Abstract
Heat shock protein 32 (Hsp32, hemoxygenase-1) is induced by reactive oxygen metabolites (ROM) and degrades heme leading to the formation of antioxidant bilirubin. Increased mucosal generation of ROM occurs in gastritis and inflammatory bowel disease. We aimed to assess mucosal expression of Hsp32 in normal stomach and colon and to test the hypothesis that disease-related differential expression occurs in inflamed tissue. Gastric body and antral mucosal biopsies were obtained from 33 patients comprising Helicobacter pylori–negative normal controls (n = 8), H pylori–negative gastritis patients (n = 11), and H pylori–positive gastritis patients (n = 14). Forty-seven archival colonic mucosal biopsies selected comprised normal histology (n = 10), active ulcerative colitis (UC) (n = 9), inactive UC (n = 8), active Crohn's disease (CD) (n = 8), inactive CD (n = 6), and other colitides (n = 6). Hsp32 expression in formalin-fixed sections was assessed by avidin-biotin peroxidase immunohistochemistry using a polyclonal rabbit anti-Hsp32 as the primary antibody. Immunohistochemical staining identified Hsp32 in all groups. Diffuse cytoplasmic staining was seen in gastric and colonic epithelial and lamina proprial inflammatory cells. Staining scores for Hsp32 were higher in antral H pylori–positive (P = 0.002) and H pylori–negative (P = 0.02) gastritis than in controls and in body H pylori–positive gastritis than in the other 2 groups (P < 0.01). Expression of Hsp32 was increased in active UC compared with inactive disease (P = 0.03) and normal controls (P = 0.02). In conclusion, Hsp32 is expressed constitutively in normal gastric and colonic mucosa, and differential expression occurs in these tissues when they are inflamed. Upregulation of Hsp32 may be an adaptive response to protect mucosa from oxidative injury in patients with gastritis and inflammatory bowel disease.
INTRODUCTION
Heat shock proteins, or stress proteins (Hsps), are ubiquitous, highly conserved intracellular proteins. Although characterized by their ability to respond to a sudden rise in temperature, in vitro they are induced not only by heat but also by a variety of other physiological stressors including inflammatory cytokines and mediators (Lindquist 1986; Polla 1988).
Hsp of molecular weight 32 kDa (Hsp32) was first observed in cells exposed to heavy metals and is now characterized as the microsomal enzyme hemoxygenase-1 (HO-1) (Maines 1988; Keyse and Tyrrell 1989; Maines and Panakian 2001; Yet et al 2002). This hemoxygenase isoenzyme is also induced by reactive oxygen metabolites (ROM) (Polla 1988; Keyse and Tyrrell 1989; Ewing and Maines 1993; Yet et al 2002) and catalyses the degradation of heme to biliverdin. Biliverdin is subsequently converted by biliverdin reductase to bilirubin, a molecule with antioxidant properties (Stocker et al 1987). Induction of HO-1 suppressed the inflammatory response in trinitrobenzene sulphonic acid (TNBS)–induced colitis in rats (Wang et al 2001) and could play a role in the control of inflammation in human gastrointestinal disease. Although hemoxygenase has been found in all eukaryotic tissues studied to date, there are no reports of the expression of HO-1 in the human stomach or colon.
Acute and chronic gastritis are common, usually asymptomatic inflammatory diseases of the stomach; their most common causes are infection with Helicobacter pylori and exogenous agents such as alcohol and nonsteroidal anti-inflammatory drugs (Weinstein 1993). Ulcerative colitis (UC) and Crohn's disease (CD) are chronic relapsing inflammatory diseases affecting the gastrointestinal tract. Although their primary cause is not yet known, pathogenetic mechanisms are being gradually unraveled (Fiocchi 1998; Podolsky 2002).
Mucosal generation of ROMs is increased and may play a pathogenic role in gastritis, particularly when due to infection with H pylori (Davies and Rampton 1994), and also in active UC and CD (Simmonds and Rampton 1993). In this immunohistochemical study, we have assessed the constitutive expression of Hsp32 in normal human gastric and colonic mucosa and tested the hypothesis that its expression in these tissues is upregulated in inflammatory diseases characterized by increased production of ROMs and other mediators.
MATERIALS AND METHODS
Patients and biopsies
Body and antral gastric mucosal biopsies taken at routine diagnostic gastroscopy from H pylori–negative histologically normal controls (n = 8), H pylori–positive gastritis patients (n = 14), and H pylori–negative gastritis patients (n = 11) were formalin fixed and embedded in paraffin. All the patients had had symptoms for at least 3 months. H pylori status was defined by the rapid urease (CLO) test and by routine histological examination of hematoxylin and eosin–stained and cresyl fast violet–stained sections. In 1 normal patient, 6 H pylori–positive patients, and 1 H pylori–negative patient, biopsies taken from the gastric body were found histologically not to contain corpus mucosa, and these were discarded.
Colonic biopsies were extracted from histopathological archives at the Department of Histopathology, Royal London Hospital. On the basis of clinical information and histological diagnosis (confirmed by a blinded histopathologist after retrieval of the specimen from the archive), 6 groups of biopsies were selected: normal histology (n = 10), active UC (n = 9), inactive UC (n = 8), active Crohn's colitis (n = 8), inactive Crohn's colitis (n = 6), and other colitides (n = 6) (radiation colitis [n = 3], pseudomembranous colitis [n = 2], and collagenous colitis [n = 1]).
Permission for the study was obtained from the East London, City and Hackney Health Authority Ethics Committee.
Grading of severity of gastritis
Haematoxylin and eosin–stained gastric mucosal sections were used to quantify gastric mucosal inflammation. For this, an experienced histopathologist (P.D.), blinded to the immunohistochemical results (see below), used a modified Sydney score for each of the 4 variables, chronic gastritis, acute gastritis, atrophy, and intestinal metaplasia (0 = none, 1 = mild, 2 = moderate, 3 = severe) (Misiewicz 1991).
Immunohistochemical staining for Hsp32
Hsp32 expression was assessed using an avidin-biotin peroxidase technique. Sections (5 μm) of formalin-fixed, paraffin-embedded biopsies were placed onto glass slides, dewaxed by immersion in xylene for 5 minutes, and rehydrated by passage through graded alcohols to water. Endogenous peroxidase activity was blocked using methanol and hydrogen peroxide and the slides washed in phosphate-buffered saline (PBS) for 5 minutes. Sections were digested using pronase (1 mg/mL) and washed serially in tap water and PBS. Polyclonal rabbit anti-rat Hsp32 (SPA 895, StressGen Biotechnologies, Bioquote Ltd, York, UK) was applied at a dilution of 1:5000 and the slides maintained overnight at room temperature. After blocking with 1% bovine serum albumin in PBS and extensive washing, Hsp32 was localized using a VECTASTAIN ABC kit (Vector Laboratories Inc, Peterborough, UK) and visualized using diaminobenzidine and hydrogen peroxide as substrates. Slides were counterstained with hematoxylin, differentiated in acid alcohol, dehydrated in graded alcohols, and finally cleared in xylene and mounted. For each biopsy, negative controls were included, omitting the primary antibody to Hsp32.
Localization of Hsp32 was assessed by light microscopy. Intensity of mucosal staining was scored on a 4-point scale by 2 observers blinded to the disease of the biopsy (0 = no staining, 1 = weak, 2 = moderate, 3 = strong).
Interobserver variation was calculated as the percentage of scores that were scored differently by the 2 observers. Intraobserver variation was assessed by repeated scoring of 20 randomly selected slides and the result expressed as a percentage of the scores differing between the first and second occasions. Inter- and intraobserver variation were each <10% for both gastric and colonic mucosa.
Statistics
Preliminary analysis of normal and disease groups' results was made by Kruskal-Wallis analysis of variance. Comparisons between groups were then made using the Mann-Whitney U-test for unpaired data, and significance levels of P < 0.05 (2-tailed) were regarded as statistically significant. The objectives were to compare disease groups with either normal controls or with each other. The raw P values obtained are shown uncorrected for multiple comparisons (Perneger 1998). Paired data were assessed using the Wilcoxon signed ranks test and correlations using Pearson's correlation coefficient.
RESULTS
Gastric mucosa
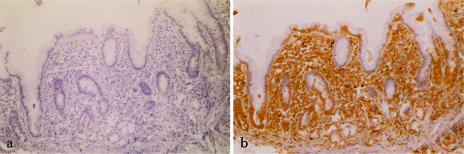
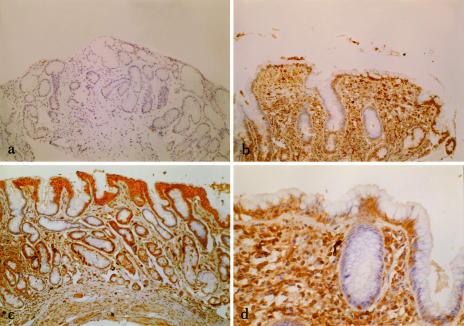
Immunoreactive Hsp32 was detected in both normal and inflamed body and antrum (Figs 1 and 2). In patients with gastritis (Figs 1b, 2b–d), diffuse cytoplasmic staining was seen in surface epithelial cells, parietal cells, and lamina proprial inflammatory cells. Staining was much less pronounced in normal gastric mucosa (Fig 2a), and there was no staining of negative controls (Fig 1a).
Fig 1.
(a) Immunohistochemical view (100× magnification) of gastric antral mucosa in a patient with Helicobacter pylori (H pylori)–positive gastritis as a negative control (ie, omitting the primary antibody to heat shock protein [Hsp32]). (b) Immunohistochemical view (100× magnification) of gastric antral mucosa in the same patient with H pylori–positive gastritis stained for Hsp32
Fig 2.
(a) Immunohistochemical view (100× magnification) of gastric antral mucosa in a normal control subject stained for heat shock protein (Hsp32). (b) Immunohistochemical view (100× magnification) of gastric antral mucosa in a patient with Helicobacter pylori (H pylori)–positive gastritis stained for Hsp32. (c) Immunohistochemical view (100× magnification) of gastric antral mucosa in a patient with H pylori–negative gastritis stained for Hsp32. (d) Immunohistochemical view (250× magnification) of gastric antral mucosa in a patient with H pylori–positive gastritis stained for Hsp32. Cytoplasmic staining (brown) is seen in superficial epithelial cells, parietal cells, and inflammatory cells of the lamina propria
In the antrum, immunohistochemical scores for Hsp32 staining of H pylori–positive gastritis patients (P < 0.002) and H pylori–negative gastritis patients (P < 0.02) were higher than normals (Table 1). In the body, scores for H pylori–positive gastritis patients were higher than those for both H pylori–negative gastritis patients (P < 0.01) and normals (P < 0.001) (Table 1). H pylori–negative gastritis patients had a higher score in the antrum than in the body (P < 0.05) (Table 1).
Table 1.
Intensity of immunohistochemical staining for Hsp32 and histological gastritis scores in gastric antral (n = 8–14) and body (n = 7–10) mucosa by disease group; median values (IQR) are shown
Histological gastritis scores were higher in patients with H pylori–positive gastritis than with H pylori–negative gastritis in the antrum (P < 0.01) but not in the body. There were no significant differences between antrum and body for each patient group. In patients with H pylori–negative, but not H pylori–positive, antral gastritis, Hsp32 staining and gastritis score showed a positive correlation (r = +0.67, P < 0.05). No correlation was seen in any other group.
Colonic mucosa
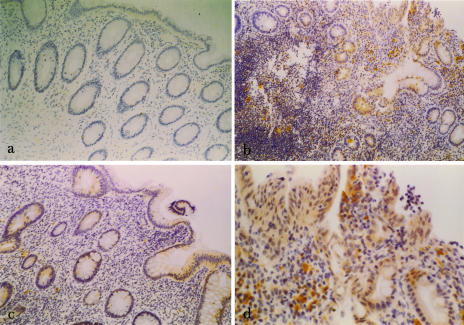
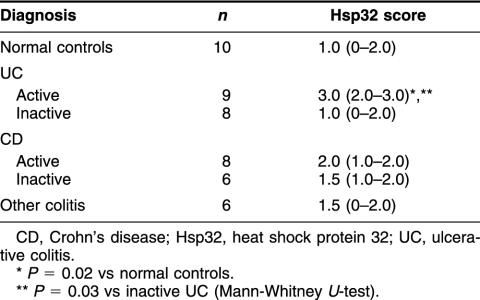
Immunoreactive Hsp32 was detected in lamina proprial inflammatory cells in all 6 patient groups including normal colonic mucosa (Fig 3). Diffuse cytoplasmic staining was also seen in surface and crypt epithelial cells in patients with UC (Fig 3b–d), CD, and other types of colitis. Scored intensity of staining for Hsp32 was significantly greater in active UC (Fig 3b,d) than in quiescent disease (P = 0.03) (Fig 3c) or normal controls (P = 0.02) (Fig 3a) (Table 2). The differences between active CD, inactive CD, and normals were not significant. Negative control sections gave no detectable staining.
Fig 3.
(a) Immunohistochemical view (100× magnification) of normal colonic mucosa stained for heat shock protein (Hsp32). Scattered positive-staining (brown) cells are seen in the lamina propria. (b) Immunohistochemical view (100× magnification) of inflamed colonic mucosa from a patient with active ulcerative colitis (UC) stained for Hsp32. (c) Immunohistochemical view (100× magnification) of colonic mucosa from a patient with inactive UC stained for Hsp32. (d) Immunohistochemical view (250× magnification) of inflamed colonic mucosa from a patient with active UC stained for Hsp32. Positive-staining (brown) superficial and crypt epithelial cells, and lamina proprial inflammatory cells are seen
Table 2.
Intensity of immunohistochemical staining for Hsp32 in colonic mucosa by disease group; median values (IQR) are shown
DISCUSSION
Although earlier reports show induction of Hsp32 (HO-1) in the gastrointestinal mucosa of rats with experimental gastric ulceration (Guo et al 2002) and colitis (Wang et al 2001), this appears to be the first report of its expression in the human stomach or colon. Hsp32 was expressed constitutively in lamina proprial inflammatory cells of normal gastric and colonic mucosa and in gastric epithelial cells. Upregulation of Hsp32 expression occurred in inflamed gastric mucosa, whether or not infected by H pylori, and in the inflamed colon, particularly in patients with UC. Because Hsp32 is induced by oxidative stress, these results support the hypothesis that increased mucosal generation of ROMs and other inflammatory mediators in inflamed gut mucosa (Simmonds and Rampton 1993; Davies and Rampton 1994) could induce local synthesis of Hsp32 (Maines 1988; Polla 1988; Maines and Panakian 2001; Yet et al 2002).
In the stomach, particularly the body, expression of Hsp32 tended to be higher in patients with H pylori–positive than H pylori–negative gastritis. However, the more severe gastritis defined histologically in infected but not uninfected biopsies (Table 1) precludes the immediate conclusion that H pylori itself specifically induces Hsp32 independently of indirect effects on lamina proprial leukocyte recruitment and activation (Davies and Rampton 1994; Bodger and Crabtree 1998). Nevertheless, the observation that gastric antral expression of Hsp32 was directly related to the severity of gastritis in H pylori–negative patients, but not H pylori–positive patients, raises the possibility that induction of Hsp32 in the latter group may depend on factors other than the intensity of the mucosal inflammatory infiltrate.
As in the stomach, increased expression of Hsp32 in inflamed large intestine is likely to be related to overproduction of ROM, proinflammatory cytokines, and other mediators including prostaglandins (Rampton and Hawkey 1984; Cantoni et al 1991; Koizumi et al 1992; Fiocchi 1998; Terry et al 1998). Hsp32 levels appeared to be highest in biopsies from patients with active UC. In these patients, the effects of treatment with corticosteroids are uncertain; although some reports suggest that steroids contribute to induction of Hsp32 (Maines 1988), other reports indicate that they downregulate its expression (Cantoni et al 1991; Lavrovsky et al 1996).
Expression of HO-1 has been described as a marker of a response to oxidative stress (Applegate et al 1991). Its increased expression in inflamed gastric and colonic mucosa in these studies could have protective consequences through increased local conversion of heme, itself often present in increased amounts in inflamed tissue, to the antioxidant bilirubin (Stocker et al 1987; Maines 1988; Maines and Panakian 2001; Yet et al 2002). Catabolism of heme by HO-1 leads also to release of free iron and carbon monoxide. Increased availability of free iron could theoretically exacerbate inflammation as a result of increased production of the toxic hydroxyl radical by way of the Fenton reaction (Simmonds and Rampton 1993). Carbon monoxide increases intracellular cyclic guanosine monophosphate (Marks et al 1991) and by binding heme moieties can modulate nitric oxide synthase activity (White and Marletta 1992), an enzyme upregulated, but of unclear pathogenic significance, in active inflammatory bowel disease (Lundberg et al 1997). Indeed, HO-1 inducers have been shown to inhibit inducible nitric oxide synthase in human epithelial cell lines (Cavicchi et al 2000). Lastly, HO-1 depletes enzymes of the arachidonic acid pathway (Martasek et al 1991), which is activated in both gastritis and inflammatory bowel disease (Rampton and Hawkey 1984; Davies and Rampton 1994).
The net result of induction of HO-1 was the attenuation of inflammation in TNBS-induced colitis in rats (Wang et al 2001). However, further studies will be required to determine the functional role of upregulated Hsp32 in more chronic human inflammatory disorders such as gastritis, UC, and CD and whether this enzyme will ever become a therapeutic target in these disorders (Immenschuh and Mamadori 2000).
REFERENCES
- Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51:974–978. [PubMed] [Google Scholar]
- Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139–150. doi: 10.1093/oxfordjournals.bmb.a011664. [DOI] [PubMed] [Google Scholar]
- Cantoni L, Rossi C, Rizzardinin M, Gadina M, Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991;279:891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavicchi M, Gibbs L, Whittle BJ. Inhibition of inducible nitric oxide synthase in the human intestinal epithelial cell line, DLD-1, by the inducers of haem oxygenase-1, bismuth salts, heme and nitric oxide donors. Gut. 2000;47:771–778. doi: 10.1136/gut.47.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies GR, Rampton DS. Helicobacter pylori, free radicals and gastroduodenal disease. Eur J Gastroenterol Hepatol. 1994;6:1–10. [Google Scholar]
- Ewing JF, Maines MD. Glutathione depletion induces heme-oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Guo JS, Cho CH, Wang JY, Koo MW. Expression and immunolocalisation of heat shock proteins in the healing of gastric ulcers in rats. Scand J Gastroenterol. 2002;37:17–22. doi: 10.1080/003655202753387293. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Mamadori G. Gene regulation of heme oxygenase as a therapeutic target. Biochem Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- Keyse SM, Tyrrell RM. Heme-oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi T, Negishi M, Ichikawa A. Induction of heme oxygenase by delta 12-prostaglandin J2 in porcine aortic endothelial cells. Prostaglandins. 1992;43:121–131. doi: 10.1016/0090-6980(92)90081-4. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Drummond GS, Abraham NG. Downregulation of the human heme oxygenase gene by glucocorticoids and identification of 56b regulatory elements. Biochem Biophys Res Commun. 1996;218:759–765. doi: 10.1006/bbrc.1996.0135. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat shock response. Ann Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lundberg JON, Lundberg JM, Alving K, Weitzburg E. Nitric oxide and inflammation: the answer is blowing in the wind. Nat Med. 1997;3:30–31. doi: 10.1038/nm0197-30. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms and clinical application. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Maines MD, Panakian M. The heme oxygenase system and cellular defense mechanisms. Do heme oxygenase-1 and heme oxygenase-2 have different functions? Adv Exp Biol Med. 2001;502:249–272. doi: 10.1007/978-1-4757-3401-0_17. [DOI] [PubMed] [Google Scholar]
- Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- Martasek P, Schwartzman ML, Goodman AI, Solangi KB, Levere RI, Abraham NG. Hemin and l-arginine regulation of blood pressure in spontaneous hypersensitive rats. J Am Soc Nephrol. 1991;2:1078–1084. doi: 10.1681/ASN.V261078. [DOI] [PubMed] [Google Scholar]
- Misiewicz JJ. The Sydney System: a new classification of gastritis. Introduction. Eur J Gastroenterol Hepatol. 1991;6:207–208. doi: 10.1111/j.1440-1746.1991.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. Br Med J. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Polla BS. A role for heat shock proteins in inflammation? Immunol Today. 1988;9:134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Rampton DS, Hawkey CJ. Prostaglandins and ulcerative colitis. Gut. 1984;25:1399–1415. doi: 10.1136/gut.25.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds NJ, Rampton DS. Inflammatory bowel disease—a radical view. Gut. 1993;34:865–868. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R, Yamamoto Y, McDonagh AF. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Terry CM, Clikeman JA, Hoidal JR, Callaghan KS. Effect of tumour necrosis factor-alpha and interleukin-1 alpha on heme oygenase-1 expression in human endothelial cells. Am J Physiol. 1998;274:H883–H991. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- Wang WP, Guo X, Koo MW, Wong BC, Lam SK, Ye YN, Cho CH. Protective role of heme oxygenase-1 in trinitrobenzene sulphonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G586–G594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- Weinstein WM 1993 Gastritis and gastropathies. In: Gastrointestinal Disease. Pathophysiology, Diagnosis, Management, ed Sleisenger MH, Fordtran JS. WB Saunders, Philadelphia, 545–571. [Google Scholar]
- White KA, Marletta MA. Nitric oxide synthase is a cytochrome P450 type hemoprotein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Yet SF, Melo LG, Layne MD, Perrelle MA. Heme oxygenase-1 in regulation of inflammation and oxidative damage. Methods Enzymol. 2002;353:163–176. doi: 10.1016/s0076-6879(02)53046-9. [DOI] [PubMed] [Google Scholar]