Abstract
Doxorubicin is an antineoplastic drug widely used in cancer treatment. However, many tumors are intrinsically resistant to the drug or show drug resistance after an initial period of response. Among the different molecules implicated with doxorubicin resistance are the heat shock proteins (Hsps). At present we do not know with certainty the mechanism(s) involved in such resistance. In the present study, to advance our knowledge on the relationship between Hsps and drug resistance, we have used peripheral blood mononuclear cells obtained from healthy nonsmoker donors to evaluate the capacity of a preliminary heat shock to elicit the Hsp response and to establish the protection against the deoxyribonucleic acid (DNA) damage induced by doxorubicin. DNA damage and repair were determined using the alkaline comet assay. We also measured the expression of Hsp27, Hsp60, Hsp70, Hsp90, hMLH1, hMSH2, and proliferating cell nuclear antigen by immunocytochemistry. The damage induced by doxorubicin was more efficiently repaired when the cells were previously heat shocked followed by a resting period of 24 hours before drug exposure, as shown by (1) the increased number of undamaged cells (P < 0.05), (2) the increased DNA repair capacity (P < 0.05), and (3) the high expression of the mismatch repair (MMR) proteins hMLH1 and hMSH2 (P < 0.05). In addition, in the mentioned group of cells, we confirmed by Western blot high expression levels of Hsp27 and Hsp70. We also noted a nuclear translocation of Hsp27 and mainly of Hsp70. Furthermore, inducible Hsp70 was more expressed in the nucleus than Hsc70, showing a possible participation of Hsp70 in the DNA repair process mediated by the MMR system.
INTRODUCTION
Doxorubicin (Adriamycin) is a member of the anthracycline family of antineoplastic drugs and is used as a first-line chemotherapy in the treatment of several solid tumor types. Previous studies have established that doxorubicin induces apoptosis of tumor cells, ie, in leukemia lymphocytes (Anand et al 1995) as well as in breast carcinomas and sarcomas (Ciocca et al 2003). The cytotoxicity of doxorubicin is due to a variety of mechanisms like Topoisomerase-II inhibition, oxygen reactive species generation, deoxyribonucleic acid (DNA) crosslinks, double-strand breaks, and the recently described inhibition of the mismatch repair (MMR) pathway (Skladanowski and Konopa 1994; Larson and Drummond 2001). Although doxorubicin is a very effective cytotoxic drug, many tumors are intrinsically resistant to the drug (innate drug resistance) or show drug resistance after an initial period of response (acquired drug resistance). Among the different molecules that have been implicated with doxorubicin resistance are the heat shock proteins (Hsps). Normal cells under constitutive conditions produce Hsps; besides, they are induced in normal and tumor cells in response to various damaging conditions including heat shock, oxidative stress, anticancer drugs, and others. The Hsps participate as molecular chaperones in a wide range of cellular processes (Georgopoulos and Welch 1993). Previous in vitro studies have involved certain Hsps with cytotoxic drug resistance, eg, elevated levels of Hsp70 and Hsp27 in breast cancer cell lines were associated with doxorubicin resistance (Ciocca et al 1992; Garrido et al 1996). Moreover, in vivo studies have demonstrated a correlation between Hsp70 and Hsp27 expression with drug resistance in breast cancer patients treated with induction chemotherapy containing doxorubicin among other drugs (Vargas-Roig et al 1998). Interestingly, in these biopsy samples there was nuclear translocation of the Hsps after chemotherapy. However, at present, we do not know which is the possible mechanism(s) implicating Hsp27 and Hsp70 with doxorubicin resistance.
In the present study, to advance our knowledge on the relationship between Hsps and drug resistance, we have used peripheral blood mononuclear cells (PBMC) obtained from healthy nonsmoker donors to evaluate the capacity of a preliminary heat shock to elicit the Hsp response and to establish the protection against the DNA damage induced by doxorubicin. In other words, we have assessed how the heat shock response may influence the DNA damage-repair capacity of the cells. The DNA repair capacity is one of the factors that could be involved in the individual phenotypic response to genotoxic agents. DNA damage and repair were determined using the alkaline comet assay. This method is very useful to measure the DNA damage in individual cells. The negatively charged broken ends of the DNA molecule are free to migrate in an electrophoretic field toward the anode, forming a comet (Fairbairn et al 1995). The technique constitutes a rapid assay for the screening of mutagen sensitivity and for the study of interindividual variations in the DNA damage susceptibility and in the DNA repair capacity (Schmezer et al 2001). We measured in control and heat shocked/doxorubicin-treated cells the expression of Hsp27, Hsp60, Hsp70, and Hsp90 by immunocytochemistry and Hsp27 and Hsp70 expression at relevant time points by Western blot. In addition, the expression of hMLH1 and hMSH2 MMR proteins and proliferating cell nuclear antigen (PCNA) also was evaluated. A deficiency of the MMR pathway has been associated with resistance to doxorubicin in cultured cells (Drummond et al 1996; Fink et al 1998). Base misincorporations occurring as a consequence of polymerase errors during DNA replication, chemical or physical DNA damages, or nonhomologous recombination are corrected by MMR. In human cells, the MMR process is mediated by hMSH2, hMSH3, hMSH6, hMLH1, and hPMS2. The repair initiation complex is adenosine triphosphate–dependent and also requires PCNA, which interacts with complexes containing hMLH1 and hMSH2 (Flores-Rozas et al 2000).
The results obtained showed the use of the comet assay to evaluate the individual response to doxorubicin and the implications of Hsps in the DNA damage-repair processes. We report here the baseline data found in PBMC from healthy individuals, and the results obtained support the further use of these assays to evaluate the individual phenotypic response in cancer patients under doxorubicin treatment.
MATERIALS AND METHODS
Cell isolation and experimental design
PBMC were obtained from 5 healthy nonsmoker donors by venous puncture. The blood was collected with heparin, and the PBMC were separated by Ficoll density gradient centrifugation (30 minutes at 1400 rpm). The PBMC were exposed to mitogen-induced blast transformation for further study of MMR proteins and PCNA. For this, the PBMC were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, 0.5 μg/mL of amphotericin B, 2 mM l-glutamine, and 10% of fetal calf serum (FCS) and incubated in the presence of 5 μg/mL of phytohemagglutinin for 48 hours. All the reagents were obtained from Sigma-Aldrich, St. Louis, MO (USA), except FCS, which was provided by Bioser (Buenos Aires, Argentina). The cellular composition of the PBMC after these treatments was characterized by immunocytochemistry (see description below). The PBMC were represented by >95% of T-lymphocytes (CD43+) and B-lymphocytes (CD20+).
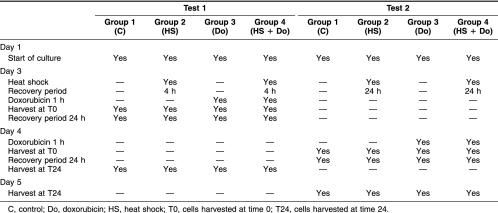
We performed 2 tests on the PBMC: test 1 and test 2. The difference between them was the recovery period after heat shock. There were 4 groups in each test: group 1 (control: C), group 2 (heat shock: HS), group 3 (doxorubicin: Do), and group 4 (heat shock+doxorubicin: HS+Do). Table 1 shows a scheme of the experimental design. Test 1: after the first 48 hours of culture, PBMC in groups 2 and 4 were treated at a nonlethal temperature of 42°C for 1 hour in a temperature-controlled waterbath, followed by a recovery period of 4 hours at 37°C. Then, groups 3 and 4 were treated for 1 hour with 21 nM of fresh doxorubicin (Filaxis Laboratory, Argentina) for 1 hour (this concentration was estimated on the basis of the percentage of damaged cells using alkaline comet assay and cell viability with trypan blue, data not shown). After treatment, the cells were washed in RPMI 1640, and half of the cells of each group were harvested at time 0 (T0). The other half of cells were resuspended in fresh culture medium for a recovery period of 24 hours at 37°C to study DNA repair and collected at time 24 (T24). Test 2: after 48 hours of culture, the groups were treated at a nonlethal temperature of 42°C for 1 hour in a temperature-controlled waterbath, followed by a recovery period of 24 hours at 37°C. When this time was completed, the groups 3 and 4 were treated with 21 nM of doxorubicin for 1 hour. At the end of this step, half of the cells of each group were collected at T0, and the other half were collected at T24. After each harvest, the cells were cryopreserved until the next studies at −80°C in a solution containing RPMI 1640 (40%), FCS (50%), and dimethyl sulfoxide (10%). All the experiments were done twice. Moreover, because we found high interindividual variations in the nuclear translocation experiments, we added 3 normal subjects for this specific set of experiments.
Table 1.
Scheme of the experimental procedure
Immunocytochemistry
PBMC were fixed in 10% buffered formalin at room temperature and smeared for immunocytochemistry. The antibodies used were: rabbit polyclonal antibody against 85 kDa caspase-cleaved fragment (p85) of human poly (adenosine 5′-diphosphate-ribose) polymerase (PARP) (Promega Corporation, Madison, WI, USA); mouse monoclonal antibody (Mab) against human CD20, B-cell (Dako Corporation, Carpinteria, CA, USA); mouse Mab against human CD43, T-cell (Dako Corporation); rabbit polyclonal antibody against Hsp25/27 provided by Dr M. Gaestel (Max-Delbrück Center for Molecular Medicine, Berlin, Germany); mouse Mab LK2 against Hsp60 (Sigma-Aldrich); mouse Mab BRM-22 against constitutive and inducible form of Hsp70 (Sigma-Aldrich); rat 1B5, against the constitutive form of Hsp70 (Hsc70), kindly provided by Dr A. Laszlo (Mallinckrodt Institute of Radiology, Washington University Medical Center, St Louis, MO, USA); mouse Mab AC88 against Hsp90 provided by Dr D.O. Toft (Mayo Clinic, Rochester, MN, USA); mouse Mab PC10 against PCNA (Novocastra, Newcastle-upon-Tyne, UK); mouse Mab against hMLH1 (Pharmingen, San Diego, CA, USA); and mouse Mab against hMSH2 (Calbiochem, San Diego, CA, USA).
For cell permeabilization, the slides were immersed 5 minutes in Nonidet-P40 (NP-40) 0.1% in phosphate-buffered saline (PBS, pH 7.4) at 4°C. PCNA, hMLH1, and hMSH2 antigen unmasking was carried out in 0.01 M citrate buffer (pH 6.0) at 100°C for 25 minutes. The cells were incubated with the primary antibodies overnight at 4°C in humidity chambers at the following dilutions: PARP, 1:200; CD20, 1:150; CD43, 1:100; Hsp25/27, 1:2000; LK2, 1:50; BRM-22, 1:1000; 1B5, 1:2000; AC88, 20 μg/mL; PC10, 1:1000; hMLH1, 20 μg/mL; hMSH2, 4 μg/mL. For Hsc70 we used a goat biotinylated anti-rat IgG antibody (Sigma-Aldrich). As second antibody we used a labeled polymer conjugated with goat anti-rabbit and goat anti-mouse immunoglobulins (DAKO EnVision System Peroxidase, Dako Corporation). Diaminobenzidine (0.5 mg/mL)-hydrogen peroxide (0.01%) was used as chromogen substrate. Slides were lightly counterstained with 0.5% methyl green and observed with an IM35 microscope (Carl Zeiss, Oberkochen, Germany). The immunostaining was evaluated according to the percentage of positive cells (cytoplasmic or nuclear staining) in 200 cells per sample under double blindness throughout the study. In every assay, a negative control was included.
Alkaline comet assay
The frozen PBMC were thawed in a thermostatic bath at 37°C, washed in PBS, and resuspended in cold PBS to a final concentration of 1 × 106 cells/mL. Cell viability was >95% in the control group. To prevent additional DNA damage, the cells were kept in the dark, at 4°C. The alkaline comet assay was performed according to a described procedure (Olive et al 1992). Cells were embedded in 1% agarose, and the suspension was spread over a frosted slide. Approximately 50 000 cells were placed on each slide. The assay was produced in duplicate. After electrophoresis, the agarose gels containing the cells were placed on microscope slides and washed twice for 2 minutes with deionized water. An improved silver staining method recently reported by us (Nadin et al 2001) was used to visualize the comets. All samples (at least 40 cells in each gel) were evaluated in duplicate under double blindness using a visual score from 0 (no damage) to 5 (total damage) (Anderson et al 1994). To facilitate the management of the data, the DNA damage was classified as: none (cells with score 0), low (cells with score 1 and 2), high (cells with score 3 and 4), and total (cells with score 5). Comets were evaluated under the 20× objective. A positive control was included in each assay, treating part of PBMC with 60 μM of hydrogen peroxide for 1 hour.
TUNEL technique
Apoptosis was evaluated by the terminal deoxyribonucleotidyl transferase–mediated dUTP-digoxigenin nick end labeling (TUNEL) method using the ApopTag Plus kit (Oncor, Gaithersburg, MD, USA; S7101-KIT), with a modification of the detection system, which significantly increased the sensitivity of the technique (Cuello-Carrión and Ciocca 1999).
Western blotting
Immunoblotting procedures were used to determine the specificity and quantity of Hsp27 and Hsp70 at relevant time points. The PBMC were washed in PBS and then resuspended in sample buffer. The proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Western blotting as described elsewhere (Ciocca et al 1989). A sample protein of 30 μL (7 × 105 cells) was loaded and separated in a resolving gel with 12.5% of acrylamide. One lane was loaded with molecular weight markers (Rainbow Marker; Amersham, Buckinghamshire, UK): Lysozyme (Mr 14 300); trypsin inhibitor (Mr 21 500); carbonic anhydrase (Mr 30 000); ovalbumin (Mr 46 400); bovine serum albumin (BSA) (Mr 69 000); and phosphorylase B (Mr 97 000). Detection of the specific Hsp27 and Hsp70 bands on the nitrocellulose paper was performed using the rabbit polyclonal antibody against Hsp25/27 at 1:2000 dilution in blocking buffer (5% BSA in PBS—0.5% Tween 20) and the mouse Mab BRM-22 against the constitutive and inducible forms of Hsp70 at 1:2000 dilution in blocking buffer. After overnight incubation at 4°C with shaking, the membranes were washed and incubated with a biotinylated swine antibody to rabbit immunoglobulins (Dako Corporation; 1:2500) and a biotinylated rabbit antibody to mouse immunoglobulins (Dako Corporation; 1:2500) for 60 minutes. After washing, the membranes were incubated with peroxidase-labeled streptavidin-biotin complex (1:5000) for 60 minutes. Washed membranes were then incubated with chemiluminescence reagents (Dupont, NEN, Boston, MA, USA) following manufacturer's instructions. The light was captured on autoradiography film (Kodak X-OMAT LS, Sigma, St Louis, MO, USA) and photographed.
Statistical analysis
The Kruskal-Wallis signed rank nonparametric test with Dunns posttest was used to compare all the groups of both tests. We also used linear regression to compare the percentage of apoptosis obtained from alkaline comet assay, TUNEL technique, and PARP cleavage. Statistical analyses were performed using the Prism computer program (GraphPad PRISM Software, San Diego, CA, USA), P < 0.05 was considered significant.
RESULTS
DNA damage produced by doxorubicin and the cytoprotective effect of heat shock
We first used the alkaline comet assay to measure the levels of DNA damage induced by doxorubicin. Previous studies have shown significant differences in the distribution of DNA damage among PBMC from each individual, between individuals according to age (Singh et al 1991), and among smokers depending to the extent of smoking (Dhawan et al 2001). Therefore, to diminish the basal level of damage, a group of nonsmoker control subjects (between 23 and 29 years of age) was selected. Figure 1 shows the different kinds of DNA damage induced by heat shock, doxorubicin alone, and doxorubicin after heat shock, in comparison with a control group. Note the significant difference found between test 1 and test 2 in group 4 (HS+Do), which constitutes the first evidence on the relationship between the recovery period after heat shock (test 1, 4 hours; test 2, 24 hours) and the cytoprotective effect on PBMC.
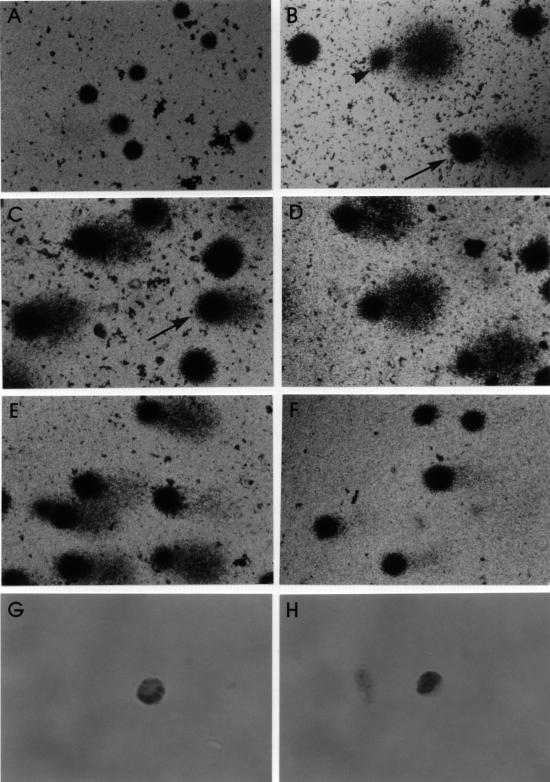
Fig 1.
Lymphocytes with silver staining to show comets and immunostained to reveal heat shock protein (Hsp)70. (A) Group 1 (control), high proportion of undamaged cells. (B) Positive control (60 μM H2O2). Note the damaged cells, one with score 3 (arrow) and the other with score 4 (arrow head). (C) Group 2 (heat shock) is represented by undamaged cells and cells with a small level of deoxyribonucleic acid (DNA) damage: score 2 (arrow). (D) Group 3 (21 nM doxorubicin) presents a high proportion of cells with score 4 (more than 50% of damage). (E) Group 4 (heat shock and doxorubicin) of test 1 at T24 presents elevated number of damaged cells, as represented by the heterogeneous sizes and intensity of the comet tails. (F) In contrast, group 4 of test 2 at T24 is characterized by a significant reduction of DNA damage. (G) Cytoplasmic immunostaining of Hsp70. (H) Nuclear expression of Hsp70. Original magnification: Fig A–F, 220×; Fig G–H, 1370×
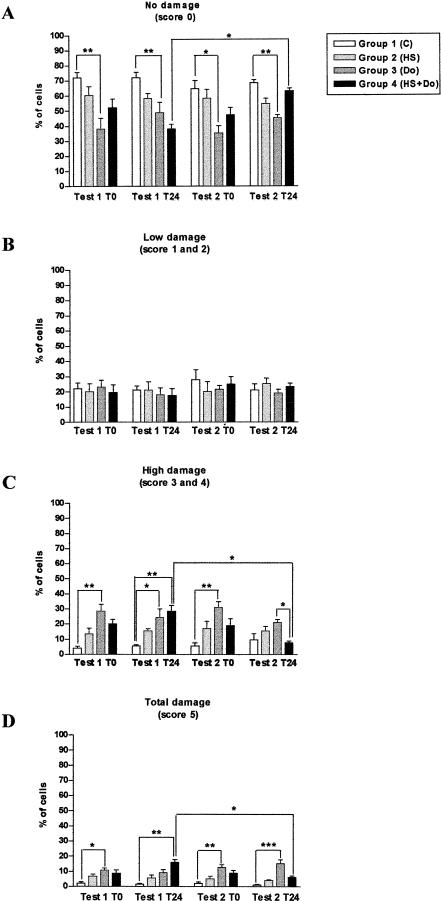
Figure 2 shows the detailed evaluation of the comets after hyperthermia and doxorubicin treatments. The heat shock caused a modest decrease in the percentage of cells without damage, with a concomitant small increase in cells with high and total damage (Fig 2A,C,D). On the other hand, doxorubicin induced a significant DNA damage (Fig 2A,C,D). This was observed in the percentage of cells with high damage for test 1 at T0, test 1 at T24, and test 2 at T0 (Fig 2C) and in the percentage of cells with total damage for test 1 at T0, test 2 at T0, and test 2 at T24 (Fig 2D). At low levels of damage, no differences were found (Fig 2B).
Fig 2.
Comet assay, deoxyribonucleic acid damage caused by hyperthermia and doxorubicin. Note in group 4 the difference in cells with none (A), high (C), and total (D) damage between test 1 and test 2 at T24 in group 4. Bars represent mean ± standard error (SEM). Significance of the differences are given by *P < 0.05, **P < 0.01, and ***P < 0.001. C: control; HS: heat shock; Do: doxorubicin; HS+Do: heat shock and doxorubicin
The heat shock protected the PBMC from the damage caused by doxorubicin (group 4), but only in test 2 (24-hour recovery period). This is supported by the significant differences found between test 1 and test 2 at T24 in the percentage of cells with no damage, with high damage, and with total damage (Fig 2A,C,D, respectively). In addition, the mentioned group in test 1 at T24 showed statistically significant differences in comparison with group 1 (control) in the percentage of undamaged cells (Fig 2A), cells with high damage (Fig 2C), and cells with total damage (Fig 2D). Furthermore, there was also an interesting significant difference in the percentage of cells with high damage (Fig 2C) in test 2 at T24 between groups 3 (Do) and 4 (HS+Do), suggesting that the heat shock protected from doxorubicin treatment.
Previous studies have shown that the nature of the cytotoxic effects of doxorubicin was mediated by the process of apoptosis. To verify whether the PBMC that presented a total damage (score 5) in the comet assay were really in apoptosis, we assessed the PARP cleavage by immunocytochemistry and by the TUNEL technique. PARP is a Mr 116 000 nuclear protein involved in the response to DNA damage. Studies of programmed cell death by genotoxic agents have demonstrated that apoptotic cells show a unique PARP cleavage pattern that is considered an early marker of apoptosis (Kaufmann et al 1993). PARP also showed a strong correlation with acridine orange, which is used to visualize the chromatin condensation pattern characteristic of apoptosis (Whitacre and Berger 1997). In the present study, we analyzed by TUNEL and PARP the group with the highest percentage of cells with total damage: group 4 of test 1 at T24 (Fig 2D). We found by linear regression a very good correlation between the comet assay and TUNEL (P = 0.0014, r2 = 0.9363), between the comet assay and PARP (P = 0.0032, r2 = 0.9052), and between PARP and TUNEL (P = 0.0001, r2 = 0.9822).
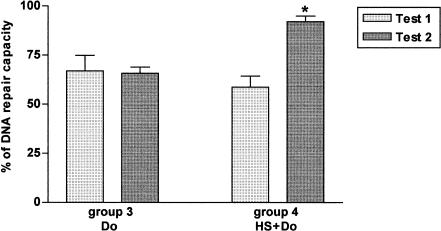
The DNA repair capacity was calculated after a fixed repair time of 24 hours as the percentage of undamaged cells in each group (groups 3 and 4) divided by the percentage of undamaged cells in group 1 (control) × 100 (Schmezer et al 2001). There was a significant difference between test 1 and test 2 in group 4 (HS+Do) only (Fig 3). These data confirm that the PBMC in group 4 (HS+Do) in test 2 repaired more efficiently the DNA damage caused by doxorubicin than in group 3 (Do). In contrast, there was no repair of the damage in group 4 in test 1, which reflects that the function of the chaperones may depend on the recovery period after heat shock.
Fig 3.
Deoxyribonucleic acid (DNA) repair capacity after a fixed repair of 24 hours. In group 3 (Do), there was no significant difference between test 1 and test 2. In contrast, in group 4 (HS+Do), the difference between both tests was statistically significant (* P < 0.05), which reflects the importance of the recovery period after the heat shock in the thermotolerance developed in response to doxorubicin. Percentages of DNA repair capacity are reported (±SEM)
Induction of Hsp27, Hsp60, Hsp70, and Hsp90 by hyperthermia and doxorubicin in vitro in PBMC
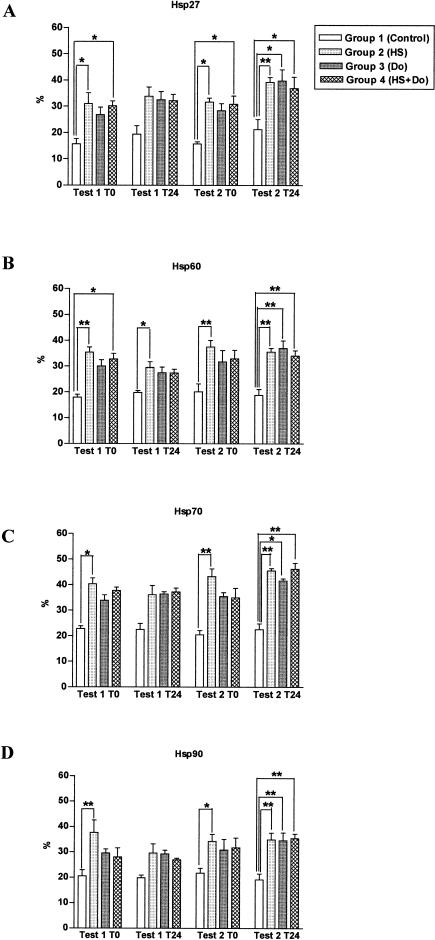
We measured by immunocytochemistry Hsp27, Hsp60, Hsp70, and Hsp90 expression in all groups of both tests at T0 and at T24. Mammalian cells are known to synthesize Hsps in vitro after brief exposures to temperatures of 3–5°C above normal (heat shock). The heat shock performed for 1 hour at 42°C induced all the Hsps with 4 as well as 24 hours of recovery in group 2 (HS), and in comparison with group 1 (control), there was a significant difference for all the Hsps measured and for test 1 and test 2 at T0 (Fig 4). The mentioned differences observed between groups 1 and 2 remained at T24 in both tests, although they were statistically significant in test 2 at T24 for all Hsps studied and in test 1 at T24 for Hsp60 only (Fig 4).
Fig 4.
Induction of heat shock proteins (Hsps) in PBMC by a heat shock at 42°C. After the thermal treatment, the lymphocytes had a recovery period of 4 hours (test 1) or 24 hours (test 2). %: Percentage of positive cells for a particular Hsp evaluating at least 200 cells per sample. * P < 0.05 and ** P < 0.01; bars represent mean ± SEM
Doxorubicin (group 3) increased the expression of the Hsps by more than 10%, and the differences with respect to group 1 (control) were significant only in test 2 at T24. The increased production of oxygen free radicals by doxorubicin may explain the increased expression of Hsp (Fig 4).
All Hsps studied increased in group 4 (HS+Do) when compared with group 1 (control). The values obtained in group 4 were very similar to those in group 2 (HS). At T0 there were significant differences with regard to the control group for Hsp27 in test 1 and test 2 (Fig 4A) and Hsp60 in test 1 (Fig 4B). In test 2, at T24, we found a significant Hsp expression with respect to group 1 (control) for Hsp27 (Fig 4A), Hsp60 (Fig 4B), Hsp70 (Fig 4C), and Hsp90 (Fig 4D).
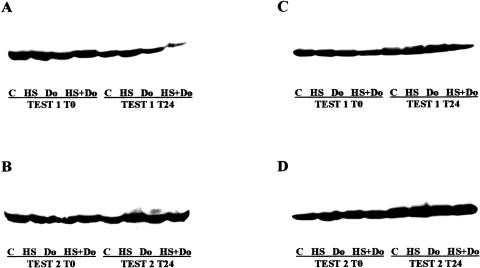
Because the purpose of the experiment was to evaluate the influence of heat treatment on doxorubicin-induced DNA damage, we paid attention to the differences in Hsps induction between group 3 (Do) and group 4 (HS+Do) and between test 1 and test 2. Nevertheless, we found no significant differences between groups 3 and 4 and between tests 1 and 2 in Hsps expression by immunocytochemistry. Because the highest mean levels of Hsps were observed in group 4 of test 2 at T24, and corresponded to Hsp27 and Hsp70, we assessed by Western blot the expression levels of the mentioned Hsps (Fig 5). In group 4 (HS+Do) we noted a higher expression of Hsp27 and Hsp70 in test 2 at T24 than in test 1 at T24. In addition, group 3 (Do) presented a modest decrease in the expression of Hsp27 and Hsp70 than group 4 (HS+Do). These data permit to conclude that the high expression levels of Hsp27 and Hsp70 found in group 4, together with the increased DNA repair capacity, suggest a clear relationship of the Hsps with the DNA repair pathway. The latter observations are consistent with previous in vitro studies in which elevated levels of Hsp27 and Hsp70 were associated with drug resistance.
Fig 5.
Western blot analysis of heat shock protein (Hsp)27 and Hsp70. (A) Hsp27 expression in test 1. Note the induction by heat shock at T0 and the reduced expression in group 4 (HS+Do) at T24. (B) Hsp27 in test 2 was more induced by heat shock (group 2) than doxorubicin (group 3). In group 4 at T24 there was an overexpression of the Hsp. (C) Hsp70 expression in test 1. Note the decreased levels in group 4 (HS+Do). (D) Hsp70 expression in test 2. The heat shock induction was more important than in test 1, and there was an overexpression in group 4. Group 1 (control: C); group 2 (heat shock: HS); group 3 (doxorubicin: Do); group 4 (heat shock+doxorubicin: HS+Do). T0: time 0, harvested immediately after doxorubicin treatment. T24: time 24, harvested 24 hours after postdoxorubicin recovery
Nuclear translocation of Hsp27 and Hsp70
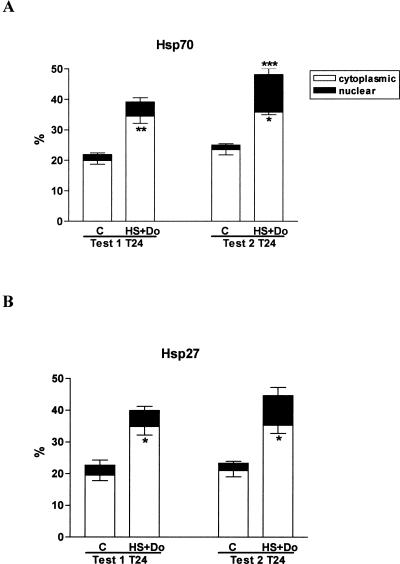
A nuclear translocation of Hsp27 and Hsp70 was found in group 4 (HS+Do) (see Fig 1G,H). This observation was more evident in test 2 than in test 1 and particularly at T24 (Fig 6). We found a statistically significant difference in test 2 at T24 between control and group 4 for nuclear Hsp70. The differences in the percentages of cytoplasmic Hsp27 and Hsp70 were significant in both tests between group 4 and control (group 1).
Fig 6.
Nuclear and cytoplasmic percentages of heat shock protein (Hsp)27 and Hsp70. The graph shows the differences found between group 1 (control: C) and group 4 (heat shock + doxorubicin: HS+Do) and between test 1 (recovery period of 4 hours after heat shock) and test 2 (recovery period of 24 hours after heat shock) at time 24 (T24). Note that the induction of Hsp70 (A) and Hsp27 (B) in test 2 was greater than in test 1, and at the same time in test 2 we observed the largest nuclear translocation of the mentioned Hsps. The latter observation correlates with the increased deoxyribonucleic acid (DNA) repair capacity verified in group 4 of test 2, suggesting that Hsp70 and Hsp27 are accomplishing a role in the DNA repair process. Significant differences in the percentage of cytoplasmic Hsp27 and Hsp70 were found between group 1 and group 4 in both tests. In addition, significant nuclear Hsp70 induction was observed in test 2 at T24 (A) in group 4 compared with group 1. *P < 0.05, **P < 0.01, and ***P < 0.001; bars represent mean ± SEM
Hsc70 was studied in group 4 of test 2, which presented a significant percentage of nuclear Hsp70. However, we verified that the nuclear translocation of Hsp70 was predominantly of inducible Hsp70 (≅70% calculated by difference between total Hsp70 and Hsc70).
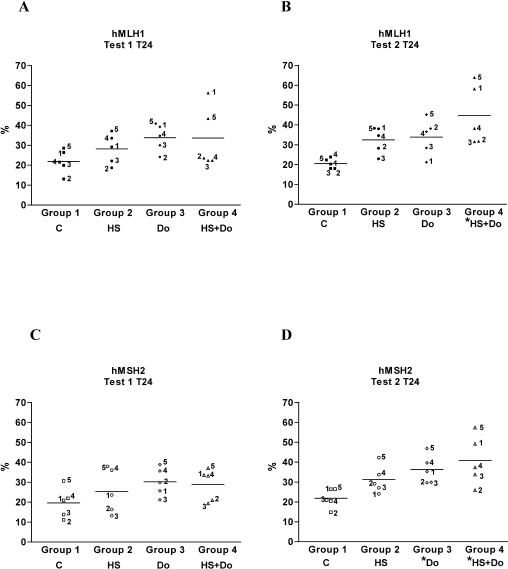
Effect of hyperthermia on the expression of hMLH1, hMSH2, and PCNA
MMR is a postreplicative DNA repair process, and PCNA is undetectable in circulating lymphocytes, therefore, for this study, the PBMC were cultured with a mitogen for 48 hours before the subsequent treatment to bring them into the cell cycle. The MMR proteins hMLH1 and hMSH2 and PCNA were studied by immunocytochemistry (Fig 7). The difference noted between both tests could be related to the increased DNA repair capacity observed in group 4 of test 2. Taken together, the data suggest that the Hsps, particularly the nuclear-translocated Hsp72, are helping certain DNA repair proteins, such as MMR proteins, in damage correction and are inhibiting the apoptotic pathways. We were interested in the action of PCNA because it is required for MMR and because this protein interacts with complexes containing hMSH2 or hMLH1 (Gu et al 1998). However, we did not observe statistically significant changes in PCNA levels between test 1 and test 2, as well as among the 4 studied groups (data not shown).
Fig 7.
Expression of the mismatch repair (MMR) proteins hMLH1 and hMSH2 at time 24 (T24). The mean values obtained from the groups 2, 3, and 4, for hMLH1 and hMSH2 and for test 1 and test 2, were greater than the basal level of group 1 (control). Note the significant increased in the mean values of the MMR proteins in group 4 of test 2, which correlates with the higher deoxyribonucleic acid repair capacity reported. There was also a significant difference between group 1 and group 3 *P < 0.05. The numbers on the figure correspond to each studied subject. C: control; HS: heat shock; Do: doxorubicin; HS+Do: heat shock and doxorubicin
DISCUSSION
The clinical outcome in cancer patients to cytotoxic drugs can be mimicked by incubation of leukocytes with drugs in culture, suggesting that this correlation reflects a cell-based phenomenon (Oshita et al 1995). Then, an important goal is to find a test that can predict the response of a patient to chemotherapy administration. In the present study, we explored the comet assay to quantify the DNA damage at individual cell level, and at the same time, to evaluate the repair proficiency using easily accessible cells like PBMC. This is our first step to further explore the usefulness of this test to predict the response of cancer patients to chemotherapy. We demonstrated that the comet assay is a highly sensitive technique to analyze the DNA damage induced by doxorubicin. In addition, the comet assay was useful to measure the number of apoptotic cells. Because apoptotic DNA fragmentation is characterized by the generation of double-strand breaks, the great extent of DNA damage in apoptotic cells could be easily distinguished by the movement of most of the DNA from the head into the tail of the comet (Fairbairn et al 1995). The data obtained with the comet assay were similar to those provided by TUNEL and PARP cleavage, indicating that the alkaline comet assay is useful not only for DNA damage estimation but also for the evaluation of the level of apoptosis in a cell population. However, like most methods, the distinction between apoptotic and advanced necrotic cells is not generally possible (Olive 1999).
In the present study, the damage produced in PBMC by doxorubicin (group 2) was measurable and statistically significant in tests 1 and 2 at T0 (immediately after doxorubicin treatment), as indicated by the decreased percentage of undamaged cells and the increased percentage of cells with high and total damage. The latter was verified more objectively by measuring the DNA repair capacity, which increased significantly when the lymphocytes were previously heat shocked (group 4) followed by a resting period of 24 hours before drug exposure, as seen in test 2 at T24. In contrast, we noted in test 1 at T24 (4 hours of recovery after heat shock) that doxorubicin increased apoptosis (data confirmed by the TUNEL technique and PARP cleavage). In addition, in test 2 at T24, group 3 (Do) was characterized by an increased number of highly damaged cells in comparison with group 4 (HS+Do), suggesting a cytoprotector effect of heat shock. These data showed that the cytoprotection of the Hsps depends on the lapse allowed after heat shock (for Hsps synthesis and activation). However, we cannot rule out that other factors of the stress response are conferring cytoprotection.
The heat shock induced at 42°C was enough to significantly increase the levels of each Hsp studied in group 2 (HS) in comparison with group 1 (control). Hsp70 was highly heat inducible, and at T0 its levels in test 1 (4 hours of recovery) were lower than those observed in test 2 (24 hours of recovery after heat shock). Hsp27 followed the same pattern as Hsp70, although with lower expression levels. In contrast, Hsp90 induction was maximal after 4 hours of recovery but with a high interindividual variation. The Hsps synthesis was also induced by doxorubicin (group 3), but it was smaller than that observed in the heat shock group and statistically not significant, except in test 2 at T24. In addition, we demonstrated by western blot in group 4 (HS+Do) a higher expression of Hsp27 and Hsp70 in test 2 at T24 than in test 1 at T24.
The low basal levels registered for all the Hsps were in accordance with previous reports (Fehrenbach et al 2000). Other authors had demonstrated that hyperthermia had only a very weak effect on Hsp70 expression in lymphocytes; this expression increased slowly and is more significant at 42°C (Oehler et al 2001).
Previous studies have shown that certain chemotherapeutic drugs are less effective on tumor cells previously exposed to hyperthermia. For example, it has been shown that elevated Hsp27 and Hsp70 levels were associated with doxorubicin resistance in MCF-7/BK and MDA-MB-231 breast cancer cells (Ciocca et al 1992) and in rodent tumors (Ciocca et al 2003). Recent studies have described the antiapoptotic role of several members of the Hsp family including Hsp27, Hsp70, and Hsp90. In the present study, we observed by the comet assay that the Hsps might contribute to cell death as well as to the repair of the DNA lesions according to the post–heat shock resting period. In other words, these results stress the importance of the heat shock response in the maintenance of the balance between cell death and survival and the time dependence of the cytoprotective effect of the Hsps. Recent studies using camptothecin-treated Jurkat cells have shown that Hsp60 is able to accelerate the maturation of procaspase-3 by different upstream activator caspases (Xanthoudakis et al 1999). It has been demonstrated that Hsp70 is able to directly inhibit caspase processing by interacting with Apaf-1 to prevent the recruitment of procaspase-9 to the apoptosome (Beere et al 2000). It has been reported that Hsp90 and Hsp27 can inhibit the formation of the competent apoptosome, whereas Hsp90 associated with Apaf-1 can prevent its oligomerization (Pandey et al 2000); Hsp27 sequesters cytochrome c preventing its binding with Apaf-1 (Bruey et al 2000).
Previous in vitro studies have shown that the nuclear translocation of Hsp70 may protect chromatin DNA from further damage or facilitate the repair of the DNA damage in unknown ways (Abe et al 1995). In addition, it has been shown that thermal preconditioning at 42°C, 24 hours before doxorubicin treatment in rat cardiac muscle cells, protects against apoptosis and that Hsp70 mediates this effect (Ito et al 1999). These observations were corroborated in vivo in breast cancer patients treated with induction chemotherapy. Those patients whose tumors expressed a high proportion of nuclear Hsp27 and Hsp70 had shorter disease-free survival (Vargas-Roig et al 1998). Interestingly, in the present study, we verified in group 4 (HS+Do) a nuclear translocation of Hsp27 and mainly of Hsp70 in test 2 at T24. Nevertheless, the nuclear Hsp70-Hsp27 translocation showed considerable interindividual variations, which may contribute to the differential disease susceptibility and response to chemotherapy (Boshoff et al 2000). The translocation of Hsc70 into the nucleus during S phase also has been reported (Zeise et al 1998). Other authors have reported a role of inducible Hsp70 in enhancing cell proliferation and providing thermoprotection in MCF-7 breast cancer cells (Barnes et al 2001). Recently, high levels of Hsp72 have been associated with lower genotoxic damage in lymphocytes of workers exposed to chemical carcinogens (Xiao et al 2002).
In the present study, we have demonstrated that the nuclear translocation of Hsp27 and more particularly of Hsp70 after doxorubicin treatment in heat shocked PBMC (group 4) was associated with both increased DNA repair capacity and with high expression of the MMR proteins hMLH1 and hMSH2. Interestingly, we noted that these findings were especially present in test 2, when the recovery period was of 24 hours after the heat shock, suggesting the importance of the protein turnover for protein effect. In addition to the described antiapoptotic function of Hsp70, we report a possible participation of Hsp70 in the DNA repair process mediated by the MMR system in human PBMC. Furthermore, Hsp70 has been recently associated with a key base excision repair enzyme providing a possible mechanism by which Hsp70 protects the cells against oxidative stress (Kenny et al 2001). We also reported a synergistic induction of cell death in group 4 (HS+Do) of test 1 at T24, which maybe due to the reduced expression of MMR proteins and to the decreased levels of Hsp27 and Hsp70. Further studies will be required to fully elucidate the molecular mechanisms by which Hsp70 and Hsp27 interact with the MMR system and their possible clinical significance in tumor progression.
Acknowledgments
This study was supported by the National University of Cuyo 06/J139, the National Research Council (CONICET), and the Argentina Foundation for Cancer Research (FAIC).
REFERENCES
- Abe T, Konishi T, Hirano T, Kasai H, Shimizu K, Kashimura M, Higashi K. Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus. Biochem Biophys Res Commun. 1995;206:548–555. doi: 10.1006/bbrc.1995.1078. [DOI] [PubMed] [Google Scholar]
- Anand S, Verma H, Kumar L, Singh N. Induction of apoptosis in chronic myelogenous leukemia lymphocytes by hydroxyurea and adriamycin. Cancer Lett. 1995;88:101–105. doi: 10.1016/0304-3835(94)03617-r. [DOI] [PubMed] [Google Scholar]
- Anderson D, Yu T-W, Phillips BJ, Shmezer P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat Res. 1994;307:261–271. doi: 10.1016/0027-5107(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW. Expression of inducible Hsp70 enhances the proliferation of MCF-7 breast cancer cells and protects against the cytotoxic effects of hyperthermia. Cell Stress Chaperones. 2001;6:316–325. doi: 10.1379/1466-1268(2001)006<0316:eoihet>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Boshoff T, Lombard F, Eiselen R, Bornman JJ, Bachelet M, Polla BS, Borman L. Differential basal synthesis of Hsp70/HSC70 contributes to interindividual variation in Hsp70/Hsc70 inducibility. Cell Mol Life Sci. 2000;57:1317–1325. doi: 10.1007/PL00000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, and Bonniaud P. et al. 2000 Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2:645–652. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Fuqua SAW, Lock-Lim S, Toft DO, Welch WJ, McGuire WL. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992;52:3648–3654. [PubMed] [Google Scholar]
- Ciocca DR, Puy LA, Fasoli LC. Study of estrogen receptor, progesterone receptor, and the estrogen-regulated Mr 24,000 protein in patients with carcinomas of endometrium and cervix. Cancer Res. 1989;49:4298–4304. [PubMed] [Google Scholar]
- Ciocca DR, Rozados VR, Cuello Carrión FD, Gervasoni SI, Matar P, Scharovsky OG. Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello-Carrión FD, Ciocca DR. Improved detection of apoptotic cells using a modified in situ TUNEL technique. J Histochem Cytochem. 1999;47:837–839. doi: 10.1177/002215549904700614. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Mathur N, Seth PK. The effect of smoking and eating habits on DNA damage in Indian population as measured in the comet assay. Mutat Res. 2001;474:121–128. doi: 10.1016/s0027-5107(00)00171-8. [DOI] [PubMed] [Google Scholar]
- Drummond JT, Anthoney A, Brown R, Modrich P. Cisplatin and adriamycin resistance are associated with MutLalpha and mismatch repair deficiency in an ovarian tumor cell line. J Biol Chem. 1996;271:19645–19648. doi: 10.1074/jbc.271.33.19645. [DOI] [PubMed] [Google Scholar]
- Fairbairn DW, Olive PL, O'Neill KL. The comet assay: a comprehensive review. Mutat Res. 1995;339:37–59. doi: 10.1016/0165-1110(94)00013-3. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Passek F, Niess AM, Pohla H, Weinstock C, Dichuth H-H, Northoff H. HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc. 2000;32:592–600. doi: 10.1097/00005768-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- Flores-Rozas H, Clark D, Kolodner RD. Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nat Genet. 2000;26:375–378. doi: 10.1038/81708. [DOI] [PubMed] [Google Scholar]
- Garrido C, Mehlen P, Fromentin A, Hammann A, Assem M, Arrigo AP, Chauffert B. Inconstant association between 27-kDa heat-shock protein (Hsp27) content and doxorubicin resistance in human colon cancer cells. Eur J Biochem. 1996;237:653–659. doi: 10.1111/j.1432-1033.1996.0653p.x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperons. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gu L, Hong Y, McCulloch S, Waranabe H, Li G-M. ATP-dependent interaction of human mismatch repair proteins and dual role of PCNA in mismatch repair. Nucleic Acids Res. 1998;26:1173–1178. doi: 10.1093/nar/26.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Shimojo T, and Fujisaki H. et al. 1999 Thermal preconditioning protects rat cardiac muscle cells from doxorubicin-induced apoptosis. Life Sci. 64:755–761. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose)polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Kenny MK, Mendez F, Sandigursky M, Kurekattil RP, Goldman JD, Franklin WA, Bases R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J Biol Chem. 2001;276:9532–9536. doi: 10.1074/jbc.M009297200. [DOI] [PubMed] [Google Scholar]
- Larson ED, Drummond JT. Human mismatch repair and G.T mismatch binding by hMutSα in vitro is inhibited by adriamycin, actinomycin D, and nogalamycin. J Biol Chem. 2001;276:9775–9783. doi: 10.1074/jbc.M006390200. [DOI] [PubMed] [Google Scholar]
- Nadin SB, Vargas-Roig LM, Ciocca DR. A silver staining method for single-cell gel assay. J Histochem Cytochem. 2001;49:1183–1186. doi: 10.1177/002215540104900912. [DOI] [PubMed] [Google Scholar]
- Oehler R, Pusch E, and Zellner M. et al. 2001 Cell type-specific variations in the induction of hsp70 in human leukocytes by fever like whole body hyperthermia. Cell Stress Chaperones. 6:306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive PL. DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int J Radiat Biol. 1999;75:395–405. doi: 10.1080/095530099140311. [DOI] [PubMed] [Google Scholar]
- Olive PL, Wlodek D, Durand RE, Banath JP. Factors influencing DNA migration from individual cells subject to gel electrophoresis. Exp Cell Res. 1992;198:259–267. doi: 10.1016/0014-4827(92)90378-l. [DOI] [PubMed] [Google Scholar]
- Oshita F, Yamamoto N, Fukuda M, Ohe Y, Tamura T, Eguchi K, Shinkai T, Saijo N. Correlation of therapeutic outcome in non-small cell lung cancer and DNA damage assayed by polymerase chain reaction in leukocytes damaged in vitro. Cancer Res. 1995;55:2334–2337. [PubMed] [Google Scholar]
- Pandey P, Saleh A, and Nakazawa A. et al. 2000 Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock. EMBO J. 19:4310–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmezer P, Rajaee-Behbahani N, and Risch A. et al. 2001 Rapid screening assay for mutagen sensitivity and DNA repair capacity in human peripheral blood lymphocytes. Mutagenesis. 16:25–30. [DOI] [PubMed] [Google Scholar]
- Singh NP, Danner DB, Tice RR, Pearson JD, Brant LJ, Morrell CH, Schneider EL. Basal DNA damage in individual human lymphocytes with age. Mutat Res. 1991;256:1–6. doi: 10.1016/0921-8734(91)90026-8. [DOI] [PubMed] [Google Scholar]
- Skladanowski A, Konopa J. Relevance of interstrand DNA crosslinking induced by anthracyclines for their biological activity. Biochem Pharmacol. 1994;47:2279–2287. doi: 10.1016/0006-2952(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer (Pred Oncol) 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Whitacre CM, Berger NA. Factors affecting topotecan-induced programmed cell death: adhesion protects cells from apoptosis and impairs cleavage of poly(ADP-ribose)polymerase. Cancer Res. 1997;57:2157–2163. [PubMed] [Google Scholar]
- Xanthoudakis S, Roy S, and Rasper D. et al. 1999 Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 18:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Chen S, and Li J. et al. 2002 Association of Hsp70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones. 7:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeise E, Kuhl N, Kunz J, Rensing L. Nuclear translocation of stress protein Hsc70 during S phase in rat C6 glioma cells. Cell Stress Chaperones. 1998;3:94–99. doi: 10.1379/1466-1268(1998)003<0094:ntosph>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]