Introduction
Ecthyma is an invasive form of impetigo that penetrates deep into the dermis. This cutaneous infection is characterized by a crusted papule or plaque beneath which a deep ulcer forms. Although typically associated with group A β-hemolytic streptococci, other pathogens have the ability to produce an ecthyma.1 Here we present a case of ecthyma from which Moraxella and Staphylococcus epidermidis were isolated. Moraxella has never been reported to cause ecthyma, and there is 1 reported case of ecthyma caused by S epidermidis.2
Case report
The patient is an 81-year-old man with hypertension and obesity who was admitted for management of acute pancreatitis. In the setting of narcotic-induced hypoventilation and obesity, the patient had respiratory failure requiring noninvasive positive pressure ventilation (NIPPV). On the seventh day of his hospital stay, he had a new 1.5-cm x 2.0-cm mildly tender necrotic ulcer on the bridge of his nose. The ulcer had an erythematous base and slightly indurated, erythematous border (Fig 1).
Fig 1.
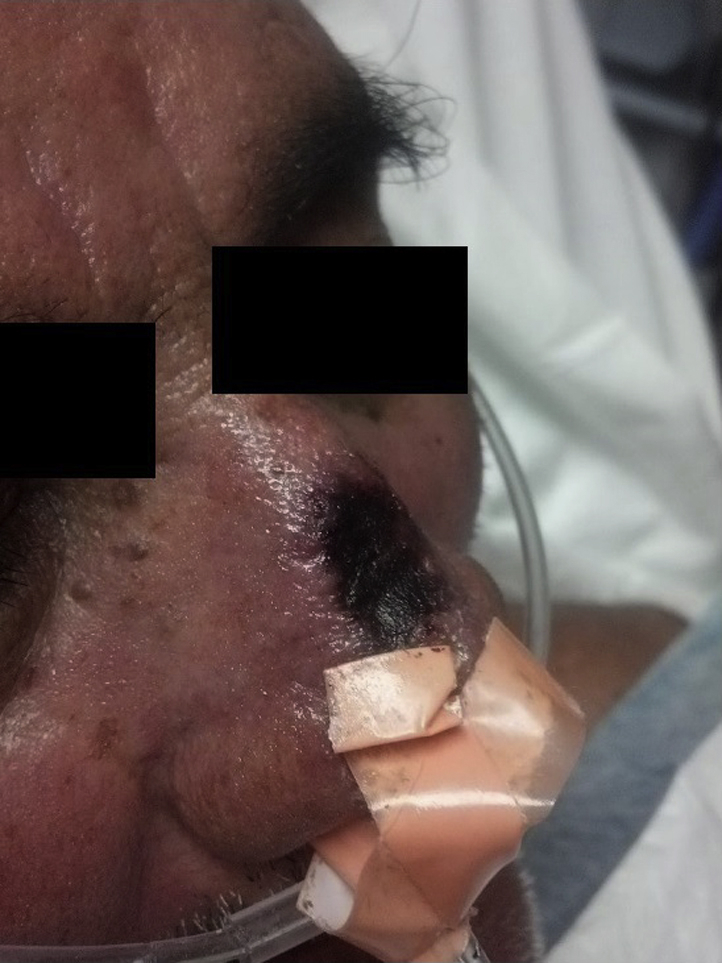
Necrotic eschar covering ulcer with an erythematous border on the nasal bridge.
The patient denied headache, visual changes, epistaxis, cough, hematemesis, fevers, chills, night sweats, ocular pain, rhinorrhea, nasal congestion, dysphagia, odynophagia, and stomatodynia. He denied recent foreign travel, insect bites, and exposure to animals. He had no history of HIV or tuberculosis infection.
Several infections were included in the differential diagnosis, including bacterial infections and mycobacterial infections. Because of the rapid progression of the lesion, the differential diagnosis included aggressive angioinvasive fungal infections such as zygomycosis. Vasculitic and vasculopathic causes were also considered in the setting of the patient's acute illness.
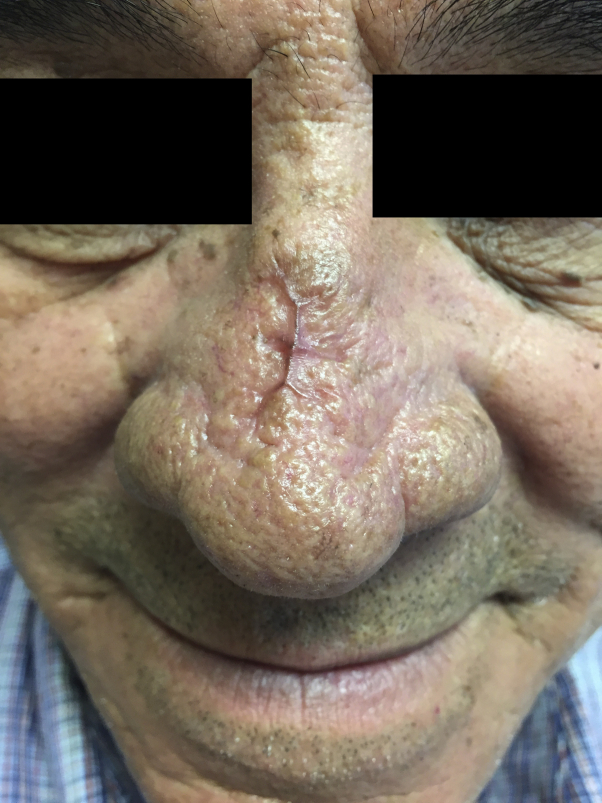
Two 3-mm punch biopsy sections were obtained from the indurated, inflamed edge of the eschar, including both affected and unaffected skin. Routine histology findings showed deep epidermal erosion, fibrin deposition, necrosis of the hair follicles and sebaceous glands with neutrophils, and evidence of bacteria within the erosion and papillary dermis. Tissue culture found heavy growth of β-lactamase-negative Moraxella species and heavy growth of S epidermidis. The patient did not have fever, bacteremia, or lymphadenopathy. Blood cultures were normal. The patient was started on a 2-week course of sulfamethoxazole-trimethoprim, 800 mg - 160 mg twice daily, and bacitracin-polymyxin B ointment topically twice daily. The ecthyma quickly resolved. He was seen in follow-up 2 months later, with a well-healed, 2-cm cicatrix without surrounding erythema (Fig 2).
Fig 2.
Nasal bridge after 2-week course of sulfamethoxazole-trimethoprim.
Discussion
To the best of our knowledge, this is the first report of ecthyma produced by Moraxella and S epidermidis. Moraxella is a gram-negative bacterium. The most common clinically significant species of Moraxella is Moraxella catarrhalis, which is part of the normal human flora of the upper respiratory tract. M catarrhalis can be transmitted via droplet spread or direct contact with contaminated secretions. It is a common cause of sinusitis and otitis media, forms a biofilm within the nasopharynx, and can cause bacteremia in patients with trans-nasal devices.3, 4 Several case reports document preseptal cellulitis caused by Moraxella in patients who are colonized by Moraxella within their nasopharynx or sinus cavities.5, 6, 7 This organism is also capable of initiating more morbid conditions, such as necrotizing fasciitis.8
Bacterial skin infections by Moraxella typically follow a 3-step process.9 First, Moraxella adheres to the extracellular matrix of epithelial cells via integral outer membrane proteins, the ubiquitous surface protein A, and the type IV pili.10 The second step is invasion of the host epithelium by Moraxella. This invasion may be facilitated by a microfilament-dependent trigger uptake mechanism, as has been shown in a tissue invasion culture assay of pulmonary epithelial cells.11 The multifunctional type IV pili also enhances Moraxellas ability to invade the host epithelium and establish a biofilm along mucous membrane surfaces.12 This active method of invasion is in contrast to the classical model of microtubule-dependent host cell–mediated endocytosis. Finally, the third step is immune system evasion and the production of inflammatory toxins. Moraxella has the unique ability to inhibit the toll-like receptor 2-mediated proinflammatory response via ubiquitous surface protein A interaction with a host cell receptor.10 Thus, Moraxella can effectively evade the immune system by means of T-cell response inhibition.
Similar to Moraxella in the respiratory tract, S epidermidis is a part of the normal human skin flora. Also like Moraxella, S epidermidis is capable of epithelial cell adhesion, invasion, and biofilm formation. These functions are facilitated by various exopolysaccharides and proteases.13 S epidermidis expresses the polysaccharide intercellular adhesin, which enables biofilm formation. S epidermidis also secretes several exotoxins, which injure host tissue and proteins. For example, the serine protease Esp degrades fibrinogen and complement factor C5, and the protease SepA degrades human antimicrobial peptides.13 In this way, S epidermidis exhibits virulent behavior.
Although uncommon, S epidermidis is reported to cause deep skin infections alone and in combination with other bacterial species. S epidermidis alone is reported to cause an ecthyma gangrenosum infection in an allogenic bone marrow transplant patient.2 Concomitant infection with S epidermidis and Pseudomonas aeruginosa was documented in a unique case of ecthyma gangrenosum in a patient with chronic renal failure.14
Thus, we present a rare case of ecthyma produced by Moraxella and S epidermidis. The patient may have been colonized by Moraxella and S epidermidis in the respiratory tract and on the skin, respectively. Just as excoriations or arthropod bites can provide a portal of entry for superinfection,15 an initial abrasion caused by the pressure and friction of the NIPPV mask likely facilitated invasion by Moraxella and S. epidermis. Moreover, the rapid formation of the ulcer, the presence of a thick hemorrhagic crust, continued progression after discontinuation of the NIPPV mask, presence of bacteria within the deep epidermal erosion, heavy growth of both organisms from the ulcer border on culture, and resolution with a thick scar support the clinical diagnosis of ecthyma.15, 16 This case suggests the ability of Moraxella and S epidermidis to produce an ecthyma and emphasizes the importance of considering both pathogens as potential causes of deeper soft tissue infections, particularly in ill patients receiving assisted ventilation or other instrumentation or manipulation of the upper respiratory tract.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Chiller K., Selkin B.A., Murakawa G.J. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc. 2001;6(3):170–174. doi: 10.1046/j.0022-202x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 2.Miyake S., Nobeyama Y., Baba-Honda H., Nakagawa H. Case of ecthyma gangrenosum in which only methicillin-resistant Staphylococcus epidermidis was detected. J Dermatol. 2015;43(4):460–462. doi: 10.1111/1346-8138.13228. [DOI] [PubMed] [Google Scholar]
- 3.Funaki T., Inoue E., Miyairi I. Clinical characteristics of the patients with bacteremia due to Moraxella catarrhalis in children: a case-control study. BMC Infect Dis. 2016;16(1):73. doi: 10.1186/s12879-016-1408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy T.F., Parameswaran G.I. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49(1):124–131. doi: 10.1086/599375. [DOI] [PubMed] [Google Scholar]
- 5.Cox N.H., Knowles M.A., Porteus I.D. Pre-septal cellulitis and facial erysipelas due to Moraxella species. Clin Exp Dermatol. 1994;19(4):321–323. doi: 10.1111/j.1365-2230.1994.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 6.Tritton T., Watts J., Sieratzki J.S. Peri-orbital cellulitis and sepsis by Branhamella catarrhalis. Eur J Pediatr. 1998;157(7):611–612. doi: 10.1007/s004310050894. [DOI] [PubMed] [Google Scholar]
- 7.Rotta A.T., Asmar B.I. Moraxella Catarrhalis Bacteremia and Preseptal Cellulitis. South Med J. 1994;87(4):541–542. doi: 10.1097/00007611-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Brittain C.J., Penwarden A., Mearza A., Verity D. Moraxella as a cause of necrotizing fasciitis of the eyelid. Eye. 2006;20:1312–1314. doi: 10.1038/sj.eye.6702173. [DOI] [PubMed] [Google Scholar]
- 9.Ki V., Coleman R. Bacterial Skin and Soft Tissue Infections in Adults: A Review of their Epidemiology, Pathogenesis, Diagnosis, Treatment and Site of Care. Can J Infect Dis Med Microbiol. 2008;19(2):173–184. doi: 10.1155/2008/846453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vries S.P., Bootsma H.J., Hays J.P., Hermans P.W. Molecular aspects of Moraxella catarrhalis pathogenesis. Microbiol Mol Biol Rev. 2009;73(3):389–406. doi: 10.1128/MMBR.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slevogt H., Seybold J., Tiwari K.N. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell Microbiol. 2007;9(3):694–707. doi: 10.1111/j.1462-5822.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 12.Luke N.R., Jurcisek J.A., Bakaletz L.O., Campagnari A.A. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun. 2007;75(12):5559–5564. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otto M. Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol. 2012;34(2):201–214. doi: 10.1007/s00281-011-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey J.D., Latkowski J.A., Louie E., Chiu E.S. Diagnosis and management of ecthyma gangrenosum in chronic renal failure patient. Arch Plast Surg. 2014;41(3):299–301. doi: 10.5999/aps.2014.41.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolognia J., Jorizzo J.L., Rapini R.P. Mosby/Elsevier; St. Louis, MO: 2008. Dermatology. [Google Scholar]
- 16.Weedon D., Strutton G., Rubin A.I., Weedon D. Churchill Livingstone/Elsevier; Edinburgh: 2010. Weedon’s Skin Pathology. [Google Scholar]