Introduction
Psoriasis is a common chronic immunologic condition of the skin and nails that affects approximately 2% of the population and is characterized by red elevated plaques with silvery scale. Various forms exist, of which, chronic plaque psoriasis is most common, occurring in 90% of patients.1 Other less common forms, including guttate, pustular, inverse, and erythrodermic, may appear spontaneously or evolve from plaque psoriasis. Psoriatic arthritis exists in around 20% to 30% of patients suffering from moderate to severe psoriasis.2
In recent years, the importance of T helper 17 cells and associated pathologic enhanced expression of the cytokine interleukin-17A (IL-17A) were discovered to be integral to the pathogenesis of psoriasis and psoriatic arthritis.1 Novel biologic therapies, such as secukinumab and ixekizumab, are monoclonal antibodies that selectively bind to and inhibit IL-17A, whereas brodalumab binds to the IL-17RA receptor. These drugs were found in clinical trials to be highly effective in treating patients with moderate-to-severe plaque psoriasis and psoriatic arthritis.3 Currently, only secukinumab from this class has received Health Canada and US Food and Drug Administration approval for treatment of moderate-to-severe psoriasis, whereas ixekizumab and brodalumab are still under clinical development.∗ Secukinumab has also recently received US Food and Drug Administration approval for treatment of psoriatic arthritis and ankylosing spondylitis but is not currently indicated for the treatment of pustular psoriasis. We report a case of severe chronic plaque psoriasis in a patient who had pustular psoriasis and new onset of psoriatic arthritis after discontinuation of brodalumab therapy.
Case report
We present a 50-year-old man with hypertension and a 14-year history of severe plaque psoriasis with no associated psoriatic arthritis or family history of psoriasis. He tried several prior treatments, including topical steroids (betamethasone valerate 0.1%, fluocinonide, clobetasol propionate, and calcipotriol/betamethasone ointment) and a 3-month course of psoralen plus ultraviolet A therapy, but there was no response. On initial visit in August 2012, his body surface area (BSA) affected was 50%, Psoriasis Area and Severity Index (PASI) score was 29.2, and Dermatology Life Quality Index (DLQI) was 23. Treatment options were discussed, and he opted to be enrolled in a new clinical trial for brodalumab, an investigational IL-17 receptor inhibitor. He began taking brodalumab, 210 mg once biweekly. After 6 months, he had complete clearance of his psoriasis.
In May 2015, Amgen decided to terminate all the brodalumab trials based on events of suicidal ideation and behavior reported by some patients during the trial.4 Six weeks after the cessation of brodalumab, there was significant return of the patient's severe plaque psoriasis; his BSA was 32% and PASI 24.8 (Fig 1, A). Additionally, pustules developed on his palms (Fig 1, B) and dactylitis and arthritis developed in his elbows, despite a previously negative history of psoriatic arthritis. The psoriatic arthritis was confirmed on radiographic imaging and by an academic rheumatologist. Treatment options were discussed, and he chose to go on secukinumab, an IL-17A cytokine inhibitor.
Fig 1.
A, Plaque psoriasis. Clinical picture of severe plaque psoriasis with sharply delineated erythematous papules and plaques with overlying silvery scale on the abdomen and right arm after cessation of brodalumab. B, Pustular psoriasis. Clinical picture of pustules in different stages of evolution on the left palm after cessation of brodalumab.
The patient was given the approved dose of secukinumab of 300 mg at week 0, 1, 2, 3, 4, and every 4 weeks thereafter. At 3 weeks of treatment, the patient received 4 doses of secukinumab, 300 mg, and his pustules resolved completely with significant improvement of his psoriatic plaques (Fig 2). His residual BSA was 22% and PASI was 9.4. His psoriatic arthritis symptoms also started to subside but were still active. Consequently, his rheumatologist added weekly subcutaneous injections of methotrexate, 15 mg at week 3 of secukinumab treatment. At his 3-month follow-up visit, his plaque psoriasis remained under excellent control with the continuation of concomitant methotrexate and secukinumab. His residual BSA was 8%, his PASI was 4.6, and he was clear of pustules. Although there was significant improvement in his psoriatic arthritis, it did not clear completely. His fingers remained mildly swollen and painful. This was, however, not entirely surprising, as psoriatic arthritis trials of secukinumab have shown that maximal drug benefit may take 26 weeks to take effect.5
Fig 2.
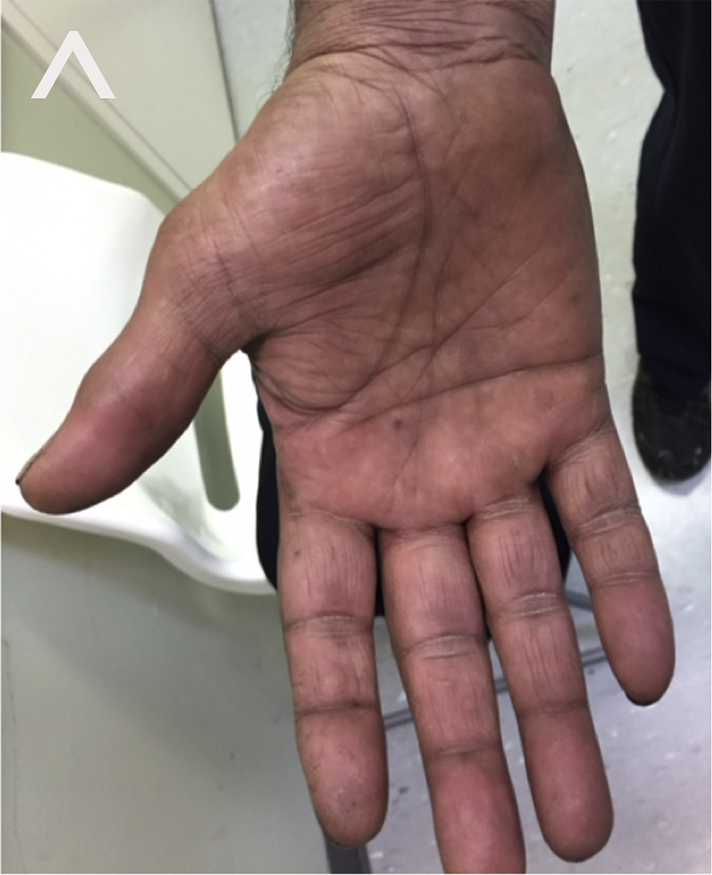
Pustular psoriasis. After 3 weeks of secukinumab therapy, palms of hands were clear of pustules, as evidenced on the right palm.
Six months after starting secukinumab, the patient was seen back in the clinic. Four weeks prior, he stopped taking methotrexate because of gastrointestinal upset and was started on apremilast by his rheumatologist. At examination, he had residual small thin psoriatic papules and plaques on his flanks (Fig 3), elbows, knees, and calves, affecting 2% BSA with PASI of 3.0 and DLQI of 5. Minimal postinflammatory hyperpigmentation was evident, and there was significant improvement of the psoriatic arthritis in his fingers, with minor pain and swelling and no pustules on his hands.
Fig 3.

Plaque psoriasis. After 6 months of secukinumab therapy, residual small psoriatic plaques and papules with overlying silvery scale remain on left flank.
Discussion
Pustular psoriasis is one of the most difficult subtypes of psoriasis to treat. Publications on successful treatment of pustular psoriasis are extremely scarce. In fact, none of the approved biologic agents for the treatment of psoriasis are indicated for pustular psoriasis.
It was remarkable that his pustules completely disappeared after 3 weeks of secukinumab treatment. His significant improvement in plaque psoriasis with secukinumab at week 3 and month 3 validate its high efficacy in this condition as has been shown in its phase III clinical trials.6 Significant psoriatic arthritis developed shortly after brodalumab was stopped. He did not have a history of psoriatic arthritis before brodalumab. We speculate that the psoriatic arthritis developed after he started brodalumab and that his arthritic symptoms were controlled with the treatment. However, this speculation could not be proven for obvious reasons.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Yeung has been a speaker, consultant, and investigator for Abbvie, Allergan, Amgen, Astellas, Baxalta, Boehringer Ingelheim, Celgene, Centocor, Coherus, Dermira, Eli Lilly, Forward, Galderma, Janssen, Leo, Medimmune, Novartis, Pfizer, Regeneron, Roche, Takeda, and Valeant. Dr Valbuena declares no conflict of interest.
Addendum: In March and June 2016, the US Food and Drug Administration and Health Canada, respectively, approved ixekizumab, another monoclonal antibody against IL-17A, for the treatment of moderate-to-severe psoriasis. Ixekizumab is a humanized IgG4 monoclonal antibody that selectively binds with IL-17A cytokine and inhibits its interaction with the IL-17 receptor.
References
- 1.Canavan T., Elmets C., Cantrell W., Evans J.M., Elewski B.E. Anti-IL-17 medications used in the treatment of plaque psoriasis and psoriatic arthritis: A comprehensive review. Am J Clin Dermatol. 2016;17(1):33–47. doi: 10.1007/s40257-015-0162-4. [DOI] [PubMed] [Google Scholar]
- 2.Gladman D., Antoni C., Mease P., Clegg D.O., Nash P. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl II):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yiu Z., Griffiths C. Interleukin 17-A inhibition in the treatment of psoriasis. Exp Rev Clin Immunol. 2016;12(1):1–4. doi: 10.1586/1744666X.2016.1112739. [DOI] [PubMed] [Google Scholar]
- 4.Amgen. Amgen to terminate participation in co-development and commercialization of brodalumab. Amgen; [cited 2016 Jan 7]; Available from: http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2015&releaseID=2052862
- 5.McInnes I., Mease P., Kirkham B. Secukinumab, a human anti-interleukin-7A monoclonal psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 6.Langley R., Elewski B., Lebwohi M. Secukinumab in plaque psoriasis – Results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]



