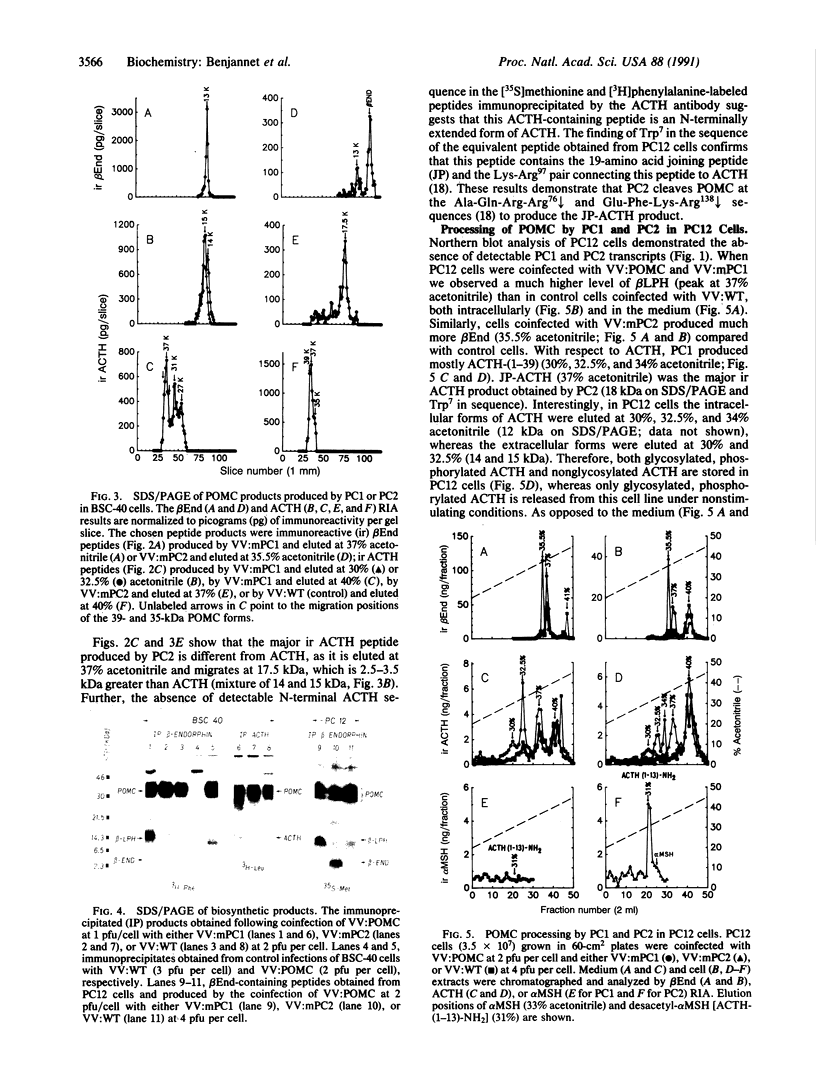
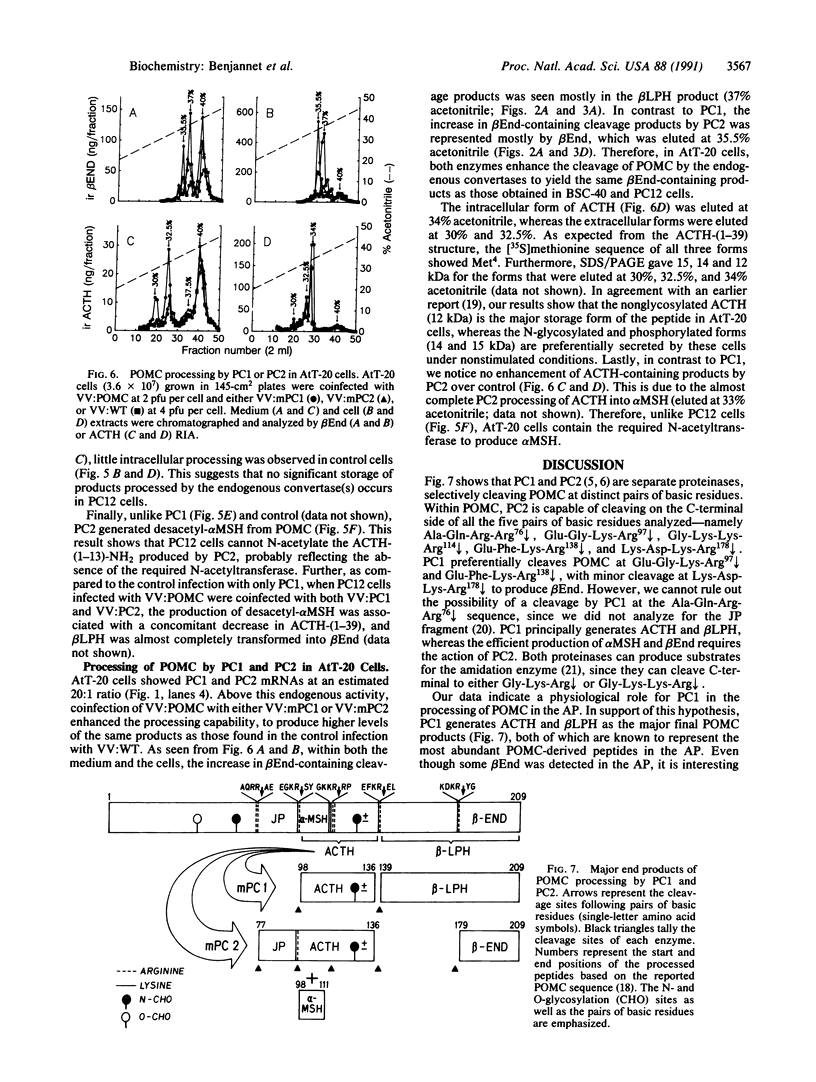
Abstract
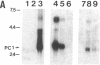
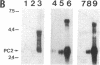
A recombinant vaccinia virus vector was used to coexpress the two candidate mouse prohormone convertases, PC1 and PC2, together with mouse proopiomelanocortin (POMC) in the constitutively secreting cell line BSC-40 and in the endocrine tissue-derived cell lines PC12 and AtT-20, which exhibit regulated secretion. Monitoring of POMC processing demonstrated the distinct cleavage specificities of PC1 and PC2, since in the cell lines analyzed (i) PC1 cleaves POMC into corticotropin and beta-lipotropin, (ii) PC2 cleaves POMC into beta-endorphin, an N-terminally extended corticotropin containing the joining peptide, and either alpha MSH or desacetyl-alpha MSH, and (iii) PC2 cleaves POMC at the five pairs of basic residues analyzed, whereas PC1 cleaves two of them preferentially, suggesting that PC2 has a broader spectrum of activity than PC1. These data are consistent with our hypothesis on the physiological role of PC1 and PC2 as distinct proprotein convertases acting alone or together to produce a set of tissue-specific maturation products in the brain and in peripheral tissues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Crine P., Lemieux E., Fortin S., Seidah N. G., Lis M., Chrétien M. Expression of variant forms of proopiomelanocortin, the common precursor to corticotropin and beta-lipotropin in the rat pars intermedia. Biochemistry. 1981 Apr 28;20(9):2475–2481. doi: 10.1021/bi00512a018. [DOI] [PubMed] [Google Scholar]
- Crine P., Seidah N. G., Routhier R., Gossard F., Chrétien M. Processing of two forms of the common precursor to alpha-melanotropin and beta-endorphin in the rat pars intermedia. Evidence for and partial characterization of new pituitary peptides. Eur J Biochem. 1980 Sep;110(2):387–396. doi: 10.1111/j.1432-1033.1980.tb04879.x. [DOI] [PubMed] [Google Scholar]
- Dickerson I. M., Mains R. E. Cell-type specific posttranslational processing of peptides by different pituitary cell lines. Endocrinology. 1990 Jul;127(1):133–140. doi: 10.1210/endo-127-1-133. [DOI] [PubMed] [Google Scholar]
- Dubé D., Lissitzky J. C., Leclerc R., Pelletier G. Localization of alpha-melanocyte-stimulating hormone in rat brain and pituitary. Endocrinology. 1978 Apr;102(4):1283–1291. doi: 10.1210/endo-102-4-1283. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A. J., Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989 Oct 27;246(4929):482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Garrett J. E., Collard M. W., Douglass J. O. Translational control of germ cell-expressed mRNA imposed by alternative splicing: opioid peptide gene expression in rat testis. Mol Cell Biol. 1989 Oct;9(10):4381–4389. doi: 10.1128/mcb.9.10.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsuzawa K., Hosaka M., Nakagawa T., Nagase M., Shoda A., Murakami K., Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990 Dec 25;265(36):22075–22078. [PubMed] [Google Scholar]
- Hruby D. E., Thomas G., Herbert E., Franke C. A. Use of vaccinia virus as a neuropeptide expression vector. Methods Enzymol. 1986;124:295–309. doi: 10.1016/0076-6879(86)24022-7. [DOI] [PubMed] [Google Scholar]
- Lazure C., Seidah N. G., Pélaprat D., Chrétien M. Proteases and posttranslational processing of prohormones: a review. Can J Biochem Cell Biol. 1983 Jul;61(7):501–515. doi: 10.1139/o83-066. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Yeast KEX2 genes encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem Biophys Res Commun. 1988 Oct 14;156(1):246–254. doi: 10.1016/s0006-291x(88)80832-5. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gianoulakis C., Crine P., Lis M., Benjannet S., Routhier R., Chrétien M. In vitro biosynthesis and chemical characterization of beta-lipotropin, gamma-lipotropin, and beta-endorphin in rat pars intermedia. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3153–3157. doi: 10.1073/pnas.75.7.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Rochemont J., Hamelin J., Benjannet S., Chrétien M. The missing fragment of the pro-sequence of human pro-opiomelanocortin: sequence and evidence for C-terminal amidation. Biochem Biophys Res Commun. 1981 Sep 30;102(2):710–716. doi: 10.1016/s0006-291x(81)80190-8. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Smyth D. G., Zakarian S., Deakin J. F., Massey D. E. Beta-endorphin-related peptides in the pituitary gland: isolation, identification and distribution. Ciba Found Symp. 1981;81:79–96. doi: 10.1002/9780470720646.ch6. [DOI] [PubMed] [Google Scholar]
- Thorne B. A., Caton L. W., Thomas G. Expression of mouse proopiomelanocortin in an insulinoma cell line. Requirements for beta-endorphin processing. J Biol Chem. 1989 Feb 25;264(6):3545–3552. [PubMed] [Google Scholar]
- Uhler M., Herbert E., D'Eustachio P., Ruddle F. D. The mouse genome contains two nonallelic pro-opiomelanocortin genes. J Biol Chem. 1983 Aug 10;258(15):9444–9453. [PubMed] [Google Scholar]
- Warren T. G., Shields D. Expression of preprosomatostatin in heterologous cells: biosynthesis, posttranslational processing, and secretion of mature somatostatin. Cell. 1984 Dec;39(3 Pt 2):547–555. doi: 10.1016/0092-8674(84)90461-6. [DOI] [PubMed] [Google Scholar]
- van de Ven W. J., Voorberg J., Fontijn R., Pannekoek H., van den Ouweland A. M., van Duijnhoven H. L., Roebroek A. J., Siezen R. J. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990 Nov;14(4):265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., van Duijnhoven H. L., Keizer G. D., Dorssers L. C., Van de Ven W. J. Structural homology between the human fur gene product and the subtilisin-like protease encoded by yeast KEX2. Nucleic Acids Res. 1990 Feb 11;18(3):664–664. doi: 10.1093/nar/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]