Abstract
BAG-1 (Bcl-2–associated athanogene) is a multifaceted protein implicated in the modulation of a large variety of cellular processes. Elucidating the molecular mechanisms that underlie the cellular functions of BAG-1 becomes an increasingly important task, particularly in light of the growing evidence connecting aberrant BAG-1 expression to certain human cancers. A common element of the remarkable functional diversity of BAG-1 appears to be the interaction with molecular chaperones of the Hsp70 family. In fact, BAG-1 functions as a nucleotide exchange factor of mammalian cytosolic Hsc70, thereby triggering substrate unloading from the chaperone. In addition, recent findings reveal an association of BAG-1 with the proteasome, which suggests a role in coordinating chaperone and degradation pathways.
INTRODUCTION
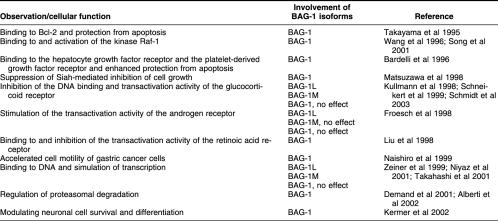
The BAG-1 protein (Bcl-2–associated athanogene) was initially identified as a binding partner of the anti–cell death protein Bcl-2 and was shown to be involved in the regulation of apoptosis (Takayama et al 1995). However, subsequent studies revealed a plethora of additional partner proteins of BAG-1, and a wide variety of cellular functions were assigned to BAG-1, ranging from transciptional regulation to the control of cell migration (reviewed in Takayama and Reed 2001; Doong et al 2002) (Table 1). The question arises whether there is a common molecular mechanism that underlies this multifunctionality of BAG-1. It is probably still too early to answer this question conclusively. Nonetheless, it is now widely accepted that BAG-1 is intimately involved in the regulation of Hsp70 chaperone proteins in the eukaryotic cytosol and nucleus. Here, we will describe the chaperone-regulating activity of BAG-1 and will discuss the diverse cellular functions ascribed to BAG-1 with regard to the cochaperone's ability to modulate Hsp70-mediated protein folding and degradation pathways.
Table 1.
Cellular functions of BAG-1 (Bcl-2–associated athanogene) isoforms
BAG-1—A NOVEL TYPE OF HSP70 NUCLEOTIDE EXCHANGE FACTOR
For a long time the prokaryotic Hsp70 reaction cycle served as a paradigm to explain the chaperone function of Hsp70 family members. The dynamic interaction of the bacterial Hsp70 homolog DnaK with polypeptide substrates depends on the concerted action of the cochaperones DnaJ and GrpE. DnaJ stimulates adenosine triphosphate (ATP) hydrolysis by DnaK to promote conversion of the chaperone to the high-affinity state for substrate binding, whereas GrpE triggers adenosine 5′ diphosphate (ADP) release and ATP rebinding, which result in substrate unloading from DnaK (Bukau and Horwich 1998). Whereas DnaJ-like proteins were also found early in the eukaryotic cytosol, cochaperones that were structurally related to GrpE remained elusive in this compartment. In fact, with the advent of genome-sequencing projects it became clear that homologs of the GrpE protein are absent from the eukaroytic cytosol. Nucleotide exchange is apparently not the rate-limiting step in the reaction cycle of eukaryotic Hsp70s but rather ATP hydrolysis itself (Höhfeld et al 1995; Minami et al 1996). Stimulation of ATP hydrolysis by DnaJ family members, therefore, seems to be sufficient to achieve a physiological relevant cycling of Hsp70s in the eukaryotic cytosol.
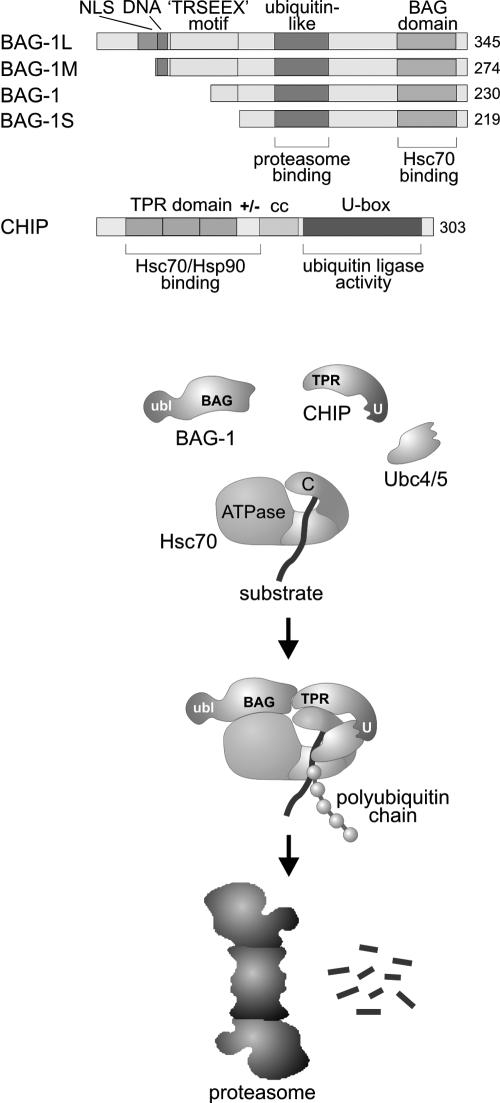
Although a general requirement for a nucleotide exchange factor can be excluded, it is still possible that such a factor (or factors) may accelerate substrate unloading from eukaryotic Hsp70 on certain chaperone pathways. This hypothesis gained ground when the apoptosis regulator BAG-1 was found to stimulate nucleotide exchange on mammalian cytosolic Hsc70 (Höhfeld and Jentsch 1997). BAG-1 exists as multiple isoforms in the mammalian cytosol, which arise from a single messenger ribonucleic acid (mRNA) by alternative translation initiation (Packham et al 1997; Coldwell et al 2001). At least 4 isoforms can be distinguished: BAG-1L (apparent molecular mass of 52 kDa), BAG-1M (46 kDa; also termed HAP46, RAP46), BAG-1 (34 kDa), and BAG-1S (29 kDa) (Takayama et al 1998). The isoforms differ with regard to the length of their amino termini (Fig 1). Several structural elements are located in this region, and their presence or absence in distinct isoforms may cause functional diversity. For example, a nuclear localization signal is present at the extreme amino terminus of BAG-1L. Accordingly, BAG-1L is located in the nucleus, whereas the shorter isoforms are primarily found in the cytosol (Takayama et al 1998). Furthermore, a deoxyribonucleic acid (DNA)–binding motif, characterized by a cluster of lysine and arginine residues, is present in BAG-1L and BAG-1M but absent in BAG-1 and BAG-1S (Zeiner et al 1999; Schmidt et al 2003). BAG-1S also lacks the hexapeptide repeat motif TRSEEX, whereas different numbers of the functionally uncharacterized motif are located at the amino termini of the longer isoforms (Fig 1). Despite this heterogeneity at the amino terminus, all isoforms share a central domain that is structurally related to the degradation marker ubiquitin and a conserved domain of about 100 amino acids at the carboxyl terminus, termed BAG domain (Fig 1). The BAG domain is sufficient for Hsc70 binding and regulation and mediates an interaction of the cochaperone with the ATPase domain of Hsc70 (Höhfeld and Jentsch 1997; Takayama et al 1997; Zeiner et al 1997). Binding to the ATPase domain results in a strong acceleration of the steady-state ATPase activity of the chaperone (Höhfeld and Jentsch 1997). This ATPase-stimulating activity of BAG-1 is critically dependent on the additional presence of Hsp40. It was therefore concluded that BAG-1 stimulates ADP release from Hsc70 after an initial Hsp40-induced conversion of the chaperone into the ADP-bound form, and experimental evidence for this notion was provided (Höhfeld and Jentsch 1997; Gässler et al 2001; Sondermann et al 2001). The findings identify BAG-1 as a nucleotide exchange factor of Hsc70. How BAG-1 triggers nucleotide exchange was revealed when the BAG domain was crystallized in association with Hsc70's ATPase domain and the structure of the complex was solved (Sondermann et al 2001). The BAG domain opens the nucleotide-binding cleft of the ATPase domain and in this way stimulates the release of bound ADP from Hsc70. The structural change induced by BAG-1 closely resembles the conformational switch forced on the ATPase domain of DnaK by GrpE (Sondermann et al 2001). Nonetheless, BAG-1 and GrpE are structurally unrelated. The BAG domain forms a 3-helix bundle that contacts Hsc70's ATPase domain mainly by electrostatic interactions. In contrast, GrpE primarily uses a β-strand subdomain to bind to the ATPase domain of DnaK through hydrophobic contacts (Sondermann et al 2001). On the basis of functional convergence, cochaperones with distinct architectures apparently evolved as nucleotide exchange factors of Hsp70 family members.
Fig 1.
Domain structure of BAG-1 (Bcl-2–associated athanogene) isoforms, of carboxyl terminus of Hsc70 interacting protein (CHIP), and of the model for the functional cooperation of the 2 cochaperones during the sorting of Hsc70-bound substrate proteins to the proteasome for degradation. Four isoforms of BAG-1 have been detected, which differ with regard to the length of their amino termini. Several structural elements are located at the amino terminus, including a nuclear localization signal (NLS), a deoxyribonucleic acid–binding motif (DNA), and multiple repeats of the hexapeptide motif TRSEEX. All isoforms possess a central ubiquitin-like domain used for proteasome binding and a carboxyl terminal BAG domain, which mediates binding and regulation of Hsc70. CHIP possesses a triple TPR domain that, together with an adjacent charged region, forms a binding site for Hsc70 and Hsp90. A central coiled coil domain (cc) may be involved in protein-protein interactions. The carboxyl terminal U-box is required for the ubiquitin ligase activity of CHIP and seems to mediate binding to ubiquitin-conjugating enzymes of the Ubc4/5 family. Association of CHIP with the carboxyl terminal domain of Hsc70 (C) gives rise to a chaperone complex that mediates sorting to the proteasome. At the same time, BAG-1 binds to the adenosine triphosphatase domain of Hsc70 (ATPase) and in addition contacts CHIP directly. The ubiquitin-like domain of BAG-1 (ubl) remains exposed in the formed complex and serves as a proteasomal sorting signal. Subunit and domain arrangement in the complex remains to be determined experimentally
BAG-1–induced conformational changes of the ATPase domain of Hsc70 are transferred to the peptide-binding pocket of the chaperone and result in the release of a bound polypeptide substrate (Lüders et al 1998, 2000a; Gässler et al 2001; Sondermann et al 2001). This parallels the findings for GrpE. The consequences for chaperone-mediated protein folding, however, can be rather different. The substrate release activity of GrpE was shown to be required for the folding of polypeptide substrates by DnaK and DnaJ (reviewed in Bukau and Horwich 1998). In contrast, folding-stimulating as well as folding-inhibiting effects were observed for BAG-1 in in vitro refolding experiments using Hsc70 and Hsp40 (Zeiner et al 1997; Lüders et al 1998, 2000a; Terada and Mori 2000; Gässler et al 2001). Furthermore, in mammalian cells BAG-1 overexpression abrogated the chaperone activity of Hsc70 (Nollen et al 2001). It, therefore, appears that BAG-1 is not a general regulator of Hsc70 during the productive folding of chaperone substrates but rather stimulates substrate unloading from Hsc70 on certain chaperone pathways on the basis of its nucleotide exchange activity.
Interestingly, human cells contain several BAG-1–related proteins: BAG-2, BAG-3 (CAIR-1; Bis), BAG-4 (SODD), BAG-5, and BAG-6 (Scythe, BAT3) (Takayama et al 1999; Takayama and Reed 2001). In addition to the conserved BAG domain required for binding and regulation of Hsc70, the BAG family members possess additional functional domains that seem to mediate targeting to diverse partner proteins and subcellular compartments. For example, BAG-3 forms a ternary complex with Hsc70 and phospholipase C-γ (PLC-γ) in response to epidermal growth factor (EGF) (Doong et al 2000). PLC-γ is contacted by BAG-3 through a domain comprising multiple PXXP motifs, whereas the BAG domain of the cochaperone remains available for binding to Hsc70. BAG-3 thus links the Hsc70 chaperone system to EGF-induced signaling pathways. Nucleotide exchange factors of the BAG family are apparently used at multiple locations to modulate Hsc70 function.
BAG-1—A COUPLING FACTOR BETWEEN HSC70 AND THE UBIQUITIN-PROTEASOME SYSTEM
A striking structural feature of all BAG-1 isoforms is the presence of a ubiquitin-like domain (Fig 1). Ubiquitin itself is a small protein that is ubiquitously expressed in eukaryotic cells and serves as a degradation signal. Covalent attachment of a chain of multiple ubiquitin molecules to a protein substrate is mediated by the concerted action of a ubiquitin-conjugating enzyme and a ubiquitin ligase and directs the modified substrate to the 26S proteasome, a heterooligomeric protease complex, for degradation (Varshavsky 1997). The ubiquitin-like domain of BAG-1 cannot be cleaved off and cannot be transferred onto other proteins. Rather the domain serves as an integral sorting signal to stimulate an interaction of BAG-1 with the proteasome (Lüders et al 2000b; Alberti et al 2002). BAG-1 associates with the proteasome in an ATP-dependent manner, probably regulated by the ATPase subunits of the 19S regulatory subcomplex of the proteasome. This association does not result in the rapid degradation of the cochaperone. Instead, BAG-1 stably associates with the proteasome, and consequently complexes of the cochaperone and the proteasome can be isolated from mammalian cells (Lüders et al 2000b). Remarkably, BAG-1 can simultaneously bind to Hsc70 and to the proteasome. This seems to enable BAG-1 to act as a coupling factor between the chaperone and the proteolytic complex. Increasing the cellular levels of BAG-1 indeed stimulates an association of Hsc70 with the proteasome (Lüders et al 2000b).
The proteasomal localization of BAG-1 prompted speculations that the cochaperone may participate in the sorting of chaperone substrates to the proteasome (Lüders et al 2000b; Höhfeld et al 2001). Based on its nucleotide exchange activity, BAG-1 may induce the release of Hsc70-bound substrates in the vicinity of the proteasome and in this way may facilitate substrate transfer into the proteolytic core of the degradation complex. Evidence for such a model was recently provided when the cooperation of BAG-1 with the carboxyl terminus of Hsc70 interacting protein (CHIP) was investigated (Demand et al 2001). Like BAG-1, CHIP combines a chaperone-binding site with a domain implicated in the regulation of the ubiquitin-proteasome system (Fig 1). A tandem arrangement of 3 tetratricopeptide repeat motifs (TPRs) at its amino terminus enables CHIP to occupy binding sites for TPR-containing cochaperones on Hsc70 and Hsp90 (Ballinger et al 1999; Scheufler et al 2000; Connell et al 2001). At the carboxyl terminus CHIP possesses a U-box, which is structurally related to RING finger domains found in many ubiquitin ligases (Jackson et al 2000). In fact, CHIP displays ubiquitin ligase activity. In conjunction with ubiquitin-conjugating enzymes of the Ubc4/5 family, CHIP mediates ubiquitin attachment to protein substrates that are presented by Hsc70 and Hsp90 (Demand et al 2001; Jiang et al 2001; Murata et al 2001). Consequently, elevating the cellular concentration of CHIP induces the degradation of known chaperone substrates by the proteasome (Connell et al 2001; Meacham et al 2001; Xu et al 2002). This function of CHIP seems to be enhanced by BAG-1. The 2 cofactors can simultaneously associate with Hsc70 because they occupy nonoverlapping binding sites on the chaperone (Höhfeld and Jentsch 1997; Ballinger et al 1999; Demand et al 2001). Moreover, a direct interaction of the 2 cofactors has been observed, which may contribute to the formation of the BAG-1/Hsc70/CHIP complex (Fig 1). A cooperation of the 2 cofactors during the sorting of chaperone substrates to the proteasome was revealed in cell culture experiments. Overexpression of BAG-1 stimulated the CHIP-mediated degradation of the glucocorticoid hormone receptor (Demand et al 2001). It seems that the 2 cochaperones are able to team up to shift the activity of Hsc70 from protein folding to protein degradation.
Intriguingly, BAG-1 itself is a substrate of the CHIP ubiquitin ligase (Alberti et al 2002). On formation of the ternary BAG-1/Hsc70/CHIP complex, CHIP mediates the attachment of a polyubiquitin chain to BAG-1 and thereby promotes the association of the chaperone complex with the proteasome. This may provide the means to regulate the chaperone-assisted degradation pathway. Multiple lines of evidence thus support a functional cooperation of BAG-1 and CHIP. However, whether this cooperation is of general character or affects only a limited set of chaperone substrates remains to be determined. Notably, similar to BAG-1, BAG-6 (Scythe/BAT3) combines a ubiquitin-like domain of unknown function and a BAG domain that is used for Hsc70 binding and regulation (Thress et al 2001). Although a proteasomal association was not yet observed for BAG-6, it is tempting to speculate that the protein may fulfill a BAG-1–like function and may cooperate with CHIP on certain degradation pathways. Furthermore, there is evidence that a ubiquitin ligase other than CHIP participates in the sorting of chaperone substrates to the proteasome (Xu et al 2002). Functional redundancy appears to exist on such sorting pathways.
BAG-1—A COCHAPERONE WITH MULTIPLE CELLULAR FUNCTIONS
The identification of BAG-1 as an Hsc70 nucleotide exchange factor and proteasome-binding partner provides a framework for our understanding of the cellular functions of BAG-1. In the past few years many such functions were assigned to BAG-1 (Table 1). For example, overexpression of BAG-1 in cell culture experiments renders cells more resistant to apoptosis, particularly in cooperation with Bcl-2 (Takayama et al 1995). Interaction of BAG-1 with Bcl-2 is ATP dependent, which may point to a participation of Hsc70 (Takayama et al 1997). Because Bcl-2 is a rather hydrophobic protein that displays its cellular activity in part in the outer mitochondrial membrane, it is conceivable that BAG-1 and Hsc70 form a chaperone complex involved in membrane insertion of Bcl-2. Such a model would be consistent with the chaperone-regulating activity of BAG-1. Yet, evidence for such a model is still missing, and an interaction between Bcl-2 and Hsc70 has not yet been demonstrated. Furthermore, it is difficult to envisage how proteasome association of BAG-1 could increase the antiapoptotic activity of Bcl-2. On the other hand, conclusions drawn from overexpression studies might be misleading. Several proteins that, like BAG-1, possess integrated ubiquitin-like domains were recently shown to assist in the sorting of protein substrates to the proteasome (reviewed in Jentsch and Pyrowolakis 2000; Hartmann-Petersen et al 2003). Nonetheless, their overexpression often blocks proteasomal degradation, possibly because their excess disturbs the stoichiometry of sorting complexes. Intriguingly, elevated levels of BAG-1 are observed in different types of tumors, and the cochaperone is therefore discussed as a novel diagnostic marker for certain cancers (Takayama et al 1998; Krajewski et al 1999; Yang et al 1999). The altered regulation of chaperone and degradation pathways that probably accompanies an elevation of cellular BAG-1 levels may significantly contribute to oncogenic transformation.
Besides apoptosis, cellular processes that are affected by BAG-1 include neuronal differentiation (Kermer et al 2002), cell motility (Naishiro et al 1999), transcription regulation (Zeiner et al 1999; Takahashi et al 2001; Schmidt et al 2003), and stress signaling (Song et al 2001). For the latter, the interaction of BAG-1 with the protein kinase Raf-1 is of relevance. The stress-signaling kinase was described to be a direct binding partner of BAG-1 (Wang et al 1996; Song et al 2001). Association with BAG-1 results in a stimulation of the kinase activity of Raf-1 and causes cell proliferation. Remarkably, the binding sites for Raf-1 and Hsc70 on BAG-1 overlap, and the 2 proteins therefore bind to BAG-1 in a mutually exclusive manner (Song et al 2001). Accordingly, it was proposed that BAG-1 functions as a molecular switch that enhances cell proliferation under normal conditions but mitigates proliferation during conditions of acute stress when chaperone levels increase (Song et al 2001).
Other important binding partners of BAG-1 are nuclear hormone receptors. Confusingly, rather diverse effects on receptor-dependent transcription have been observed on overexpression of BAG-1 in cell cultures. This may in part depend on the isoform of BAG-1 that was investigated. For instance, interaction with BAG-1M and BAG-1 negatively regulates the DNA binding and transactivation activities of the retinoic acid receptor and the glucocorticoid receptor (Kullmann et al 1998; Liu et al 1998; Schneikert et al 2000). BAG-1L was shown to enhance the activity of the androgen receptor (Froesch et al 1998; Knee et al 2001), whereas stimulating as well as suppressing effects were observed for the interaction of BAG-1L with vitamin D receptor (Guzey et al 2000; Witcher et al 2001). The data reveal a regulatory role of BAG-1 in hormone receptor action, although it currently appears impossible to draw a consistent model to explain this regulatory role. In any case, it might be helpful to distinguish between cytoplasmatic and nuclear actions of BAG-1 in this respect. Before nuclear hormone receptors are activated, they interact with molecular chaperones in the cytosol to attain a folding state capable of binding their corresponding ligands (Frydman and Höhfeld 1997). The first steps of receptor maturation depend on the action of Hsc70, suggesting that BAG-1 might interfere with these initial folding events by virtue of its substrate release activity (Kanelakis et al 1999). In fact, the regulatory role of BAG-1 on nuclear receptors depends on the presence of the BAG domain and thus most likely involves a modulation of Hsc70 function (Zeiner et al 1999; Schmidt et al 2003). The situation may change when BAG-1 enters the nucleus. A cluster of lysine and arginine residues present at the amino terminus of BAG-1M and BAG-1L was recently shown to mediate a nonsequence-specific binding of the cochaperone to DNA. The DNA-binding domain enables BAG-1M and apparently also BAG-1L to activate transcription (Zeiner et al 1999; Niyaz et al 2001; Takahashi et al 2001; Schmidt et al 2003). Moreover, the DNA-binding domain must be present on the cochaperone together with the Hsc70-interacting BAG domain to achieve an inhibition of glucocorticoid receptor–dependent transcription (Schmidt et al 2003). The cochaperone appears to use its DNA-binding domain to recruit Hsc70 to chromosomal loci and to modulate chaperone-assisted steps during transcription. These findings are particularly exciting with respect to a recent report implicating chaperones in transcription regulation (Freeman and Yamamoto 2002). In this report molecular chaperones were shown to promote the disassembly of transcriptional regulatory complexes, thus enabling regulatory machineries to detect and respond to signaling changes. BAG-1 may participate in such processes.
CONCLUDING REMARKS
Many of the cellular functions of BAG-1 may be explained by its chaperone-regulating activity and in some cases may involve the modulation of degradation pathways. Still, several of the ways in which BAG-1 acts are not yet understood. In this regard it appears necessary to broaden the experimental strategies for analyzing BAG-1 function. New answers will certainly arise from experiments that involve downregulation of the enigmatic cochaperone by small inhibiting RNAs and from the characterization of corresponding knockout animals that are currently generated.
Acknowledgments
Work in the authors' laboratory was supported by grants of the Deutsche Forschungsgemeinschaft.
REFERENCES
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Höhfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem. 2002;277:45920–45927. doi: 10.1074/jbc.M204196200. [DOI] [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–6212. [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Coldwell MJ, deSchoolmeester ML, Fraser GA, Pickering BM, Packham G, Willis AE. The p36 isoform of BAG-1 is translated by internal ribosome entry following heat shock. Oncogene. 2001;20:4095–4100. doi: 10.1038/sj.onc.1204547. [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Höhfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93–96. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Demand J, Alberti S, Patterson C, Höhfeld J. Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol. 2001;11:1569–1577. doi: 10.1016/s0960-9822(01)00487-0. [DOI] [PubMed] [Google Scholar]
- Doong H, Price J, and Kim YS. et al. 2000 CAIR-1/BAG-3 forms an EGF-regulated ternary complex with phospholipase C-γ and Hsp70/Hsc70. Oncogene. 19:4385–4395. [DOI] [PubMed] [Google Scholar]
- Doong H, Vrailas A, Kohn EC. What's in the ‘BAG‘?—a functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32. doi: 10.1016/s0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051. [DOI] [PubMed] [Google Scholar]
- Froesch BA, Takayama S, Reed JC. BAG-1L protein enhances androgen receptor function. J Biol Chem. 1998;273:11660–11666. doi: 10.1074/jbc.273.19.11660. [DOI] [PubMed] [Google Scholar]
- Frydman J, Höhfeld J. Chaperones get in touch: the hip-hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Gässler CS, Wiederkehr T, Brehmer D, Bukau B, Mayer MP. Bag-1M accelerates nucleotide release for human Hsc70 and Hsp70 and can act concentration-dependent as positive and negative cofactor. J Biol Chem. 2001;276:32538–32544. doi: 10.1074/jbc.M105328200. [DOI] [PubMed] [Google Scholar]
- Guzey M, Takayama S, Reed JC. BAG1L enhances trans-activation function of the vitamin D receptor. J Biol Chem. 2000;275:40749–40756. doi: 10.1074/jbc.M004977200. [DOI] [PubMed] [Google Scholar]
- Hartmann-Petersen R, Seeger M, Gordon C. Transferring substrates to the 26S proteasome. Trends Biochem Sci. 2003;28:26–31. doi: 10.1016/s0968-0004(02)00002-6. [DOI] [PubMed] [Google Scholar]
- Höhfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Höhfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PK, Eldridge AG, Freed E, Furstenthal L, Hsu JY, Kaiser BK, Reimann JD. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–439. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- Jentsch S, Pyrowolakis G. Ubiquitin and its kin: how close are the family ties? Trends Cell Biol. 2000;10:335–342. doi: 10.1016/s0962-8924(00)01785-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Kanelakis KC, Morishima Y, Dittmar KD, Galigniana MD, Takayama S, Reed JC, Pratt WB. Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J Biol Chem. 1999;274:34134–34140. doi: 10.1074/jbc.274.48.34134. [DOI] [PubMed] [Google Scholar]
- Kermer P, Krajewska M, Zapata JM, Takayama S, Mai J, Krajewski S, Reed JC. Bag1 is a regulator and marker of neuronal differentiation. Cell Death Differ. 2002;9:405–413. doi: 10.1038/sj.cdd.4400972. [DOI] [PubMed] [Google Scholar]
- Knee DA, Froesch BA, Nuber U, Takayama S, Reed JC. Structure-function analysis of Bag1 proteins. Effects on androgen receptor transcriptional activity. J Biol Chem. 2001;276:12718–12724. doi: 10.1074/jbc.M010841200. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, and Turner BC. et al. 1999 Prognostic significance of apoptosis regulators in breast cancer. Endocr Relat Cancer. 6:29–40. [DOI] [PubMed] [Google Scholar]
- Kullmann M, Schneikert J, Moll J, Heck S, Zeiner M, Gehring U, Cato AC. RAP46 is a negative regulator of glucocorticoid receptor action and hormone-induced apoptosis. J Biol Chem. 1998;273:14620–14625. doi: 10.1074/jbc.273.23.14620. [DOI] [PubMed] [Google Scholar]
- Liu R, Takayama S, Zheng Y, Froesch B, Chen GQ, Zhang X, Reed JC, Zhang XK. Interaction of BAG-1 with retinoic acid receptor and its inhibition of retinoic acid-induced apoptosis in cancer cells. J Biol Chem. 1998;273:16985–16992. doi: 10.1074/jbc.273.27.16985. [DOI] [PubMed] [Google Scholar]
- Lüders J, Demand J, Schönfelder S, Frien M, Zimmermann R, Höhfeld J. Cofactor-induced modulation of the functional specificity of the molecular chaperone Hsc70. Biol Chem. 1998;379:1217–1226. doi: 10.1515/bchm.1998.379.10.1217. [DOI] [PubMed] [Google Scholar]
- Lüders J, Demand J, Papp O, Höhfeld J. Distinct isoforms of the cofactor BAG-1 differentially affect Hsc70 chaperone function. J Biol Chem. 2000a;275:14817–14823. doi: 10.1074/jbc.275.20.14817. [DOI] [PubMed] [Google Scholar]
- Lüders J, Demand J, Höhfeld J. The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem. 2000b;275:4613–4617. doi: 10.1074/jbc.275.7.4613. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Takayama S, Froesch BA, Zapata JM, Reed JC. p53-inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol. 2001;3:100–105. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- Minami Y, Höhfeld J, Ohtsuka K, Hartl FU. Regulation of the heat-shock protein 70 reaction cycle by the mammalian DnaJ homolog, Hsp40. J Biol Chem. 1996;271:19617–19624. doi: 10.1074/jbc.271.32.19617. [DOI] [PubMed] [Google Scholar]
- Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naishiro Y, Adachi M, and Okuda H. et al. 1999 BAG-1 accelerates cell motility of human gastric cancer cells. Oncogene. 18:3244–3251. [DOI] [PubMed] [Google Scholar]
- Niyaz Y, Zeiner M, Gehring U. Transcriptional activation by the human Hsp70-associating protein Hap50. J Cell Sci. 2001;114:1839–1845. doi: 10.1242/jcs.114.10.1839. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Kabakov AE, Brunsting JF, Kanon B, Höhfeld J, Kampinga HH. Modulation of in vivo HSP70 chaperone activity by Hip and Bag-1. J Biol Chem. 2001;276:4677–4682. doi: 10.1074/jbc.M009745200. [DOI] [PubMed] [Google Scholar]
- Packham G, Brimmell M, Cleveland JL. Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J. 1997;328:807–813. doi: 10.1042/bj3280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Wochnik GM, Rosenhagen MC, Young JC, Hartl FU, Holsboer F, Rein T. Essential role of the unusual DNA-binding motif of BAG-1 for inhibition of the glucocorticoid receptor. J Biol Chem. 2003;278:4926–4931. doi: 10.1074/jbc.M212000200. [DOI] [PubMed] [Google Scholar]
- Schneikert J, Hubner S, Langer G, Petri T, Jaattela M, Reed J, Cato AC. Hsp70-RAP46 interaction in downregulation of DNA binding by glucocorticoid receptor. EMBO J. 2000;19:6508–6516. doi: 10.1093/emboj/19.23.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneikert J, Hubner S, Martin E, Cato AC. A nuclear action of the eukaryotic cochaperone RAP46 in downregulation of glucocorticoid receptor activity. J Cell Biol. 1999;146:929–940. doi: 10.1083/jcb.146.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Höhfeld J, Hartl FU, Moarefi I. Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science. 2001;291:1553–1557. doi: 10.1126/science.1057268. [DOI] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol. 2001;3:276–282. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sasaki R, Takahashi J, Takayama S, Reed JC, Andoh T. BAG-1M, an isoform of Bcl-2-interacting protein BAG-1, enhances gene expression driven by CMV promoter. Biochem Biophys Res Commun. 2001;286:807–814. doi: 10.1006/bbrc.2001.5473. [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Krajewski S, and Krajewska M. et al. 1998 Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res. 58:3116–3131. [PubMed] [Google Scholar]
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80:279–274. doi: 10.1016/0092-8674(95)90410-7. [DOI] [PubMed] [Google Scholar]
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- Terada K, Mori M. Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70. J Biol Chem. 2000;275:24728–24734. doi: 10.1074/jbc.M002021200. [DOI] [PubMed] [Google Scholar]
- Thress K, Song J, Morimoto RI, Kornbluth S. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J. 2001;20:1033–1041. doi: 10.1093/emboj/20.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system. Trends Biochem Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- Wang HG, Takayama S, Rapp UR, Reed JC. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci U S A. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher M, Yang X, Pater A, Tang SC. BAG-1 p50 isoform interacts with the vitamin D receptor and its cellular overexpression inhibits the vitamin D pathway. Exp Cell Res. 2001;265:167–173. doi: 10.1006/excr.2001.5176. [DOI] [PubMed] [Google Scholar]
- Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A. 2002;99:12847–12852. doi: 10.1073/pnas.202365899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Pater A, Tang SC. Cloning and characterization of the human BAG-1 gene promoter: upregulation by tumor-derived p53 mutants. Oncogene. 1999;18:4546–4553. doi: 10.1038/sj.onc.1202843. [DOI] [PubMed] [Google Scholar]
- Zeiner M, Gebauer M, Gehring U. Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 1997;16:5483–5490. doi: 10.1093/emboj/16.18.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M, Niyaz Y, Gehring U. The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc Natl Acad Sci U S A. 1999;96:10194–10199. doi: 10.1073/pnas.96.18.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]