Abstract
Damage to the peripheral vestibular system can result in debilitating postural, perceptual, and visual symptoms. A potential new treatment for this clinical problem is to replace some aspects of peripheral vestibular function with an implant that senses head motion and provides this information to the brain by stimulating branches of the vestibular nerve. In this review I consider animal studies performed at our institution over the past 15 years, which have helped elucidate how the brain processes information provided by a vestibular (semicircular canal) implant and how this information could be used to improve the problems experienced by patients with peripheral vestibular damage.
Keywords: vestibular system, vertigo, prosthesis, implant, ataxia
the peripheral vestibular system consists of five sensors located within each labyrinth and their afferent innervation (the 8th cranial nerves) that connects them to the brain. The sensors are divided into two categories—the three semicircular canals transduce angular head velocity, and two otolith organs transduce gravity and the inertial force produced by linear acceleration. The brain uses the rotational and gravito-inertial information provided by the vestibular periphery to estimate angular head velocity, head orientation relative to gravity, and linear acceleration (Laurens and Angelaki 2011), and these three signals contribute to the reflexive eye movements elicited by head motion (the vestibuloocular reflex or VOR), the vestibulo-spinal projections involved in postural control, and the ascending projections to the thalamus and cerebral cortex that underlie vestibular perception. Patients with extensive peripheral vestibular damage therefore experience postural instability, spatial misperceptions, and motion of visual images during head movements (oscillopsia) (Minor 1998). Peripheral vestibular damage is usually irreversible, and while the brain has extensive adaptive capabilities, central compensation is typically inadequate when the damage is moderate or severe, even when it is augmented with vestibular physical therapy (Whitney et al. 2016). In particular, most patients with bilateral vestibular damage (e.g., due to aminoglycoside toxicity) improve only modestly and are often left with debilitating symptoms due to inadequate peripheral vestibular function.
Improving Central Vestibular Signals After Peripheral Damage
Several therapeutic approaches have the potential to improve the veracity of central vestibular signals after the periphery has been damaged and could thereby alleviate patient symptomatology. Peripheral hair cells or afferent nerves could be regenerated (Li et al. 2016); medications could improve the central compensation process (Günther et al. 2015); reliable nonvestibular information (“sensory substitution”) about motion and/or orientation could be provided through other sensory channels such as tactile or tongue stimulation (Yamanaka et al. 2016); and finally a prosthetic device [“vestibular implant” (VI)] could be developed that transduces the physical parameters normally sensed by the canals and otolith organs and provides this information to the brain by electrically stimulating branches of the vestibular afferent nerve (Dakin et al. 2013). While considerable effort has focused on each of these approaches, this review concentrates on the VI, which builds on the groundbreaking work of Cohen and collaborators (Cohen et al. 1964; Suzuki et al. 1969), who demonstrated five decades ago that electrical stimulation of individual canal ampullary nerves produced eye movements in the plane of the canal and therefore mimicked the VOR response produced by head rotation in the canal's plane. These results demonstrated that individual canals could be selectively activated with electrical stimulation, and they underlie the subsequent development of the VI, which is based on long-term restoration of canallike signals by transducing angular head velocity with sensors and using this information to modulate electrical stimulation of canal afferents.
The Harvard/Mass Eye and Ear (MEE) group was the first to explore VI stimulation in animal models, but several other groups around the world have similarly focused their efforts on this topic. The present article, however, has a limited focus, specifically the MEE experience developing and testing a semicircular canal implant over the past 15 years in animal models ranging from rodents to rhesus monkeys. While I refer to the work of other labs as needed, I refrain from discussing their work in detail.
The Harvard/MEE Vestibular Implant—Background
The prototype MEE VI transduces angular head velocity about one rotational axis and provides this information to the brain by modulating the rate of electrical pulses applied to the corresponding canal ampullary nerve. While this basic approach is potentially applicable to the canals and otolith organs, all studies to date have focused on semicircular canal implantation. This is because the stereocilia of the hair cells in the canal's cristae are aligned so head rotation either increases or decreases the firing rate of all primary afferents that innervate the canal, depending on the direction of head rotation. This basic process can be simulated by a canal implant that senses angular head velocity and modulates the rate of the current pulses applied to the canal ampullary nerve. In contrast, the stereocilia in the otolithic maculae are oriented radially and reverse direction at the striola. The primary otolith afferents therefore modulate in a complex pattern when the head tilts or translates, and this cannot be readily simulated by increasing or decreasing the rate or amplitude of current pulses applied to the otolithic afferents.
The initial MEE implant (Gong and Merfeld 2000, 2002) consisted of an angular velocity sensor, an electrode implanted near the ampulla of one semicircular canal, and the power and circuitry needed to modulate the rate of biphasic current pulses supplied by the electrode as a function of angular head velocity in the plane of the stimulated canal. To encode head rotation in either direction with a unilateral implant, the tonic rate of stimulation was chosen to be well above the normal tonic discharge rate of canal afferents such that it could modulate up when the head rotated toward the stimulated ear and down when it rotated away from that ear. Although eye movement responses can be generated by modulating the amplitude or rate of the current pulses (Gong and Merfeld 2000), the prototype prosthesis used rate modulation since that recapitulates how the normal canal encodes angular head velocity. We recognize that this type of stimulation provides only a rough facsimile of the normal vestibular afferent signal delivered by the functioning canal, and other groups have significantly modified this approach (for example, by lowering the tonic stimulation rate and using an asymmetric transfer function between angular head velocity and stimulation rate) in an attempt to reduce habituation effects and widen the dynamic stimulation range (Dai et al. 2011). While the optimal way to transfer angular velocity information to the brain via electrical stimulation of ampullary nerve fibers remains to be determined, as outlined below we found that, analogous to the relatively simple electrical signal provided by a cochlear implant, the prototype VI could generate a number of critical and sophisticated behavioral responses despite the nonphysiological characteristics of the stimulation protocol.
While the initial implants consisted of a stripped wire electrode with a single remote return, all labs working on VIs now use electrodes with multiple stimulation and return sites (typically adapted from cochlear implant designs), which allow for much greater control over current paths. Furthermore, the initial studies were one-dimensional since only the lateral canal was implanted, but subsequently work in all labs has generalized this approach to the three angular velocity dimensions by implanting each of the three canals in one ear with an electrode controlled by input from an angular velocity sensor aligned with the sensitive rotational axis of the given canal. Additional advances achieved by other laboratories have included software that corrects the direction (axis) of the eye rotation and different stimulation approaches to determine how angular head motion information can most effectively be transmitted to the brain (cf. Davidovics et al. 2012, 2013; Fridman and Della Santina 2013).
Studying the Vestibular Implant—Overview
We began our work on the VI by considering several important functions we felt the VI must be capable of achieving if it is to potentially prove clinically efficacious. These functions relate to two limitations of the VI as it was conceptualized: 1) the nonphysiological aspects of the canal afferent signal produced by the VI mandate investigation to determine if the brain can utilize this information to generate qualitatively normal behavioral responses, and 2) the utility of providing angular velocity information to the brain without appropriate gravito-inertial cues must be determined, specifically the potential of the VI to improve nonoculomotor behaviors that are reliant on vestibular information such as perception of head orientation and postural control. Regarding the nonphysiological aspects of the angular motion signal provided by the VI, the implant uses a high baseline rate of stimulation, so it is important that the brain adapt or habituate to this tonic stimulation rate so nystagmus, imbalance, and vertigo that are associated with a vestibular tone imbalance do not persist, but at the same time the brain must remain sensitive to modulations in stimulation rate so that the motion information encoded by these modulations can be utilized. In addition, most likely (see supportive data below) the stimulated vestibular afferents are all entrained at the same firing rate, coincident with the rate of pulses supplied by the implanted electrode. This is another important nonphysiological constraint, since normally afferent firing is not constrained to single rates within or between fibers (Goldberg and Fernández 1982). Finally, a canal implant can ideally provide a signal that encodes angular velocity of the head in three dimensions, but the absence of relevant otolith information is potentially problematic. For example, imbalance is a crucial problem in patients with severe peripheral vestibular damage, and since gravity (the force that must be overcome to remain upright) is sensed by the otoliths and not the canals, it is not intuitive that angular velocity information provided by a VI could potentially alleviate postural instability.
Given these limitations on the type and quality of information provided by the VI, we felt it was crucial to determine whether the brain could utilize the VI in a manner that approximates the response to normal canal rotational information. First, we examined the capacity of the VI to improve the VOR and nonoculomotor behaviors in vestibulopathic animals; second, we investigated whether the brain was capable of adapting the central motion signals provided by the VI; and third, we examined whether the brain could integrate VI information with other sensory (e.g., otolith) and motor cues. Adaptation is critical because the behaviors driven by implant cannot be perfectly calibrated de novo, so the brain must be able to optimize behavioral responses through adaptation driven by feedback of sensory errors (e.g., motion of images on the retina during head movements must be used to optimize the VOR's kinematic features; cf. Robinson 1982). The capacity for sensory integration is also critical because the vestibular canals are inherently part of a multimodal system (Angelaki and Cullen 2008) that synthesizes angular velocity information with other signals, so the information provided by the VI must be recognized by the brain as a suitably physiological rotational signal so that it will be appropriately incorporated into the motion and orientation signals calculated by the brain.
With these principles in mind, we performed a series of studies to determine if the VI could perform adequately with regard to the following: 1) Static vestibular function—can the brain acclimate to the chronic, high-frequency electrical stimulation provided by the implant? 2) Simple angular VOR responses—by modulating the strength of electrical stimulation as a function of angular head velocity, can an implant generate a compensatory VOR response when the head is rotated? 3) Sensory integration, vision—can the brain integrate the prosthetic rotational signal with visual inputs? 4) Sensory integration, otolith—does prosthetic stimulation engage the velocity storage mechanism in the brain that is responsible for the synthesis of canal and otolith inputs? 5) Perceptual responses, head orientation—can the brain temporally integrate the angular velocity information provided by the prosthesis to generate an estimate of head orientation relative to gravity? 6) Postural responses—does angular velocity information (or its integral) provided by the prosthesis improve postural control during head movements?
Below these questions are considered in detail in terms of their practical and scientific implications, and then I consider potential future directions for vestibular implantation studies in human subjects. Before presenting this work, however, it is important to emphasize that our research on the VI has been based on measuring behavioral responses rather than neuronal activity and therefore we were required to deduce the effects of the VI on the brain from the resultant VOR, perceptual, and postural responses. While we could attempt to extrapolate in a qualitative manner from behavior to neuronal responses, the absence of neural recordings has been an important constraint on the work performed by our laboratory and by other laboratories in this field. Neural recordings of vestibular afferents and central vestibular neurons during electrical stimulation provided by a VI, which help clarify the neuronal mechanisms underlying behavioral observations, have only become available recently (Mitchell et al. 2016) and will prove crucial to advancing understanding of the brain's response to electrical stimulation provided by a VI.
Studying the Vestibular Implant—Animal Models
Before considering specific experiments, I will review the three animal models we used in this work. For studies of static behavior, namely, nystagmus produced by electrical stimulation with the head stationary, we used guinea pigs with an electrode implanted in one lateral canal ampulla. These animals were otherwise normal and did not undergo an ablative procedure. Since they are lateral-eyed and afoveate, guinea pigs are poor models for dynamic oculomotor behavior but are adequate to study static vestibular tone balance. To study the horizontal angular VOR during yaw-axis rotation, we shifted to frontal-eyed foveate animals that more closely resemble humans and used squirrel monkeys that had both lateral canals inactivated through a standard canal plugging procedure (Angelaki et al. 1996) and had an electrode implanted in one lateral canal ampulla. The advantage of this model, which has been widely used to study changes in VOR after canal inactivation, is that it does not affect the static canal afferent activity but simply renders the canal insensitive to angular velocity over the range of frequencies we tested. Finally, to study perception, posture, and the three-dimensional VOR, we shifted to rhesus monkeys since they could be trained on the complex behavioral tasks we required. For these animals, we used aminoglycosides (gentamicin, streptomycin) to destroy hair cells in the vestibular labyrinth in both ears and all end organs (although as noted below the end-organ damage is not equally distributed). These animals had electrodes implanted in one posterior canal ampulla or the ampullae of all three canals in one ear. The vestibular ablation in these animals differed from the squirrel monkey preparation in several ways—rather than just the lateral canals, all vestibular end organs were damaged, and the hair cells were killed rather than being spared as they were with the squirrel monkeys (see Lewis et al. 2010 for sample pathology of an implanted squirrel monkey ear). Despite the extensive hair cell damage, however, aminoglycosides appear to have only minor effects on the integrity and activity of the primary canal afferents and the nerves remain excitable in response to electrical stimulation (Hirvonen et al. 2005). One key point to stress is that the VI appears to directly stimulate the primary canal afferent fibers (Goldberg et al. 1984) and does not require intact hair cells to produce its effects, so the mode of stimulation was presumably very similar in all three of these animal models despite the differences in peripheral vestibular integrity.
Static vestibular function.
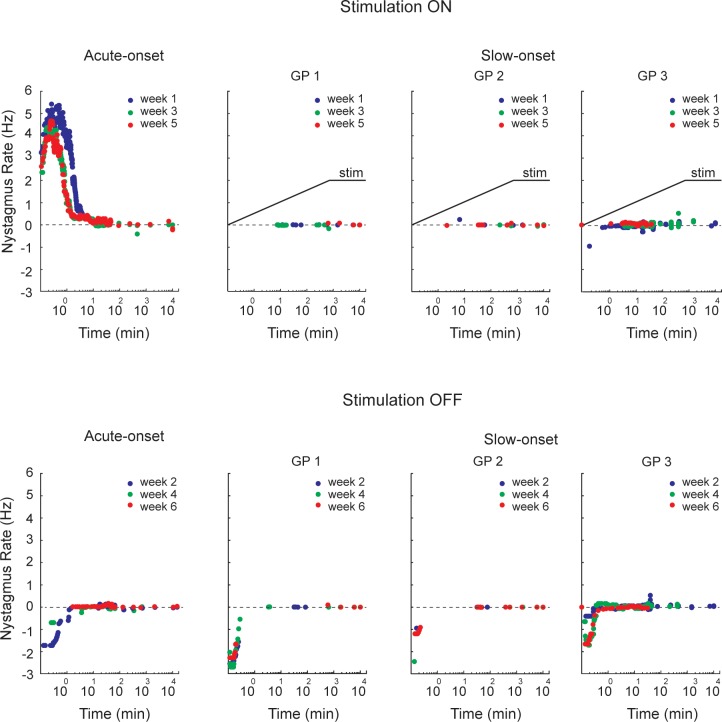
A critical first step was to determine if the brain could acclimate to the high (200–250 pulses/s) tonic rate of stimulation provided by the implant. One concern was that the eyes would move with each current pulse and therefore would oscillate at the rate of stimulation. This has never been observed in normal subjects, presumably because firing of individual afferent fibers is independent and activity in single nerve fibers is inadequate to produce a measurable eye movement. Since we activated the afferents with electrical pulses, however, our presumption was that all affected afferents would fire synchronously and that this synchronous activity could generate small high-frequency eye movements that were time-locked to the stimulation and potentially could degrade vision. Indeed, eye movement recordings showed that the eyes did oscillate at the tonic stimulation frequency but these movements resolved rapidly when the stimulation was maintained (Saginaw et al. 2011). The sustained, supranormal discharge rate of vestibular afferents in one ear (due to the high-frequency, tonic electrical stimulation) results in a tone imbalance that is evidenced by both nystagmus (which had a frequency of ∼5 Hz in our animals at stimulation onset) and abnormal percepts of motion (vertigo). A second important concern was therefore to determine if the nystagmus (which is the oculomotor correlate of vestibular tone imbalance and roughly correlates in humans with vertigo severity; Cousins et al. 2013) would also resolve rapidly after stimulation was initiated. We found that the nystagmus largely abated within ∼30 min, even when the animals were kept in darkness (Fig. 1, top left; Merfeld et al. 2006). These results demonstrate that in response to tonic high-frequency electrical stimulation either habituation (decreased responsiveness to a repeated stimulus) and/or adaptation (central recalibration that serves to minimize a behavioral error) was capable of minimizing the nystagmus response fairly rapidly.
Fig. 1.
Rate of nystagmus quick phases vs. time when electrical stimulation of the lateral canal was turned on and off, collected in 4 guinea pigs. One pig had the stimulation turned on and off abruptly, while the other 3 pigs (GP 1–3) had the stimulation ramped on slowly but turned off abruptly. Each on and off state was maintained for 1 wk. For the pig with acute on and off transitions, the quick phase rate declined rapidly after the stimulation was first begun (week 1) but more quickly for subsequent stimulus-on transitions (weeks 3 and 5). Similarly, when the stimulation was turned off acutely for this pig, the quick phase rate also declined rapidly for the first stimulus-off transition (week 2) but more quickly for subsequent off transitions (weeks 4 and 6). In contrast, the 3 pigs that had the stimulation ramped on had virtually no nystagmus during stimulus onset and did not demonstrate a progressive reduction in quick phase rate during multiple acute stimulus offsets. Adapted from Lewis et al. (2013b) with permission from Springer.
A related issue was the concern that suddenly turning the high-frequency stimulation off (e.g., when bathing or sleeping) or on (e.g., after waking) would generate relatively long, repeated episodes of vertigo (and associated nystagmus). We examined this question by measuring the rate and duration of nystagmus produced by repeated stimulation-on and stimulation-off transitions and found that the nystagmus response became progressively smaller with repeated transitions until it nearly was extinguished (Fig. 1, left; Merfeld et al. 2006). These results suggest that patients should have less vertigo after they experience multiple on-off transitions, but they also raised an interesting scientific question: is the attenuation of the nystagmus response after multiple on-off transitions due to habituation (because of prolonged exposure to electrical stimulation) or to dual-state (context dependent) adaptation, whereby subjects become adapted to both the stimulation-on and -off states and are able to switch between these two states using a cue linked directly to the stimulation (Lewis et al. 2003). This question was investigated by comparing nystagmus responses in guinea pigs that experienced multiple abrupt on-off transitions with those that received the same stimulation exposure (thereby controlling for habituation effects) with different dynamics, a gradual increase in the stimulation rate. We found that the reduction in nystagmus was due to the pattern of stimulation transitions, not just the exposure to stimulation (Fig. 1), implying that dual-state adaptation contributed to the very rapid attenuation of nystagmus that occurred after several on-off transitions were experienced (Lewis et al. 2013b).
In sum, studies of static vestibular function in guinea pigs showed that the eye oscillations synched to the stimulation pulses as well as the more characteristic pathological nystagmus resulting from the vestibular tone imbalance resolved relatively quickly after stimulation was initiated, which implies that both the vertigo and the visual disturbance produced by these eye movements should attenuate rapidly after each on-off stimulus transition. Furthermore, the nystagmus resolved progressively more rapidly after repeated stimulation on-off transitions in a manner that appeared to reflect dual-state adaptation to the two stimulation conditions.
The one-dimensional angular VOR.
To evaluate the capabilities of the unilateral implant to generate VOR responses, squirrel monkeys had both lateral canals inactivated (plugged) and then one lateral canal was instrumented with an implant that sensed head rotation in yaw and stimulated the lateral canal's ampullary nerve. A skull implant held the velocity sensor, power supply, and prosthesis circuity, and the animal could move freely in its cage during prolonged periods of stimulation that lasted more than a year in some cases (Merfeld et al. 2007). The angular velocity signal transduced by the sensor was high-pass filtered with a cutoff frequency of 0.03 Hz (consistent with a time constant of 5 s) to simulate the high-pass characteristics of the normal semicircular canals. The transfer function used to relate the filtered head velocity to the rate of pulses provided by the implanted electrode was a hyperbolic tangent, a function that was linear over much of its range but saturated at the upper and lower extremes. Using this approach, the baseline stimulation rate was 200–250 Hz with the head stationary and modulated up or down, based on the direction of head rotation, in an essentially linear manner over the physiological range of head angular velocities. The slope of the linear portion, whose units were pulse per second/degree per second, could be increased or decreased to modify the sensitivity of the implant, and typically slopes near 1 or 2 were employed. Similarly, the time constant of the high-pass filtered could also be modified (Merfeld et al. 2007).
After the prosthesis was activated, a dynamic vestibular response could be elicited with head rotation about the yaw axis, evidenced by a compensatory VOR (Fig. 2; Lewis et al. 2010). This response was initially robust but rapidly attenuated over the first 2 h with the gain (eye velocity/head velocity) dropping from values that approximate normal function (e.g., 0.4) to values as low as 0.1. Similar to the static condition, the rapid reduction in dynamic eye movement responses appeared to reflect a combination of habituation (reduced sensitivity after repetitive stimulation) and adaptation (the brain's attempt to minimize a behavioral error, in this case the tone imbalance that produced the spontaneous nystagmus). When the monkeys wore the prosthesis chronically (up to 1 yr) in their cages and were tested intermittently on the VOR task, however, the gain gradually increased but the phase lead remained elevated, associated with a VOR time constant (∼3 s) that was shorter than the input provided by electrical stimulation (5 s).
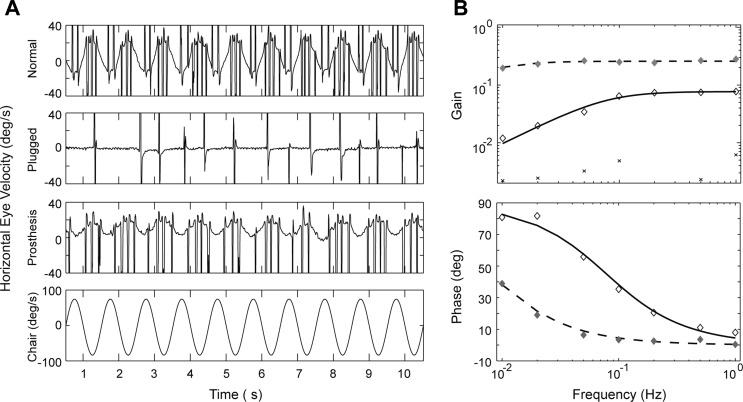
Fig. 2.
Horizontal VOR responses during yaw axis, sinusoidal rotation in a squirrel monkey. A: eye movement responses in the normal monkey (1st row), after bilateral plugging of the lateral canals (2nd row), and with the canal implant subsequently activated (3rd row). Fourth row shows angular velocity of the head during en bloc rotation in the rotatory chair. B: VOR gain and phase vs. frequency for this monkey in the normal state (⧫), after canal plugging (×), and during prosthetic stimulation (◇). VOR gain and phase during prosthetic stimulation showed the expected high-pass characteristics, but the gains were smaller than normal and the phase leads were larger. Phase could not be calculated in the plugged state because of the low gain values. Adapted from Lewis et al. (2010) with permission.
Overall these results demonstrate that motion-modulated stimulation was able to generate a compensatory VOR response and imply that the brain was able to adaptively increase the VOR gain during chronic stimulation. This adaptive change could be driven by visual feedback cues based on image motion on the retina during head movements as occurs normally (Lisberger and Pavelko 1986), or via some form of central sensitization of the VOR pathways engendered by the chronic electrical stimulation, and could include contributions from the normal vertical canals (which were not plugged and are weakly activated during yaw rotation; Tusa et al. 1996) as well as the VI. The VOR phase, in contrast, was not amenable to adaptation and showed no evidence of velocity storage, the central process that normally reduces the low-frequency VOR phase lead (Raphan and Cohen 1985). As discussed below, one potential explanation for the abnormal phase lead is that afferent noise was coherent in the stimulated fibers rather than being randomly distributed within and between fibers. It has been speculated that the velocity storage time constant is determined by the noise characteristics of the afferent fibers (Laurens and Angelaki 2011), and this conceptualization predicts that coherent noise would shorten the VOR time constant as we observed.
Sensory integration—vision.
As previously noted, it is highly unlikely that the stimulation provided by the VI could generate VOR responses with perfectly compensatory kinematic features. The normal VOR, in particular, is highly reliant on central adaptation to adjust the amplitude and direction (axis) of the eye movements that occur in response to head rotations. It has been shown that under normal conditions, for example, a compensatory VOR axis requires the brain to combine information from all three canals in each ear (Robinson 1982) and that the axis is amenable to adaptive recalibration when the direction of head rotation and retinal slip are misaligned (Schultheis and Robinson 1981). Conversely, the VOR axis is inappropriate when the cerebellum is damaged (Shaikh et al. 2011), presumably reflecting a loss of central optimization.
To evaluate whether the brain is able to use visual feedback to modify the VOR produced by the implant, we sought evidence of adaptive changes in the VOR axis during the period of prosthetic stimulation—if observable, this would provide a clear indication that the prosthetic rotational cue could engage in normal sensory integration with the visual system. Two pieces of information provide evidence that the brain can indeed use retinal slip information to adjust the axis of the VOR produced by the implant. First, in the monkeys that underwent nearly 1 yr of prosthetic stimulation, the VOR axis was not perfectly compensatory when stimulation began (e.g., horizontal head movements produced VOR responses with small vertical components in addition to the much larger horizontal components). Over time, however, the VOR axis became more closely aligned with the axis of head rotation as the vertical VOR component diminished while the horizontal component increased (Lewis et al. 2010). A much more dramatic form of axis adaptation was observed in a monkey that had the stimulating electrode placed in the posterior canal even though the angular velocity sensor remained aligned with the lateral canals (Lewis et al. 2002–2003). When the implant was activated, horizontal head rotations produced eye movements that were primarily vertical and torsional (reflecting the posterior canal activation), but over 1 wk the VOR axis shifted substantially as the horizontal response increased in amplitude while the vertical response slightly decreased (Fig. 3).
Fig. 3.
VOR responses during yaw-axis sinusoidal rotation in a squirrel monkey with bilateral lateral canal plugs that had the stimulating electrode implanted in 1 posterior canal while the angular velocity sensor was aligned with the lateral canal's sensitive axis. A and B show minimal VOR responses after plugging but without prosthetic stimulation. C and D show responses when the implant was first activated and display relatively large vertical but minimal horizontal eye movements (e.g., VOR axis close to pitch in the frontal plane). E and F show the VOR after 1 wk of chronic stimulation and demonstrate a substantial increase in the horizontal response and a slight decrease in the vertical response, yielding a VOR rotational axis that is closer to the head's yaw rotational axis. Bottom row shows angular velocity of the head during en bloc rotation in the centrifuge motion device. Reprinted from Lewis et al. (2002–2003) with permission from IOS Press.
These results provide clear evidence that the brain can use visual information to adjust the axis of the VOR that is generated with electrical stimulation of canal afferents, with the goal of minimizing retinal image motion during head motion. More generally, these findings indicate that the brain can integrate visual information with prosthesis-mediated angular velocity signals to parametrically adapt the VOR in a manner that recapitulates normal visually guided adaptation.
Sensory integration—otoliths.
Synthesis of angular velocity information provided by the canals and the gravito-inertial information provided by the otolith organs and the other linear acceleration sensors in the body (which together I will refer to as graviceptive sensors) is a crucial element of normal central vestibular processing. A major question, therefore, is whether the brain is able to integrate normal graviceptive signals with the angular velocity information provided by the canal implant. One reason for skepticism is that the velocity storage mechanism in the brain stem that prolongs the VOR time constant is also responsible for synthesizing canal and graviceptive inputs (Wearne et al. 1998), and we previously showed that the VOR generated by electrical stimulation displays an abnormally short time constant with no evidence that the velocity storage mechanism was engaged.
Since the otolith organs are the primary graviceptors, it may seem moot to determine whether the VI rotational signals can integrate with otolith signals, as presumably whatever disorder damaged the canals in both ears will destroy the otolith organs as well. This assessment is incorrect for three reasons: 1) Patients with bilateral vestibular damage will be the most appropriate group to receive VI in the future, and some of the most common labyrinthine disorders that cause bilateral vestibular damage [such as aminoglycoside toxicity (Tsuji et al. 2000) and idiopathic bilateral vestibular hypofunction (Priesol et al. 2014)] can differentially affect the canal and otolith organs. In particular, both of these disorders demonstrate relative sparing of the otolith organs. 2) As noted above, the otoliths are only one element of the body's graviceptive system, albeit a dominant one in many instances. 3) Integration of VI rotational cues with otolith signals, even under unusual experimental circumstances, would demonstrate that the brain is capable of synthesizing the VI signal with other sensory inputs (in this instance, otolithic cues that encode the direction of gravity), which is one of the keys to demonstrating that the relatively simple rotational signal provided by the VI can function in a qualitatively normal fashion within the multimodal processing environment that is inherent to vestibular function.
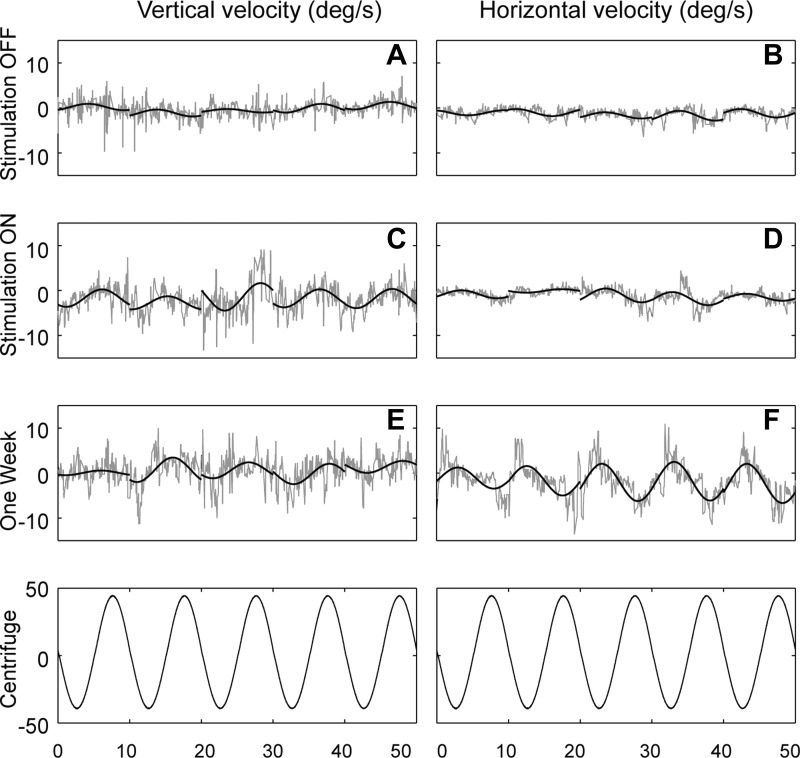
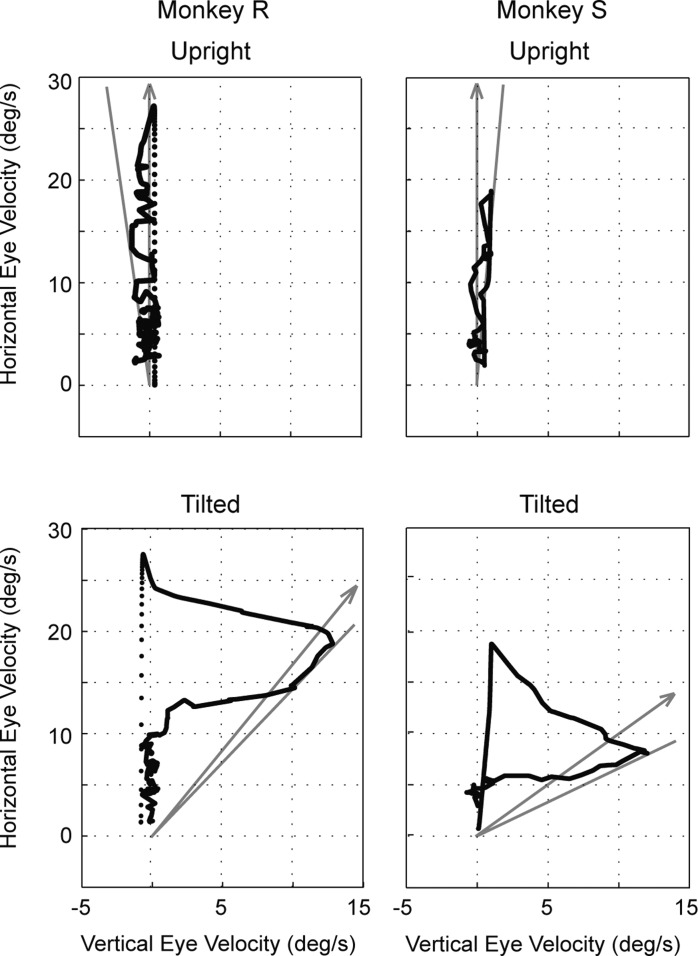
To determine whether canal-otolith interaction occurs with the prosthetic canal input, we used a paradigm similar to postrotational tilt, which is a common way to assess canal-otolith interactions. In this test, the head is rotated at a constant angular velocity about an Earth-vertical yaw axis, the rotation is suddenly stopped, and then the head is tilted away from the vertical axis. Because of their high-pass filter characteristics, when the head rotation is suddenly stopped the canals provide a signal to the brain indicating that the head is rotating in the opposite direction. The canal-otolith interaction following postrotational tilt is evidenced by attenuation (“dumping”) of the VOR response and a shift in the eye's rotational axis toward the direction of gravity (Raphan and Cohen 1985). Although the prosthesis VOR time constant was not prolonged in a manner that demonstrates velocity storage, we found that when the lateral canal was electrically stimulated by the implant with the animal's head tilted (with the stimulation rate and tilt angle determined empirically), the VOR response was attenuated and its rotational axis shifted toward alignment with gravity (Fig. 4; Lewis et al. 2012).
Fig. 4.
VOR responses in 2 squirrel monkeys when 1 lateral canal was stimulated electrically while the head was upright or statically tilted in the roll plane. With the head upright, VOR responses were horizontal with little vertical component; when the head was tilted, a substantial vertical eye velocity developed that shifted the VOR's rotational axis toward alignment with gravity (arrows). Solid lines indicate the peak axis shift. Monkey R was tilted 30° in roll, while monkey S was tilted 45°. Adapted from Lewis et al. (2012) with permission.
These results have considerable practical importance because they demonstrate that the angular velocity information provided by the implant can interact in the brain with the gravitational information provided by the otolith organs (and any other operative graviceptors) in a qualitatively normal manner. Furthermore, this work also demonstrates that the three properties of velocity storage can be dissociated with the gravity-sensitive dumping and spatial orientation properties occurring despite the absence of the characteristic dynamic effects on the low-frequency VOR. The reason for this dissociation is unknown, but we suspect that it reflects the nonphysiological activation of canal afferents by the electrical stimulation, in particular the apparent synchronous firing of these afferents (Saginaw et al. 2011), which could correlate the noise in the afferent signal and therefore shorten the velocity storage time constant (Lewis et al. 2012).
Perceptual responses—head orientation.
As discussed above, the canals provide information about the head's angular velocity and the otolith organs provide gravitational and linear acceleration information. When the inner ears are damaged both types of inputs can be degraded, and while the VI provides angular velocity information (in 1 or more dimensions), no information is provided about head orientation relative to gravity. What can the brain accomplish in this situation? It is clear that the electrical stimulation provided by the implant can generate compensatory VOR responses and can therefore improve vision while moving, but are there any other beneficial effects?
Even though gravity is directly sensed by the otolith organs, it has been hypothesized (cf. Angelaki et al. 1999) that the brain actually calculates head orientation relative to gravity by temporally integrating the angular velocity signals provided by the canals. The reason for this hypothesis is that the otolith organs sense the vector sum of gravity and linear acceleration but the brain is able to separate this signal into its gravitational and translational components. One way this could be accomplished is by temporally integrating angular velocity information for rotations whose axes are not aligned with the Earth vertical. By tracking these rotations the brain can estimate head orientation relative to gravity, and then linear acceleration of the head can be calculated by subtracting the estimate of gravity from the gravito-inertial force (GIF) information provided by the otolith organs. This conceptualization predicts that a VI that provides three-dimensional angular velocity information will do more than generate reflexive eye movements—it could provide the rotational information required to calculate head orientation relative to gravity.
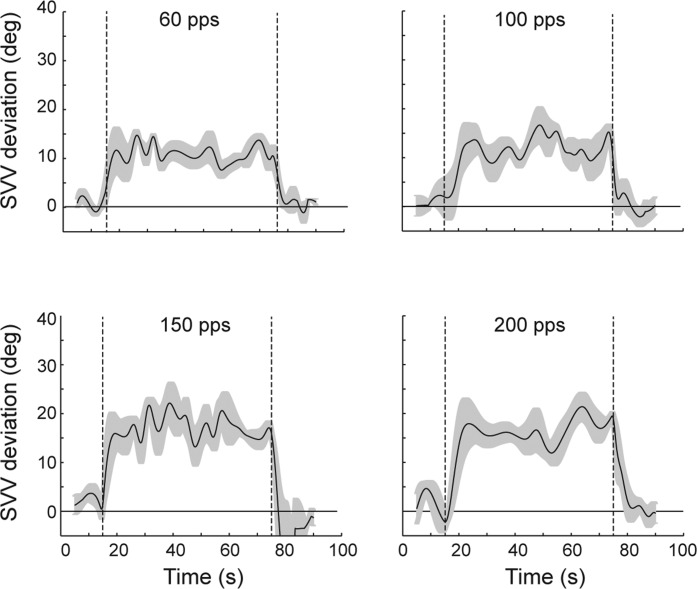
We investigated this question by training rhesus monkeys to perform a subjective visual vertical (SVV) task, which requires turning a steering wheel to align a visual light bar with the perceived direction of gravity (Lewis et al. 2008), and found that normal monkeys perform this task in a manner that is qualitatively similar to humans, with perception of gravity based on the frequency of head motion and on the integration of canal and otolith signals. We then tested monkeys on this task with and without electrical stimulation provided by an electrode implanted in one posterior canal. When the stimulation was present the SVV responses deviated away from the stimulated ear (Fig. 5), consistent with a misperception of roll tilt toward the canal that was activated (Lewis et al. 2013a).
Fig. 5.
Subjective visual vertical (SVV) responses for a rhesus monkey tested with the head upright before, during, and after electrical stimulation of 1 posterior canal at rates ranging from 60 to 200 pulses per second (pps). Before and after stimulation the SVV responses were near the Earth-vertical (defined as 0°), but during stimulation (period between 15 and 75 s) the SVV shifted away from the stimulated ear, consistent with a misperception of roll tilt toward that ear. SVV shifts increased monotonically with the rate of stimulation. Adapted from Lewis et al. (2013a) with permission.
This finding demonstrates the important scientific point that the brain can estimate head orientation relative to gravity by integrating angular velocity information from the canals and also provides the critical practical finding that a canal prosthesis can modulate the perceived orientation of the head relative to gravity. A more definitive test would be to measure SVV responses during dynamic head tilts in the presence and absence of motion-modulated canal stimulation in animals with severe bilateral vestibular deficits. Our preliminary results from performing this experiment suggest that bilateral vestibular damage degrades the perception of head orientation but that this improves when motion-modulated stimulation is provided (Lewis 2015). Additional work must be performed to verify this finding, but it is an exciting result since it implies that a canal implant could improve percepts of head orientation in vestibulopathic patients.
Postural responses.
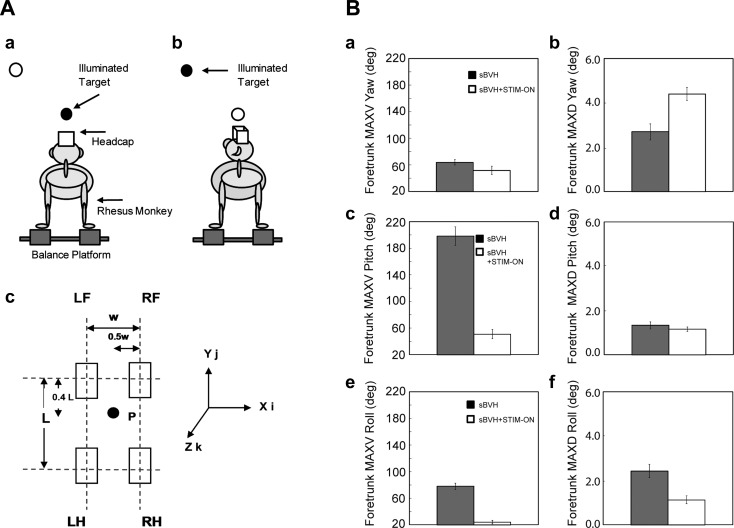
Patients with severe peripheral vestibular damage are unsteady while standing and walking and are at an increased risk of falling. Vestibulopathic patients are particularly imbalanced when visual cues are minimal (e.g., walking in the dark), proprioceptive signals from the leg are degraded (e.g., walking on sand), the support surface moves (e.g., standing on a moving subway), and the head turns quickly. The latter situation is the most common in normal daily life and is the one situation when angular head velocities are routinely high. A VI that responds to head rotation would presumably be most helpful during head turns, and we therefore investigated its effects on postural stability in a vestibulopathic rhesus monkey. Specifically, we measured head and trunk motion and forces applied to the support surface while the monkey stood loosely tethered on a balance platform and made voluntary head turns between two targets (Fig. 6) and found that the trunk was more stable during head turns when the canal implant was activated (Thompson et al. 2016). One mechanism that could potentially explain how information about head angular velocity supplied by the implant could improve postural stability during head turns relates to the concept that body orientation relative to gravity is an important controlled variable during standing (Stapley et al. 2006). Body orientation relative to gravity could be calculated by the brain by combining information about head orientation relative to gravity (derived from vestibular information) with information about head position relative to the trunk (derived primarily from cervical proprioceptive signals). Since our perceptual studies suggest that the first step of this process (estimating head orientation relative to gravity) can be accomplished by the prosthesis, it is logical to assume that the prosthesis may similarly be able to help improve the brain's estimate of body position relative to gravity, thereby improving postural control.
Fig. 6.
Effects of prosthetic stimulation on postural stability in a rhesus monkey with severe bilateral vestibular ablation. A: schematic drawing of the rhesus monkey standing on the balance platform (a) and turning its head between 2 visual targets (b). c: Schematic diagram of the balance platform when viewed from above, with the distance between the 2 left and 2 right limbs (w) and the distance between the 2 forelimbs and the 2 hindlimbs (L) indicated. The coordinate system used to measure rotation and translation of the head and trunk is also shown. LF, left forelimb; RF, right forelimb; LH, left hindlimb; RH, right hindlimb. B: mean and SE for the peak velocity (MAXV, left) and peak displacement (MAXD, right) of the foretrunk in yaw (a and b), pitch (c and d), and roll (e and f) associated with head turns between the target located straight ahead and the obliquely displaced target. Filled bars show results with stimulation off, and open bars show results with stimulation on. Principal effects of stimulation were reductions in the peak velocity of foretrunk in pitch and roll. sBVH, severe bilateral vestibular hypofunction. Adapted from Thompson et al. (2016) with permission from Springer.
Future Directions/Human Studies
A number of intriguing and complex questions can be studied with VIs (Lewis 2015), particularly since the implanted subjects are beginning to shift from animals to human patients. Below I have divided these questions into “clinical” and “scientific” categories, but it is apparent from the above discussion that most work in this field will concurrently provide both practical knowledge that will contribute to the clinical implementation of VIs in human subjects and scientific knowledge about how the brain processes vestibular information.
Clinical issues.
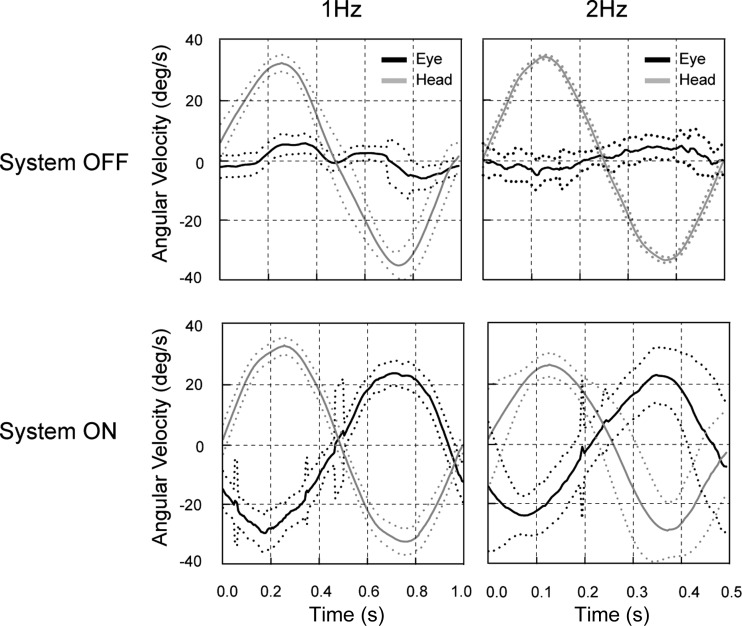
Vestibular implantation in humans is in a nascent stage at several centers around the world. The most important practical goals at present are to demonstrate that the three-dimensional canal implant can improve vestibular-mediated behaviors in patients with severe peripheral vestibular damage, including the VOR, balance, and motion perception. While these experiments require quantification of these behaviors in vestibulopathic patients with and without chronic, motion-modulated stimulation, a first step is to focus on the effects of acute canal stimulation using the implant. In this regard, the experiments performed to date in humans are highly promising since angular VOR responses can clearly be elicited (Perez Fornos et al. 2014; Fig. 7), and other studies have also demonstrated perceptual (Phillips et al. 2015) and postural (Phillips et al. 2013) effects of short-term canal stimulation.
Fig. 7.
Horizontal VOR response in a human patient with bilateral vestibular damage during yaw axis sinusoidal rotation. With the stimulation off (top), en bloc rotation (gray) produced only minimal VOR responses (black). With the implant activated (bottom), however, large compensatory VOR responses were evoked by the same rotational stimuli. Adapted from Perez Fornos et al. (2014) with permission.
One problem to consider, however, is that implants are currently limited to the semicircular canals, and while these appear perfectly capable of generating compensatory three-dimensional angular VOR responses that can improve vision while moving, it is uncertain whether the behavioral benefits in the perceptual and postural realms will be nearly as dramatic as the oculomotor results. Indeed, the effect of providing an angular velocity signal to the brain without a concomitant otolith signal could prove quite problematic. In a normal subject, if the head rotates about an axis not aligned with gravity, the canals provide the angular velocity signal whose integral is used to estimate the shift in head orientation and the otolith organs measure the associated shift in the orientation of the GIF. In a patient with severe vestibular damage who is utilizing the canal prosthesis, a similar head rotation would provide a facsimile of the three-dimensional angular velocity signal from the prosthetic stimulation but damaged otolith organs would not provide the appropriate signal indicating a shift in GIF. In normal experience, a change in the orientation of gravity relative to the head that is unaccompanied by a shift in GIF can only occur if a linear acceleration takes place at the same instant and effectively cancels the effect of the head tilt on the GIF sensed by the otolith organs. In other words, the brain may well make a misestimate of linear acceleration in this situation (Merfeld et al. 1999), which would be expected to impair perception and postural stability. Our preliminary results in rhesus monkeys suggest that an aberrant estimate of translation (as reflected in the eye movement response) is generated by the prosthesis-mediated rotational signal. It is unclear, however, if this situation would persist in patients with chronic loss of peripheral vestibular function (the more relevant situation for patients with vestibular damage), as the linkage between the components of the GIF may degrade when their tight association is chronically decoupled (Angelaki et al. 2000). Further work is clearly needed to assess the benefits and potential adverse effects of three-dimensional canal stimulation, and ultimately the field of VIs may require a combination of canal implants with prosthetics that simulate the more complex behavior of the otolith organs.
Another ongoing problem is to determine how head motion information transduced by an implant can most effectively be encoded to optimize the transmission of information to the brain. Now that recordings in primary vestibular afferents and neurons in the vestibular nuclei are being obtained during prosthetic stimulation (Mitchell et al. 2016), the nonphysiological characteristics of the afferent signals generated by the implant can be fully characterized and approaches can be devised to improve the quality of the afferent signal by modifying stimulation techniques. Very high-frequency (5 kHz) background stimulation has been suggested (Rubinstein et al. 1999), for example, as a method to desynchronize firing of vestibular afferents within and between fibers that could serve to normalize the pattern of afferent activity produced by the implant. Similarly, this type of stimulation appears to produce random discharges in vestibular (and auditory) afferents (Litvak et al. 2003) and presumably by titrating the amplitude of the 5 kHz stimulation stochastic resonance (Collins et al. 1995) could be induced, improving the ability of the implant to transmit information to the brain.
Scientific issues.
The canal implant offers several unique capabilities that could facilitate vestibular research, particularly since complex psychophysical studies will become feasible as the implanted population transitions from animals to human patients. In normal circumstances activation of the vestibular system requires motion, and for research purposes this is inherently problematic because the vestibular signal takes time to reach threshold levels; other sensory systems (e.g., somatosensory) will always be activated to some degree; and activity in both inner ears is modulated together since the end organs function as pairs. In addition, the reliability of vestibular inputs cannot be modified with motion stimuli. The VI bypasses these problems because the signal from one ear can be turned on and off with precise timing; it can be activated in isolation without the involvement of other sensory systems; and it can be activated in one ear without affecting the contralateral ear. Furthermore, the motion-modulated stimulation provided by the implant can be chronically modified by turning it on or off or by changing its sensitivity, and the noise characteristics of the afferent signal can be altered by adding a stochastic (Goel et al. 2015) or a 5 kHz (Rubinstein et al. 1999) pattern of background stimulation.
Working with colleagues at the University of Geneva, we intend to address a number of scientific questions in implanted humans, focusing in particular on higher cognitive functions. In brief, we plan to 1) investigate how the brain extracts information from vestibular afferents by measuring perceptual and VOR thresholds when the noise characteristics of the vestibular signal input are modified; 2) study vestibular-visual integration in the spatial domain to determine if sensory integration is statistically optimal (Ernst and Banks 2002) when the reliability of the vestibular cue is modified; 3) study sensory integration in the time domain (Vroomen and Keetels 2010), taking advantage of the unique capabilities offered by implant subjects who can experience precisely timed vestibular and cochlear signals (by activating their vestibular and cochlear implants with a range of stimulus onset asynchronies); and 4) study how vestibular information contributes to spatial orientation and navigation (Yoder and Taube 2014) by measuring path integration as well as virtual and motoric navigation when the implant is chronically active or inactive in patients with bilateral vestibular hypofunction who lack normal hippocampal spatial codes (Brandt et al. 2005). Overall, this work should provide crucial information at the scientific and translational levels, which will contribute to both the implementation of a VI in humans and understanding of sensorimotor processing ranging from basic (e.g., signal extraction) to complex (e.g., navigational) levels.
GRANTS
This work was funded by National Institute on Deafness and Other Communication Disorders Grants DC-006909, DC-008362, and DC-13069 to R. F. Lewis and DC-003066 and DC-008167 to Daniel Merfeld. Additional funding was provided by EU Contract 225929 (“CLONS”).
DISCLOSURES
This work has been supported in part by the Med El Corporation.
AUTHOR CONTRIBUTIONS
R.F.L. conceived and designed research; R.F.L. performed experiments; R.F.L. analyzed data; R.F.L. interpreted results of experiments; R.F.L. prepared figures; R.F.L. drafted manuscript; R.F.L. edited and revised manuscript; R.F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dan Merfeld, Csilla Haburcakova, Wangsong Gong, Daniel Lee, Nils Guinand, Angelica Perez Fornos, J.-P. Guyot, and David Balkwill.
REFERENCES
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31: 125–150, 2008. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Hess BJ, Arai Y, Suzuki J. Adaptation of primate vestibuloocular reflex to altered peripheral vestibular inputs. I. Frequency-specific recovery of horizontal VOR after inactivation of the lateral semicircular canals. J Neurophysiol 76: 2941–2953, 1996. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19: 316–327, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Merfeld DM, Hess BJ. Low-frequency otolith and semicircular canal interactions after canal inactivation. Exp Brain Res 132: 539–549, 2000. [DOI] [PubMed] [Google Scholar]
- Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain 128: 2732–2741, 2005. [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI, Bender MB. Eye movements from semicircular canal nerve stimulation in the cat. Ann Otol Rhinol Laryngol 73: 153–169, 1964. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Chow CC, Imhoff TT. Stochastic resonance without tuning. Nature 376: 236–238, 1995. [DOI] [PubMed] [Google Scholar]
- Cousins S, Kaski D, Cutfield N, Seemungal B, Golding JF, Gresty M, Glasauer S, Bronstein AM. Vestibular perception following acute unilateral vestibular lesions. PLoS One 8: e61862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Fridman GY, Davidovics NS, Chiang B, Ahn JH, Della Santina CC. Restoration of 3D vestibular sensation in rhesus monkeys using a multichannel vestibular prosthesis. Hear Res 281: 74–83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Elmore LC, Rosenberg A. One step closer to a functional vestibular prosthesis. J Neurosci 33: 14978–14980, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovics NS, Fridman GY, Della Santina CC. Co-modulation of stimulus rate and current from elevated baselines expands head motion encoding range of the vestibular prosthesis. Exp Brain Res 218: 389–400, 2012. [DOI] [PubMed] [Google Scholar]
- Davidovics NS, Rahman MA, Dai C, Ahn J, Fridman GY, Della Santina CC. Multichannel vestibular prosthesis employing modulation of pulse rate and current with alignment precompensation elicits improved VOR performance in monkeys. J Assoc Res Otolaryngol 14: 233–248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. [DOI] [PubMed] [Google Scholar]
- Fridman GY, Della Santina CC. Safe direct current stimulation to expand capabilities of neural prostheses. IEEE Trans Neural Syst Rehabil Eng 21: 319–328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R, Kofman I, Jeevarajan J, De Dios Y, Cohen HS, Bloomberg JJ, Mulavara AP. Using low levels of stochastic vestibular stimulation to improve balance function. PLoS One 10: e0136335, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Fernández C. Eye movements and vestibular-nerve responses produced in the squirrel monkey by rotations about an earth-horizontal axis. Exp Brain Res 46: 393–402, 1982. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith M, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51: 1236–1256, 1984. [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng 28: 572–581, 2000. [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular canal prosthesis. IEEE Trans Biomed Eng 49: 175–181, 2002. [DOI] [PubMed] [Google Scholar]
- Günther L, Beck R, Xiong G, Potschka H, Jahn K, Bartenstein P, Brandt T, Dutia M, Dieterich M, Strupp M, la Fougère C, Zwergal A. N-acetyl-l-leucine accelerates vestibular compensation after unilateral labyrinthectomy by action in the cerebellum and thalamus. PLoS One 10: e0120891, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol 93: 643–655, 2005. [DOI] [PubMed] [Google Scholar]
- Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res 210: 407–422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF. Advances in the diagnosis and treatment of vestibular disorders: psychophysics and prosthetics. J Neurosci 35: 5089–5096, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF. Vestibular prosthesis: perception and posture in animal models. 3rd Congress of European ORL-HNS, Prague. RT-051, 2015. [Google Scholar]
- Lewis RF, Clendaniel RA, Zee DS. Vergence-dependent adaptation of the vestibulo-ocular reflex. Exp Brain Res 152: 335–340, 2003. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Gong W, Ramsey M, Minor L, Boyle R, Merfeld DM. Vestibular adaptation studied with a prosthetic semicircular canal. J Vestib Res 12: 87–94, 2002–2003. [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Karmali F, Merfeld DM. Spatial and temporal properties of eye movements produced by electrical stimulation of semicircular canal afferents. J Neurophysiol 108: 1511–1520, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Lee D, Merfeld D. Electrical stimulation of semicircular canal afferents affects the perception of head orientation. J Neurosci 33: 9530–9535, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Makary C, Merfeld DM. Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J Neurophysiol 103: 1066–1079, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Merfeld DM. Roll tilt psychophysics in rhesus monkeys during vestibular and visual stimulation. J Neurophysiol 100: 140–153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Nicoucar K, Gong W, Haburcakova C, Merfeld DM. Adaptation of vestibular tone studied with electrical stimulation of semicircular canal afferents. J Assoc Res Otolaryngol 14: 331–340, 2013b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You D, Chen Y, Chai R, Li H. Regeneration of hair cells in the mammalian vestibular system. Front Med 10: 143–151, 2016. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA. Vestibular signals carried by pathways subserving plasticity of the vestibulo-ocular reflex in monkeys. J Neurosci 6: 346–354, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak LM, Smith ZM, Delgutte B, Eddington DK. Desynchronization of electrically evoked auditory-nerve activity by high-frequency pulse trains of long duration. J Acoust Soc Am 114: 2066–2078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng 53: 2362–2372, 2006. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Haburcakova C, Gong W, Lewis RF. Chronic vestibulo-ocular reflexes evoked by a vestibular prosthesis. IEEE Trans Biomed Eng 54: 1005–1015, 2007. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature 398: 615–618, 1999. [DOI] [PubMed] [Google Scholar]
- Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA 279: 541–544, 1998. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Della Santina CC, Cullen KE. Plasticity within non-cerebellar pathways rapidly shapes motor performance in vivo. Nat Commun 7: 11238, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Fornos A, Guinand N, van de Berg R, Stokroos R, Micera S, Kingma H, Pelizzone M, Guyot JP. Artificial balance: restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front Neurol 5: 66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, Defrancisci C, Ling L, Nie K, Nowack A, Phillips JO, Rubinstein JT. Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp Brain Res 229: 181–195, 2013. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Nie K, Jameyson E, Phillips CM, Nowack AL, Golub JS, Rubinstein JT. Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J Neurophysiol 113: 3866–3892, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priesol AJ, Valko Y, Merfeld DM, Lewis RF. Motion perception in patients with idiopathic bilateral vestibular hypofunction. Otolaryngol Head Neck Surg 150: 1040–1042, 2014. [DOI] [PubMed] [Google Scholar]
- Raphan T, Cohen B. Velocity storage and the ocular response to multidimensional vestibular stimuli. Rev Oculomot Res 1: 123–143, 1985. [PubMed] [Google Scholar]
- Robinson DA. The use of matrices in analyzing the three-dimensional behavior of the vestibulo-ocular reflex. Biol Cybern 46: 53–66, 1982. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res 127: 108–118, 1999. [DOI] [PubMed] [Google Scholar]
- Saginaw MA, Gong W, Haburcakova C, Merfeld DM. Attenuation of eye movements evoked by a vestibular implant at the frequency of the baseline pulse rate. IEEE Trans Biomed Eng 58: 2732–2739, 2011. [DOI] [PubMed] [Google Scholar]
- Schultheis LW, Robinson DA. Directional plasticity of the vestibuloocular reflex in the cat. Ann NY Acad Sci 374: 504–512, 1981. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, Carey JP, Nguyen KD, Zee DS. Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J Neurophysiol 105: 3034–3041, 2011. [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Ting LH, Kuifu C, Everaert DG, Macpherson JM. Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns in the standing cat. J Neurophysiol 95: 3783–3797, 2006. [DOI] [PubMed] [Google Scholar]
- Suzuki JI, Goto K, Tokumasu K, Cohen B. Implantation of electrodes near individual vestibular nerve branches in mammals. Ann Otol Rhinol Laryngol 78: 815–826, 1969. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Haburcakova C, Lewis RF. Vestibular ablation and a semicircular canal prosthesis affect postural stability during head turns. Exp Brain Res 234: 3245–3257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Velázquez-Villaseñor L, Rauch SD, Glynn RJ, Wall C 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Aminoglycoside ototoxicity. Ann Otol Rhinol Laryngol Suppl 181: 20–25, 2000. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Grant MP, Buettner UW, Herdman SJ, Zee DS. The contribution of the vertical semicircular canals to high-velocity horizontal vestibulo-ocular reflex (VOR) in normal subjects and patients with unilateral vestibular nerve section. Acta Otolaryngol 116: 507–512, 1996. [DOI] [PubMed] [Google Scholar]
- Vroomen J, Keetels M. Perception of intersensory synchrony: a tutorial review. Atten Percept Psychophys 72: 871–884, 2010. [DOI] [PubMed] [Google Scholar]
- Wearne S, Raphan T, Cohen B. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol 79: 2690–2715, 1998. [DOI] [PubMed] [Google Scholar]
- Whitney SL, Alghadir AH, Anwer S. Recent evidence about the effectiveness of vestibular rehabilitation. Curr Treat Options Neurol 18: 13, 2016. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Sawai Y, Murai T, Nishimura T, Kitahara T. Long-term effects of electrotactile sensory substitution therapy on balance disorders. Neuroreport 27: 744–748, 2016. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Taube JS. The vestibular contribution to the head direction signal and navigation. Front Integr Neurosci 8: 32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]