Abstract
Purpose of review
This report examines the mechanism(s) by which each protein of the contact activation system - factor XII (FXII), high molecular weight kininogen (HK), and prekallikrein (PK) - influence thrombosis risk.
Recent findings
FXII generates thrombin through contact activation via interaction with artificial surfaces as on medical instruments such as indwelling catheters, mechanical values, stents and ventricular assist devices. Inhibition of FXIIa-mediated contact activation prevents thrombosis under contact activation circumstances without affecting hemostasis. Current studies suggest that HK deficiency parallels that of FXII and inhibits contact activation. PK inhibition contributes to thrombosis prevention by contact activation inhibition in the nylon monofilament model of transient middle cerebral artery occlusion. However, in arterial thrombosis models where reactive oxygen species are generated, PK deficiency results in down-regulation of vessel wall tissue factor generation with reduced thrombin generation. Exploiting this latter PK pathway for thrombosis risk reduction provides a general, overall reduced tissue factor, antithrombotic pathway without risk for bleeding.
Summary
These investigations indicate that 1) the proteins of the contact activation and kallikrein/kinin systems influence thrombosis risk by several mechanisms and 2) understanding of these pathway provides insight into several novel targets to prevent thrombosis without increase in bleeding risk.
Keywords: Factor XII, High Molecular Weight Kininogen, Prekallikrein, Bradykinin B2 Receptor, Mas Receptor
Introduction
Emerging data indicate that the proteins of the contact activation system (CAS) [factor XII (FXII), high molecular weight kininogen (HK), prekallikrein (PK)] influence thrombosis risk without effect on hemostasis, the cessation of bleeding. The CAS and its interacting kallikrein/kinin system (KKS) (See a recent review characterizing these two proteolytic systems) are participants in modulating thrombosis risk in the intravascular compartment and also interact closely with the renin-angiotensin system (RAS)1. Mice deficient of FXII (f12−/−), HK (kgn1−/−), or PK (klkb1−/−) have a delayed thrombosis time in arterial injury models2–4. Additionally, mice deficient in the bradykinin B2 receptor (B2R) (bdkrb2−/−) in the KKS also have delayed thrombosis5,6. However, mice deficient in prolylcarboxyxpeptidase (prcpgt/gt), a protein involved in PK activation, and the angiotensin-(1–7) receptor, Mas (mas1−/−), of the RAS have shorter arterial thrombosis times7,8. In all cases, the change in thrombosis risk in these knockout mice has no effect hemostasis and bleeding risk. Recently a novel general hypothesis for the initiation of thrombosis has been proposed suggesting that vessel wall injury occurs first leading to subsequent platelet thrombus formation9,10. Although this model was developed as a means to understand tissue factor-thrombin actions in clot formation, it also serves as a guide to better understand how the proteins of the CAS/KKS, to be discussed below, influence thrombosis risk in physiologic states. The present review examines what is known about the mechanisms of how FXII, HK, and PK influence thrombosis risk using the data derived from study of murine deletion models. In doing so, several commonly used murine thrombosis models are interpreted as to how they induce vessel occlusion. Supporting proteins like the B2R, Mas receptor and prolylcarboxypeptidase are discussed in order to clarify mechanism(s) for thrombosis risk alteration associated with CAS proteins. The overall message of this review is that the mechanisms for thrombosis risk reduction for CAS/KKS proteins extend beyond contact pathway activation via physiologic or pathophysiologic surfaces in the intravascular compartment. The CAS/KKS interacts with additional pathways to modulate thrombosis risk and further understandings of these pathways should lead to more precise antithrombotic treatments.
Factor XII
Since its discovery in 1955, FXII deficiency was recognized to prolong surface-activated blood coagulations assays without a clinical increase in bleeding or abnormal hemostasis. For 5 decades since discovery, most investigators dismissed FXII as unimportant. However, recognition that f12−/− mice have delayed thrombosis on several murine models without an increase in bleeding has generated renewed interest in this enzyme2. FXII has the peculiar ability to bind to artificial surfaces and in doing so change shape such that its single chain zymogen becomes a two chain active enzyme [factor XIIa (FXIIa)]. The mechanism of this phenomenon called “autoactivation” is unknown. Recent studies suggest that via polyphosphates (polyP), inorganic polymers of orthophosphate units linked by phosphoanhydride bonds, FXII, like single chain urokinase, elaborates proteolytic activity for small molecule substrates, without becoming a two chain enzyme11. PolyP is present in all prokaryotes and eukaryotes and becomes available on cell lysis. Additionally, aggregated proteins, amyloid beta peptide1–42, vessel wall collagen, RNA and DNA extruded from dying cells or neutrophils in the active process of NETosis also serve as charged surfaces for FXII autoactivation1. It is commonly believed that the mechanism by which FXII contributes to thrombus formation in vivo in pathophysiologic and, perhaps, physiologic states is through contact autoactivation on exposed biologic surfaces leading to factor XI activation and subsequent thrombin formation (contact activation thrombin generation)1.
Animal thrombosis studies show that FXII deficiency affords thrombosis protection in injury models that are believed to depend on the contact activation pathway. F12−/− mice are protected from stroke induced by a silicon rubber-coated nylon monofilament injury-induced middle cerebral artery (MCA)12. It is important to recall that the first indication for biologic contact activation was the observation that bird plasma (now known to be deficient in FXII) did not have an electronegative thread accelerated blood clotting like other animals’ plasmas13. F12−/− mice are protected from collagen-epinephrine- and long-chain polyP-induced venous thromboembolism, constriction-induced venous thrombosis and a rat arteriovenous shunt model2,3,14–17. Most investigators agree that artificial surface exposure is an initiating event for thrombosis in these models although the physiochemical process of autoactivation of FXII in these models has not been demonstrated in vivo. F12−/− mice also have delayed arterial thrombosis on ferric chloride and Rose Bengal-induced carotid artery thrombosis18,19. Understanding the mechanism(s) for how FXII deficiency leads to thrombosis delay in these latter models is less clear. Both the ferric chloride and Rose Bengal thrombosis models elevate vessel reactive oxygen species (ROS). Although it is claimed that ferric chloride treatment physically disrupts endothelium interfering with adhesion mechanisms and exposing collagen, other data suggest that after ferric chloride or Rose Bengal treatment, the endothelium is physically intact20,21. It is harder to make an argument that loss of FXII contact activation on the chemically altered endothelium allows for the thrombosis delay. To my knowledge today, there are no studies examining FXII deficient animals on the cremasteric artery laser injury model for thrombosis.
Are there potential other mechanisms by which FXII contributes to the constitutive nature of arterial thrombosis in the intravascular compartment? FXII deficient patients have decreased leukocyte migration into skin windows22. To the extent that leukocytes participate in venous and arterial thrombosis, reduced leukocyte function associated with FXII deficiency may additionally reduce thrombosis potential. Such a mechanism may be occurring in stroke where FXII deficiency is protective23. In addition to altering leukocyte function, FXII’s absence reduces BK formation and BK’s effect on leukocyte function through the B1R could be contributory24,25. Finally FXIIa has a direct role in fibrin stabilization and its loss may alter formed fibrin such that it is less resistant to fibrinolysis26.
Although to date there is no direct evidence in vivo of FXII autoactivation on an abnormal biologic surface promoting thrombosis, indirect evidence suggests that such may be occurring. The amyloid peptide Aβ42 that forms in Alzheimer’s disease (AD) is a FXII contact activation surface27. AD is associated with microvessel thrombosis and patients with AD have evidence in plasma of prior in vivo activation of the CAS as indicated by increased circulating FXIIa and decreased factor XII and C1 inhibitor levels28,29. Also, AD mice have evidence for prior activation of contact proteins and Aβ42 peptide injected into wild type mice induces in vivo contact activation28,29.
Independent of disease states, inhibition of contact activation may be the best means to prevent medical artificial surface activation of blood coagulation. Recent investigations show that a phage display-produced Fab 3F7 to the light chain of FXIIa provides anti-thrombosis protection in rabbits attached to an extracorporeal membrane oxygenator (ECMO) equivalent to unfractionated heparin but with less fibrin formation30. The use of this unique Fab to FXIIa introduces a novel anti-thrombotic agent that has no influence on hemostasis31. In these animal subjects, activated coagulation time is not measurable, but there is no risk for bleeding. This animal study is the best showing that the mechanisms for thrombosis are separable from blood coagulation and hemostasis. The ability to inhibit contact activation-induced thrombosis by FXIIa inhibition also is supported by the use of a rHA-Infestin-4 in a rat model of ischemic stroke32. In this latter investigation, infarct area and brain edema formation are reduced with better neurologic scores in treated animals32. rHA-Infestin-4 also prevents occlusion of an arteriovenous shunt model in mice and rabbits33.
Inhibition of FXII contact activation also is an important mechanism for catheter-induced thrombosis34. The observations above on the effect of anti-FXIIa inhibitors on medical device thrombosis are applicable to commonly-used catheter-induced thrombosis34. There is limited protection from catheter-induced thrombin generation with anti-FXa (e.g. fondaparinux) and anti-FIIa (e.g. dabigatran) therapies35,36. Enoxaparin affords better protection from catheter-associated thrombus formation than fondaparinux because some low molecular weight heparin chains are of sufficient length to forms stable enzyme-antithrombin complexes with FXIIa, FXIa, and FIXa. Unfractionated heparin provides the best protection for this reason34.
Thus, precise indications for FXIIa inhibition for thrombosis prevention are developing from the array of in vivo studies in progress37. Most investigations suggest that FXIIa inhibition in animals results in reduced contact activation-induced thrombin generation (Figure 1), but this mechanism is harder to reconcile with the protection seen in f12−/− mice in the Rose-Bengal and ferric chloride injury models. Since FXIIa inhibition does not influence hemostasis, its use is highly suited for thrombosis prevention with any implanted medical device or indwelling artificial surface such as catheters and stents. This population of patients is enormous but its introduction into medical trials will be delayed by the cost of the agent and the appropriate inherent conservatism of the cardiopulmonary bypass community’s comfort with the use of unfractionated heparin. However, there are some cautions for the use of FXIIa inhibitors. Loss of FXIIa is presently not known to influence tissue factor-induced thrombosis, but there are insufficient studies addressing this question. Further, absent FXIIa may adversely influence the stability of formed fibrin. Also it is not known what long-term FXII/FXIIa inhibition will have on its role as a promoter of cell growth, angiogenesis and neutrophil function in wound healing and injury repair38.
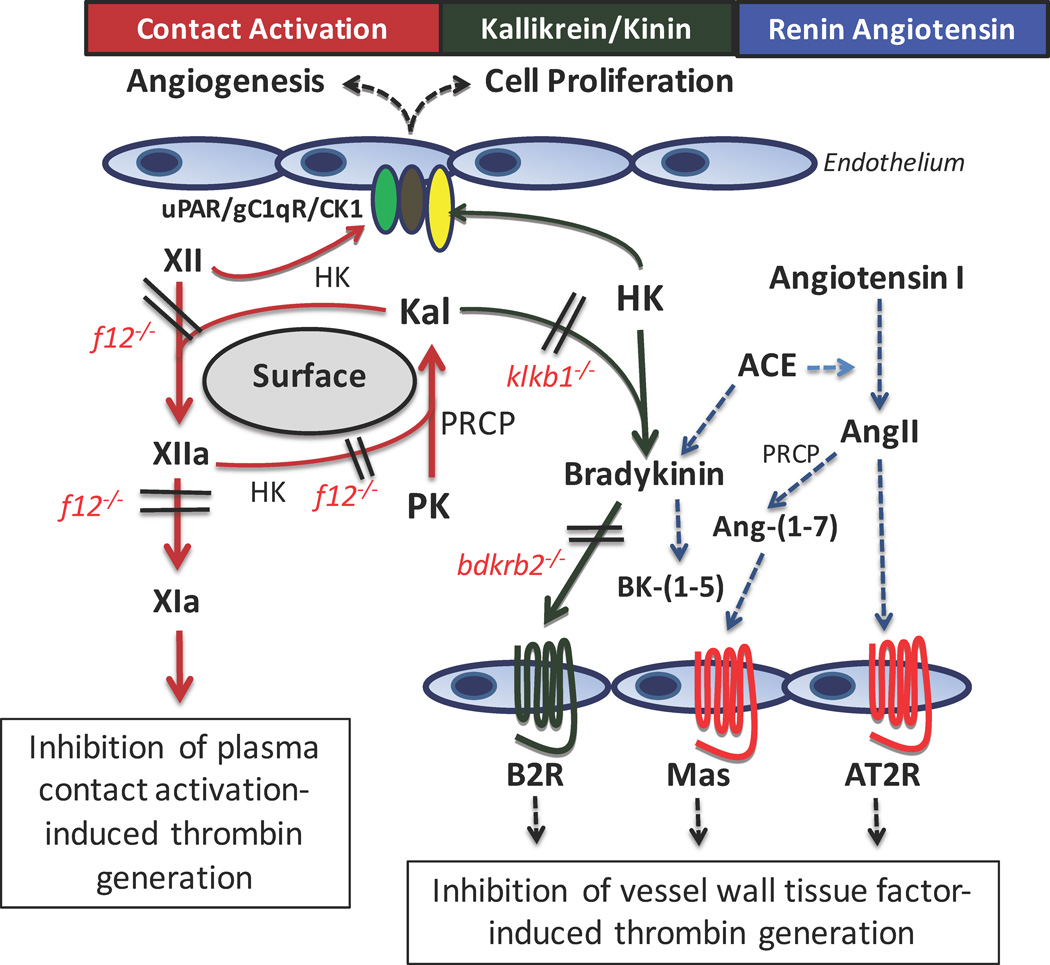
Figure 1.
Antithrombotic potential of the contact activation and kallikrein/kinin systems. The contact activation system (CAS) consists of factor XII (XII) auto-activating on surfaces (biologic or artificial) to form factor XIIa (XIIa) that then reciprocally activates prekallikrein (PK) to form plasma kallikrein (KAL) with amplification of activation of each zymogen. The system is accelerated by the presence of the cofactor to XII and PK activation, high molecular weight kininogen (HK). Another serine protease, prolycarboxypeptidase, (PRCP) also activates PK to KAL independent of factor XIIa. Formed factor XIIa also activates factor XI (XIa) leading to contact activation-induced thrombin generation. Deletion of FXII (f12−/− mice) or inhibition of FXIIa blocks contact activation-induced thrombin generation. This pathway is most operative with device-related thrombosis. FXII also has a multiprotein receptor system on endothelium that consists of uPAR, gC1qR, and cytokeratin 1 (CK1) that when stimulated induces cell proliferation and angiogenesis. HK binds to the same receptor system to block these functions of FXII.
The kallikrein/kinin system (KKS) consists of formed plasma kallikrein (Kal) by any mechanism (XIIa, PRCP) cleaving HK to liberate bradykinin (BK). BK binds to its constitutively expressed receptor, the bradykinin B2 receptor (B2R). In PK deficient (klkb1−/−) mice, reduced BK formation and B2R expression is associated with increased amounts of the G-protein coupled receptor Mas. Similarly, but to a greater extent, in B2R deleted (bdkrb2−/−) mice, the absent receptor is associated with increased expression of the G-protein coupled receptors Mas and angiotensin receptor 2 (AT2R). Further, it has elevated BK that is metabolized by angiotensin converting enzyme (ACE) of the renin angiotensin system (RAS) to produce bradykinin-(1–5) (RPPGF). The increased ACE activity also produces more angiotensin II (AngII) from angiotensin I. The excess AngII binds the AT2R or is metabolized by PRCP or ACE2 (not shown) to angiotensin-(1–7) [Ang-(1–7)] to stimulate the Mas receptor. Ang-(1–7) is the natural ligand of Mas. The combined AngII and Ang-(1–7) agonism on the AT2R and Mas, respectively, results in a 2–3 fold increase in prostacylin. In klkb1−/− mice, constitutive levels of Ang-(1–7) is sufficient to stimulate the over-expressed Mas receptor to increase prostacyclin production 1.5–2.0-fold. The increased prostacyclin in both animal models then leads to a cascade of downstream signals that first results in reduction of vessel wall tissue factor in addition to reduced contact activation.
High Molecular Weight Kininogen
HK is a cofactor for plasma kallikrein’s (KAL) activation of FXII and FXIIa’s activation of factor XI. In plasma, almost all of plasma prekallikrein and factor XI circulate bound to HK. HK functions as the endothelial cell receptor for PK and factor XI. It is also a substrate of factor XIIa, plasma kallikrein, and factor XIa and results in the liberation of bradykinin (BK) from HK1,39. F12−/− mice have plasma BK levels 50% of normal whereas prekallikrein deficient (klkb1−/−) mice have markedly reduced plasma BK levels approaching that of HK-deficient mice (kgn1−/−)3,4,24. These studies indicate that plasma kallikrein is responsible for the majority of BK release from HK observed at baseline in vivo. Kgn1−/− mice have delayed thrombosis on the Rose Bengal model for carotid artery thrombosis4. On a ferric chloride carotid artery thrombosis assay, kgn1−/− mice occlude faster in response to 5% and 7.5% ferric chloride compared to klkb1−/− mice. These data suggest that the absence of HK affords less thrombosis protection than absence of PK40. To put this comment in perspective, klkb1−/− mice (PK deficient) appear less protected from 5–10% ferric chloride-induced carotid artery thrombosis than f12−/− mice40. Thus on the ferric chloride assay, loss of FXII provides the largest protection from thrombosis, followed by loss of PK, and HK-deficiency affords the least protection in the ferric chloride model – with the caveat that this model may not be an ideal assessment of contact activated mediated thrombosis.
However, comparative inhibition studies are not investigations of the fundamental mechanistic basis for thrombosis protection. The kgn1−/− mice have been studied the least regarding their mechanism(s) for thrombosis delay. Kgn1−/− mice are protected from stroke induced by nylon filament injury of the MCA – a model that appears to be a contact activation pathway-mediated thrombosis41. In a prostate cancer prostasome-induced model for pulmonary embolism, kgn1−/− mice, like f12−/− and f11−/− mice, are resistant to pulmonary emboli and have a high rate of survival15. Prostate cancer prostasomes are a form of polyP and its intravenous injection is considered a contact activation model resulting in acute pulmonary embolism, inflammation, and death3,14. These results are distinctly different than that seen with klkb1−/−, bdkrb2−/− and wild type mice where the deficient proteins confer no protection from polyP-induced pulmonary embolism and death3,15. These results confirm prior studies where klkb1−/− mice, unlike f12−/− mice, are not protected from long chain polyP- or collagen-epinephrine-induced pulmonary embolism and death3. In sum, these studies suggest that the mechanism(s) by which HK deficiency contributes to thrombosis delay are more like those for f12−/− mice than those involved in klkb1−/− (PK deficient) mice (see the next section below). This assessment suggests that kininogen deficiency influences in vivo contact activation potential and its contribution to thrombin generation similar to FXII and factor XI deficiency. Additional studies are needed to ascertain if HK deficiency has any effect on leukocyte function and influences the constitutive anti-thrombotic nature of the vessel wall like PK loss3,22 (see below). Presently, too little is known about the mechanism(s) for thrombosis delay in HK deficiency in experimental studies to suggest it as a target for development of anti-thrombosis therapies.
Prekallikrein
Using the silicon rubber-coated nylon monofilament occlusion model in the MCA, klkb1−/− mice are protected from transient brain ischemia43. Reconstitution of the klkb1−/− with purified plasma PK such that the plasma levels of BK were normalized restores their thrombotic potential to normal. These data are consistent with the findings of f12−/− and kgn1−/− mice and indicate that the mechanism that decreases ischemia in klkb1−/− mice is reduced contact activation-induced thrombin generation12,41–43. PK deficiency is associated with reduced contact activation of plasma, but if one lets plasma sit on the bench for 3 h, the contact activation deficiency self corrects44. PK increases the rate of contact activation but is not absolutely required for it like FXII. These data also are good evidence that the transient ischemia model MCA occlusion is basically a contact activation-induced thrombosis model. Additionally, PK deficiency is recognized to have delayed arterial thrombosis on ferric chloride and Rose Bengal assays3,42.
However, when the standard silicon rubber-coated nylon monofilament is permanently left in the MCA of klkb1−/− mice, these animals are also protected from stroke43. These data are significantly different from those of permanent monofilament MCA occlusion experiments in f12−/− and kgn1−/− mice12,41. This observation suggests that another mechanism is occurring for thrombosis inhibition. This observation also supports the epidemiologic data that PK has an additional role in accelerating cardiovascular disease45–47. Several recent epidemiologic studies indicate that PK contributes to cardiovascular disease especially in diabetics45–47. The mechanism(s) by which PK contributes to stroke protection is not completely known but it may be related to an influence of PK on leukocyte function43. Klkb1−/− mice have reduced leukocyte migration and Il-1b mRNA in ischemic cerebral cortex and these parameters are corrected by reconstitution with plasma PK43. Rebuck observed that PK deficient patients have reduced leukocyte migration into skin windows22. The influence of PK on brain parenchyma may be unique, however, because when we examined those observations extensively in skin wound biopsies and thioglycolate-induced peritoneal exudates, we did not see reduced leukocyte migration in klkb1−/− mice tissue. As a result, we too sought other mechanisms to explain the constitutive, deleterious cardiovascular effects of PK and how its absence is thromboprotective.
Since PK deficient plasma in humans and mice has reduced contact activation-induced thrombin generation, we expected that the collagen-epinephrine- or long chain polyP-induced pulmonary embolism models in klkb1−/− mice would yield results similar to those seen in f12−/− mice3,42. Surprisingly, we obtained opposite results3. Klkb1−/− mice like wild type mice have no protection from death in these models, unlike f12−/− mice, despite inhibition of contact activation3,43. In the collagen-epinephrine-induced pulmonary embolism model, klkb1−/− mice have significantly less pulmonary edema as seen by Evans blue staining3. There was no survival advantage or reduced thrombi for klkb1−/− mice like wild type mice and unlike f12−/− mice. These data suggest several things. First, PK deficiency influences thrombosis risk differently than FXII deficiency. In support of that assessment, we observed that reconstitution of klkb1−/− mice such that their plasma concentration of PK was 450 nM does not correct the delayed time to thrombosis in the Rose Bengal thrombosis assay unlike the MCA occlusion model3,43. Second, it also suggests that the collagen-epinephrine pulmonary embolism model may not be completely a contact activation-induced thrombosis model. Last, it suggests that FXII nulls may reduce thrombosis risk by mechanisms in addition to contact activation inhibition, but different from PK deficiency.
Investigations to find if PK influences thrombosis risk by mechanisms in addition to contact activation inhibition revealed that klkb1−/− mice have a 1.5–2-fold increase in plasma prostacyclin3. This elevation occurs as result of a reduced B2R activation due to less plasma BK release with a concomitant increase in the expression of the endothelial cell receptor Mas3. Increased endothelial cell and plasma prostacyclin results in the expression of two vasculo-protective transcription factors, Krupple-like factor 4 (KLF4) in endothelium and sirtuin (silent mating type information regulation 2 homolog) 1 (Sirt1) in vascular smooth muscle. Both KLF4 and Sirt1 act to dampen NFκB function down-regulating vascular tissue factor (TF) expression3. Plasma from klkb1−/− mice has significantly reduced TF-induced thrombin generation that becomes particularly manifest at low dose (0.6 pM) TF concentrations3. Further TF activity and antigen are reduced in the vessel wall3. These data indicate that unlike f12−/− and, perhaps, kgn1−/− mice, klkb1−/− have reduced thrombosis due to less vessel wall TF. These studies indicate that in PK deficiency, the major mechanism for thrombosis delay may be due to the reduction of vessel wall tissue factor-induced thrombin generation (Figure 1). This mechanism for thrombosis protection in klkb1−/− mice is an in vivo example of the recent novel hypothesis that vessel wall TF and thrombin generation are the initial events in thrombus formation leading to platelet recruitment9,10.
The thrombosis reduction mechanism seen in klkb1−/− mice is not unique to this knockout animal alone. Bdkrb2−/− (bradykinin B2 receptor null) mice have a similar mechanism for thrombosis delay5,6. In the absence of the B2R, both Mas and the angiotensin receptor 2 (AT2R) become increased. These animals have elevated prostacyclin and endogenous nitrate formation. Unlike klkb1−/− mice, bdkrb2−/− have long tail bleeding times that lead to a selective platelet function defect6. The platelet function defect is in part mediated by decreased collagen-induced platelet activation via GPVI and integrins α2β16. Bdkrb2−/− mice have normal thrombin- and ADP-induced platelet activation. The difference in between klkb1−/− and bdkrb2−/− mice is the degree in elevation of plasma prostacyclin. Klkb1−/− mice have a 1.5–2-fold increase in prostacyclin that alters vessel wall TF but is not high enough to inhibit platelets3. Bdkrb2−/− mice, on the other hand, have a 2–3-fold increase in plasma prostacyclin that reduces both vessel wall TF and collagen-induced platelet activation3,6. Like klkb1−/− mice, bdkrb2−/− mice have elevated vessel wall KLF4 and Sirt1 and reduced TF-induced thrombin generation of plasma5,6.
The mechanism for thrombosis delay in klkb1−/− mice is the mirror image of the thrombotic state in cyclooxygenase 2 deleted (COX2−/−) mice that have decreased prostacyclin, Sirt1, elevated vessel wall TF, and accelerated thrombosis48. Like COX2−/− mice, Mas1−/− and agtr2−/− (AT2R null) mice have shortened times to arterial thrombosis8,48,49. Finally, prekallikrein is a zymogen that becomes activated to plasma kallikrein by FXIIa through the process of contact activation and by the endothelial cell S28 membrane serine protease prolylcarboxypeptidase (PRCP) independent of FXIIa50,51. In endothelium, PRCP acts as a growth factor, stimulates angiogenesis, and promotes vascular repair52. Prcpgt/gt mice have increased vascular reactive oxygen species formation associated with constitutive hypertension and shortened times to arterial thrombosis7,53. Prcpgt/gt mice have a cardiovascular thrombosis phenotype that is opposite to klkb1−/− mice. These data suggest that the influence of PK on thrombosis is more complex than FXII. In the brain middle cerebral artery transient wire placement model, reduced contact activation appears to be the mechanism for stroke reduction. In the Rose Bengal and ferric chloride carotid artery models, reduced vessel wall TF is the major pathway for thrombosis reduction. This latter mechanism involves interactions with receptors in the vascular renin angiotensin system. Although plasma kallikrein inhibitors (such as ecallantide) have been useful in the management of acute attacks of hereditary angioedema, to my knowledge, we do not know if these agents also decrease thrombosis risk. The complexity of PK’s actions suggest that its downstream targets may be best to modulate for thrombosis reduction than plasma PK itself.
Conclusion
There has been a significant increase in understandings of the contributions and importance of the proteins of the CAS and KKS. These proteins, FXII, HK, and PK, all reduce thrombosis risk without effect on hemostasis. Presently there are good data to recommend targeting FXIIa for thrombosis reduction under conditions when human blood interfaces with medical artificial surfaces. More information is needed to determine if FXII/FXIIa inhibitors are effective anti-thrombosis agents in other conditions. Plasma PK deficiency prevents thrombosis through both inhibition of contact activation and reduction of vessel wall TF expression. This bimodal mechanism broadens the potential of PK inhibition even though compared to FXII deletion it is a less potent inhibitor in certain models such as the ferric chloride thrombosis assay. Further investigations may find analogous non-contact activation thrombosis inhibition pathways for FXII and HK. Additionally, targets mediating the delayed thrombosis of PK deficient mice also may be viable anti-thrombosis agents. In sum, the CAS and KKS pathways have matured in understanding and may turn out be important targets for new antithrombotic therapies. After 50 years, the unimportant proteins of the CAS have become very important with great therapeutic potential.
Key points.
Factor XII increases thrombin generation by contact activation on artificial medical technology surfaces.
Prekallikrein deficiency decreases thrombosis by inhibiting contact activation and stimulating the vascular renin-angiotensin system to reduce vessel wall tissue factor.
Both FXII and PK or its related pathways are good targets to prevent thrombosis on artificial medical surfaces or the natural vessel wall, respectively.
Acknowledgments
Funding: NHLBI HL052779-18 (AHS), HL109561-04 (MEM), HL126645-01 (DIS), and a grant from the Department of Defense BC150596P1 (AHS).
I would like to thanks Drs. Gregory Adams, Chao Fang, and Evi Stavrou and Ms. Alona Merkulova for their contributions towards the development of this work and Alec Schmaier and Evi Stavrou for their critical reviews of this manuscript.
Footnotes
Conflicts of interest.
None to report.
References
- 1. Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14:28–39. doi: 10.1111/jth.13194. *An up-to-date review on this field.
- 2.Renné T, Pozgajová M, Grüner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stavrou EX, Fang C, Merkulova A, Alhalabi O, Grobe N, Antoniak S, Mackman N, Schmaier AH. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125:710–719. doi: 10.1182/blood-2014-01-550285. **The first study showing prekallikrein deficiency modulates risk to thrombosis by altering vessel wall tissue factor.
- 4.Merkulov S, Zhang WM, Komar AA, Schmaier AH, Barnes E, Zhou Y, Lu X, Iwaki T, Castellino FJ, Luo G, McCrae KR. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood. 2008;111:1274–1281. doi: 10.1182/blood-2007-06-092338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shariat-Madar Z, Mahdi F, Warnock M, Homeister JW, Srikanth S, Krijanovski Y, Murphey LJ, Jaffa AA, Schmaier AH. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006 Jul 1;108(1):192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1–7 and Mas decrease thrombosis in Bdkrb2−/− mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams GN, LaRusch GA, Stavrou E, Zhou Y, Nieman MT, Jacobs GH, Cui Y, Lu Y, Jain MK, Mahdi F, Shariat-Madar Z, Okada Y, D'Alecy LG, Schmaier AH. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. 2011;117:3929–3937. doi: 10.1182/blood-2010-11-318527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furie B. Pathogenesis of thrombosis. Hematology Am Soc Hematol Educ Program. 2009:255–258. doi: 10.1182/asheducation-2009.1.255. [DOI] [PubMed] [Google Scholar]
- 10.Ivanciu L, Krishnaswamy S, Camire RM. New insights into the spatiotemporal localization of prothrombinase in vivo. Blood. 2014;124:1705–1714. doi: 10.1182/blood-2014-03-565010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12:1513–1522. doi: 10.1111/jth.12663. ***This is the first paper to suggest that factor XII elaborates enzymatic activity when complexed to polyP without becoming a two-chain enzyme FXIIa. An analogy to this phenomena is single chain urokinase that as a protein is very similar to FXII.
- 12.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renné C, Gailani D, Nieswandt B, Renné T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering JW, Hewitt JA. Studies of the coagulation of the blood: Part I. Some physio-chemical aspects of coagulation. Biochem J. 1921;15:710–724. doi: 10.1042/bj0150710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nickel KF, Ronquist G, Langer F, Labberton L, Fuchs TA, Bokemeyer C, Sauter G, Graefen M, Mackman N, Stavrou EX, Ronquist G, Renné T. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood. 2015;126:1379–1389. doi: 10.1182/blood-2015-01-622811. *This articles compares all the contact activation and kallikrein/kinin system deletion mice for their “activability” on the long chain polyP model for pulmonary embolism.
- 16.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai TQ, Wu W, Shin MK, Xu Y, Jochnowitz N, Zhou Y, Hoos L, Bentley R, Strapps W, Thankappan A, Metzger JM, Ogletree ML, Tadin-Strapps M, Seiffert DA, Chen Z. Factor XII full and partial null in rat confers robust antithrombotic efficacy with no bleeding. Blood Coagul Fibrinolysis. 2015;26:893–902. doi: 10.1097/MBC.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–399. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, Cheng Q, Sun MF, McCarty OJ, Tucker EI, Kataoka H, Renné T, Morrissey JH, Gruber A, Gailani D. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood. 2014;123:1739–1746. doi: 10.1182/blood-2013-04-499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 21.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121:3733–3741. doi: 10.1182/blood-2012-11-468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rebuck JW. The skin window as a monitor of leukocytic functions in contact activation factor deficiencies in man. Am J Clin Pathol. 1983;79:405–413. doi: 10.1093/ajcp/79.4.405. [DOI] [PubMed] [Google Scholar]
- 23.Pham M, Stoll G, Nieswandt B, Bendszus M, Kleinschnitz C. Blood coagulation factor XII--a neglected player in stroke pathophysiology. J Mol Med (Berl) 2012;90:119–126. doi: 10.1007/s00109-011-0812-9. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki T, Castellino FJ. Plasma levels of bradykinin are suppressed in factor XII-deficient mice. Thromb Haemost. 2006;95:1003–1010. doi: 10.1160/TH06-03-0128. [DOI] [PubMed] [Google Scholar]
- 25.Araújo RC, Kettritz R, Fichtner I, Paiva AC, Pesquero JB, Bader M. Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol Chem. 2001;382:91–95. doi: 10.1515/BC.2001.014. [DOI] [PubMed] [Google Scholar]
- 26.Konings J, Govers-Riemslag JW, Philippou H, Mutch NJ, Borissoff JI, Allan P, Mohan S, Tans G, Ten Cate H, Ariëns RA. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–3951. doi: 10.1182/blood-2011-03-339572. [DOI] [PubMed] [Google Scholar]
- 27.Schmaier AH. Alzheimer's Disease is In-Part a Thrombo-Hemorrhagic Disorder. J Thromb Haemost. 2016 Jan 28; [Google Scholar]
- 28.Zamolodchikov D, Chen Z-L, Conti BA, Renné T, Strickland S. Activation of the factor XII-driven contact system in Alzheimer’s disease patient and mouse model plasma. Proc Natl Acad Sci. 2015;112:4068–4073. doi: 10.1073/pnas.1423764112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamolodchikov D, Renné T, Strickland S. The Alzheimer's disease peptide Aβ promotes thrombin generation through activation of coagulation factor XII. J Thromb Haemost. 2015 Nov 28; doi: 10.1111/jth.13209. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsson M, Rayzman V, Nolte MW, Nickel KF, Björkqvist J, Jämsä A, Hardy MP, Fries M, Schmidbauer S, Hedenqvist P, Broomé M, Pragst I, Dickneite G, Wilson MJ, Nash AD, Panousis C, Renné T. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014 Feb 5;6(222):222ra17. doi: 10.1126/scitranslmed.3006804. **The first in vivo study that clearly shows thrombosis protection on artificial surfaces with FXIIa inhibition without effect on hemostasis.
- 31.Schmaier AH. Extracorporeal circulation without bleeding. Sci Transl Med. 2014 Feb 5;6(222):222fs7. doi: 10.1126/scitranslmed.3008497. [DOI] [PubMed] [Google Scholar]
- 32.Krupka J, May F, Weimer T, Pragst I, Kleinschnitz C, Stoll G, Panousis C, Dickneite G, Nolte MW. The Coagulation Factor XIIa Inhibitor rHA-Infestin-4 Improves Outcome after Cerebral Ischemia/Reperfusion Injury in Rats. PLoS One. 2016 Jan 27;11(1):e0146783. doi: 10.1371/journal.pone.0146783. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May F, Krupka J, Fries M, Thielmann I, Pragst I, Weimer T, Panousis C, Nieswandt B, Stoll G, Dickneite G, Schulte S, Nolte MW. FXIIa inhibitor rHA-Infestin-4: Safe thromboprotection in experimental venous, arterial and foreign surface-induced thrombosis. Br J Haematol. 2016 Mar 27; doi: 10.1111/bjh.13990. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015 Jun 13;(Suppl 1):S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 35.Yau JW, Stafford AR, Liao P, Fredenburgh JC, Roberts R, Weitz JI. Mechanism of catheter thrombosis: comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood. 2011;118:6667–6674. doi: 10.1182/blood-2011-07-364141. [DOI] [PubMed] [Google Scholar]
- 36.Yau JW, Liao P, Fredenburgh JC, Roberts RS, Weitz JI. Only high levels of dabigatran attenuate catheter thrombosis in vitro and in rabbits. Thromb Haemost. 2014;112:79–86. doi: 10.1160/TH13-12-1047. [DOI] [PubMed] [Google Scholar]
- 37.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost. 2015;13:1383–1395. doi: 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaRusch GA, Mahdi F, Shariat-Madar Z, Adams G, Sitrin RG, Zhang WM, McCrae KR, Schmaier AH. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood. 2016;115:5111–5120. doi: 10.1182/blood-2009-08-236430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 40.Kokoye Y, Ivanov I, Cheng Q, Matafonov A, Dickeson SK, Mason S, Sexton DJ, Renné T, McCrae K, Feener EP, Gailani D. A comparison of the effects of factor XII deficiency and prekallikrein deficiency on thrombus formation. Thromb Res. 2016;140:118–124. doi: 10.1016/j.thromres.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langhauser F, Göb E, Kraft P, Geis C, Schmitt J, Brede M, Göbel K, Helluy X, Pham M, Bendszus M, Jakob P, Stoll G, Meuth SG, Nieswandt B, McCrae KR, Kleinschnitz C. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120:4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bird JE, Smith PL, Wang X, Schumacher WA, Barbera F, Revelli JP, Seiffert D. Effects of plasma kallikrein deficiency on haemostasis and thrombosis in mice: murine ortholog of the Fletcher trait. Thromb Haemost. 2012;107:1141–1150. doi: 10.1160/th-11-10-0682. [DOI] [PubMed] [Google Scholar]
- 43.Göb E, Reymann S, Langhauser F, Schuhmann MK, Kraft P, Thielmann I, Göbel K, Brede M, Homola G, Solymosi L, Stoll G, Geis C, Meuth SG, Nieswandt B, Kleinschnitz C. Blocking of plasma kallikrein ameliorates stroke by reducing thromboinflammation. Ann Neurol. 2015;77:784–803. doi: 10.1002/ana.24380. [DOI] [PubMed] [Google Scholar]
- 44.Abildgaard CF, Harrison J. Fletcher factor deficiency: family study and detection. Blood. 1974;43:641–644. [PubMed] [Google Scholar]
- 45.Jaffa AA, Durazo-Arvizu R, Zheng D, Lackland DT, Srikanth S, Garvey WT, Schmaier AH DCCT/EDIC Study Group. Plasma prekallikrein: a risk marker for hypertension and nephropathy in type 1 diabetes. Diabetes. 2003;52:1215–1221. doi: 10.2337/diabetes.52.5.1215. [DOI] [PubMed] [Google Scholar]
- 46.Jaffa MA, Luttrell D, Schmaier AH, Klein RL, Lopes-Virella M, Luttrell LM, Jaffa AA DCCT/EDIC Research Group. Plasma Prekallikrein Is Associated With Carotid Intima-Media Thickness in Type 1 Diabetes. Diabetes. 2016;65:498–502. doi: 10.2337/db15-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gittleman HR, Merkulova A, Alhalabi O, Stavrou EX, Veigl ML, Barnholtz-Sloan JS, Schmaier AH. A Cross-Sectional Study of KLKB1 and PRCP Polymorphisms in Patient Samples with Cardiovascular Disease. Frontiers in Hematology. 2016 doi: 10.3389/fmed.2016.00017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbieri SS, Amadio P, Gianellini S, Tarantino E, Zacchi E, Veglia F, Howe LR, Weksler BB, Mussoni L, Tremoli E. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation. 2012;126:1373–1384. doi: 10.1161/CIRCULATIONAHA.112.097295. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Ricciotti E, Scalia R, Tang SY, Grant G, Yu Z, Landesberg G, Crichton I, Wu W, Puré E, Funk CD, FitzGerald GA. Vascular COX-2 modulates blood pressure and thrombosis in mice. Sci Transl Med. 2012 May 2;4(132):132ra54. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277:17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- 51.Shariat-Madar Z, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004;103:4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- 52.Marangoni RA, Santos RA, Piccolo C. Deficient prolylcarboxypeptidase gene and protein expression in left ventricles of spontaneously hypertensive rats (SHR) Peptides. 2014;61:69–74. doi: 10.1016/j.peptides.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Adams GN, Stavrou EX, Fang C, Merkulova A, Alaiti MA, Nakajima K, Morooka T, Merkulov S, LaRusch GA, Simon DI, Jain MK, Schmaier AH. Prolylcarboxypeptidase promotes angiogenesis and vascular repair. Blood. 2013;122:1522–1531. doi: 10.1182/blood-2012-10-460360. [DOI] [PMC free article] [PubMed] [Google Scholar]