Abstract
Previous studies have suggested that protein kinase C (PKC) is involved in heat shock protein (Hsp)–mediated cardioprotection. Therefore, we wanted to determine whether overexpression of Hsps modulates PKC expression, which will give us further insight into understanding the mechanism by which Hsps and PKC interact to protect cells from stress-induced injury. Specifically, we overexpressed the inducible form of Hsp70 (Hsp70i) or Hsp90 in rat neonatal cardiomyocytes and evaluated PKCδ or PKCɛ expression by immunoblotting and immunofluorescent confocal microscopy. Western analysis showed that overexpression of Hsp70i or Hsp90 decreased PKCɛ expression. However, overexpression of Hsp70i or Hsp90 did not modify PKCδ expression over control levels. Overexpression of constitutively active PKCδ or PKCɛ increased Hsp70i expression over control levels. The data suggest that overexpression of Hsps differentially modulates expression of PKC isoforms in rat neonatal cardiomyocytes. Furthermore, PKC may directly play a role in Hsp-mediated cardioprotection by upregulating Hsp70i expression.
INTRODUCTION
Heat shock proteins (Hsps), which are also known as molecular chaperones, allow cells to adapt and tolerate stressful insults, such as heat (Benjamin and McMillan 1998). Hsps are known to be cardioprotective during stress (Lau et al 1997; Martin et al 1997; Lin et al 2001). Specifically, reports have indicated that Hsp70 is cardioprotective against ischemic injury (Mestril et al 1994; Marber et al 1995; Plumier et al 1995); however, studies still need to be conducted to examine factors that regulate Hsp-mediated cardioprotection. Previous studies have suggested that protein kinase C (PKC) may mediate protection induced by heat stress (Joyeux et al 1997; Kukreja et al 1999; Meldrum et al 2001). However, the mechanism for PKC involvement in Hsp-mediated cardioprotection is not fully understood.
PKC isoforms have distinct functions in the heart. Two of the novel isoforms, PKCδ and PKCɛ, have been shown to be cardioprotective (Gray et al 1997; Zhao et al 1998; Dorn et al 1999; Liu et al 1999; Fryer et al 2001). In contrast, some recent studies have reported that PKCδ activation increases damage during ischemic injury and is associated with apoptosis (Chen et al 2001; Heidkamp et al 2001). There is some evidence that suggests an association between PKC and Hsps. For example, it was recently reported that the overexpression of PKCɛ in transgenic mice upregulates Hsp70 when compared with nontransgenic mice (Ping et al 2001). Furthermore, it was reported that overexpression of Hsp70 inhibits PKC activity and subsequently inhibits heat shock factor 1 (HSF1) phosphorylation in human epidermoid A-431 cells (Ding et al 1998); however, it would be interesting to know which PKC isoforms are modulated by Hsps in cardiomyocytes.
Because we know that Hsps and PKC are factors that separately mediate cardioprotection during stressful insult, we believe that it was important to evaluate their relationship. Specifically, we wanted to determine whether Hsp overexpression modulated PKC levels in rat neonatal cardiomyocytes, which will help us determine whether the PKC signaling cascade is involved in this form of Hsp-mediated cardioprotection. Therefore, we overexpressed inducible form of Hsp70 (Hsp70i) or Hsp90 in rat neonatal cardiomyocytes and examined expression levels of PKCδ and PKCɛ. We also overexpressed PKC isoforms in cardiomyocytes and analyzed Hsp70i expression to establish whether a feedback mechanism exists between Hsps and PKC. We found that overexpression of Hsp70i and Hsp90 inhibited PKCɛ expression but did not modulate PKCδ expression. Furthermore, we demonstrate that overexpression of either PKCδ or PKCɛ increased Hsp70i expression in cardiomyocytes.
RESULTS
Western analysis was used to determine whether overexpression of Adhsp70i or Adhsp90 modulates expression of PKCδ or PKCɛ. Protein extracts from cardiomyocytes infected with AdcaPKCδ (Fig 1A) were used as positive controls for identifying PKCδ. Cardiomyocytes overexpressing Adhsp70i or Adhsp90 did not alter PKCδ levels when compared with uninfected cells or cells infected with the control infected vector, AdSR (Fig 1A). Figure 1B illustrates quantitatively the densitometric measurements of PKCδ expression from multiple Western blots. Immunofluorescent microscopy illustrated the effects of Hsp70 or Hsp90 on PKCδ expression in rat neonatal cardiomyocytes. Overexpression of Adhsp70i or Adhsp90 (Fig 1C, panels c and d) in cardiomyocytes displayed similar fluorescence intensity for PKCδ when compared with uninfected and AdSR-infected cells (Fig 1C, panels a and b).
Fig 1.
Analysis of PKCδ expression in rat neonatal cardiomyocytes. Rat neonatal cardiomyocytes were isolated as previously described (Iwaki et al 1993). Cardiomyocytes were plated at a density of 1 million cells/well in 6-well plates. On day 1 after isolation, neonatal cardiomyocytes were infected with adenoviruses at a multiplicity of infection (MOI) of 10:1. After 1 hour of infection, the cells were incubated in serum-free medium for 48 hours before protein analysis. The viruses were prepared as previously described (Mestril et al 1996; Heidkamp et al 2001; Strait et al 2001). In brief, the rat Hsp70 gene and Hsp90 gene, and the constitutively active PKCδ cDNA containing adenovirus (provided by Drs Peter Parker and Peter Sudgen, Imperial College of Technology and Medicine, Cambridge, UK), were produced via homologous recombination. The recombinant adenoviruses were amplified into 293 cells and CsCl purified, and titer was determined by a plaque assay. (A) Cardiomyocytes infected for 48 hours with AdcaPKCδ, AdSR (control adenoviral construct), Adhsp70i, or Adhsp90 were washed with PBS and scraped in solution B containing 1% Triton X-100, 0.5% deoxycholate, and 5 μM 2-mercaptoethanol. Protein concentrations were determined using the BCA protein assay (Pierce Chemical Co., Rockford, IL, USA). Protein extracts (40 μg) were electrophoresed through 8% linear gradient sodium dodecyl sulfate–polyacrylamide gels and transferred to nitrocellulose membranes. Membranes incubated with rabbit polyclonal PKCδ antibody (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) followed by incubation with horseradish peroxidase–conjugated anti-rabbit IgG (1:1000). Immunoblotting for actin was used to verify equal loadings. (B) Densitometric quantitation of PKCδ expression. Autoradiographic results were scanned and then quantified with UN-SCAN-IT program (Silk Scientific, Inc., Orem, UT, USA). Values are mean arbitrary units ± SE from 3 independent experiments. Statistical significance between control and experimental groups was assessed with 1-way analysis of variance, followed by Tukey posttest. *P < 0.05 was considered significant. (C) Immunofluorescence analysis of PKCδ in rat neonatal cardiomyocytes. Cardiomyocytes (3 × 105) cultured in chamber slides (Nalge Nunc International Corp., Naperville, IL, USA) were infected with adenoviral constructs for 48 hours. Cells were fixed in cold acetone for 5 minutes, washed in PBS, and then permeabilized with Triton X-100. Cells were blocked with 1% goat serum in PBS for 15 minutes. Cells were then incubated with rabbit polyclonal nPKCδ antibody (1:50, Santa Cruz), followed by incubation with fluorescein-conjugated goat anti-rabbit antibody (1:50, Santa Cruz). Slides were mounted with glycerol and analyzed using the Bio-Rad Radiance 2000 Confocal Imaging System. Panel a: uninfected (control); panel b: control adenoviral construct (AdSR); panel c: Adhsp70i; and panel d: Adhsp 90. PKC, protein kinase C; Hsp, heat shock protein; cDNA, complementary deoxyribonucleic acid; PBS, phosphate-buffered saline
In contrast, cardiomyocytes infected with Adhsp70i or Adhsp90 demonstrated decreased PKCɛ levels when compared with uninfected or AdSR-infected cells (Fig 2A). Protein extracts from cardiomyocytes overexpressing AdcaPKCɛ were used as a positive control (Fig 2A). Figure 2B illustrates the pooled densitometric measurements of PKCɛ levels obtained from the Western blots. Immunofluorescent analysis for PKCɛ indicated that cardiomyocytes overexpressing Adhsp70i or Adhsp90 (Fig 2C, panels c and d) displayed a weaker fluorescence intensity for PKCɛ when compared with uninfected or AdSR-infected cells (Fig 2C, panels a and b).
Fig 2.
Analysis of PKCɛ expression in rat neonatal cardiomyocytes. (A) Adenoviruses were constructed as described in Fig 1. Cardiomyocytes were uninfected or infected for 48 hours with AdcaPKCɛ (provided by Drs Peter Parker and Peter Sudgen, Imperial College of Technology and Medicine, Cambridge, UK), AdSR, Adhsp70i, or Adhsp90. Protein extracts were separated by 8% SDS-PAGE, transferred to nitrocellulose membrane, and incubated with rabbit polyclonal PKCɛ antibody (1:1000, Santa Cruz). Immunoblotting for actin was used to verify equal loadings. (B) Densitometric quantitation of PKCɛ expression. Values are mean arbitrary units ± SE from 3 independent experiments. (C) Immunofluorescence analysis of PKCɛ in rat neonatal cardiomyocytes. Cells were infected in chamber slides with adenoviral constructs for 48 hours. Cardiomyocytes were fixed and then stained for PKCɛ (1:50, Santa Cruz). Panel a: uninfected (control); panel b: control adenoviral construct (AdSR); panel c: Adhsp70i; and panel d: Adhsp90. *P < 0.05 vs control cells. PKC, protein kinase C; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis
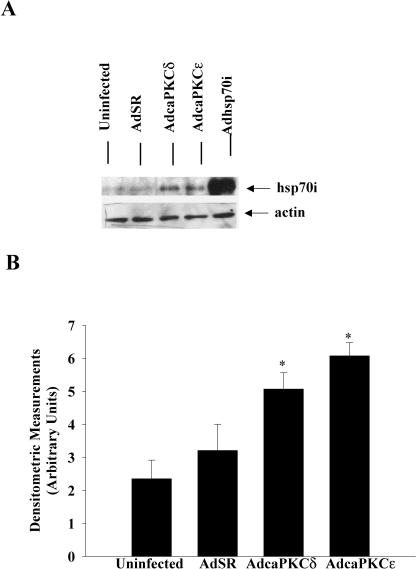
Because overexpression of Hsp70i decreased PKCɛ expression in rat neonatal cardiomyocytes, but did not alter expression of PKCδ, we wanted to determine whether overexpression of PKCδ or PKCɛ differentially modulates Hsp70i expression. Therefore, we infected rat neonatal cardiomyocytes with AdcaPKCδ or AdcaPKCɛ for 48 hours. Western blot analysis was used to evaluate Hsp70i expression. Protein extracts from cells that were infected with Adhsp70i were used as positive controls for identifying Hsp70i (Fig 3A). As illustrated in Figure 3A, cardiomyocytes overexpressing PKCδ or PKCɛ increased Hsp70i expression when compared with cells that were uninfected or cells infected with the control vector AdSR. Figure 3B illustrates quantitatively the densitometric measurements of Hsp70i expression from Western blots.
Fig 3.
Analysis of Hsp70i expression in rat neonatal cardiomyocytes. (A) Adenoviruses were constructed as described in Figure 1. Cardiomyocytes were uninfected or infected for 48 hours with control adenoviral construct (AdSR), AdcaPKCδ, AdcaPKCɛ, or Adhsp70i. Protein extracts were separated by 8% SDS-PAGE and transferred to nitrocellulose membranes. The antibody used was rabbit polyclonal Hsp70i (1:1000, Stressgen Biotechnologies Inc., Victoria, BC, Canada). Immunoblotting for actin was used to verify equal loadings. (B) Densitometric quantitation of Hsp70i expression. Values are mean arbitrary units ± SE from 3 independent experiments. *P < 0.05 vs control cells. Hsp, heat shock protein; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis
DISCUSSION
Hsps are primarily known for their ability to assist proteins in folding properly (Benjamin and McMillan 1998). The mechanisms by which Hsps protect cells under stressful conditions are not fully understood; however, recent evidence indicates that these proteins interact with kinases, such as PKC, that have also been shown to be protective. Overexpression of PKCɛ in transgenic mice demonstrated an increase in Hsp70, and furthermore, it was shown that Hsp70 resides in PKCɛ complexes (Ping et al 2001). Our results, using an alternate approach, confirm that PKC induces upregulation of Hsp70i.
The mechanism by which PKC is involved in Hsp-mediated cardioprotection may be due to Hsp autoregulation. Our data establish that overexpression of constitutively active PKCδ or PKCɛ increases Hsp70i expression (Fig 3). In addition, when we overexpress Hsp70i, we detect a decrease in PKCɛ expression (Fig 2A). However, overexpression of Hsp70i does not affect PKCδ expression (Fig 1A), which implies that Hsp70i will be upregulated when this PKC isoform is increased (as seen in Fig 3). Therefore, we have a phenomenon wherein PKC upregulates expression of Hsps; yet, elevated levels of Hsp70i decrease expression of selective PKC isoforms. Although previous studies have documented that PKCδ is cardioprotective (Zhao et al 1998; Fryer et al 2001), it should be noted that studies have also revealed that PKCδ may cause further damage induced by ischemia (Chen et al 2001). Based on these findings, one can understand why it is important to study the various isoforms of PKC and determine how these isoforms may differentially modulate effectors such as Hsps, which have been shown to be cardioprotective.
Because overexpression of PKC isoforms may lead to phosphorylation of many proteins, there are likely several mechanisms by which PKC upregulates Hsp70. We believe that PKC may potentially upregulate Hsp70 through a mechanism that involves HSF1 activation. Studies conducted in human epidermoid A-431 cells demonstrated that phorbol 12-myristate 13-acetate (PMA) (PKC stimulator) increases HSF1 phosphorylation in cells transfected with Hsp70 complementary deoxyribonucleic acid (Ding et al 1998). Therefore, it is a possibility that PKC may lead to phosphorylation of HSF1 and subsequently modulate expression of Hsps.
In summary, our data suggest that overexpression of Hsps modulates PKC isoforms differentially. In addition, our study implies that Hsps may be autoregulated through a process that involves PKC. Further studies will have to be conducted to determine the mechanism of this interaction. It will also be interesting to examine this phenomenon in other physiologic models, which may also help elucidate mechanisms by which PKC mediates cardioprotection during heat stress.
Acknowledgments
This study was supported by NIH grant HL-61339, a grant-in-aid from the American Heart Association to R.M., a scientist development grant from AHA to J.L.M., and the John and Marian Falk Trust for Medical Research. We thank Tina Griffin and Tina Valdez for their technical support.
REFERENCES
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Chen L, Hahn H, and Wu G. et al. 2001 Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 98:11114–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XZ, Tsokos GC, Kiang JG. Overexpression of HSP-70 inhibits the phosphorylation of HSF1 by activating protein phosphatase and inhibiting protein kinase C activity. FASEB J. 1998;12:451–459. doi: 10.1096/fasebj.12.6.451. [DOI] [PubMed] [Google Scholar]
- Dorn GW II, Souroujon MC, and Liron T. et al. 1999 Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci U S A. 96:12798–12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-delta in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol. 2001;280:H1333–H1346. doi: 10.1152/ajpheart.2001.280.3.H1346. [DOI] [PubMed] [Google Scholar]
- Gray MO, Karliner JS, Mochly-Rosen D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J Biol Chem. 1997;272:30945–30951. doi: 10.1074/jbc.272.49.30945. [DOI] [PubMed] [Google Scholar]
- Heidkamp MC, Bayer AL, Martin JL, Samarel AM. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res. 2001;89:882–890. doi: 10.1161/hh2201.099434. [DOI] [PubMed] [Google Scholar]
- Iwaki K, Chi SH, Dillman WH, Mestril R. Induction of HSP70 in cultured rat neonatal cardiomyocytes by hypoxia and metabolic stress. Circulation. 1993;87:2023–2032. doi: 10.1161/01.cir.87.6.2023. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Baxter GF, Thomas DL, Ribuot C, Yellon DM. Protein kinase C is involved in resistance to myocardial infarction induced by heat stress. J Mol Cell Cardiol. 1997;29:3311–3319. doi: 10.1006/jmcc.1997.0556. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Qian YZ, Okubo S, Flaherty E. Role of protein kinase C and 72 kDa heat shock protein in ischemic tolerance following heat stress in the rat heart. Mol Cell Biochem. 1999;195:123–131. doi: 10.1023/a:1006977311448. [DOI] [PubMed] [Google Scholar]
- Lau S, Patnaik N, Sayen MR, Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–2294. doi: 10.1161/01.cir.96.7.2287. [DOI] [PubMed] [Google Scholar]
- Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillman WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787. [DOI] [PubMed] [Google Scholar]
- Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-epsilon is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–1948. doi: 10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillman WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Investig. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillman WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;16:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- Meldrum KK, Meldrum DR, Sezen SF, Crone JK, Burnett AL. Heat shock prevents simulated ischemia-induced apoptosis in renal tubular cells via a PKC-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2001;281:R359–R364. doi: 10.1152/ajpregu.2001.281.1.R359. [DOI] [PubMed] [Google Scholar]
- Mestril R, Chi SH, Sayen MR, O'Reilly D, Dillman WH. Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. J Clin Investig. 1994;93:759–767. doi: 10.1172/JCI117030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestril R, Giordano FJ, Conde AG, Dillman WH. Adenovirus-mediated gene transfer of a heat shock protein 70 (hsp 70i) protects against simulated ischemia. J Mol Cell Cardiol. 1996;28:2351–2358. doi: 10.1006/jmcc.1996.0228. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Pierce WM Jr., Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Investig. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait JB III, Martin JL, Bayer A, Mestril R, Eble DM, Samarel AM. Role of protein kinase C-ɛ in hypertrophy of cultured neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H756–H766. doi: 10.1152/ajpheart.2001.280.2.H756. [DOI] [PubMed] [Google Scholar]
- Zhao J, Renner O, Wightman L, Sugden PH, Stewart L, Miller AD, Latchman DS, Marber MS. The expression of constitutively active isotypes of protein kinase C to investigate preconditioning. J Biol Chem. 1998;273:23072–23079. doi: 10.1074/jbc.273.36.23072. [DOI] [PubMed] [Google Scholar]