Abstract
Hsp27 is considered a potential marker for cell differentiation in diverse tissues. Several aspects linked to the differentiation process and to the transition from high to low metastatic potential were analyzed in melanoma cells transfected with Hsp27. E-cadherin plays a central role in cell differentiation, migration, and normal development. Loss of expression or function of E-cadherin has been documented in a variety of human malignancies. We observed by fluorescence-activated cell sorter (FACS) as well as immunofluorescence (IF) analysis a pronounced expression of E-cadherin in Hsp27-transfected A375 melanoma cells compared with control melanoma cells. The expression of the adhesion molecule MUC18/MCAM correlates directly with the metastatic potential of melanoma cells. In contrast to wild-type and neotransfected melanoma cells, in Hsp27-transfected cells the expression of MUC18/MCAM could not be detected by FACS and IF analysis. The plasminogen activator (PA) system plays a central role in mediating extracellular proteolysis and also in nonproteolytic events such as cell adhesion, migration, and transmembrane signaling. Hsp27 transfectants revealed elevated messenger ribonucleic acid expression of the urokinase-type PA (uPA) and its inhibitor, PA inhibitor type 1, which might indicate a neutralization effect of the proteolytic activity of uPA. Control cells failed to express both these molecules. The influence of Hsp27 expression on uPA activity and the involvement of E-cadherin could be demonstrated by use of anti–E-cadherin–blocking antibody. Our data provide evidence for an inhibitory-regulatory role of Hsp27 in tumor progression as found in our system.
INTRODUCTION
Small heat shock proteins (sHsps) form an abundant and ubiquitous family of stress proteins that have been found in all organisms studied so far (Hightower 1991; Morimoto et al 1994). sHsps range in monomer size from 15 to 30 kDa and from oligomeric complexes of 200–800 kDa. Phosphorylation, which is a common feature of mammalian sHsps (Lavoie et al 1993), seems to lead to changes in the oligomeric structure of these proteins (Kato et al 1994) but does not interfere with chaperone activity (Buchner 1996).
Among sHsps, human Hsp27 and its murine homolog Hsp25 are the most thoroughly investigated members of the family. sHsps can function as molecular chaperones by preventing irreversible aggregation of other proteins and increasing the yield of renaturation after heat or chemical denaturation. In addition, they are involved in several cellular processes such as signal transduction, growth regulation (Spector et al 1992, 1993; Knauf et al 1993; Kindas-Mügge et al 1996, 1998; Richards et al 1996), development (Pauli et al 1990; Gernold et al 1993; Michaud et al 1997; Jantschitsch et al 1998), differentiation (Shakoori et al 1992; Stahl et al 1992; Kindas-Mügge and Trautinger 1994; Spector et al 1995; Trautinger et al 1995; Hell-Pourmojib et al 2002), and tumorigenesis (Ciocca et al 1993). Hsp27 is considered a potential marker of differentiation in mammalian osteoblasts (Shakoori et al 1992), leukemia, cells (Spector et al 1995), P16 embryonal carcinoma and BLC6 stem cells (Stahl et al 1992), as well as in epidermal keratinocytes (Trautinger et al 1995). Recently, we found that Hsp27 could influence the malignant phenotype of a human melanoma cell line A375 in vitro (Aldrian et al 2002). Using matrigel-coated filters we found decreased cell invasiveness in Hsp27-overexpressing cells and reduced secretion of matrix metalloproteinases (MMP-2 and MMP-9) as detected by zymograms as well as by gelatinase activity assays. The most striking characteristic of the Hsp27-transfected cell line was the absolute loss or lack of expression of the avβ3 integrin (fluorescence-activated cell sorter [FACS] analysis and immunofluorescence [IF]), which is most frequently expressed in invasive melanoma cells. It is not expressed in normal melanocytes, and its appearance coincides with progression to the invasive phase. Hsp27-transfected cells failed to express this adhesion molecule. We, therefore, set out to further investigate in Hsp27-overexpressing melanoma cells cellular aspects linked to the transition from high to low metastatic potential.
Cell adhesion molecules are surface structures that bind specifically to ligands on other cells and are important in organogenesis and tissue remodeling. Perturbation in cell adhesion in tumor cells can lead to an interruption of cell-cell interactions as well as the development of new interactions, both of which are important for metastasis formation (Buck 1995).
Cadherins comprise a family of calcium-dependent cellular adhesion molecules expressed on most cell types that form solid tissues (Bracke et al 1996). In the human skin, melanocytes are located in the epidermis, which enables intercellular communication with keratinocytes. Cadherins, which appear to determine the position of the melanocytes in the skin, are critical for melanocyte development. During melanocyte transformation the communication with the keratinocytes is lost (Herlyn et al 2000), and loss or down-regulation of E-cadherin expression occurs. This has consequences for the regulatory cross-talk between various types of cells in the skin (Seline et al 1996). Forced expression of E-cadherin in melanoma cells can reverse the malignant phenotype. The control of keratinocytes over the melanoma cells is reestablished (Herlyn et al 2000).
It has been shown that loss of E-cadherin function is associated with up-regulation or induction of invasion-related receptors. The melanoma cell adhesion molecule MCAM, also known as MUC18, is a cell surface glycoprotein of 113 kDa. It has been shown to be involved in the development and progression of malignant melanoma (Shih et al 1994; Johnson 1999). In normal adult tissues MUC18/MCAM is expressed on smooth muscle (Lehmann et al 1987) and on activated T lymphocytes (Pickl et al 1997).
The urokinase-type plasminogen activator receptor (uPAR) is located on the cell surface and plays a role in mediating extracellular degradative and invasive processes but also in nonproteolytic events such as cell adhesion, migration, and transmembrane signaling (Andreasen et al 1997; Preissner et al 2000). The activity of uPA bound to uPAR is regulated by inhibitors, which hold a central position in suppressing both uPAR-mediated cell adhesion and proteolysis (Deng et al 1996).
In the present study, we investigated the importance of E-cadherin, MUC18/MCAM, and the regulation of the PA system mediating the reduced malignant phenotypes observed in Hsp27 transfectants from the melanoma cell line A375.
MATERIALS AND METHODS
Transfection and cell culture
The melanoma cell line A375 was obtained from the American Type Culture Collection (ATCC, Rockville, MD). Stably transfected clones were selected as previously described (Kindas-Mügge et al 1996). Briefly, A375 cells were transfected with the Hsp27 expression vectors pSG-2711 and pWLneo, conferring neomycin resistance to G418 using the calcium phosphate precipitation procedure. Clones A375 2A and 11A expressed high levels of Hsp27. A375/neo is the mock transfected control cell line, and A375/wt designates the nontransfected (wild type) cells.
Cells were cultured in Dulbecco modified Eagle medium (DMEM; Life Technologies Inc, Paisley, UK) supplemented with 10% fetal calf serum, penicillin (110 U/mL), and streptomycin (100 μg/mL) at 37 °C in 7.5% CO2. Selective media used for cultivation of transfected cell lines were further supplemented with G418 (0.4 mg/mL; Sigma, St Louis, MO, USA).
Western blotting
Cells were lysed by repeated freeze-thawing cycles in distilled water containing 1 mM phenylmethylsulfonylfluoride (Sigma), aprotinin (1–2 μg/mL, Sigma), and leupeptin (1–2 μg/mL, Sigma). Supernatants were collected, and total protein content was measured (Bio-Rad protein assay, Bio-Rad Lab, München, Germany) and subjected (10 μg) to 14% sodium dodesyl sulfate–polyacrylamide gels. Hsp27 was observed by Western blotting, using a specific monoclonal antibody (Neo Markers, Fremont, CA, USA) and incubating the blot overnight at 4°C. Subsequent incubation was performed with peroxidase-conjugated rabbit anti-mouse antibody (Dako Diagnostics Ag, Vienna, Austria) and detected by chemoluminiscence according to instructions of the manufacturer (ECL, Amersham Pharmacia Biotech, Buckinghamshire, UK).
Ribonucleic acid extraction and reverse transcription
Total ribonucleic acid (RNA) was purified from A375 cells according to the procedure supplied with the RNAzol™ B reagent (CS-104, Biomedica, Vienna, Austria). HepG2 cells were used as a positive control. The RNA pellet was washed with 70% ethanol, air-dried, and dissolved in water. Two micrograms of the RNA preparation was incubated for 5 minutes at 95°C and then quickly chilled in an ice bath. Samples were reverse transcribed into complementary deoxyribonucleic acid (cDNA) in a final volume of 50 μL using random hexamers (2.5 μL, 100 pM/μL) (Roche Diagnostics GmbH, Vienna, Austria) and Moloney murine leukemia virus reverse transcriptase (1.5 μL, 150 U) as directed by the manufacturer (Promega, Mannheim, Germany).
Amplification of specific cDNA by polymerase chain reaction
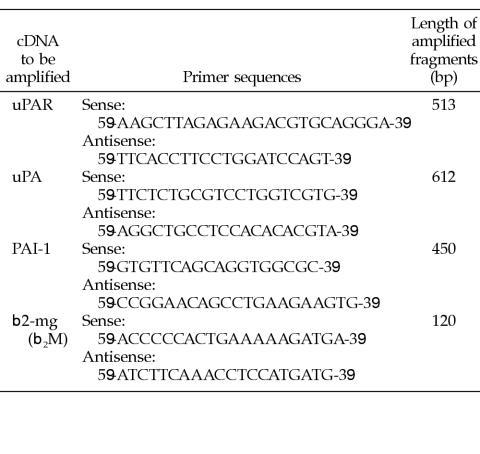
A total of 5 μL of the cDNA was used in the polymerase chain reaction (PCR). Oligonucleotide primers were obtained from Vienna Biocenter (VBC-Genomics Bioscience Research, Vienna, Austria). Amplifications were as follows: polymerase buffer (Roche Diagnostics) with 1.5 mM MgCl2 (β2-microglobulin) or 0.75 mM MgCl2 (uPA, PA inhibitor type 1 [PAI-1], uPAR), 0.2 mM of each deoxynucleoside triphosphate (Roche Diagnostics), 10 pmol (β2-microglobulin) and 20 pmol (uPA, PAI-1, uPAR) of sense and antisense primers (the sequences are listed in the table), and 1 U Taq DNA-polymerase (Roche Diagnostics) in a final volume of 50 μL.
PCR condition was an initial denaturation step at 95°C for 5 minutes; 30 cycles of 30 seconds denaturation at 94°C, 30 seconds primer annealing at 60°C, and 30 seconds extension at 72°C; and a final elongation step at 72°C for 7 minutes. PCR was performed on a DNA thermal cycler 2400 (Perkin Elmer, Norwalk, Connecticut, USA).
Negative controls without DNA were included to assess cross-contamination of the samples. The efficiency of cDNA synthesis from each sample was estimated by PCR with β2-microglobulin–specific primers.
Twenty microliters of the PCR products was resolved on a 2% ethidium bromide–stained agarose gel electrophoresis.
 |
FACS and IF analysis
Cell suspensions (1 × 106 cells/100 μL phosphate-buffered saline [PBS]) were incubated with anti–E-cadherin and anti–MUC18/MCAM antibody for 45 minutes at 4°C (5 μg E-cadherin, HECD-1, Alexis Biochemicals, Eubio, Vienna, Austria; 1 μg MUC18 [CD146]), kindly supplied by Dr. Majdic (Pickl et al 1997). The cells were washed and incubated with secondary phycoerythrin (PE)–labeled anti-immunoglobulin (Ig) (20 μL, Becton Dickinson, San Jose, CA) for 45 minutes at 4°C. After a final washing, the cells (500 μL PBS) were analyzed with a flow cytometer (FACS Calibur, Becton Dickinson, immunocytometry systems). Control serum, subclass-matched Ig, was used as the negative control (Becton Dickinson).
For IF staining, cytospin preparations, incubated with anti–E-cadherin or anti–MUC18/MCAM antibodies, followed by secondary PE-labeled anti-IgG, were analyzed with indirect IF confocal microscopy.
Enzyme-linked immunosorbent assay measurements of secreted uPA and PAI-1
Cell lines (7 × 105 cells) were incubated for 24 hours in medium (2 mL, T12.5 cm2) without serum. Culture medium was collected and separated from residual cells by centrifugation. The samples were stored at −80°C until analysis.
Enzyme-linked immunosorbent assays (ELISAs) were performed according to instructions of the manufacturer (IMUBIND uPA ELISA kit no. 894, IMUBIND PAI-1 ELISA kit no. 822, American Diagnostics, Greenwich, CT, USA).
Growth on fibrin matrix
Hsp27-transfected cells (7 × 105) were harvested and suspended in serum-free DMEM (200 μL) containing control Ig (5 μg, Becton Dickinson) or anti–E-cadherin antibody (5 μg, Alexis Biochemicals). To support the interaction between antibodies and antigen on the basolateral region of the cells (Maillet and Buc-Caron 1985), the cell suspensions were incubated by gentle shaking for 2 hours at 37°C. Serum-free medium was added (1.8 mL), and the cells were seeded on fibrin matrix gels (4 mL) in T12.5 cm2 flasks and incubated at 37°C in 7.5% CO2 for 48 hours. After incubation, images of cell aggregates were captured with a Nikon E300 inverted microscope (magnification 100×). Fibrin matrix was prepared by adding fibrinogen (sterile-filtered, 3 mg/mL, Sigma-Aldrich, Vienna, Austria) to the flasks followed by addition of thrombin (0.4 U/mL, Sigma-Aldrich).
Enzyme-linked immunosorbent assay measurements of secreted uPA after incubation with E-cadherin antibodies
To support the interaction between antibodies and antigen on the basolateral region of the cells (Maillet and Buc-Caron 1985), Hsp27-transfected cells (7 × 105) were incubated by gentle shaking for 2 hours at 37°C with either control Ig or anti–E-cadherin antibody (5 μg) in serum-free media (200 μL). After incubation, serum-free media (1.8 mL) were added to the cell suspensions, and the cells were further incubated in flasks (T12.5 cm2) for 24 hours. Conditioned media were collected and analyzed for uPA secretion. Cells treated with control Ig were used as positive controls.
RESULTS
To assess the influence of Hsp27 expression on cellular processes linked to differentiation and to the transition of melanoma cell from high to low metastatic potential (Aldrian et al 2002), Hsp27 transfectants from the A375 melanoma cell line and the cell lines transfected with the plasmid for neo as well as wild-type cells were analyzed by Western blotting. High expression levels of Hsp27 were only found in Hsp27-transfected cells (Fig 1). The protein band of lower molecular weight indicates that Hsp27 is processed or degraded, which is in accordance with other reports (Brenner et al 1995).
Fig 1.
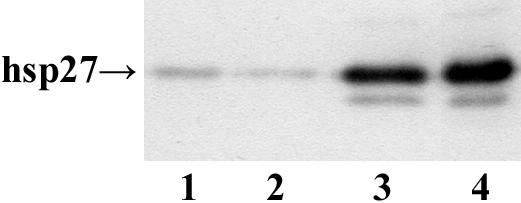
Western blot analysis of Hsp27-transfected and control cells (A375). Cell lysates (10 μg) were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto the nitrocellulose membrane, and analyzed by using the anti-human Hsp27 antibody. Lanes 1–4: A375/wt, A375/neo, A375 Cl 2A, and A375 Cl 11A
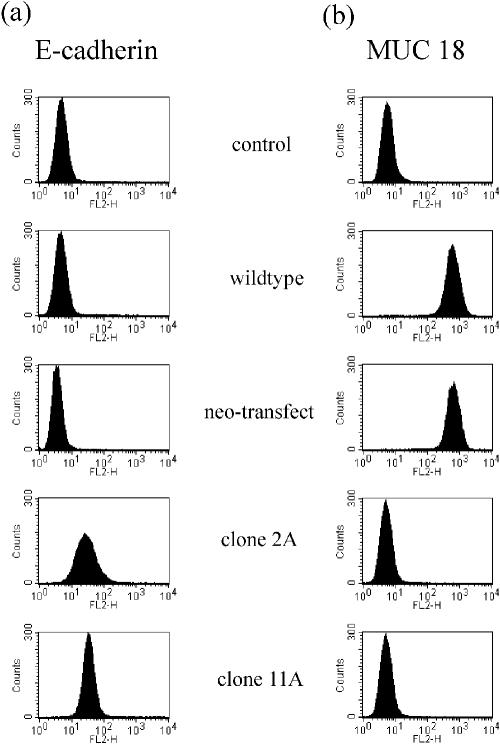
Because it is known that E-cadherin is a regulator of differentiation, we investigated whether Hsp27 expression may have an influence on the E-cadherin expression pattern. Hsp27-transfected cell lines as well as control cells were incubated with antibodies raised against the extracellular domain of the E-cadherin subunit and analyzsed by FACS. Elevated expression of E-cadherin was found in Hsp27 transfectant, whereas control cells failed to express this subunit (Fig 2a).
Fig 2.
Fluorescence-activated cell sorter (FACS) analysis of E-cadherin (a) and MUC18/MCAM (b) expression in Hsp27-transfected and control cells (A375). Cells were incubated with antibodies to the respective adhesion molecule and analyzed by FACS. Control serum, subclass-matched immuno-globulin, was used as control
It has been shown that loss of E-cadherin is associated with an induction of the surface adhesion molecule MUC18/MCAM (Johnson 1999). Thus, expression of this cell adhesion molecule correlates with metastatic potential in melanoma cells. MUC18/MCAM expression was determined by FACS analysis, using antibodies reacting with the extracellular subunit. Wild-type cells as well as neo control cells expressed high levels of MUC18/MCAM, whereas Hsp27-transfected cells did not stain positively with this specific antibody (Fig 2b).
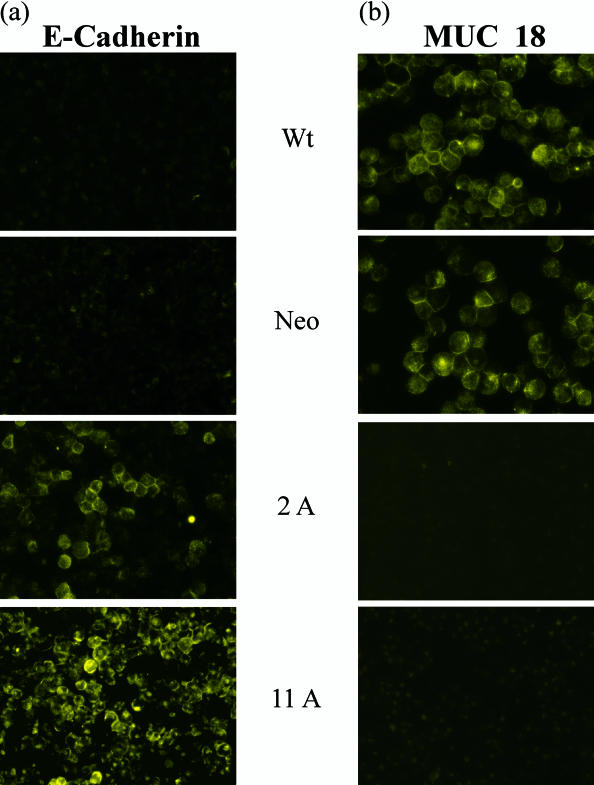
To confirm the results of FACS analysis (Fig 2), cytospin preparations of the respective clones and control cells, incubated with anti–E-cadherin and anti–MUC18/MCAM antibodies, were analyzed with indirect IF confocal microscopy. As compared with FACS analysis, E-cadherin was localized on the surface of Hsp27 transfectants, whereas control cells were not stained (Fig 3a). Cytospin preparations, incubated with anti–MUC18/MCAM antibody, localized the expression of MUC18/MCAM at the cell membrane of the control cells (Fig 3b), whereas no staining was found in Hsp27-transfected cells, which is in agreement with the data of FACS analysis (Fig 2).
Fig 3.
Immunofluorescence (IF) analysis of E-cadherin (a) and MUC18/MCAM (b) in Hsp27-transfected and control cells (A375). Cytospin preparations incubated with anti–E-cadherin and anti-MUC18 antibodies were analyzed by indirect IF confocal microscopy
The uPA system regulates the rate of tissue degradation and cell migration and invasion, and the activity can be determined according to a proteolytic as well as nonproteolytic mechanism (Andreasen et al 1997).
The uPAR is a cell membrane–binding protein for uPA, accumulating plasminogen activation activity at cell surfaces. Using reverse transcriptase–PCR (RT-PCR) we found that the uPAR gene expression revealed a high expression of uPAR messenger RNA (mRNA) in control cells as well as in Hsp27-transfected cells (Fig 4a). With regard to the levels of uPA and its inhibitor PAI-1, RT-PCR analysis revealed a complete absence of mRNA in both wild-type and neo-transfected cells (Fig 4 b,c). However, in Hsp27-transfected cells a high level of uPA mRNA was found. An increase was also shown in PAI-1, which suggests a neutralization of the proteolytic activity of uPA in these cells.
Fig 4.
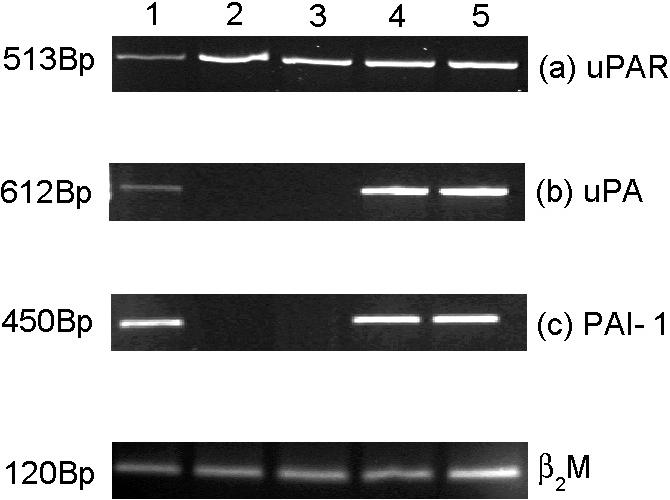
Reverse transcriptase–polymerase chain reaction (PCR) analysis of urokinase-type plasminogen activator receptor (uPAR) (a), uPA (b), and PAI-1 (c) in Hsp27-transfected and control cells (A375). Lanes 1–5: HepG2-positive control cells, A375/wt, A375/neo, A375/2A, A375/11A. The efficiency of complementary deoxyribonucleic acid synthesis of each sample was estimated by PCR with β2-microglobulin–specific primers (d)
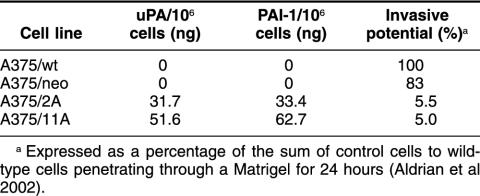
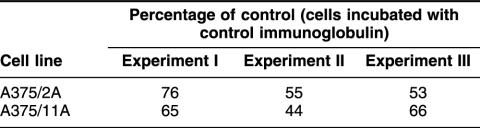
Because of the striking difference in uPA and PAI-1 mRNA expression between control cells and Hsp27 transfectants, an ELISA was also performed. Control cells failed to express uPA and PAI-1 in contrast to Hsp27-transfected cells (Table 1), which released considerable amounts of uPA and PAI-1, thus confirming the results of the RT-PCR analysis shown in Fig 4.
Table 1.
Expression of urokinase-type plasminogen activator (uPA) and its inhibitor, PA inhibitor type 1 (PAI-1), in Hsp27-transfected and control cells (A375)
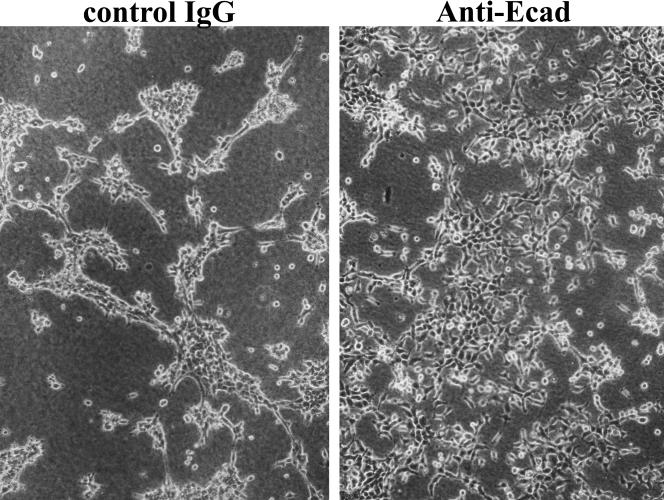
uPA has been shown to have fibrinolytic activity, resulting in a formation of cell aggregates on fibrin (Gold et al 1989; Sasaki et al 1999). Moreover, it has been demonstrated by IF analysis that uPA colocalizes with E-cadherin at cell-cell junctions and also that a blocking antibody to E-cadherin inhibits the fibrinolytic activity of uPA. To determine whether E-cadherin is involved in the activation of uPA in A375 Hsp27-transfected cells, positive for E-cadherin expression, cells were seeded overnight at 37°C, on fibrin in the presence of control Ig or anti–E-cadherin antibodies. Cells incubated with control Ig showed fibrinolytic activity by the ability to form cell aggregates consisting of compacted cells on fibrin. This aggregation of cells on fibrin was partly inhibited by anti–E-cadherin antibody, indicating an inhibition of uPA activity by blockade of E-cadherin (Fig 5).
Fig 5.
E-cadherin and fibrinolytic activity. Inhibition of cellular compaction on fibrin by anti–E-cadherin antibody. Hsp27-transfected cells (A375/11A) were seeded on fibrin in the presence of either control immunoglobulin or anti–E-cadherin antibodies and incubated for 48 hours at 37°C
To further test for regulation of uPA expression by E-cadherin, Hsp27-transfected cells were incubated with control Ig or anti–E-cadherin antibodies overnight. Conditioned media were collected, and uPA levels were analyzed by ELISA. Compared with control Ig, the uPA secretion was reduced by the addition of anti–E-cadherin antibody (Table 2). These findings indicate that in Hsp27-transfected cells E-cadherin has an effect on uPA expression in these cells.
Table 2.
Urokinase-type plasminogen activator secretion in Hsp27-transfected cells incubated with anti–E-cadherin antibodies
DISCUSSION
Recently we have demonstrated that Hsp27 overexpression resulted in a reversal of the malignant phenotype of the A375 cell line in vitro (Aldrian et al 2002). The lost integrin receptor αvβ3 and the reduction of MMP-2 and MMP-9 secretion correlated with decreased invasive potential. In the present study, the role of Hsp27 transfectants in the melanoma cell line A375 was examined in relation to the expression and regulation of cell adhesion molecules E-cadherin, MUC18/MCAM, and PA system.
The role of cell adhesion molecules in carcinogenesis has been ambiguous because they can act as anchors to stabilize the cell's position or as grips for moving cancer cells. Thus, their function may be interpreted as both invasion suppressor and invasion promotor molecules.
E-cadherin is a member of the cadherin family, cell adhesion proteins that are expressed in a variety of tissues. E-cadherin is expressed in epithelial cells, where it is localized to the adherence junctions, a belt-like structure surrounding the cell and binding to its neighbors (Bracke et al 1996). It is also able to stimulate gap junctional communication between adjacent cells. E-cadherin plays a role in cell differentiation, normal development, and cell migration (Herlyn et al 2000). Loss of expression or function of E-cadherin has been observed in several different human carcinomas (Buck 1995; Bracke et al 1996; Guilford 1999). This seems to represent one of the earliest changes in the development of the metastatic process, releasing the cells from adhesion-mediated controls of neighboring cells and enabling them to separate from primary tumor masses (Birchmeier and Behrens 1994).
In a transgenic mouse model Perl et al (1998) showed that loss of E-cadherin expression coincides with the transition from well-differentiated adenoma to invasive carcinoma. Forced expression of E-cadherin by melanoma cells through gene transduction has been shown to have profound effects on the phenotype of melanoma cells. Cells lose their invasive capacity by no longer expressing the invasion-related adhesion receptors, namely β3 integrin subunit and MUC18/MCAM (Hodivala and Watt 1994; Hsu et al 2000).
We have reported (Kindas-Mügge et al 1996) that Hsp27 overexpression in A375 melanoma cells was accompanied by the inhibition of the growth rate in vitro and by a delay and a reduced rate of tumor appearance in athymic nude mice. In the present study, using FACS analysis as well as IF, we found that Hsp27 transfectants displayed an increased expression of E-cadherin as compared with control cells. These data suggest that Hsp27-mediated signal transduction may also contribute to regulation of the expression of adhesion molecules, particularly of E-cadherin. The cell surface adhesion molecule MUC18/MCAM is expressed on metastatic melanoma but not on normal melanocytes (Shih et al 1994; Xie et al 1997), and this correlates directly with the metastatic potential of human melanoma cells. MUC18/MCAM, analyzed by FACS and IF, was highly expressed in wild-type and neo transfected cells. These cells were shown to be negative for E-cadherin expression. In Hsp27-transfected cells, positive for E-cadherin, no expression of MUC18/MCAM was observed.
As mentioned before, we have shown a decreased invasiveness, down-regulation of αvβ3 integrin expression, and loss of metastatic potential in Hsp27-overexpressing A375 cell clones (Aldrian et al 2002). The association of a decreased invasive phenotype with gain of E-cadherin and loss of MUC18/MCAM is in accordance with the data from Johnson (1999) and Hsu et al (2000). Both found that dysregulation of E-cadherin function is associated with induction of MUC18/MCAM and αvβ3 in melanoma development and may indicate a critical event in melanoma progression.
The uPAR present on the surface of most cell types is a key component in the control of cell adhesion, cell migration, and extracellular proteolysis. There are two mechanisms contributing to the effect of uPA on cell migration (Andreasen et al 1997), a proteolytic action as well as a nonproteolytic action, which is coupled to cell adhesion and transmembrane signaling.
The involvement of constituents of the plasmin activation system was investigated in our model system. mRNA for the urokinase receptor (uPAR) was detected in all the cell lines analyzed. In contrast, striking differences were observed in the expression of uPA and its inhibitor, PAI-1. Hsp27 transfectants, which have a decreased aggressive growth behavior in vitro (Aldrian et al 2002), showed enhanced mRNA expression of uPA as well as its inhibitor, PAI-1. uPA and its inhibitor (PAI-1) bind to uPAR. PAI-1 is known to regulate the activity of uPA, thus suppressing uPAR-mediated cell adhesion and proteolysis (Deng et al 1996). One might speculate that the enzymatic activity of uPA is regulated by its inhibitor, PAI-1, also indicating a nonproteolytic role for the uPA system in Hsp27-transfected cells.
Hsp27 seems to up-regulate both uPA and its inhibitor simultaneously. This is in accordance with the study of Tran-Thang et al (1996). These authors demonstrated that the induction with inflammatory cytokines results in an up-regulation of uPA as well as PAI-1 in SW 620 colon cancer cells. No effect on induction of PAI-1 was found in SW 1116 cells.
It has been demonstrated that E-cadherin plays a role in organizing the cellular distribution of uPA in keratinocytes (Jensen and Wheelock 1992). Sasaki et al (1999) have shown that in noninvasive mammary epithelia cells the regulation of uPA was mediated by E-cadherin. By the addition of anti–E-cadherin antibody, the activation of uPA was altered.
uPA has been shown to mediate fibrinolytic activity, which results in cell aggregation on fibrin (Gold et al 1989; Sasaki et al 1999). Anti–E-cadherin antibody inhibited this activation. The effect of E-cadherin on uPA expression in the Hsp27-positive cells was investigated in a fibrinolytic activity assay. Interruption of cell-cell contacts by anti–E-cadherin antibodies prevented at least partly cellular aggregation on fibrin. We also found a decreased level of uPA secretion in conditioned medium, collected from Hsp27-transfected cells, incubated overnight with anti–E-cadherin antibody. These findings indicate an effect of E-cadherin on uPA activity in our cell model.
Our data provide evidence of a pronounced influence of Hsp27 expression on the tumor progression of melanoma. This effect might be based on an induction of E-cadherin expression and the consequent reduction of MUC18 and changes in the PA system. This observation might lead to promising novel targeted strategies for the therapy of human melanoma.
Acknowledgments
The technical assistance of Eva Sandor is gratefully acknowledged.
REFERENCES
- Aldrian S, Trautinger F, Fröhlich I, Micksche M, Kindas-Mügge I. Overexpression of Hsp27 affects the metastatic potential in vitro in a melanoma cell line. Cell Stress Chaperones. 2002;7:177–185. doi: 10.1379/1466-1268(2002)007<0177:oohatm>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen P, Kjoller L, Christensen L, Duffy M. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochem Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Bracke M, Van Roy F, Mareel M. The E-cadherin/catenin complex in invasion and metastasis. Curr Top Microbiol Immunol. 1996;213:123–161. doi: 10.1007/978-3-642-61107-0_9. [DOI] [PubMed] [Google Scholar]
- Brenner B, Tao Y, Pearson E, Remer I, Wainberg M. Altered constitutive and stress-regulated heat shock protein 27 expression in HIV type 1-infected cell lines. AIDS Res Hum Retrovir. 1995;11:713–717. doi: 10.1089/aid.1995.11.713. [DOI] [PubMed] [Google Scholar]
- Buchner J. Supervising the fold: functional principles of molecular chaperones. FASEB J. 1996;10:10–19. [PubMed] [Google Scholar]
- Buck C 1995 Adhesion mechanisms controlling cell-cell and cell-matrix interactions during the metastatic process. In: The Molecular Basis of Cancer, ed Mendelsohn J, Howley P, Israel M, Liotta L. W.B. Saunders, Philadelphia, PA, 172–205. [Google Scholar]
- Ciocca D, Oesterreich S, Chamness G, McGuire W, Fugua A. Biological and clinical implications of heat shock protein 27 000(hsp27). A review. J Natl Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- Deng G, Curriden S, Wang S, Rosenberg S, Loskutoff D. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernold M, Knauf U, Gaestel M, Stahl J, Koetzel P. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14:103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- Gold L, Schwimmer R, Quigley J. Human plasma fibronectin as a substrate for human urokinase. Biochem J. 1989;262:529–534. doi: 10.1042/bj2620529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P. E-cadherin downregulation in cancer: fuel on the fire? Mol Med Today. 1999;5:172–177. doi: 10.1016/s1357-4310(99)01461-6. [DOI] [PubMed] [Google Scholar]
- Hell-Pourmojib M, Neuner P, Fischer H, Rezaie S, Kindas-Mügge I, Knobler R, Trautinger F. Differential expression of a novel gene in response to hsp27 and cell differentiation in human keratinocytes. J Investig Dermatol. 2002;119:154–159. doi: 10.1046/j.1523-1747.2002.01793.x. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Berking C, Li G, Satyamoorthy K. Lesions from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. 2000;10:303–312. doi: 10.1097/00008390-200008000-00001. [DOI] [PubMed] [Google Scholar]
- Hightower L. Heat shock proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hodivala K, Watt F. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Meier F, Nesbit M, Hsu J, Van Belle P, Elder D, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156(5):1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantschitsch CH, Kindas-Mügge I, Metze D, Amann G, Micksche M, Trautinger F. Expression of the small heat shock protein hsp27 in developing human skin. Br J Dermatol. 1998;139:247–253. doi: 10.1046/j.1365-2133.1998.02361.x. [DOI] [PubMed] [Google Scholar]
- Jensen P, Wheeloock M. Regulation of urokinase plasminogen activator localization in keratinocytes by calcium ion and E-cadherin. Exp Cell Res. 1992;202:190–198. doi: 10.1016/0014-4827(92)90419-9. [DOI] [PubMed] [Google Scholar]
- Johnson J. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- Kato K, Hasegawa K, Goto S, Inaguma Y. Dissociation as a result of phosphorylation of an aggregated form of the small heat shock protein hsp27. J Biol Chem. 1994;269:11274–11278. [PubMed] [Google Scholar]
- Kindas-Mügge I, Herbacek I, Jantschitsch Ch, Micksche M, Trautinger F. Modification of growth and tumorigenicity in epidermal cell lines by DNA-mediated gene transfer of 27 000 heat shock protein (hsp27) Cell Growth Differ. 1996;7:1167–1174. [PubMed] [Google Scholar]
- Kindas-Mügge I, Micksche M, Trautinger F. Modification of growth in small heat shock (hsp27) gene transfected breast carcinoma. Anticancer Res. 1998;18:413–418. [PubMed] [Google Scholar]
- Kindas-Mügge I, Trautinger F. Increased expression of the 27 000 heat shock protein (hsp27) in in vitro differentiated normal human keratinocytes. Cell Growth Differ. 1994;5:777–781. [PubMed] [Google Scholar]
- Knauf U, Bielka H, Gaestel M. Overexpression of the small heat shock protein hsp25 inhibits growth of Ehrlich ascites tumor cells. J Cell Physiol. 1993;156:619–625. [Google Scholar]
- Lavoie J, Hickey E, Weber L, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- Lehmann J, Holzmann B, Breitbart E, Schmiegelow P, Rietthmüller G, Johnson J. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113.000 and a protein with a moelcular weight of 76.000. Cancer Res. 1987;47:841–847. [PubMed] [Google Scholar]
- Maillet L, Buc-Caron M. Modulation of specific protein expression in teratocarcinoma cell aggregates by antibodies affecting cell-cell interactions. Dev Biol. 1985;111:1–7. doi: 10.1016/0012-1606(85)90429-4. [DOI] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay R. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R, Tissieres A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperons. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1–30. [Google Scholar]
- Pauli D, Tonka CH, Tissieres A, Arrigo A. Tissue-specific expression of the heat shock protein hsp27 during Drosophila melanogaster development. J Cell Biol. 1990;111:817–828. doi: 10.1083/jcb.111.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Pickl W, Majdic O, and Fischer G. et al. 1997 MUC18/MCAM: an activation associated surface molecule of human T lymphocytes. J Immunol. 158:2107–2115. [PubMed] [Google Scholar]
- Preissner K, Kanse S, May A. Urokinase receptor: a molecular organiser in cellular communication. Curr Opin Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- Richards E, Hickey E, Weber L, Master J. Effect of overexpression of the small heat shock protein hsp27 on the heart and drug sensitivities of human testis tumor cells. Cancer Res. 1996;56:2446–2451. [PubMed] [Google Scholar]
- Sasaki C, Lin H, Passaniti A. Regulation of urokinase plasminogen activator (uPA) activity by E-cadherin and hormones in mammary epithelial cells. J Cell Phys. 1999;1881:1–13. doi: 10.1002/(SICI)1097-4652(199910)181:1<1::AID-JCP1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Seline P, Norris D, Horkawa T, Fujita M, Middleton M, Morelli J. Expression of E and P-cadherin by melanoma cells decreases in progressive melanomas and following ultraviolet radiation. J Investig Dermatol. 1996;106:1320–1324. doi: 10.1111/1523-1747.ep12349048. [DOI] [PubMed] [Google Scholar]
- Shakoori A, Obersdorf A, Weber L, Hickey E, Stein J, Lian J, Stain G. Expression of heat shock protein genes during differentiation of mammalian osteoblasts and promyelocytic leukaemia cells. J Cell Biochem. 1992;48:277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- Shih I, Elder D, Hsu M, Herlyn M. Regulation of Mel-CAM/MUC18 expression on melanocytes of different stages of tumor progression by normal keratinocytes. 1994. Am J Pathol. 1994;145:837–845. [PMC free article] [PubMed] [Google Scholar]
- Spector N, Hardy L, Ryan C, Miller W Jr, Humes J, Nadler L, Luedke E. 28-kDa mammalian heat shock protein, a novel substrate of a growth regulatory protease involved in differentiation of human leukaemia cells. J Biol Chem. 1995;270:1003–1006. doi: 10.1074/jbc.270.3.1003. [DOI] [PubMed] [Google Scholar]
- Spector N, Ryan C, Levine H, Nadler L, Arrigo A. Heat shock protein is a unique marker of growth arrest during differentiation of HL-60 cells. J Cell Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- Spector N, Samson W, Ryan C, Gribben J, Urba W, Welch W, Nadler L. Growth arrest of human B lymphocytes is accompanied by induction of the low molecular weight mammalian heat shock protein (hsp28) J Immunol. 1992;148:1668–1673. [PubMed] [Google Scholar]
- Stahl J, Wobus A, Ihrig S, Lutsch G, Bielka H. The small heat shock protein hsp25 is accumulated in P19 embryonal carcinoma cells and embryonic stem cells of line BLC6 during differentiation. Differentiation. 1992;51:33–37. doi: 10.1111/j.1432-0436.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Tran-Thang C, Kruithof E, Lahm E, Schuster W, Tada M, Sordat B. Modulation of the plasminogen activation system by inflammatory cytokines in human colon carcinoma cells. Br J Cancer. 1996;74:846–852. doi: 10.1038/bjc.1996.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautinger F, Kindas-Mügge I, Dekrout B, Knobler R, Metze D. Expression of the 27 kDa heat shock protein in human epidermis and in epidermal neoplasms: an immuno-histological study. Br J Dermatol. 1995;133:194–202. doi: 10.1111/j.1365-2133.1995.tb02615.x. [DOI] [PubMed] [Google Scholar]
- Xie S, Luca M, Huang S, Gutman M, Reich R, Johnson J, Bar-Eli M. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res. 1997;57:2295–2303. [PubMed] [Google Scholar]