Abstract
Objective:
The purpose of the current study was to evaluate parent–offspring resemblance for alcohol consumption and dependence symptoms, including sex-specific effects, and how these patterns change across adolescence and early adulthood.
Method:
Three cohorts of twins were assessed longitudinally at five time points between ages 14 and 29 years, with parents directly assessed at intake, using structured interviews. Twin offspring and parents from the population-based Minnesota Twin Family Study were included for a total sample size of 3,762 offspring (52% female) and their parents. Alcohol use was measured using an index based on drinking quantity, frequency, maximum drinks, and number of intoxications. Alcohol dependence symptom counts were also used.
Results:
Parent–offspring correlations for alcohol consumption increased from age 14 (r = .12) to age 17 (r = .25), remained stable from ages 17 through 24, and then decreased slightly by age 29 (r = .19). Familial resemblance for symptoms of alcohol dependence peaked at age 17 (r = .18) then decreased through age 29 (r = .11). Parent–offspring correlations of both measures did not vary significantly by sex of offspring or sex of parent.
Conclusions:
Overall, parent–offspring resemblance for alcohol use and problems is relatively stable after early adulthood, with resemblance for alcohol use at higher magnitudes across offspring development. Evidence for differential resemblance based on sex of offspring or parents was lacking.
There is a strong progression of alcohol use and problems over adolescence and early adulthood. In 2013, approximately 35% of 15-year-olds in the United States reported drinking in the past month, increasing to 51% of young adults (Substance Abuse and Mental Health Services Administration [SAMHSA], 2014). Prevalence of heavy episodic drinking follows a similar increase from 14% of 12- to 20-year-olds to 33% of young adults. Furthermore, approximately 7% of all adults (16.6 million) and 3% of adolescents (697,000) meet criteria for an alcohol use disorder (SAMHSA, 2014). In addition to its high prevalence, adolescent alcohol use is associated with future wide-ranging consequences including adverse neurological and health-related outcomes, as well as social and family-related consequences (Crowe et al., 2011). There is ample evidence that targeting risk factors for excessive alcohol use and problems can reduce these negative outcomes (O’Connell et al., 2009). Thus, understanding which adolescents are at risk for heavy alcohol use and problems is important in improving long-term prospects across multiple domains of functioning.
Previous research has shown that levels of alcohol use in adolescence are significantly associated with future use patterns (Brook et al., 2010; Irons et al., 2015; Shortt et al., 2007). Of additional importance is whether parental alcohol use and problems are related to offspring alcohol use. Parental alcohol use has been shown to predict the onset of and overall offspring alcohol use (Alati et al., 2014; Armstrong et al., 2013; Brook et al., 2010; Kelly et al., 2011; Latendresse et al., 2008; Shortt et al., 2007), whereas parental alcohol problems have been shown to predict both alcohol use and problems in offspring (Kendler et al., 2013; Latendresse et al., 2008). There is some inconsistency in these findings, however, depending on offspring age, suggesting that the association of offspring alcohol use and problems with respective parental measures may increase across adolescent development (Kendler et al., 2013; Latendresse et al., 2008). Koning et al. (2010) found that parental alcohol use was not associated with offspring alcohol use in early adolescence (mean age of 12.6 years), whereas studies finding a significant association include an offspring sample in middle to late adolescence (Armstrong et al., 2013; Latendresse et al., 2008). There is, however, a dearth of research evaluating how this pattern changes from adolescence to adulthood. The few studies that use longitudinal data include only two time points in adolescence, so it is unclear whether this pattern would hold across broader age ranges, encompassing early adolescence through young adulthood.
An additional question of interest is whether there are differential effects based on the sex of the parent or the sex of the offspring. Although there are many studies investigating the relationship between parental diagnosis of alcoholism and adolescent offspring drinking (Chassin et al., 1993; Lieb et al., 2002; Poelen et al., 2007), the few studies that have evaluated sex-specific resemblance in parent–offspring drinking patterns have yielded inconsistent results. Kelly et al. (2011) assessed adolescent drinking in 6th-grade and 8th-grade offspring, as well as in their parents, and found a complex pattern of differential effects based on age and sex. In contrast to social learning theory (Bandura, 1977), which might expect greater like-sex similarity, younger males’ alcohol use was significantly associated with mother’s drinking, whereas older males’ alcohol use was significantly associated with both parents’ alcohol use. For females, both mother’s and father’s drinking was significantly related to offspring drinking at both ages. In contrast, several studies have reported no significant sex-of-offspring effect (Alati et al., 2014; Shortt et al., 2007; Van Zundert et al., 2006).
A final question is whether levels of parental alcohol use, versus parental alcohol problems, are more strongly associated with offspring alcohol use. Very few studies have assessed whether normative or problematic parental alcohol use is more predictive of offspring adolescent use. Kendler et al. (2013) evaluated both alcohol consumption and problems in offspring at ages 15 and 18, and their parents. Alcohol consumption and problems in offspring were significantly related to both maternal and paternal alcohol problems, but parental alcohol consumption did not independently relate to offspring use.
Although there are previous studies comparing parent and offspring drinking behaviors, they have some notable limitations. Nearly all have used a cross-sectional design, making it difficult to obtain an accurate picture of whether the relationship of parent to offspring drinking changes over time. Moreover, most studies have been confined to samples of offspring in mid-adolescence; we know very little about whether the parent–offspring drinking resemblance that exists in adolescence endures into adulthood. Another major limitation of existing research is that assessment of parental drinking is most often based on offspring reports. Although this is convenient, it introduces a potential for systematic error in the measurement of parental drinking behaviors. In the current study, we combined several large community- based samples, assessed alcohol phenotypes multiple times between ages 14 and 29, and incorporated direct interviews of both parents and offspring to address these limitations and extend the scope of research on parent–offspring resemblance for drinking. Specifically, we investigated the following: (a) does the degree of parent–offspring resemblance for drinking change from early adolescence through early adulthood, (b) does parent–offspring resemblance for drinking vary by parent and offspring sex, and (c) are parental drinking problems more strongly associated with offspring drinking than patterns of parental alcohol use?
Method
Participants
The combined sample consisted of 3,762 offspring (52% female) and their parents (N = 3,508; 53% female) from three cohorts of twins involved in a longitudinal study of risk for substance misuse at the Minnesota Center for Twin and Family Research (MCTFR): two younger cohorts (first assessed at a target age of 11) and an older cohort (first assessed at a target age of 17). All cohorts consisted of both male and female like-sex twin pairs, with a total of 1,205 monozygotic and 676 dizygotic pairs. Twin offspring were ascertained through Minnesota state birth records and were excluded if they were adopted, did not live within driving distance of Minneapolis, or had a physical or mental disability that prevented completion of the assessment. A family design was used, with in-person assessments of both mothers and fathers at the initial offspring assessment. All adult participants gave informed consent, and minor participants gave assent with their parents providing consent. Participants received an honorarium for participating. A complete description of the three cohorts can be found in Iacono et al. (1999) and Keyes et al. (2009). Consistent with the demographics of the state of Minnesota at the time of ascertainment of each cohort, approximately 98% of participants were White.
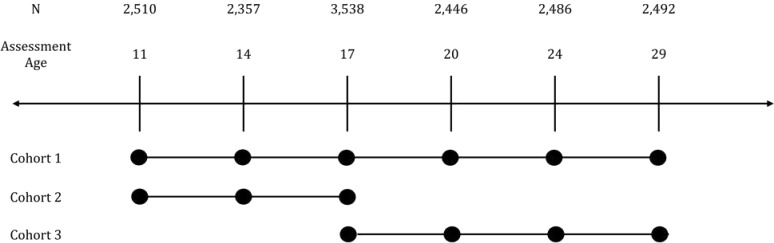
The original age-11 cohort consisted of offspring born between 1977 and 1984 (n = 1,512; age at first assessment, M [*SD] = 11.7 [0.4]). More recently, an additional cohort of age 11 offspring, born between 1988 and 1994, were assessed (n = 998; age at first assessment, M [SD] = 11.9 [0.4]), a subset of whom were selected because they had high levels of behavioral disinhibition, a risk factor for early adolescent substance use. Finally, the age-17 cohort consisted of offspring born between 1972 and 1979 (n = 1,252; age at first assessment, M [SD] = 17.5 [0.5]). Through an overlapping cohort design (Figure 1), each cohort has been assessed approximately every 3 years from their first assessment, with up to five follow-up assessments at target ages of 11, 14, 17, 20, 24, and 29. Offspring data from the age-11 assessment were not used in the analysis, as there was virtually no alcohol use at this age. Follow-up participation rates of the combined cohorts ranged from 88.5% to 94.0% across assessments. Within the overall sample, 86.6% of offspring had complete data available for both mothers and fathers on all measures.
Figure 1.
Participants of the different cohorts were eligible for different assessments, so the total sample size changes across the target assessment ages. The largest sample size occurs at the target age-17 assessment, where all three cohorts participated.
Measures
Measures of alcohol use and problems were used for both parents and offspring. The measure of alcohol use was a composite index consisting of four self-report alcohol use items: frequency of alcohol use (scored from 0 = never to 5 = at least once per day), average number of drinks per drinking event (scored from 0 = never drank to 6 = 30 or more), maximum number of drinks in a 24-hour period (scored from 0 = never drank to 6 = 30 or more), and number of times intoxicated (scored from 0 = never to 6 = 50 or more). The alcohol index questions were asked in two formats. At the target age 14, 17, and 20 assessments, these questions were computer administered and covered the last 12 months. At the target age 17, 20, 24, and 29 assessments (and for parents at intake), they were administered by trained interviewers, covering the time since the last assessment, with an expanded version of the Substance Abuse Module (Robins et al., 1987), an interview supplement to the Composite International Diagnostic Interview (Robins et al., 1988) to which these four items had been added. The items were summed for both the computerized and interviewer-administered formats to form an initial index. The overlapping assessments at ages 17 and 20 allowed us to examine the consistency across the assessment types. The correlation between the two types of assessments was .88 at age 17 and .81 at age 20. To make the indices comparable, they were harmonized using moderated nonlinear factor analysis. The index was then linearly transformed such that nondrinkers had an index score of zero, and there was a standard deviation of 1.0 at the target age 17 assessment. This results in a range of alcohol index scores from 0 to approximately 4.5.
Because the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-IIIR; American Psychiatric Association, 1987), was the diagnostic system in place when most of the parents were assessed, alcohol problems were measured as a symptom count of DSM-III-R alcohol dependence criteria, assessed at each wave through a structured clinical interview. Following each interview, DSM symptoms since the previous assessment (or lifetime symptoms for parents and older cohort males at age 20 only) were determined by consensus of two individuals with advanced clinical training, supervised by a Ph.D. clinical psychologist. The reliability of the consensus diagnoses (kappa) exceeded .92 for all substance disorders. Including offspring who participated at intake but did not participate in all subsequent assessment waves, the drinking index was available for 87.7%–90.3% of the original offspring sample at each assessment between ages 17 and 29, and dependence symptoms were available at each assessment for 88.4%–94.0% of the sample. At age 14, roughly 15% of participants completed assessments over the phone and thus did not complete the computerized alcohol measure, resulting in fewer offspring (81.6%) with available drinking index scores at age 14.
The effects of sample attrition were evaluated by comparing participants and nonparticipants at a given assessment with their responses at the previous assessment. There were no significant differences in either the drinking index score or number of alcohol dependence symptoms between offspring who participated and those who did not at any assessment between ages 14 and 29 (standardized mean differences from 0.06 to 0.20, all p > .05), with two exceptions. Age 20 participants had significantly fewer dependence symptoms than nonparticipants at their prior age 17 assessment (standardized mean difference of 0.25, p = .02), and age 24 participants had lower drinking index scores at their prior age 20 assessment than did nonparticipants (standardized mean difference of 0.25, p = .01). Overall, all standardized mean differences were less than or equal to 0.25, indicating minimal bias in the sample, with a slight overrepresentation of those with fewer alcohol dependence symptoms and lower alcohol consumption between ages 20 and 24.
Analyses
Parent–offspring correlations were estimated using maximum likelihood methods in OpenMx (Boker et al., 2016; Neale et al., 2016; Pritikin et al., 2015). Various models were fit to test whether the correlations varied as a function of sex of offspring, sex of parent, or by time. Models were compared using the chi-square likelihood ratio test and Akaike Information Criterion. To account for the correlated nature of twins, intraclass covariance structures were used (Carey, 2005). This incorporates both individuals in a twin pair using the within-pair and between-pair covariances. Twins of different zygosity were allowed to have different within-pair covariance structures.
Results
Descriptive results
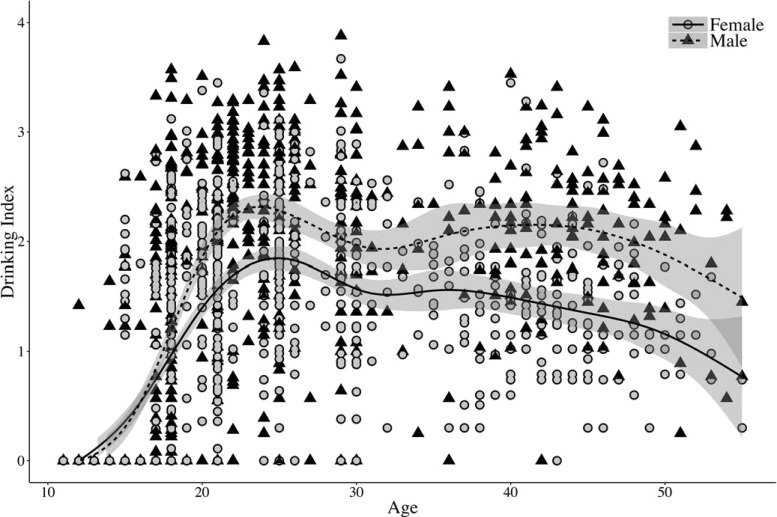
Table 1 provides descriptive statistics of the drinking index and alcohol dependence symptoms for the offspring across all assessments used for analyses (ages 14-29), as well as for the parents at intake. The mean (SD) age of the parents at the time of the intake assessment was 43.98 (5.7) years for fathers and 41.55 (5.2) years for mothers. There were significant sex differences on both measures, with males consistently having higher mean drinking index scores and a greater number of dependence symptoms, beginning at age 17. A plot of the drinking index across all ages for offspring and parents, split by sex, is shown in Figure 2. For clarity, the plot shows only a random 10% of the data at each age. Consistent with previous research, this figure illustrates the sex difference in drinking behaviors (Khan et al., 2013), as well as the peak in drinking during the early 20s (Schulenberg et al., 1996). A visual inspection of the scatter plot of parental drinking index scores versus offspring scores indicates a linear relationship.
Table 1.
Descriptive information on age, drinking index,a and alcohol dependence symptomsb in offspring at all assessments and parents at twins’ first assessment
| Target assessment age | Males | Females |
| Age 14 | ||
| n | 1,154 | 1,203 |
| Age, in years, M (SD) | 14.86 (0.5) | 14.82 (0.6) |
| Index, M (SD) | 0.34 (0.7) | 0.34 (0.7) |
| Symptoms | – | – |
| Age 17 | ||
| n | 1,692 | 1,846 |
| Age, in years, M (SD) | 17.79 (0.6) | 17.89 (0.7) |
| Index, M (SD) | 1.17 (1.1) | 1.0*** (0.9) |
| Symptoms, M (SD) | 0.67 (1.4) | 0.34*** (1.1) |
| Age 20 | ||
| n | 1,111 | 1,335 |
| Age, in years, M (SD) | 21.42 (0.9) | 20.84 (0.6) |
| Index, M (SD) | 2.12 (0.9) | 1.59*** (0.8) |
| Symptoms, M (SD) | 1.49 (1.9) | 0.44*** (1.1) |
| Age 24 | ||
| n | 1,170 | 1,316 |
| Age, in years, M (SD) | 24.88 (1.0) | 25.13 (0.8) |
| Index, M (SD) | 2.25 (0.8) | 1.80*** (0.7) |
| Symptoms, M (SD) | 1.37 (1.7) | 0.54*** (1.2) |
| Age 29 | ||
| n | 1,179 | 1,313 |
| Age, in years, M (SD) | 29.53 (0.7) | 29.34 (0.7) |
| Index, M (SD) | 1.89 (0.8) | 1.55*** (0.7) |
| Symptoms, M (SD) | 0.92 (1.6) | 0.30*** (1.0) |
| Parental intake | ||
| n | 1,641 | 1,867 |
| Age, in years, M (SD) | 43.98 (5.7) | 41.55 (5.2) |
| Index, M (SD) | 2.05 (0.7) | 1.52*** (0.6) |
| Symptoms, M (SD) | 1.97 (2.3) | 0.63*** (1.4) |
Notes: Sample size and mean age at each assessment incorporate participants with either the drinking index or dependence symptoms available at that assessment. Age 14 dependence symptoms are not included, as they were not used in the analysis because of low variance.
The drinking index has a range of 0 to approximately 4.5, with 0 indicating no alcohol use and larger values indicating heavier alcohol consumption;
dependence symptoms range from 0 to 9, with 0 indicating no dependence symptoms and increasing values indicating a greater number of symptoms.
Significant sex differences within assessment age (p < .0001).
Figure 2.
Plot of drinking index by sex across all offspring and parent assessments. The plot shows only a random 10% of the full sample for clarity. Loess curves were added to graphically display the relationship between the drinking index and age by gender. Age 11 drinking index data were included for completeness but were not used in the analysis.
Parent–offspring correlations
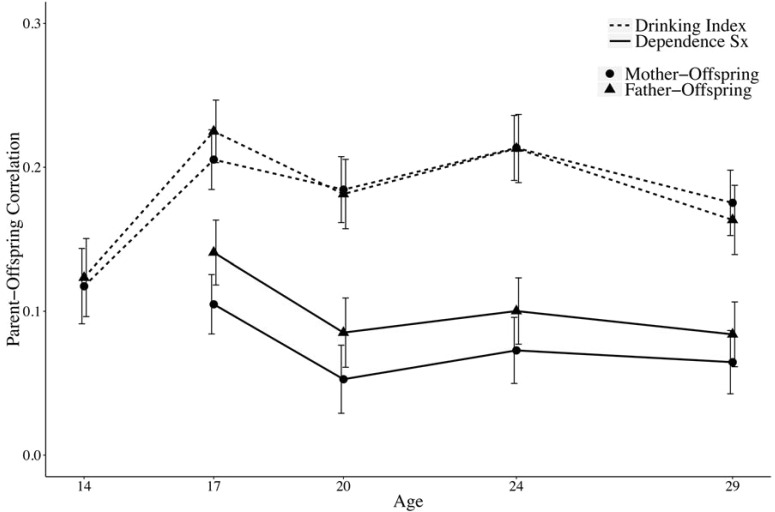
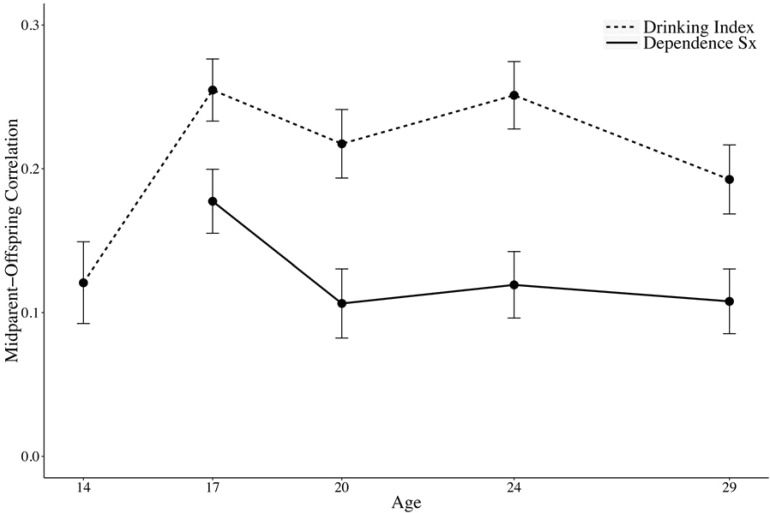
The parent–offspring and midparent–offspring correlations for the drinking index and alcohol dependence symptoms are shown in Figures 3a and 3b, respectively. The midparent values are simply the means between mother and fathers. The correlations between the drinking index and alcohol dependence symptoms generally follow the same pattern, although the drinking index correlations are consistently higher than for the dependence symptoms. The midparent–offspring correlations for the drinking index ranged from .12 at age 14 to .25 at ages 17 and 24; for alcohol dependence symptoms, this range was from .11 at ages 20 and 29 to .18 at age 17. The sharpest change in the drinking index correlations occurs from age 14 to age 17. The magnitude of the correlations is generally highest at the age 17 assessment for both the drinking index and dependence symptoms. There is stability or decreasing correlations after age 17, through age 29.
Figure 3a.
Estimated parent–offspring correlations for both the drinking index and alcohol dependence symptoms as a function of age. The error bars indicate 1 SE.
Figure 3b.
Estimated midparent–offspring correlations for both the drinking index and alcohol dependence symptoms as a function of age. The midparent correlations are based on the simple means between mother and fathers. The error bars indicate 1 SE.
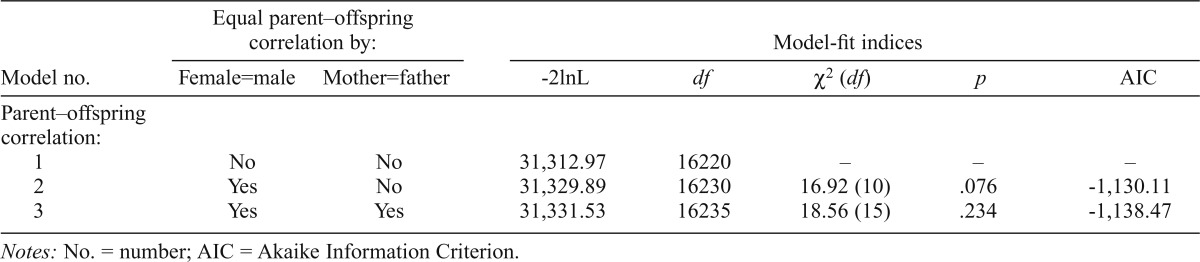
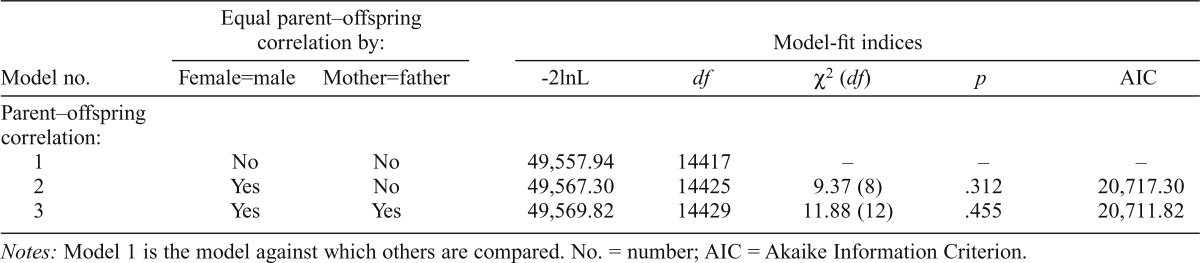
All parent–offspring and midparent–offspring correlations were estimated in OpenMx. First, we tested for sex-of-offspring and sex-of-parent effects. Table 2a shows the parent–offspring correlation model fit indices for the drinking index. Model 1 allows the correlation estimates to vary freely across male and female offspring with each parent and is the base model against which others are compared. Model 2 constrains the correlation estimates to be equal across male and female offspring. If this model fits significantly worse than the base model, indicated by a p value less than .05, this would suggest that the parent–offspring correlations are significantly different between males and females. Female offspring showed consistently higher correlations with both parents on the drinking index than males, although this did not achieve statistical significance (p = .08). Model 3 constrained both male and female offspring estimates to be equal, as well as mother–offspring and father–offspring estimates. This did not result in significantly reduced model fit, indicating no evidence for a sex-of-parent effect (p = .23). The model fit indices for alcohol dependence symptoms are shown in Table 2b. Models 1 and 2 were constrained in the same way as described above. Again, there was no evidence for a sex-of-offspring effect (Model 2, p = .31) or sex-of- parent effect (Model 3, p = .46).
Table 2a.
Model fit statistics for parent–offspring correlations of the drinking index
| Equal parent–offspring correlation by: |
Model-fit indices |
||||||
| Model no. | Female=male | Mother=father | -2lnL | df | X2(df) | p | AIC |
| Parent–offspring correlation: | |||||||
| 1 | No | No | 31,312.97 | 16220 | – | – | – |
| 2 | Yes | No | 31,329.89 | 16230 | 16.92 (10) | .076 | -1,130.11 |
| 3 | Yes | Yes | 31,331.53 | 16235 | 18.56 (15) | .234 | -1,138.47 |
Notes: No. = number; AIC = Akaike Information Criterion.
Table 2b.
Model fit statistics for parent–offspring correlations of dependence symptoms
| Equal parent–offspring correlation by: |
Model-fit indices |
||||||
| Model no. | Female=male | Mother=father | -2lnL | df | X2(df) | p | AIC |
| Parent–offspring correlation: | |||||||
| 1 | No | No | 49,557.94 | 14417 | – | – | – |
| 2 | Yes | No | 49,567.30 | 14425 | 9.37 (8) | .312 | 20,717.30 |
| 3 | Yes | Yes | 49,569.82 | 14429 | 11.88(12) | .455 | 20,711.82 |
Notes: Model 1 is the model against which others are compared. No. = number; AIC = Akaike Information Criterion.
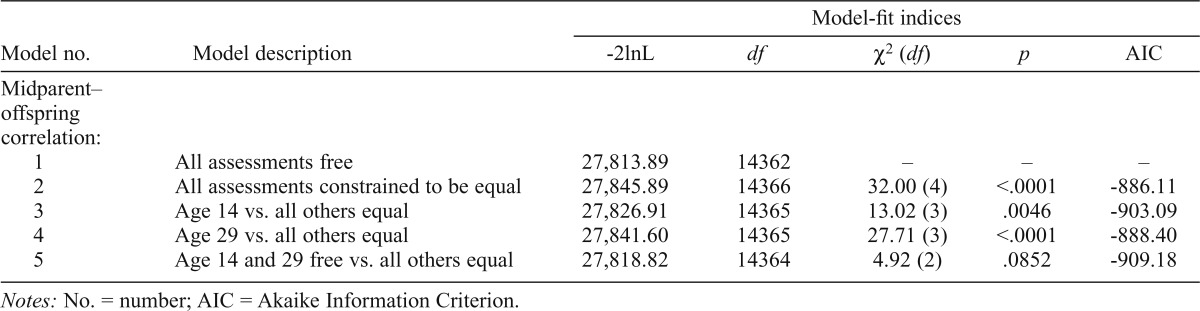
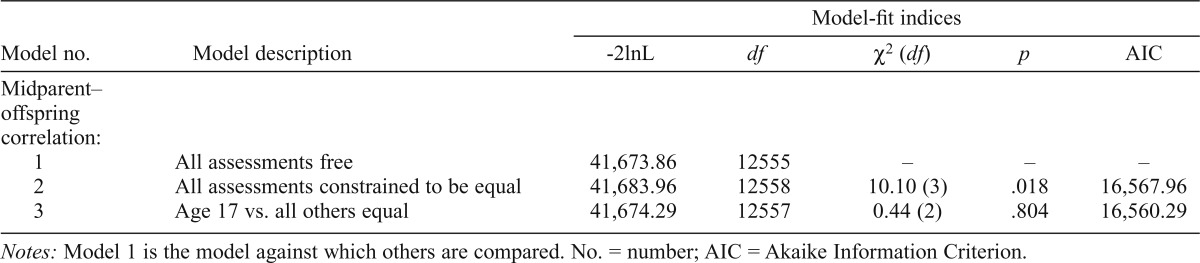
In summary, there was no significantly reduced model fit for either measure when constraining male and female offspring correlations to be equal or when adding an additional mother–offspring and father–offspring equality constraint. Because of this lack of gender effect for both measures, male and female data were combined to model midparent–offspring correlations. This simplifies the structure of the overall model to allow a clearer picture of the correlational patterns over time. We then tested whether the pattern of resemblance changed with offspring age. The midparent–offspring correlation model fit indices for the drinking index are shown in Table 3a. Model 1 allows the correlation estimates to vary freely across all offspring assessment ages and is the base model against which others are compared. Model 2 constrains the estimates to be the same across all ages and results in a significant loss of model fit (p < .01). We then started with the outermost ages, comparing age 14 to all other ages (Model 3) and age 29 to all other ages (Model 4). In Model 3, the correlation estimate at age 14 was allowed to vary, whereas the estimates at ages 17, 20, 24, and 29 were constrained to be equal. In Model 4, the correlation estimate at age 29 was allowed to vary freely and the estimates at all other assessment ages were constrained to be equal. Both of these models resulted in a significant decrease in fit. The best-fitting model allowed age 14 and age 29 correlations to vary freely while constraining age 17, 20, and 24 estimates to be equal (Model 5). The midparent–offspring correlation model fit indices for dependence symptoms (Table 3b) also varied by age. Similar to the drinking index, constraining the correlations to be equal across all ages resulted in a significant loss of model fit (Model 2). We again started with the outermost age and found the best-fitting model allowed the age 17 correlation estimates to vary freely while constraining age 20, 24, and 29 correlations to be equal (Model 3).
Table 3a.
Model fit statistics for midparent–offspring correlations of the drinking index
| Model-fit indices |
||||||
| Model no. | Model description | -2lnL | df | X2(df) | P | AIC |
| Midparent–offspring correlation: | ||||||
| 1 | All assessments free | 27,813.89 | 14362 | – | – | – |
| 2 | All assessments constrained to be equal | 27,845.89 | 14366 | 32.00 (4) | <.0001 | -886.11 |
| 3 | Age 14 vs. all others equal | 27,826.91 | 14365 | 13.02(3) | .0046 | -903.09 |
| 4 | Age 29 vs. all others equal | 27,841.60 | 14365 | 27.71 (3) | <.0001 | -888.40 |
| 5 | Age 14 and 29 free vs. all others equal | 27,818.82 | 14364 | 4.92 (2) | .0852 | -909.18 |
Notes: No. = number; AIC = Akaike Information Criterion.
Table 3b.
Model fit statistics for midparent–offspring correlations of dependence symptoms
| Model-fit indices |
||||||
| Model no. | Model description | -2lnL | df | X2(df) | P | AIC |
| Midparent–offspring correlation: | ||||||
| 1 | All assessments free | 41,673.86 | 12555 | – | – | – |
| 2 | All assessments constrained to be equal | 41,683.96 | 12558 | 10.10(3) | .018 | 16,567.96 |
| 3 | Age 17 vs. all others equal | 41,674.29 | 12557 | 0.44 (2) | .804 | 16,560.29 |
Notes: Model 1 is the model against which others are compared. No. = number; AIC = Akaike Information Criterion.
Given that multiple cohorts were used in the analysis, we also tested whether the mother–, father–, and midparent–offspring correlations varied by cohort at each age. For the drinking index, of 21 possible cohort comparisons, only 1, the midparent–offspring correlation at age 29, was significantly different (p = .03), whereas all others could be constrained to be equal across cohorts. For dependence symptoms, of 18 possible cohort comparisons, only 1, the father–offspring correlation at age 17, was significantly different (p = .03), whereas all others could be constrained to be equal across all cohorts. The failure to observe a greater-than-chance number of cohort differences, given the total number of comparisons, and the lack of consistency in the observed differences suggests that there is little evidence for differential parent–offspring correlations based on birth cohort.
Discussion
The purpose of the current study was to determine parent–offspring similarity on measures of alcohol use and alcohol dependence across a 15-year time span. The sample included 3,762 offspring (and their parents), prospectively followed from early adolescence (age 14) through young adulthood (age 29) via an overlapping cohort design, assessed on alcohol use with a composite drinking index and on alcohol dependence symptoms using DSM criteria. Overall, parent–offspring resemblance was highest for the drinking index, a measure of alcohol consumption. Similarity on both measures was significantly different than zero and varied significantly based on offspring age. There was little evidence for sex-of-parent or sex-of-offspring effects.
Overall, the patterns of parent–offspring resemblance on alcohol use and alcohol dependence symptoms were similar. However, the difference in magnitude of the correlations between the two measures was unexpected. At all assessments, the parent–offspring correlations for the drinking index were larger than for alcohol dependence symptoms. Because prior research has generally focused on how either parental alcohol use or parental alcohol problems alone relate to offspring use, how parent–offspring resemblance on these two different alcohol phenotypes might compare was unclear. Our results imply that offspring are more similar to their parents in their patterns of alcohol use than they are for symptoms of alcohol dependence.
The pattern of parent–offspring resemblance over offspring age was slightly unexpected and only partially supported our hypothesis. For the drinking index, resemblance increased from age 14 to age 17, where it stabilized. This increase in resemblance for alcohol use coincides with the age when offspring are both initiating alcohol use and living with their parents. This may be a maximal time in which they are modeling parental behaviors and would be consistent with prior research (Armstrong et al., 2013; Koning et al., 2010; Latendresse et al., 2008). There were no significant differences in the parent–offspring correlations from age 17 to age 24, followed by a significant decrease in resemblance at age 29. These ages coincide with times in which the offspring likely have moved out and are increasingly becoming independent from their parents through age 29, lessening the impact of the parental shared environment. In addition, they are undergoing developmental transitions that have been associated with subsequent increases (dissolution of romantic relationships) and decreases (having children) in drinking (Fergusson et al., 2012; Fleming et al., 2010). These more proximal, nonshared environmental factors may be exerting a stronger influence on drinking patterns at ages 24-29 than the more distal parental environment.
For alcohol dependence symptoms, parent–offspring resemblance peaked at age 17 before decreasing. The resemblance remained stable from age 20 to age 29. This is consistent with previous findings from an MCTFR study of adopted adolescents that indicated that exposure to parental alcohol misuse represents an environmental risk factor for alcohol use, at least during adolescence (King et al., 2009). However, we have also found that parent–child resemblance for substance use disorders appears primarily due to genetic transmission (Hicks et al., 2013), and the importance of genetic factors on many behavioral phenotypes, including alcohol, increases from early to late adolescence (Bergen et al., 2007; McGue et al., 2014). Thus, both genetic and environmental contributors to alcohol dependence that are shared between parents and offspring may be particularly salient during late adolescence.
It is important to note some limitations of the current study. First, the sample is overwhelmingly White. The results may not generalize to other racial or ethnic groups. Second, complete data on parental alcohol use and symptoms for all three cohorts were only available at intake. Lack of repeated measures of parental drinking behaviors preclude us from evaluating the effect of change in parental alcohol use and symptoms on adolescent drinking patterns. In addition, for dependence symptoms, males at age 20 reported lifetime symptoms, whereas females and males at all other assessments reported symptoms since the last assessment. Although we do not believe this discrepancy greatly affects the results, it is important to consider. Last, we are unable to draw conclusions of the causal relationship between parental and offspring alcohol use and symptoms from this analysis. Nonetheless, because of the longitudinal design coupled with broad offspring ages, the results indicate that parental drinking behaviors, whether through genetic or environmental effects, have an important role in offspring alcohol use and problems.
Parent–offspring resemblance for dependence symptoms should be interpreted with a note of caution. Both the drinking index and alcohol dependence symptoms were treated as continuous variables in this analysis. Although an ordinal model may be more appropriate for alcohol dependence symptoms, we were unable to estimate parameters in this way because of convergence problems Although assuming a normal distribution of alcohol dependence symptoms may not be ideal, prior simulation studies suggest that doing so results in relatively little bias of the parameter point estimates (Kirkpatrick & Neale, 2016). It can, however, inflate likelihood ratio type I error rates.
In conclusion, parent–offspring similarity for measures of alcohol use and symptoms were significant and showed generally similar patterns across adolescence and early adulthood. The resemblance for symptoms of alcohol dependence was of a lower magnitude than for the measure of alcohol use. Offspring resemble their parents in alcohol use and symptoms most in late adolescence and early adulthood. Our results are consistent with previous research showing that the prediction of offspring alcohol use and symptoms from the respective parental measures increases across adolescent development (Kendler et al., 2013; Latendresse et al., 2008). These results extend the prior literature, however, by following offspring further into adulthood. We found that although parent–offspring resemblance does increase over early to mid-adolescence, this similarity stabilizes or decreases thereafter. Finally, the results did not support a significant sex-of-parent or sex-of-offspring effect in terms of resemblance for alcohol use or symptoms. The lack of sex-specific transmission we observed is consistent with several previous studies (Alati et al., 2014; Shortt et al., 2007; Van Zundert et al., 2006), as well as findings from earlier analyses of a subset of the samples used here, which found that paternal and maternal maximum drinks consumed in a 24-hour period predicted offspring maximum consumption similarly for males and females (Malone et al., 2002, 2010).
Footnotes
This research was supported by the National Institute on Alcohol Abuse and Alcoholism Grant AA009367 (to Matthew McGue), National Institute on Drug Abuse (NIDA) Grants DA005147 and DA013240 (to William G. Iacono), and NIDA Grant DA038065 (to Irene Elkins).
References
- Alati R., Baker P., Betts K. S., Connor J. P., Little K., Sanson A., Olsson C. A. The role of parental alcohol use, parental discipline and antisocial behaviour on adolescent drinking trajectories. Drug and Alcohol Dependence. 2014;134:178–184. doi: 10.1016/j.drugalcdep.2013.09.030. doi:10.1016/j.drugalcdep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., rev. Washington, DC: Author; 1987. [Google Scholar]
- Armstrong J. M., Ruttle P. L., Burk L. R., Costanzo P. R., Strauman T. J., Essex M. J. Early risk factors for alcohol use across high school and its covariation with deviant friends. Journal of Studies on Alcohol and Drugs. 2013;74:746–756. doi: 10.15288/jsad.2013.74.746. doi:10.15288/jsad.2013.74.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- Bergen S. E., Gardner C. O., Kendler K. S. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. doi:10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Boker S. M., Neale M. C., Maes H. H., Wilde M. J., Spiegel M., Brick T. R., Kirkpatrick R. M. OpenMx User Guide, Release 2.6.7. 2016. Retrieved from http://openmx.psyc.virginia.edu/documentation. [Google Scholar]
- Brook J. S., Balka E. B., Crossman A. M., Dermatis H., Galanter M., Brook D. W. The relationship between parental alcohol use, early and late adolescent alcohol use, and young adult psychological symptoms: A longitudinal study. American Journal on Addictions. 2010;19:534–542. doi: 10.1111/j.1521-0391.2010.00083.x. doi:10.1111/j.1521-0391.2010.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey G. The intraclass covariance matrix. Behavior Genetics. 2005;35:667–670. doi: 10.1007/s10519-005-5877-1. doi:10.1007/s10519-005-5877-1. [DOI] [PubMed] [Google Scholar]
- Chassin L., Pillow D. R., Curran P. J., Molina B. S. G., Barrera M., Jr Relation of parental alcoholism to early adolescent substance use: A test of three mediating mechanisms. Journal of Abnormal Psychology. 1993;102:3–19. doi: 10.1037//0021-843x.102.1.3. doi:10.1037/0021-843X.102.1.3. [DOI] [PubMed] [Google Scholar]
- Crowe A. H., Mullins T. G., Cobb K. A., Lowe N. C. Underage drinking: Intervention principles and practice guidelines for community corrections. 2011. Report prepared by the Council of State Governments and American Probation and Parole Association. Retrieved from https://www.appa-net.org/eweb/docs/appa/pubs/UDIPPGCC.pdf. [Google Scholar]
- Fergusson D. M., Boden J. M., John Horwood L. Transition to parenthood and substance use disorders: Findings from a 30-year longitudinal study. Drug and Alcohol Dependence. 2012;125:295–300. doi: 10.1016/j.drugalcdep.2012.03.003. doi:10.1016/j.drugalcdep.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Fleming C. B., White H. R., Catalano R. F. Romantic relationships and substance use in early adulthood: An examination of the influences of relationship type, partner substance use, and relationship quality. Journal of Health and Social Behavior. 2010;51:153–167. doi: 10.1177/0022146510368930. doi:10.1177/0022146510368930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks B. M., Foster K. T., Iacono W. G., McGue M. Genetic and environmental influences on the familial transmission of externalizing disorders in adoptive and twin offspring. JAMA Psychiatry. 2013;70:1076–1083. doi: 10.1001/jamapsychiatry.2013.258. doi:10.1001/jamapsychiatry.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono W. G., Carlson S. R., Taylor J., Elkins I. J., McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. doi:10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- Irons D. E., Iacono W. G., McGue M. Tests of the effects of adolescent early alcohol exposures on adult outcomes. Addiction. 2015;110:269–278. doi: 10.1111/add.12747. doi:10.1111/add.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. B., Toumbourou J. W., O’Flaherty M., Patton G. C., Homel R., Connor J. P., Williams J. Family relationship quality and early alcohol use: Evidence for gender-specific risk processes. Journal of Studies on Alcohol and Drugs. 2011;72:399–407. doi: 10.15288/jsad.2011.72.399. doi:10.15288/jsad.2011.72.399. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Gardner C. O., Edwards A., Hickman M., Heron J., Macleod J., Dick D. M. Dimensions of parental alcohol use/problems and offspring temperament, externalizing behaviors, and alcohol use/problems. Alcoholism: Clinical and Experimental Research. 2013;37:2118–2127. doi: 10.1111/acer.12196. doi:10.1111/acer.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes M. A., Malone S. M., Elkins I. J., Legrand L. N., McGue M., Iacono W. G. The enrichment study of the Minnesota Twin Family Study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Research and Human Genetics. 2009;12:489–501. doi: 10.1375/twin.12.5.489. doi:10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Okuda M., Hasin D. S., Secades-Villa R., Keyes K., Lin K.-H., Blanco C. Gender differences in lifetime alcohol dependence: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcoholism: Clinical and Experimental Research. 2013;37:1696–1705. doi: 10.1111/acer.12158. doi:10.1111/acer.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Keyes M., Malone S. M., Elkins I., Legrand L. N., Iacono W. G., McGue M. Parental alcohol dependence and the transmission of adolescent behavioral disinhibition: A study of adoptive and non-adoptive families. Addiction. 2009;104:578–586. doi: 10.1111/j.1360-0443.2008.02469.x. doi:10.1111/j.1360-0443.2008.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick R. M., Neale M. C. Applying multivariate discrete distributions to genetically informative count data. Behavior Genetics. 2016;46:252–268. doi: 10.1007/s10519-015-9757-z. doi:10.1007/s10519-015-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning I. M., Engels R. C. M. E., Verdurmen J. E. E., Vollebergh W. A. M. Alcohol-specific socialization practices and alcohol use in Dutch early adolescents. Journal of Adolescence. 2010;33:93–100. doi: 10.1016/j.adolescence.2009.05.003. doi:10.1016/j.adolescence.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Latendresse S. J., Rose R. J., Viken R. J., Pulkkinen L., Kaprio J., Dick D. M. Parenting mechanisms in links between parents’ and adolescents’ alcohol use behaviors. Alcoholism: Clinical and Experimental Research. 2008;32:322–330. doi: 10.1111/j.1530-0277.2007.00583.x. doi:10.1111/j.1530-0277.2007.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb R., Merikangas K. R., Hofler M., Pfister H., Isensee B., Wittchen H.-U. Parental alcohol use disorders and alcohol use and disorders in offspring: A community study. Psychological Medicine. 2002;32:63–78. doi: 10.1017/s0033291701004883. doi:10.1017/S0033291701004883. [DOI] [PubMed] [Google Scholar]
- Malone S. M., Iacono W. G., McGue M. Drinks of the father: Father’s maximum number of drinks consumed predicts externalizing disorders, substance use, and substance use disorders in preadolescent and adolescent offspring. Alcoholism: Clinical and Experimental Research. 2002;26:1823–1832. doi: 10.1097/01.ALC.0000042222.59908.F9. doi:10.1111/j.1530-0277.2002.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Malone S. M., McGue M., Iacono W. G. Mothers’ maximum drinks ever consumed in 24 hours predicts mental health problems in adolescent offspring. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2010;51:1067–1075. doi: 10.1111/j.1469-7610.2010.02219.x. doi:10.1111/j.1469-7610.2010.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M., Malone S., Keyes M., Iacono W. G. Parent–offspring similarity for drinking: A longitudinal adoption study. Behavior Genetics. 2014;44:620–628. doi: 10.1007/s10519-014-9672-8. doi:10.1007/s10519-014-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. C., Hunter M. D., Pritikin J. N., Zahery M., Brick T. R., Kirkpatrick R. M., Boker S. M. OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika. 2016;81:535–549. doi: 10.1007/s11336-014-9435-8. doi: 10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell M. E., Boat T., Warner K. E. Preventing mental, emotional, and behavioral disorders among young people: Progress and possibilities. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Poelen E. A. P., Scholte R. H. J., Willemsen G., Boomsma D. I., Engels R. C. M. E. Drinking by parents, siblings, and friends as predictors of regular alcohol use in adolescents and young adults: A longitudinal twin-family study. Alcohol and Alcoholism. 2007;42:362–369. doi: 10.1093/alcalc/agm042. doi:10.1093/alcalc/agm042. [DOI] [PubMed] [Google Scholar]
- Pritikin J. N., Hunter M. D., Boker S. Modular open-source software for item factor analysis. Educational and Psychological Measurement. 2015;75:458–474. doi: 10.1177/0013164414554615. doi:10.1177/0013164414554615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L. N., Babor T. F., Cottier L. B. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis, MO: Washington University; 1987. [Google Scholar]
- Robins L. N., Wing J., Wittchen H. U., Helzer J. E., Babor T. F., Burke J., Towle L. H. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. doi:10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Schulenberg J., O’Malley P. M., Bachman J. G., Wadsworth K. N., Johnston L. D. Getting drunk and growing up: Trajectories of frequent binge drinking during the transition to young adulthood. Journal of Studies on Alcohol. 1996;57:289–304. doi: 10.15288/jsa.1996.57.289. doi:10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- Shortt A. L., Hutchinson D. M., Chapman R., Toumbourou J. W. Family, school, peer and individual influences on early adolescent alcohol use: First-year impact of the Resilient Families programme. Drug and Alcohol Review. 2007;26:625–634. doi: 10.1080/09595230701613817. doi:10.1080/09595230701613817. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings (HHS Publication No. (SMA) 14–4863) Rockville, MD: Author; 2014. [Google Scholar]
- Van Zundert R. M. P., Van DerVorst H., Vermulst A. A., Engels R. C. M. E. Pathways to alcohol use among Dutch students in regular education and education for adolescents with behavioral problems: The role of parental alcohol use, general parenting practices, and alcohol- specific parenting practices. Journal of Family Psychology. 2006;20:456–467. doi: 10.1037/0893-3200.20.3.456. doi:10.1037/0893-3200.20.3.456. [DOI] [PubMed] [Google Scholar]