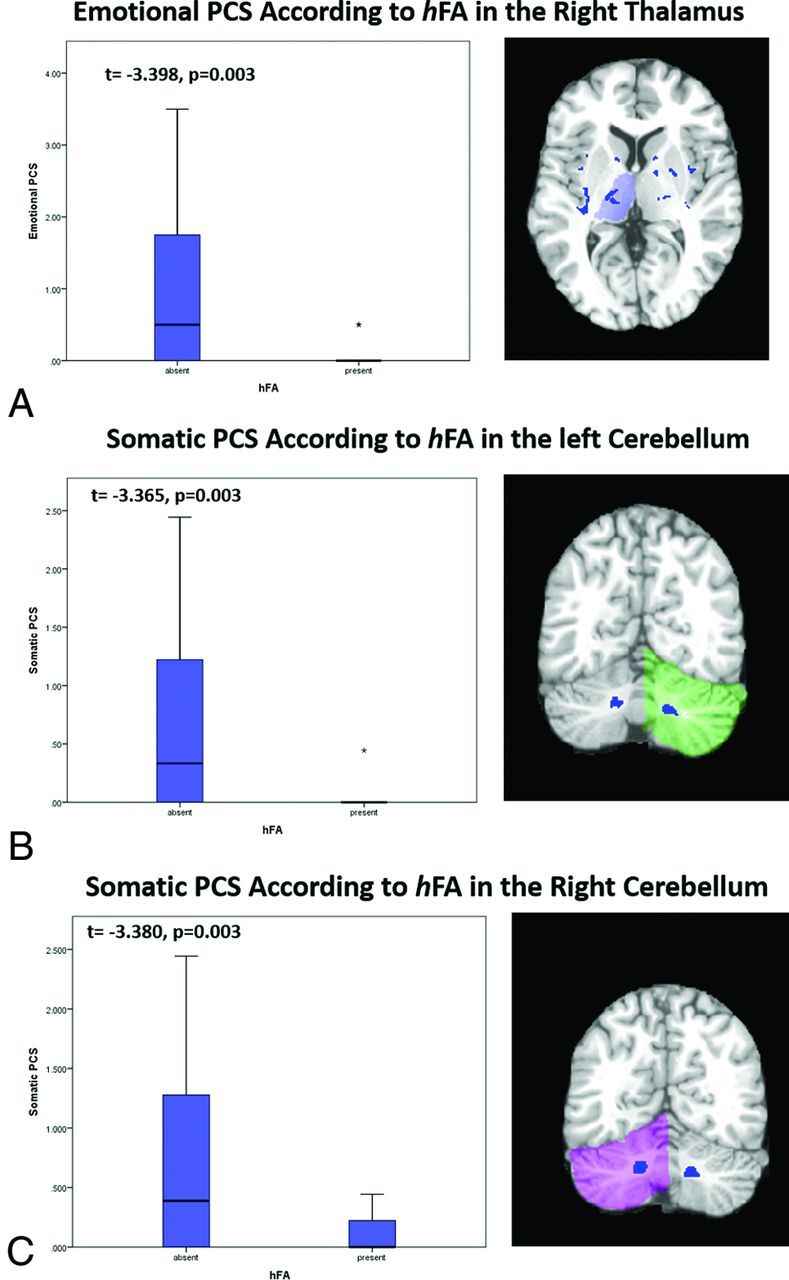
Associations between regional imaging measures and long-term functional outcomes. A, Subjects with hFA in the right thalamus have significantly fewer emotional postconcussion symptoms at 1 year than those without it (t = −3.398, P = .003). Of those with hFA in the right thalamus, only 2 subjects reported emotional postconcussion symptoms. The asterisk indicates an outlier. B, Subjects with hFA in the left cerebellar white matter have significantly fewer somatic postconcussion symptoms at 1 year than those without hFA in the left cerebellum (t = −3.365, P = .003). Of those with hFA in the left cerebellum, only 1 subject reported somatic postconcussion symptoms. The asterisk indicates an outlier. C, Subjects with hFA in the right cerebellar white matter have significantly fewer somatic postconcussion symptoms at 1 year than do those without high FA in the right cerebellum (t = −3.380, P = .003). Of those with hFA in the left cerebellum, only 2 reported somatic postconcussion symptoms.
Regional Imaging Measures and HRQoL. There were no significant differences in HRQoL between individuals with and without hFA or lFA within individual regions.
Discussion
This prospective, longitudinal study leverages individualized assessment of DTI16,17 to demonstrate associations between early imaging and 1-year mTBI outcomes.
Imaging studies of mTBI outcomes generally use either a priori ROI or group-level voxelwise comparisons of subjects and controls to extract imaging measures for study. Most important, neither of these approaches acknowledges nor has the ability to characterize the widely recognized substantial spatial variation in injury location, which is a principal feature of mTBI.33 As a result, these standard approaches will include areas with and without tissue injury in the imaging measures they study. We therefore used a technique that specifically identifies abnormalities in each individual patient (EZ-MAP), without dilution by values from normal tissue (inevitable with group-level delineation).
We have previously demonstrated the robustness17 and application16 of the EZ-MAP to the detection of microstructural abnormalities in patients with mTBI. In this study, we further demonstrate that the EZ-MAP method is highly effective in identifying brain pathology related to long-term outcomes. Similar to using the EZ-MAP, various other studies have used individual subject-level procedures33,34 to detect pathology in DTI datasets from patients with mTBI. Our approach differs in that we used regression adjustment for covariates and EZ-MAP bootstrap resampling, to better characterize the population variance. Imaging measures summarized over brain subregions, such as the frontal lobe, surpassed whole-brain summary measures (eg, mean FA across all abnormal white matter voxels) in identifying relationships between DTI measures and functional outcomes. Although this result may be related to differences in the statistical approach between the whole-brain measures and subregion measures (correlation analysis versus dichotomous analysis, respectively), it potentially reveals an important phenomenon: Structure-function relationships are more effectively detected when measures are extracted from delimited brain regions relevant to a particular function and not diluted by measures from regions not specifically supporting that function.
Using the EZ-MAP approach, we found regions of hFA, in addition to regions of lFA, in almost all subjects. A minority of prior studies, most of which used group-level techniques to identify abnormalities, also reported high FA and have attributed this finding to cytotoxic edema, altered myelin sheath water composition, or inflammation. Thus, hFA has been considered an additional biomarker for injury pathology due to mTBI.6,9,15 In contrast, our use of individual-level delineation of abnormalities reveals associations of hFA with better long-term outcomes. These associations may reflect differences in the type of abnormality identified by using individual-level versus group-level approaches. Whereas hFA delineated at the group level might represent damage to common areas injured across subjects, regions of abnormally hFA identified at the individual level might reflect subject-specific compensatory mechanisms that enhance diffusion anisotropy through structural or functional changes, such as myelination or increased synaptogenesis at the level of the dendritic spine, which is mediated by actin polymerization.35 Notably, studies have shown that short-term reversible increases in anisotropy develop with cognitive training, presumably through similar neuroplastic mechanisms.36
Relating structural changes to their functional consequences is important in understanding the import of imaging findings and characterizing injury effects in patients with TBI. Thus, the regional analyses we performed are most salient and reveal several interesting structure-function relationships. Notably, most literature on neuroanatomic structure-function relationships focuses on gray matter. However, white matter axons form the infrastructure of distributed neural networks, which underlie the domains of higher functioning so commonly impacted by the white matter injury that follows TBI.37,38 We found significant regional associations of frontal and temporal hFA with performance on tasks of attention, tested by using the Digit Span Forward, Continuous Performance Task, and switching of attention (digits) tasks; the latter 2 depend on processing speed and therefore index neural network function. Moreover, the frontal39–41 and temporal lobes42 both directly play an important role in attention. The significant association of hFA in the thalamus with emotional PCS is not surprising, given the susceptibility of the thalamus to mTBI and its role as the major relay network of the brain, with extensive limbic and prefrontal connectivity.43,44 We also identified associations of cerebellar abnormalities with somatic PCS, including dizziness, nausea/vomiting, double vision, and blurry vision, which reflect the role of the cerebellum in balance and spatial orientation.
In addition to FA, which characterizes overall coherence of diffusion direction, we explored the utility of AD, RD, and MD. Preclinical experimental studies posit low AD as a marker for intra-axonal injury and high RD as a marker for transaxonal/axolemmal injury and demyelination.4 We found that high RD, but not low AD, significantly correlated with worse outcomes. We may not have identified abnormally low AD as a correlate of outcomes because the expected decrease in AD may be masked by increased AD related to gliosis and edema, which evolve in the late acute setting, the timeframe during which our subjects underwent DTI (mean, 7 days).45 In a postmortem analysis of patients with multiple sclerosis, increased RD correlated with the severity of demyelination and decreased axonal attenuation, presumably because the 2 are interrelated.46 We found that mean RD, but not mean AD, was significantly associated with memory and somatic autonomy in correlation analyses, consistent with this pattern. Because high RD may be a manifestation of transaxonal injury, as suggested by preclinical studies,47 it may indicate more severe and irreparable axonal pathology and therefore might serve as a better early predictor of long-lasting dysfunction.
Several limitations of this study should be considered. Anisotropic voxels with 4.5-mm section thickness limit spatial resolution and may lead to spuriously low FA, particularly when multiple crossing fibers are included within a single voxel.48 Nonetheless, this limitation applies equally to mTBI and control subjects, who underwent identical imaging procedures; therefore, we do not expect a systematic bias to result. Despite the standardization of imaging, this limitation may partially account for the absence of significant associations between the presence of low FA and outcomes on the regional level. Moreover, the nature of the interdependence of the various DTI parameters should be noted because for instance, increased AD and RD might lead to high MD but not low FA, and an increase in AD alone would affect MD and FA. As such, primary identification of abnormality based on a summary measure such as FA may ultimately be of less value than a measure such as RD and may serve as the basis for future study. In our study, of the 39 subjects who underwent acute imaging, 26 completed PCS and HRQoL assessment (33% attrition) and 20 were available for cognitive testing (49% attrition). Attrition in mTBI cohorts is a particular challenge to research in head trauma because subjects are disinclined to follow-up either because they do not perceive themselves as injured or because of injury-related dysfunction and stress. The rate of attrition we encountered is well within the range reported in mTBI studies (35%–88%),49–51 and we did not identify demographic or injury-severity differences between those who did or did not complete follow-up. Nonetheless, the possibility of bias due to selective attrition cannot be completely excluded. PCS and HRQoL are assessed by self-report, and studies have shown that symptoms related to postconcussion syndrome are common in other disease conditions, such as chronic pain,52 and in healthy controls.53 Thus, a further potential limitation is that symptom measures were not tested in the control sample. We addressed this issue by excluding subjects with preexisting conditions that might cause symptoms, including prior head injury, and by using tools that have been extensively validated and widely applied to TBI.20,21 Ultimately, the shortcomings of subjective assessment tools underscore the importance of other, more objective indicators of impairment that might not otherwise be appreciated. This limitation is thus a major motivation for the current study. Finally, a future step might be to evaluate similar effects in a larger cohort of patients with mTBI and in a group of healthy controls.
Conclusions
In this prospective, longitudinal study of urban subjects with mTBI, we confirm that both brain-wide and regional individualized quantification of microstructural changes shortly following mTBI are associated with important outcomes at 1 year after injury. We further identify abnormally high FA and support the hypothesis that it may be a marker of compensatory neural mechanisms and harbinger of favorable outcome, which may open new avenues toward TBI treatment.
Supplementary Material
ABBREVIATIONS:
- AD
axial diffusivity
- EZ-MAP
Enhanced Z Score Microstructural Assessment of Pathology
- FA
fractional anisotropy
- HRQoL
health-related quality of life
- hFA
high fractional anisotropy
- lFA
low fractional anisotropy
- MD
mean diffusivity
- mTBI
mild traumatic brain injury
- PCS
postconcussion symptoms
- RD
radial diffusivity
- TBI
traumatic brain injury
Footnotes
Disclosures: Mimi Kim—RELATED: Grant: National Institutes of Health*; UNRELATED: Consultancy: Lupus Foundation of America; Grants/Grants Pending: National Institutes of Health.* Richard B. Lipton—RELATED: Grant: National Institutes of Health R01*; UNRELATED: Consultancy: serves as consultant and advisory board member or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Avanir Pharmaceuticals, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Colucid Pharmaceuticals, Dr Reddy's Laboratories, ElectroCore, Eli Lilly, eNeura Therapeutics, Informa, Merck & Co, Novartis, Pfizer, Teva, and Vedanta; Grants/Grants Pending: receives research support from the National Institutes of Health*: PO1AG003949 (Program Director), PO1AG027734 (Project Leader), RO1AG025119 (Investigator), RO1AG022374–06A2 (Investigator), RO1AG034119 (Investigator), RO1AG12101 (Investigator), 23NS05140901A1 (Mentor), and K23NS47256 (Mentor), and the National Headache Foundation*; Payment for Lectures (including service on Speakers Bureaus): lecture honoraria for Continuing Medical Education–accredited lectures from the American Headache Society, the American Academy of Neurology, and the Headache Cooperative of New England; Payment for Development of Educational Presentations: American Headache Society, Comments: Chronic Migraine Education Program; Stock/Stock Options: eNeura Therapeutics. Michael L. Lipton—UNRELATED: Expert Testimony: Various (on behalf of defendants and plaintiffs); Grants/Grants Pending: Resurrecting Lives Foundation,* Comments: grant for research on imaging in blast TBI; Patents (planned, pending or issued): image-processing methodology (patent pending)*; Royalties: Springer (MRI physics book); Other: the Dana Foundation,* Comments: grant for study of imaging in sports TBI. *Money paid to the institution.
This work was supported by a National Institutes of Health grant NS082432-03 (M.L.L.).
References
- 1. Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J Head Trauma Rehabil 2005;20:5–18 10.1097/00001199-200511000-00009 [DOI] [PubMed] [Google Scholar]
- 2. Bigler ED. Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc 2004;10:794–806 [DOI] [PubMed] [Google Scholar]
- 3. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013;246:35–43 10.1016/j.expneurol.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mac Donald C, Dikranian K, Song S, et al. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol 2007;205:116–31 10.1016/j.expneurol.2007.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shenton M, Hamoda H, Schneiderman J, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–92 10.1007/s11682-012-9156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bazarian JJ, Zhong J, Blyth B, et al. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 2007;24:1447–59 10.1089/neu.2007.0241 [DOI] [PubMed] [Google Scholar]
- 7. Niogi S, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol 2008;29:967–73 10.3174/ajnr.A0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 2009;252:816–24 10.1148/radiol.2523081584 [DOI] [PubMed] [Google Scholar]
- 9. Mayer A, Ling J, Mannell M, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010;74:643–50 10.1212/WNL.0b013e3181d0ccdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smits M, Houston GC, Dippel DW, et al. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 2011;53:553–63 10.1007/s00234-010-0774-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alhilali LM, Yaeger K, Collins M, et al. Detection of central white matter injury underlying vestibulopathy after mild traumatic brain injury. Radiology 2014;272:224–32 10.1148/radiol.14132670 [DOI] [PubMed] [Google Scholar]
- 12. Grossman EJ, Ge Y, Jensen JH, et al. Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 2012;29:2318–27 10.1089/neu.2011.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma 2010;27:683–94 10.1089/neu.2009.1073 [DOI] [PubMed] [Google Scholar]
- 14. Sbordone RJ. The hazards of strict reliance on neuropsychological tests. Appl Neuropsychol Adult 2014;21:98–107 10.1080/09084282.2012.762630 [DOI] [PubMed] [Google Scholar]
- 15. Wilde E, McCauley S, Hunter J, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 2008;70:948–55 10.1212/01.wnl.0000305961.68029.54 [DOI] [PubMed] [Google Scholar]
- 16. Lipton ML, Kim N, Park YK, et al. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav 2012;6:329–42 10.1007/s11682-012-9175-2 [DOI] [PubMed] [Google Scholar]
- 17. Kim N, Branch CA, Kim M, et al. Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PLoS One 2013;8:e59382 10.1371/journal.pone.0059382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams LM, Simms E, Clark CR, et al. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery:“neuromarker.” Int J Neurosci 2005;115:1605–30 10.1080/00207450590958475 [DOI] [PubMed] [Google Scholar]
- 19. Yuspeh RL, Vanderploeg RD. Spot-the-Word: a measure for estimating premorbid intellectual functioning. Arch Clin Neuropsychol 2000;15:319–26 10.1093/arclin/15.4.319 [DOI] [PubMed] [Google Scholar]
- 20. King N, Crawford S, Wenden F, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995;242:587–92 10.1007/BF00868811 [DOI] [PubMed] [Google Scholar]
- 21. Bergner M, Bobbitt RA, Pollard WE, et al. The Sickness Impact Profile: validation of a health status measure. Med Care 1976;14:57–67 [DOI] [PubMed] [Google Scholar]
- 22. Potter S, Leigh E, Wade D, et al. The Rivermead Post Concussion Symptoms Questionnaire: a confirmatory factor analysis. J Neurol 2006;253:1603–14 10.1007/s00415-006-0275-z [DOI] [PubMed] [Google Scholar]
- 23. Bergner M, Bobbitt RA, Carter WB, et al. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981;19:787–805 [DOI] [PubMed] [Google Scholar]
- 24. Smith SM, Johansen-Berg H, Jenkinson M, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2007;2:499–503 10.1038/nprot.2007.45 [DOI] [PubMed] [Google Scholar]
- 25. Holmes CJ, Hoge R, Collins L, et al. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 1998;22:324–33 10.1097/00004728-199803000-00032 [DOI] [PubMed] [Google Scholar]
- 26. Ardekani BA, Guckemus S, Bachman A, et al. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods 2005;142:67–76 10.1016/j.jneumeth.2004.07.014 [DOI] [PubMed] [Google Scholar]
- 27. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–19 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 28. Oishi K, Zilles K, Amunts K, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 2008;43:447–57 10.1016/j.neuroimage.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011;364:2091–100 10.1056/NEJMoa1008069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 1995:289–289 [Google Scholar]
- 31. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–78 10.1006/nimg.2001.1037 [DOI] [PubMed] [Google Scholar]
- 32. Sterr A, Herron KA, Hayward C, et al. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol 2006;6:7 10.1186/1471-2377-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer AR, Bedrick EJ, Ling JM, et al. Methods for identifying subject-specific abnormalities in neuroimaging data. Hum Brain Map 2014;35:5457–70 10.1002/hbm.22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouix S, Pasternak O, Rathi Y, et al. Increased gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PLoS One 2013;8:e66205 10.1371/journal.pone.0066205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonhoeffer T, Yuste R. Spine motility: phenomenology, mechanisms, and function. Neuron 2002;35:1019–27 10.1016/S0896-6273(02)00906-6 [DOI] [PubMed] [Google Scholar]
- 36. Scholz J, Klein MC, Behrens TE, et al. Training induces changes in white-matter architecture. Nat Neurosci 2009;12:1370–71 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Filley CM. White matter: beyond focal disconnection. Neurol Clin 2011;29:81–97, viii 10.1016/j.ncl.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 38. Filley CM. White matter: organization and functional relevance. Neuropsychol Rev 2010;20:158–73 10.1007/s11065-010-9127-9 [DOI] [PubMed] [Google Scholar]
- 39. Foster J, Eskes G, Stuss D. The cognitive neuropsychology of attention: a frontal lobe perspective. Cogitive Neuropsychology 1994;11:133–47 10.1080/02643299408251971 [DOI] [Google Scholar]
- 40. Olton DS, Wenk GL, Church RM, et al. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia 1988;26:307–18 10.1016/0028-3932(88)90083-8 [DOI] [PubMed] [Google Scholar]
- 41. Nagahama Y, Okada T, Katsumi Y, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 2001;11:85–92 10.1093/cercor/11.1.85 [DOI] [PubMed] [Google Scholar]
- 42. Bocquillon P, Dujardin K, Betrouni N, et al. Attention impairment in temporal lobe epilepsy: a neurophysiological approach via analysis of the P300 wave. Hum Brain Mapp 2009;30:2267–77 10.1002/hbm.20666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: a longitudinal diffusional kurtosis and perfusion imaging study. AJNR Am J Neuroradiol 2013;34:951–57, S1–3 10.3174/ajnr.A3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grossman EJ, Inglese M. The role of thalamic damage in mild traumatic brain injury. J Neurotrauma 2016;33:163–67 10.1089/neu.2015.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mac Donald CL, Dikranian K, Bayly P, et al. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci 2007;27:11869–76 10.1523/JNEUROSCI.3647-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klawiter EC, Schmidt RE, Trinkaus K, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2011;55:1454–60 10.1016/j.neuroimage.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–22 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- 48. Oouchi H, Yamada K, Sakai K, et al. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR Am J Neuroradiol 2007;28:1102–06 10.3174/ajnr.A0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paniak C, Toller-Lobe G, Reynolds S, et al. A randomized trial of two treatments for mild traumatic brain injury: 1 year follow-up. Brain Injury 2000;14:219–26 10.1080/026990500120691 [DOI] [PubMed] [Google Scholar]
- 50. Emanuelson I, Andersson Holmkvist E, Björklund R, et al. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand 2003;108:332–38 10.1034/j.1600-0404.2003.00155.x [DOI] [PubMed] [Google Scholar]
- 51. Corrigan JD, Harrison-Felix C, Bogner J, et al. Systematic bias in traumatic brain injury outcome studies because of loss to follow-up. Arch Phys Med Rehabil 2003;84:153–60 10.1053/apmr.2003.50093 [DOI] [PubMed] [Google Scholar]
- 52. Smith-Seemiller L, Fow NR, Kant R, et al. Presence of post-concussion syndrome symptoms in patients with chronic pain vs mild traumatic brain injury. Brain Inj 2003;17:199–206 10.1080/0269905021000030823 [DOI] [PubMed] [Google Scholar]
- 53. Chan RC. Base rate of post-concussion symptoms among normal people and its neuropsychological correlates. Clin Rehabil 2001;15:266–73 10.1191/026921501675253420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.