Abstract
Overexpression of heat shock protein (Hsp) 70 and Hsp27 in vivo was proclaimed as a potential tool in therapy of ischemia-reperfusion injury. However, it was so far not known whether these Hsps can beneficially act when increased in cells just at the stage of postischemic reperfusion. This issue was examined in a model of ischemia-reperfusion stress when cultures of endothelial cells (EC) from human umbilical vein were infected with virus-based vectors expressing Hsp70 or Hsp27, or Hsp56, or green fluorescent protein (GFP) and exposed to 20 hours of hypoxia followed by reoxygenation. The infection was performed either 10 hours before hypoxia or immediately after hypoxia, or at different time points of reoxygenation. Only low cell death was detected during hypoxia, but later, up to 40% of the treated cells died via caspase-dependent apoptosis between 6 and 12 hours of reoxygenation. The percentage of apoptotic cells was 1.6- to 3-fold greater in Hsp56- and GFP-infected EC than in Hsp70- or Hsp27-infected EC. The last 2 groups exhibited a lesser extent of procaspase-9 and procaspase-3 activation within 6–9 hours of reoxygenation. The cytoprotective effects of overexpressed Hsp70 and Hsp27 were observed not only in the case of infection before hypoxia but also when EC were infected at the start of reoxygenation or 1–2 hours later. An increase in the Hsp70 and Hsp27 levels in infected EC correlated well with their resistance to apoptosis under reoxygenation. These findings suggest that overexpression of Hsp70 or Hsp27, if it occurs in the involved cells at the early stage of postischemic reperfusion, can still be cytoprotective.
INTRODUCTION
Acute attacks of myocardial or cerebral ischemia evoke massive cell death in the affected tissues, which may be fatal for the patient. Such acute attacks mainly result from spasm of arteries or their occlusion by thrombi, which halt blood supply to a part of the heart or brain. The ischemic episode changes into the phase of reperfusion when blood flow is renewed after vasorelaxation or thrombolysis. In spite of the recovering blood circulation herein, many affected cells can afterward die through reperfusion injury and stimulating the apoptotic pathways, which was shown in animal models (Sunnergren and Rovetto 1987; Fliss and Gattinger 1996; Scarabelli et al 2001, 2002). Because apoptosis in the involved tissue regions is a delayed event usually peaking in several hours after manifestation of acute ischemia, there is some time for intervention when special therapy could prevent or at least minimize the cell death after the ischemic insult.
In this respect, in vivo overexpression of certain heat shock proteins (Hsps) seems to be a promising approach aimed at alleviating ischemia-reperfusion lesions in patients' tissues. Actually, it was demonstrated in numerous models with various types of cells that a transient increase in the intracellular Hsp70, Hsp60/10, and Hsp27 levels can protect against cell death resulting from ischemia-reperfusion and improve the postinsult functional recovery (Latchman 2001a; Snoeckx et al 2001). Gene vectors delivering these Hsps and special drugs inducing or enhancing the endogenous Hsp expression were therefore considered as potential tools in gene therapy and pharmacotherapy of ischemic injury in humans, respectively (Morris et al 1996; Vigh et al 1997; Latchman 2001a, 2001b; Ooie et al 2001; Polakowski et al 2002).
The therapeutic use of such tools in a clinical setting is, however, limited by a number of problems. In fact, all the published reports proving cytoprotective features of Hsps describe situations when intracellular accumulation of either Hsp was triggered in advance, ie, when the Hsp induction or overexpression preceded the delayed stress. In reality, most acute ischemic attacks happen suddenly, and their start is practically unpredictable, so that it seems impossible to create excess Hsp(s) in human cells before the unexpected stroke. The question arises: will the “late” upregulation of the intracellular Hsp levels (eg, by means of gene therapy or special drugs) be beneficial while a severe ischemic insult was already taking place? As for the stage of ischemia, targeting of Hsp-expressing vectors or Hsp-inducing drugs to the stressed cells along with blood flow will be complicated by failing blood circulation within ischemia-affected regions of tissue. It should be noted that urgent therapy with thrombolytic and vasodilatory remedies, which are usually applied on acute ischemia, may restore blood circulation in the affected region. In this case, the postischemic reperfusion generates additional cell-damaging factors (oxidative stress, Ca2+ overload), but on the other hand, the stressed cells become more available for any therapeutic agents coming along with renewed blood flow. Hence, it seems to be of importance to elucidate whether transient Hsp overexpression will be beneficial when artificially triggered during reperfusion.
The latter issue is addressed in the present study performed on cultured endothelial cells (EC) undergoing posthypoxic reoxygenation that mimics reperfusion in vivo. Vascular EC were chosen as a relevant object because these cells being obligatory components of blood vessels strongly suffer during postischemic reperfusion that can aggravate ischemic lesions and dysfunction of all the surrounding tissue (Sunnergren and Rovetto 1987; Svendsen et al 1991; Scarabelli et al 2001). Moreover, the vascular endothelium appears to be one of the primary targets for any infective agent or drug introduced into the bloodstream for therapeutic intervention. Because overexpressed Hsp70 was previously shown to protect EC from hypoxic injury (Suzuki et al 1998), whereas a cytoprotective role for Hsp27 was suggested after studies on similar models (Loktionova et al 1998; Razandi et al 2000), the effects of overexpression of these Hsps were particularly examined in this study. The data obtained demonstrate that overexpression of Hsp70 or Hsp27, being initiated in human vascular EC at the start of posthypoxic reoxygenation or during its early period (till 3 hours), attenuates the delayed cell death (apoptosis) resulting from hypoxia-reoxygenation. This finding may promote development of new approaches to more effective therapy of ischemia-reperfusion injury in humans.
MATERIALS AND METHODS
Cells
Venous EC were isolated from human umbilical cords and cultured according to routine techniques previously described (Loktionova et al 1998). The cultures were grown on gelatin-coated substrates at 37°C in a humidified air atmosphere with 5% CO2. The culture growth medium consisted of Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum (both from HyClone, Cramlington, UK), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μg/mL heparin, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid, and 50 U/mL penicillin. Homogeneity of isolated and grown EC populations was confirmed by their total staining with an antibody to factor VIII–related antigen. The EC cultures of passages 1–3 were used for the experiments with viral infection and hypoxia-reoxygenation.
Virus construction
Hsp complementary deoxyribonucleic acid (cDNA) or a control gene encoding the green fluorescent protein (GFP) under the control of the cytomegalovirus immediate early-promoter was inserted into the herpes simplex virus (HSV-1) genome as previously described (Wagstaff et al 1999). Hsp cDNAs were from human inducible Hsp70 (Wu et al 1985), rabbit Hsp56 (a gift of M.-C. Lebeau), and hamster Hsp27 (Lavoie et al 1990). Each Hsp cDNA was introduced into the Lat region of a defective HSV vector lacking functional ICP27 and therefore unable to replicate lytically (Coffin et al 1996). All viruses were grown in baby hamster kidney cells artificially engineered to express ICP27 and therefore allowing propagation of the ICP27-deficient virus (Howard et al 1998).
Hypoxia and reoxygenation
Culture dishes or plates with EC adherent to gelatin-coated plastic or coverslips were incubated at 37°C in the hypoxic chamber in a humidified atmosphere of 5% CO2 and 95% N2. Residual O2 concentration was monitored using a special O2 electrode (Kendro Laboratory Products, Bishops Stortford, Hertfordshire, UK) and did not exceed 1%. The cells were exposed to such hypoxia for 20 hours and then placed into a CO2 incubator under normal conditions of cell cultivation. Herein, immediately after the hypoxic treatment, the culture medium over the treated cells was replaced with normal growth medium (or the serum-free one to perform the viral infection) previously conditioned in petri dishes for 4 hours at 37°C under normoxic (21% O2) environment to sharply initiate reoxygenation.
Treatments of EC with specific inhibitors of caspase-9 and caspase-3
To elucidate a role of caspase activation in the mechanism of delayed EC death after hypoxia, the cells were treated with cell-permeable inhibitors of caspase-9 and caspase-3. Under the medium change immediately after hypoxia, the specific inhibitor of either caspase-9 (Z-LEHD.fmk; C9i) or caspase-3 (Ac-DEVD.cmk; C3i) was added to some EC samples at concentration 0.07 μM (Scarabelli et al 2002). Both the inhibitors were from Calbiochem (La Jolla, CA, USA).
Infection of EC with virus-based vectors expressing Hsp70, Hsp56, Hsp27, or GFP
The EC cultures at the stage of 80% confluence were used for infection with the viral vectors. Before the virus treatment, the culture medium was removed from EC, and they were washed twice with sterile phosphate-buffered saline (PBS). Then, PBS was replaced with serum-free RPMI 1640 in which 40 pfu/mL of each virus were injected. The cells were in contact with the virus-containing medium for 0.5 hour at 37°C in a CO2 incubator. After this period, the infecting medium was harvested and replaced with normal growth medium. Using such a protocol, the cells were infected either 10 hours before the hypoxic treatment or immediately after its ending (ie, at the start of reoxygenation) or 1, 2, and 3 hours after the start of reoxygenation. In all cases of the infection after hypoxia, the serum-free medium, before the virus addition, was previously conditioned under normoxic environment as described above.
In situ determination of apoptosis
Apoptotic nuclei were revealed by labeling of DNA 3′ ends with fluorescein-conjugated deoxyuridine triphosphate (dUTP) using terminal deoxynucleotidyl transferase (Boehringer Mannheim, Mannheim, Germany), according to the manufacturer's protocol. For terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) assays, EC were grown onto round coverslips in 24-well culture plates (Nunc, Roskilde, Denmark). The assays were performed after the cells were fixed with 4% paraformaldehyde for 3 minutes at 25°C and washed with PBS 3 times. The reaction solution containing 2 mM fluorescein-conjugated dUTP and 10 U of terminal deoxynucleotidyl transferase was added to the fixed cells for 2 hours in a humidified incubator at 37°C. After washing with PBS, the cell preparations were examined under a fluorescence microscope to determine the fluorescein-labeled (apoptotic) nuclei as a percentage of total cell nuclei.
In parallel, the percentage of dead EC was determined using double-label staining of the nonfixed cells with acridine orange (AO, 3 μg/mL) and propidium iodide (PI, 10 μg/mL) (Gabai et al 2000). The fraction of apoptotic EC was identified by fluorescence microscopy as the AO-labeled cells displaying patterns of condensed chromatin or fragmented nuclei and intact plasma membrane (the absence of PI staining). In each experiment, at least 10 microscopic fields per sample (400–600 cells) were counted, and the experiments were reproduced 3–4 times.
3-(4,5-Dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assay of EC viability
The poststress EC viability was also assessed in the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide (MTT) assay (Stempien-Otero et al 1999). After the hypoxia-reoxygenation treatment, the cells were incubated in the presence of 0.5 mg/mL MTT at 37°C for 3 hours. Then, the reagent-containing medium was aspirated, the cells were washed with PBS, and the formazan product was solubilized by adding dimethyl sulfoxide. Absorbance at 630 nm (background) was subtracted from absorbance at 570 nm for each well on a plate reader (Hewlett Packard, Geneva, Switzerland).
Sodium dodecyl sulfate electrophoresis and Western blotting
To analyze Hsp expression in EC, they were washed with PBS, harvested, and lysed into a reducing Laemmli sample buffer supplemented with inhibitors of proteases (Loktionova et al 1998). Aliquots of these lysates were run in sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and separated proteins were then electrotransferred from gel slabs onto nitrocellulose membrane (Hybond C, Amersham, Buckinghamshire, UK). After routine blocking step, blots were probed with specific anti-Hsp antibodies. Hsp27 was detected with rabbit antibodies recognizing both rodent and human Hsp27 (kindly provided by Prof. M. Gaestel). Hsp56 was detected using a monoclonal antibody KN382/EC1 (Stressgen). Hsp70 was revealed with a monoclonal antibody N27F3-4 (Stressgen, Victoria, BC, Canada) reacting with both the inducible (Hsp70) and constitutive form (Hsc70).
To assess the procaspase activation, EC lysates were electrophoretically run, and immunoblotting was performed with rabbit antibodies to caspase-9 and caspase-3 (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), which recognize both the inactive precursors and the activated (cleaved) forms of either caspase. In addition, the caspase-3 activation in reoxygenated EC was evaluated on specific cleavage of poly–adenosine diphosphate ribose polymerase (PARP) using rabbit anti-PARP antibodies (Santa Cruz Biotechnology Inc). Immunoblotting with antibodies to actin (Sigma Chemical Co, St. Louis, MO, USA) was used to equalize protein loading in all the samples probed. The cell lysates for Western blotting with the anticaspase and anti-PARP antibodies were prepared according to the manufacturer's instruction.
The immunoreactive bands on blots were visualized on an imaging film using respectively anti-mouse or anti-rabbit IgG peroxidase conjugates and an enhanced chemiluminescence kit (all from Amersham). The films with developed tracks were scanned and quantitatively analyzed as earlier described (Kabakov et al 2002).
Statistics
All quantitative data are expressed as means ± SE of 5–6 separate experiments. Significance of Hsp-mediated cytoprotection from apoptosis has been compared with the control (GFP-infected EC) for each case of the Hsp overexpression and each group of the cells using analysis of variance (a multiway ANOVA or covariance). ANOVA significance when P < 0.01 (*) or P < 0.05 (‡) was additionally confirmed with the F-test.
RESULTS
GFP and Hsp accumulation in EC infected before or after hypoxia
The cells were infected with the HSV-1–based vector expressing GFP or Hsp70, or Hsp56, or Hsp27 as described in Materials and Methods. Then, the in situ overexpression of each transgenic product was experimentally confirmed. GFP accumulation in the GFP-infected cells was established by fluorescence microscopy, which showed bright green fluorescence in 85–90% of EC in the treated cell culture. The efficiency of infection with the Hsp vectors and the intracellular Hsp levels were assessed by flow cytometry analysis after immunofluorescent staining of the fixed and permeabilized EC suspensions. In each case of the infection, this analysis revealed 80–85% of EC with similar intensity of fluorescence, which was severalfold higher than that in the uninfected control (data not shown), thus indicating a relatively even level of specific Hsp overexpression within the infected cell populations.
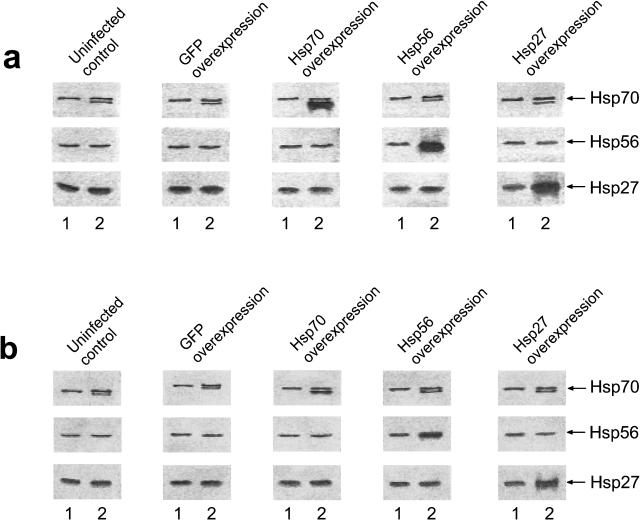
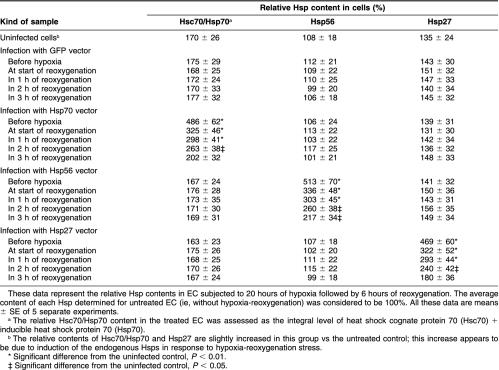
To evaluate a contribution of each overexpressed Hsp to the total content of this Hsp in the treated cells, we used Western blotting with the antibodies recognizing both the overexpressed product and the endogenous Hsp including its constitutive form. Figure 1 shows that the endogenous Hsp expression is not altered in GFP-infected EC. The quantitative analysis of the blots demonstrates a statistically significant increase in the total content of either Hsp in the cells infected with the respective Hsp vector vs control (uninfected or GFP-infected EC) (see Table 1). A slight increase in the intracellular Hsp70 content revealed in the control cells after hypoxia-reoxygenation (Fig 1) appears to be due to induction of the stress response. Meanwhile, EC infected with the Hsp27-, Hsp56-, or Hsp70-expressing vectors before hypoxia exhibited a stable 2.8- to 4-fold rise in the total intracellular content of either Hsp at 6 hours of reoxygenation (Fig 1; Table 1). Importantly, EC infected with these vectors immediately after hypoxia or 1–2 hours after the start of reoxygenation also exhibited consistently increased (1.5- to 2-fold) levels of the respective Hsps at 6 hours of reoxygenation. In contrast, analogous infection procedure initiated 3 hours after hypoxia yielded only insignificant Hsp accumulation in the treated EC at 6 hours of reoxygenation (Fig 1; Table 1).
Fig 1.
ECL-revealed images of immunoblots showing bands of Hsc70/Hsp70, Hsp56, and Hsp27 in uninfected EC and in EC infected with the HSV-1–based vector expressing GFP or Hsp70, or Hsp56, or Hsp27. Each Hsp was detected in the cell lysates obtained before hypoxia (lanes 1) and after 20 hours of hypoxia followed by 6 hours of reoxygenation (lanes 2). These immunoblots demonstrate 2 different situations when the cells were infected with the respective vector 10 hours before hypoxia-reoxygenation (a) and when the cells were infected 2 hours after the start of reoxygenation (b). The average intracellular Hsp content determined after quantitative analyses of these and the other blots are presented in Table 1. ECL, enhanced chemiluminescence; EC, endothelial cells; HSV, herpes simplex virus; GFP, green fluorescent protein; Hsp, heat shock protein
Table 1.
Relative heat shock protein (Hsp) content in endothelial cells (EC) after hypoxia-reoxygenation and pre- or posthypoxic infection with the virus-based vectors expressing green fluorescent protein (GFP) or Hsp70, Hsp56, or Hsp27
Posthypoxic overexpression of Hsp70 or Hsp27 in EC can reduce delayed cell death during reoxygenation
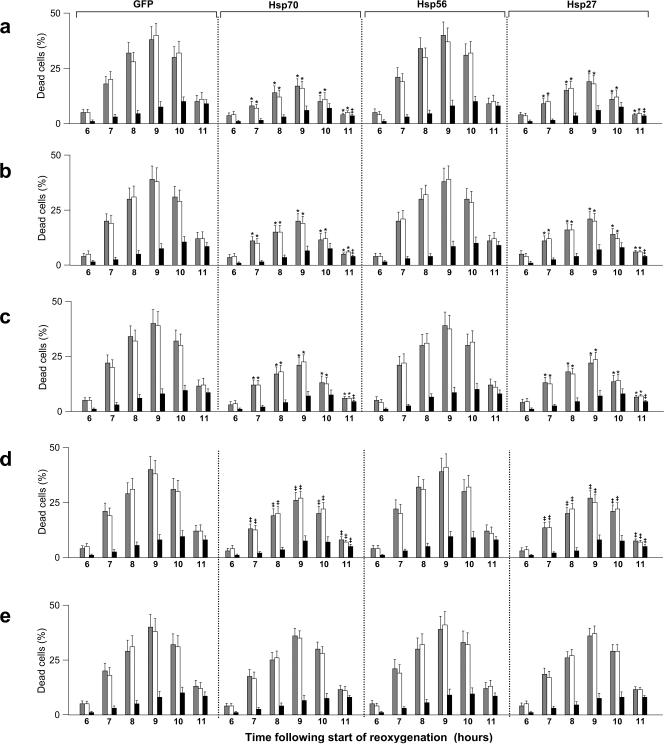
The staining of EC with PI and AO revealed rather low percentage of dead cells in the untreated control (∼1–2%) as well as in the treated samples immediately after 20 hours of hypoxia (2–3%) and at first 6 hours of subsequent reoxygenation (4–5%). No cell detachment was observed in the hypoxia-treated EC cultures. This is in accordance with data of Stempien-Otero et al (1999), who reported that human umbilical vein–derived EC are exclusively resistant to sustained hypoxia. However, probing of the cell death at 7, 8, 9, and 10 hours of the posthypoxic reoxygenation demonstrates significant percentages of dead EC (Fig 2). This delayed cell death exhibited characteristic features of classical apoptosis including annexin V labeling on the outer surface of intact plasma membrane (not shown), caspase activation (see below), TUNEL-labeled cell nuclei (Fig 2), condensation of the chromatin or nuclear fragmentation (Fig 2), and internucleosomal cleavage of DNA (not shown). The percentage of necrotic (PI positive) EC in the treated cultures was insignificant (2–3%) till 9–10 hours of reoxygenation. Some decrease in the relative number of apoptotic cells at 9 and 10 hours of reoxygenation is due to their partial transition into so-called secondary necrosis and also detachment or destruction of a part of the dead cells; the secondary necrosis was indeed determined on a marked increase in the fraction of PI-positive EC (see Fig 2, time points 9 and 10 hours). In 18–20 hours after the start of reoxygenation, the treated cultures began to proliferate and gradually repair the initial cell density.
Fig 2.
Effects of the overexpression of GFP, Hsp70, Hsp56, and Hsp27 on the intensity of delayed cell death in EC subjected to 20 hours of hypoxia followed by reoxygenation. The percentages of dead cells were determined at 6, 7, 8, 9, 10, and 11 hours of reoxygenation after counting apoptotic EC with the TUNEL-positive nuclei (gray bars) and, in parallel, apoptotic AO-stained EC with the condensed chromatin or fragmented nuclei (white bars) and necrotic (PI positive) EC (black bars). These data demonstrate 5 different situations when the cells were infected with the respective vector 10 hours before hypoxia (a) or at the start of reoxygenation (b) or 1 hour (c), 2 hours (d), and 3 hours (e) after the start of reoxygenation. The product overexpressed in the infected cells is indicated for each group at the top of this figure. *Significant difference from the GFP control, P < 0.01. ‡Significant difference from the GFP control, P < 0.05. GFP, green fluorescent protein; Hsp, heat shock protein; EC, endothelial cells; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling; AO, acridine orange; PI, propidium iodide
It is clearly seen in Figure 2 that, under all protocols used, overexpressed Hsp56 does not protect EC from delayed apoptosis after hypoxia-reoxygenation. In contrast, Hsp70 as well as Hsp27 being overexpressed in EC did reduce the numbers of dead EC in the reoxygenated cultures. Importantly, statistically significant protection of the treated EC from apoptosis took place when the cells were infected with the Hsp70 or Hsp27 vectors before hypoxia (Fig 2A) or at the start of reoxygenation (Fig 2B), or 1–2 hours after the start of reoxygenation (Fig 2C, D). These cytoprotective effects conferred by the late (posthypoxic) overexpression of Hsp70 and Hsp27 were revealed by both the TUNEL and AO-PI staining. However, “too late” infection of EC with the Hsp70 and Hsp27 vectors being initiated at 3 hours of reoxygenation did not confer reliable cytoprotection (Fig 2E). This correlates with poor intracellular accumulation of either overexpressed product for such a short postinfection period (see Table 1).
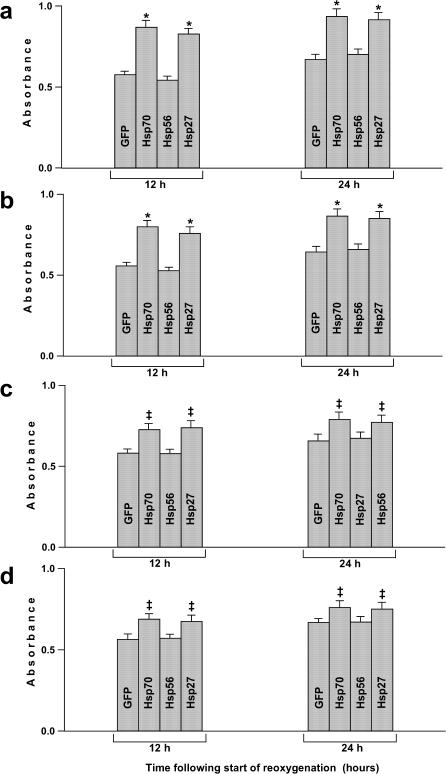
In parallel, we assessed cell viability in the reoxygenated EC cultures using the MTT assay. Because at 12 hours of reoxygenation the data of MTT assay may reflect not only differences in the density of viable EC but also differences in functioning (or damage-repair) of mitochondria in either cell sample, we additionally probed the situation at a more delayed time point: 24 hours after the start of reoxygenation. Figure 3 demonstrates that the treated cultures overexpressing Hsp70 or Hsp27 exhibit elevated cell viability after hypoxia-reoxygenation. The cytoprotective influence of overexpressed Hsp70 and Hsp27 was also marked when infection of EC with the respective vectors was performed at the start or at 1–2 hours of posthypoxic reoxygenation (Fig 3). This is in good agreement with the above-presented data on delayed cell death (see Fig 2). Likewise, viewing of the treated EC under a phase-contrast microscope revealed the higher cell density and accelerated recovery of the EC monolayer integrity in the cultures overexpressing Hsp70 or Hsp27 (not shown).
Fig 3.
Effects of the overexpression of GFP, Hsp70, Hsp56, and Hsp27 on the EC viability determined in the MTT assay at 12 and 24 hours after the start of posthypoxic reoxygenation. These data demonstrate 4 different situations when the cells were infected with the respective vector 10 hours before hypoxia (a) or at the start of reoxygenation (b) or 1 hour (c) and 2 hours (d) after the start of reoxygenation. The specific Hsp overexpressed in the infected cells is indicated within each bar. *Significant difference from the GFP control, P < 0.01. ‡Significant difference from the GFP control, P < 0.05. GFP, green fluorescent protein; Hsp, heat shock protein; EC, endothelial cells; MTT, 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide
Caspase activation resulting from hypoxia-reoxygenation can be impaired by posthypoxic overexpression of Hsp70 or Hsp27
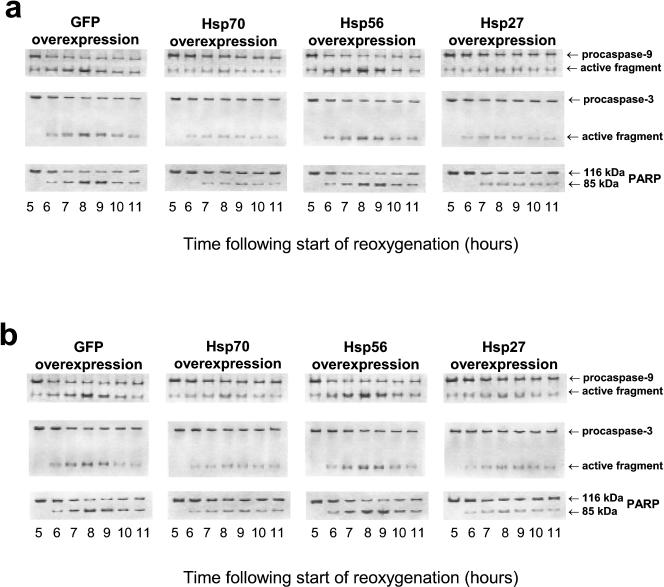
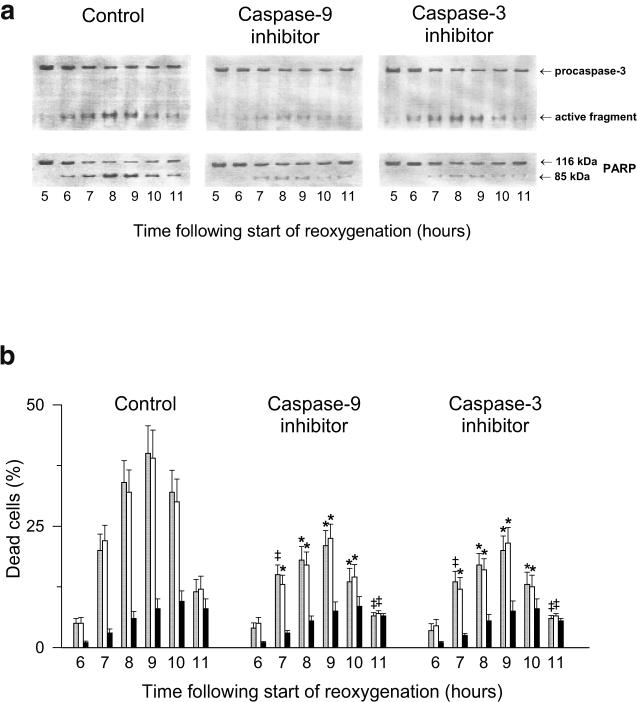
Figures 4 and 5A illustrate how procaspase-9 and procaspase-3 are activated in EC undergoing posthypoxic reoxygenation. The activating cleavage of procaspase-9 yields its 37-kDa (active) form; the latter, in turn, cleaves procaspase-3 thereby generating active caspase-3. PARP, which is 1 of critical (directly associated with apoptosis) substrates of caspase-3, is also cleaved in the reoxygenated EC (Figs 4 and 5A). To be sure that it is the caspase-dependent apoptosis that is a cause of the delayed cell death in our model, we used specific inhibitors of caspase-9 (Z-LEHD.fmk; C9i) and caspase-3 (Ac-DEVD.cmk; C3i). Each of these inhibitors, when added to the incubation medium, suppressed the characteristic PARP cleavage (Fig 5A) and reduced apoptosis (Fig 5B) in the reoxygenated EC, which is in good agreement with the previous in situ results obtained on isolated and perfused rat hearts (Scarabelli et al 2002).
Fig 4.
ECL-revealed images of immunoblots showing the hypoxia-reoxygenation–induced activation of caspase-9 and caspase-3 in EC infected with the HSV-1–based vector expressing either GFP or Hsp70, or Hsp56, or Hsp27. All the immunoreactive forms of the caspases and PARP were detected in the cell lysates obtained at different time points of reoxygenation after 20 hours of hypoxia. These immunoblots demonstrate 2 different situations when the cells were infected with the respective vector 10 hours before hypoxia-reoxygenation (a) and when the cells were infected 2 hours after the start of reoxygenation (b). Besides the blots presented, very similar results have also been obtained in four other independent experiments. ECL, enhanced chemiluminescence; EC, endothelial cells; HSV, herpes simplex virus; GFP, green fluorescent protein; Hsp, heat shock protein; PARP, poly–adenosine diphosphate ribose polymerase.
Fig 5.
Effects of specific cell-permeable inhibitors of caspase-9 and caspase-3 on the hypoxia-reoxygenation–induced caspase activation (a) and the intensity of delayed cell death (b) in EC subjected to 20 hours of hypoxia followed by reoxygenation. After the start of reoxygenation, the cells were incubated in the presence of 0.07 μM Z-LEHD.fmk (an inhibitor of caspase-9) or 0.07 μM Ac-DEVD.cmk (an inhibitor of caspase-3). The immunoreactive forms of caspase-3 and PARP were detected by Western blotting of the cell lysates obtained at different time points of the posthypoxic reoxygenation (a); besides these blots, very similar results have also been obtained in three other independent experiments. The percentages of dead cells were determined at 6, 7, 8, 9, 10, and 11 hours of reoxygenation after counting apoptotic EC with the TUNEL-positive nuclei (gray bars) and, in parallel, apoptotic AO-stained EC with the condensed chromatin or fragmented nuclei (white bars) and necrotic (PI positive) EC (black bars) (b). *Significant difference from the inhibitor-untreated control, P < 0.01. ‡Significant difference from the inhibitor-untreated control, P < 0.05. EC, endothelial cells; PARP, poly–adenosine diphosphate ribose polymerase; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling; AO, acridine orange; PI, propidium iodide
Comparing Figures 4 and 5A one can see that infection with the vectors expressing GFP or Hsp56 does not affect the caspase activation in EC undergoing posthypoxic reoxygenation. Contrary to that, infection with the vectors expressing Hsp70 or Hsp27, when performed before hypoxia, considerably attenuates the activation of both caspase-9 and caspase-3 occurring within 6–9 hours of reoxygenation (Fig 4A). The late (posthypoxic) infection with the Hsp70- or Hsp27-expressing vector, when initiated at the start of reoxygenation (not shown) or 1–2 hours later (Fig 4B), also results in apparent suppression of the caspase activation in the treated EC. These effects of either Hsp overexpressed correlate well with the data on cytoprotection observed on the pre- and posthypoxic infection (see Figs 2 and 3). Meanwhile, no inhibition of the caspase activation was found when EC were infected with the Hsp70- or Hsp27-expressing vector at 3 hours of reoxygenation (not shown) because no reduced apoptosis was observed on such a protocol of infection (Fig 2E). Thus, the cytoprotection conferred by the overexpression of Hsp70 or Hsp27 appears to be associated with an ability of either Hsp to inhibit the activation of caspase-9 or caspase-3 (or both) in EC undergoing posthypoxic reoxygenation.
DISCUSSION
Salvage of the vascular endothelium from the cell-killing effects of ischemia-reperfusion appears to be 1 of the most important tasks in therapy of ischemic insults. Actually, minimization of the EC death in cardiac or cerebral blood vessels during ischemia-reperfusion could alleviate the tissue injury and then facilitate functional recovery after the stroke. Our data presented here show that in the in vitro model mimicking cytotoxic influence of ischemia-reperfusion on human vascular EC, overexpression of Hsp70 or Hsp27 protects the treated cells from delayed apoptosis and improves the poststress cell survival (see Figs. 2 and 3). The overexpressed Hsp56 did not protect the treated EC consistent with the previous data obtained on rat cardiomyocytes and neuronal cells subjected to simulated ischemia (Brar et al 1999; Wagstaff et al 1999). Importantly, we found that the late (posthypoxic) infection of EC with the Hsp70- or Hsp27-expressing vector can still confer reliable cytoprotection. Taking into account the data of Western blot analysis (see Fig 1; Table 1) this means that even a slight rise (∼50–70% vs GFP control) in intracellular Hsp70 and Hsp27 achieved at 6 hours of posthypoxic reoxygenation can be sufficient to significantly reduce delayed apoptosis in the treated EC. Such a phenomenon is not unique solely for EC because the posthypoxic infection of rat neonatal cardiomyocytes with the same Hsp70- or Hsp27-expressing vectors protected from the delayed cell death as well (B. Brar and D.S. Latchman, personal communication).
As for a mechanism of the Hsp70- and Hsp27-mediated protection from apoptosis, this appears to be based on the inhibitory action of either Hsp toward activation of caspase-9 or caspase-3 (or both) occurring in the reoxygenated EC (see Fig 4). Such a suppression of the caspase activation in the Hsp70- and Hsp27-overexpressing EC was in fact expected because analogous results were earlier obtained on other cell lines with different models of caspase-dependent apoptosis (Beer et al 2000; Bruey et al 2000; Mosser et al 2000; Pandey et al 2000; Saleh et al 2000; Paul et al 2002). It is possible that excess Hsp70 and Hsp27 protect against release of cytochrome c from mitochondria damaged by hypoxia-reoxygenation or, otherwise, these Hsps somehow block other key links in the apoptotic pathway. As for Hsp70, this Hsp was shown to inhibit the cytochrome c release from mitochondria of heat-stressed cells that correlated with attenuation of caspase-dependent apoptosis (Mosser et al 2000). Moreover, in experiments in vitro, Hsp70 was found to interact with Apaf-1, which is discussed as a cytoprotective mechanism preventing recruitment of procaspase-9 to the apoptosome (Beer et al 2000; Saleh et al 2000). In turn, excess Hsp27 is able to suppress the cytochrome c release from mitochondria (Paul et al 2002) and bind to the released cytochrome c (Bruey et al 2000); both these activities can interfere with the apoptosome formation and execution of apoptotic cell death. Other researchers reported that Hsp27 blocks apoptosis by inhibiting activation of procaspase-3 (Pandey et al 2000). Thus, there are at least several potential mechanisms by which either Hsp could protect reoxygenated EC from caspase-dependent apoptosis.
It is indeed intriguing that relatively poor (∼50–70% vs GFP control) upregulation of the intracellular Hsp70 or Hsp27 level achieved artificially by the posthypoxic infection confers reliable cytoprotection against delayed apoptosis. Herein, it should be taken into consideration that hypoxia and reoxygenation exert proteotoxic effects, ie, cause damage of many cellular proteins. Because both Hsp70 and Hsp27 are molecular chaperones recognizing and binding nonnative proteins, the cytosolic pools of Hsp70 and Hsp27 are probably depleted during hypoxia-reoxygenation through the chaperone targeting to the stress-damaged proteins. While Hsp70 and Hsp27 are engaged in chaperoning, either Hsp may be required for the inhibitory interaction with components of the apoptotic signaling pathway (eg, mitochondrial membranes, cytochrome c, Apaf-1) to prevent cell death. At least for Hsp70, the chaperone activity has been shown to be essential for protection of the heat-stressed cells from caspase-dependent apoptosis (Mosser et al 2000). We suggest that the stress-damaged proteins compete with some protein effectors of apoptosis for binding to Hsp70 or Hsp27 (or both). If this occurs, this competition can define the stressed cell's fate: in dependence on the severity of the stress-induced protein damage, the pools of Hsp70 and Hsp27 are either sufficient or insufficient to halt the cell death signal transduction. Such a quality control allows the stressed cell to “choose” between recovery (if damage is still reparable) and apoptosis (if damage is too severe). Thus, even slight enhancement of the intracellular Hsp levels may sometimes determine the stressed cell's choice in favor of recovery and survival against triggering the suicide program (apoptosis).
Our finding that the late (posthypoxic) overexpression of Hsp70 and Hsp27 in human EC can considerably improve survival of the treated cells hopefully discovers novel options in strategy of therapy of ischemia-reperfusion injury. Indeed, in the case of acute ischemia, it seems practically impossible to upregulate the intracellular Hsp levels in patient's tissues before the sudden attack. However, based on the data presented here, the Hsp overexpression in the involved tissue can also be beneficial when triggered after the insult. In this respect, artificial vectors (eg, virus-based ones) delivering Hsp70 or Hsp27 genes (or both) into the affected human cells (may be quite effective therapeutic tools against ischemia-reperfusion injury). Besides gene therapy with the Hsp-transgenic vectors, there is another way to artificially increase the intracellular Hsp level in tissues suffering from ischemia: a use of pharmacological inducers or enhancers of the endogenous Hsp expression in the target cells. In particular, such inducers of Hsp70 as Herbimycin A and Geranylgeranylacetone have already been shown to possess a cytoprotective potential on testing in models with simulated ischemia (Morris et al 1996; Ooie et al 2001). Likewise, Bimoclomol seems very attractive for the same purpose as a clinically applicable drug enhancing the endogenous Hsp70 expression in ischemia-stressed cells and thereby protecting them from lethal injury (Vigh et al 1997; Polakowski et al 2002).
It is generally accepted that one of the most serious problems in pharmacotherapy (as well as in gene therapy) of ischemic insults is targeting of drugs (or transgenic vectors) to the damaged tissue region. Actually, usual delivery of either therapeutic agent along with blood flow is complicated here through the broken circulation within the ischemic area. Our “proof-of-principle” results suggest that both the Hsp-expressing vectors and the Hsp-inducing drugs can effectually be delivered to the target cells when the interrupted circulation is renewed, ie, at the stage of reperfusion. Because thrombolytic and vasodilatory remedies are the most popular and widely used kinds for urgent treating of patients with acute ischemia, the phase of reperfusion, in many cases, follows the phase of ischemia in the patient's tissue. If so, because of the restored blood circulation, the involved cells are rendered more available for interaction with any therapeutic agent injected into the bloodstream. However, postischemic reperfusion per se is cytotoxic and can kill the involved cells. From this it follows that in the case of ischemic stroke, a combination of the therapy aimed at restoration of blood circulation (thrombolytic and vasodilatory remedies) with therapy aimed at intracellular Hsp upregulation (Hsp-expressing vectors or Hsp-inducing drugs) could yield more benefit than each one without the other. In this situation, the improved therapeutic effect may be achieved by introducing a catheter into an occluded artery to inject the thrombolytic or vasodilatory drugs and the Hsp-upregulating agents near the ischemia-affected region. As another approach herein, the Hsp-expressing vectors may be directly injected into loci of ischemic lesions similarly to what was realized in a model with kainic acid–induced neuronal cell death in vivo (Kalwy et al 2003).
Sometimes, physiologically unfavorable conditions resembling ischemia-reperfusion are forcedly created in the course of specific surgical procedures such as transplantation of donor organs or operations with bypass perfusion. Under such a “surgical ischemia,” the treated organ can undergo the cytotoxic influence of acute reperfusion when normal circulation is renewed after the surgical intervention; in a number of cases, this harmful influence may result in serious complications and unsuccessful outcome of the operation. We believe that, in these cases, the artificial (eg, transgenic vector induced or drug induced) upregulation of intracellular Hsp70 or Hsp27 (or both) could protect from reperfusion injury the donor organ after its implantation into a recipient as well as the patient's organ being operated with involving of bypass perfusion.
Acknowledgments
We are grateful to Dr S.A. Loktionova for her assistance in the isolation of human EC and Dr B. Brar for her contribution to the development of the experimental approaches used here. This study was supported by the Wellcome Trust (grant 062891) and INTAS (grant 01-0409).
REFERENCES
- Beer HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Brar B, Stephanou A, Wagstaff MJD, Coffin RS, Marber MS, Engelman G, Latchman DS. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Coffin RS, Howard MK, and Cumming DVE. et al. 1996 Gene delivery to cardiac cells in vitro and in vivo using herpes simplex virus vectors. Gene Ther. 3:560–566. [PubMed] [Google Scholar]
- Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Wey JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in Hsp72-mediated protection of myogenic cells from transient energy deprivation. J Biol Chem. 2000;275:38088–38094. doi: 10.1074/jbc.M006632200. [DOI] [PubMed] [Google Scholar]
- Howard MK, Kershaw T, and Gibb B. et al. 1998 High efficiency gene transfer to the central nervous system of rodents and primates using herpes virus vectors lacking functional ICP27 and ICP34.5. Gene Ther. 5:1137–1147. [DOI] [PubMed] [Google Scholar]
- Kabakov AE, Budagova KR, Latchman DS, Kampinga HH. Stressful preconditioning and HSP70 overexpression attenuate proteotoxicity of cellular ATP depletion. Am J Physiol. 2002;283:C521–C534. doi: 10.1152/ajpcell.00503.2001. [DOI] [PubMed] [Google Scholar]
- Kalwy SA, Akbar MT, Coffin RS, de Belleroche J, Latchman DS. Heat shock protein 27 delivered via a herpes simplex virus vector can protect neurons of the hippocampus against kainic-acid-induced cell loss. Brain Res Mol Brain Res. 2003;111:91–103. doi: 10.1016/s0169-328x(02)00692-7. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001a;51:637–646. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Gene delivery and gene therapy with herpes simplex virus-based vectors. Gene. 2001b;264:1–9. doi: 10.1016/s0378-1119(01)00322-5. [DOI] [PubMed] [Google Scholar]
- Lavoie J, Chretien P, Landry J. Sequence of the Chinese hamster small heat shock protein hsp27. Nucleic Acids Res. 1990;18:1637. doi: 10.1093/nar/18.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktionova SA, Ilyinskaya OP, Kabakov AE. Early and delayed tolerance to simulated ischemia in heat-preconditioned endothelial cells: a role for HSP27. Am J Physiol. 1998;275:H2147–H2158. doi: 10.1152/ajpheart.1998.275.6.H2147. [DOI] [PubMed] [Google Scholar]
- Morris SD, Cumming DVE, Latchman DS, Yellon DM. Specific induction of the 70-kD heat stress protein by the thyrosine kinase inhibitor Herbimycin-A protects rat neonatal cardiomyocytes—a new pharmacological route to stress protein expression? J Clin Investig. 1996;97:706–712. doi: 10.1172/JCI118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooie T, Takahashi N, and Saikawa T. et al. 2001 Single oral dose of Geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 104:1837–1843. [DOI] [PubMed] [Google Scholar]
- Pandey P, Farber R, and Nakazawa A. et al. 2000 Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakowski JS, Wegner CD, Cox BF. Bimoclomol elevates heat shock protein 70 and cytoprotects rat neonatal cardiomyocytes. Eur J Pharmacol. 2002;435:73–77. doi: 10.1016/s0014-2999(01)01551-5. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J Biol Chem. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri EA. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2002;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Scarabelli T, Stephanou A, and Pasini E. et al. 2002 Different signaling pathways induce apoptosis in endothelial cells and cardiac myocytes during ischemia/reperfusion injury. Circ Res. 90:745–748. [DOI] [PubMed] [Google Scholar]
- Scarabelli T, Stephanou A, and Rayment N. et al. 2001 Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation. 104:253–256. [DOI] [PubMed] [Google Scholar]
- Snoeckx LHEH, Cornelussen RN, van Nieuwenhoven FA, Reneman RS, van der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- Stempien-Otero A, Karsan A, and Cornejo CJ. et al. 1999 Mechanisms of hypoxia-induced endothelial cell death. J Biol Chem. 274:8039–8045. [DOI] [PubMed] [Google Scholar]
- Sunnergren KP, Rovetto MJ. Myocyte and endothelial injury with ischemia reperfusion in isolated rat hearts. Am J Physiol. 1987;252:H1211–H1217. doi: 10.1152/ajpheart.1987.252.6.H1211. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sawa Y, Kaneda Y, Ichikawa H, Shirakura R, Matsuda H. Overexpressed heat shock protein 70 attenuates hypoxic injury in coronary endothelial cells. J Mol Cell Cardiol. 1998;30:1129–1136. doi: 10.1006/jmcc.1998.0678. [DOI] [PubMed] [Google Scholar]
- Svendsen JH, Bjerrum PJ, Haunso S. Myocardial capillary permeability after regional ischemia and reperfusion in the in vivo canine heart. Circ Res. 1991;68:174–184. doi: 10.1161/01.res.68.1.174. [DOI] [PubMed] [Google Scholar]
- Vigh L, Literati PN, and Horvath I. et al. 1997 Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 3:1150–1154. [DOI] [PubMed] [Google Scholar]
- Wagstaff MJD, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J Biol Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- Wu B, Hunt R, Morimoto R. Structure and expression of the human gene encoding major heat shock protein hsp70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]