Abstract
Nodals are signaling factors of the transforming growth factor-β (TGFβ) superfamily with a key role in vertebrate development. They control a variety of cell fate decisions required for the establishment of the embryonic body plan. We have identified two highly conserved transmembrane proteins, Nicalin and Nomo (Nodal modulator, previously known as pM5), as novel antagonists of Nodal signaling. Nicalin is distantly related to Nicastrin, a component of the Alzheimer's disease-associated γ-secretase, and forms a complex with Nomo. Ectopic expression of both proteins in zebrafish embryos causes cyclopia, a phenotype that can arise from a defect in mesendoderm patterning mediated by the Nodal signaling pathway. Accordingly, downregulation of Nomo resulted in an increase in anterior axial mesendoderm and the development of an enlarged hatching gland. Inhibition of Nodal signaling by ectopic expression of Lefty was rescued by reducing Nomo levels. Furthermore, Nodal- as well as Activin-induced signaling was inhibited by Nicalin and Nomo in a cell-based reporter assay. Our data demonstrate that the Nicalin/Nomo complex antagonizes Nodal signaling during mesendodermal patterning in zebrafish.
Keywords: Lefty, mesoderm, Nicastrin, Nodal, pM5
Introduction
The transforming growth factor-β (TGFβ) superfamily consists of a large number of structurally related, secreted proteins, which regulate a wide range of biological functions in developing and adult tissues including cell proliferation, differentiation, apoptosis and cell fate specification (Massague, 1998). In addition to their structural similarity, TGFβ ligands share a common signaling pathway, which includes the binding and activation of a serine/threonine kinase receptor complex, the phosphorylation and activation of intracellular signal transducers of the Smad family and the translocation of the Smad complex to the nucleus, where it associates with other transcriptional regulators to activate target gene transcription (Shi and Massague, 2003). Based on sequence similarity and the specific signaling pathway that they activate, the TGFβ proteins are divided into several subgroups including the Nodal subfamily, which plays a key role in the embryonic development of vertebrates (Schier and Shen, 2000; Schier, 2003). Nodals are required for processes as different as the formation of mesoderm and endoderm, positioning of the anterior–posterior body axis, specification of left–right symmetry and patterning of the nervous system. In contrast to other TGFβ ligands, Nodals require the presence of coreceptors of the EGF-CFC family in addition to TGFβ-type receptors to initiate signaling (Shen and Schier, 2000). The Nodal signal is then relayed to the nucleus, where specific transcription factors, most notably FoxHI (FAST2), are activated.
In the zebrafish, mesendoderm formation and patterning are primarily controlled by the two Nodal-related genes cyclops and squint (Schier, 2001; Whitman, 2001). cyclops;squint double mutants lack all endoderm and head and trunk mesoderm including notochord, heart, kidney, blood, liver, pancreas and gut (Feldman et al, 1998). Gene expression and fate map analyses have revealed that Nodals act before gastrulation to specify the mes- and endodermal progenitors. A lack of Nodal signaling causes the abnormal specification of these cells followed by their abnormal movement (Feldman et al, 2000; Carmany-Rampey and Schier, 2001). Instead of internalizing to give rise to mesoderm and endoderm, cells stay on the outside and contribute to neuroectoderm. The induction and patterning of mes- and endoderm by Nodals largely depend on graded signaling: different signal strengths are achieved by the generation of an activity gradient within a field of cells through the interaction of Nodals and Nodal inhibitors (Chen and Schier, 2002; Feldman et al, 2002; Solnica-Krezel, 2003). This provides positional information to each cell resulting in the establishment of different cell fates, with endoderm requiring the highest Nodal levels, followed by the anterior mesendoderm (prechordal plate) and mesoderm. Several extracellular and intracellular Nodal inhibitors have been identified including the Lefty proteins, divergent members of the TGFβ family (Schier, 2003). Leftys are considered classical feedback inhibitors, since their expression depends on Nodal signaling. They represent long-range inhibitors contributing to the generation of Nodal activity gradients (Chen and Schier, 2002; Feldman et al, 2002). Leftys can directly bind and inhibit Nodals or compete with them for binding to EGF-CFC coreceptors (Chen and Shen, 2004; Cheng et al, 2004). Other extracellular Nodal inhibitors like Cerberus, Coco and Charon belong to the DAN/Cerberus family (Balemans and Van Hul, 2002; Hashimoto et al, 2004). These proteins act by binding directly to Nodals inhibiting their interaction with the receptor. Intracellular Nodal inhibitors include tomoregulin-1, which might also inhibit EGF-CFC coreceptors (Harms and Chang, 2003), and DRAP, which binds FoxH1 and prevents its DNA binding (Iratni et al, 2002).
The Notch pathway is another signaling pathway regulating cell fate decisions during embryonic development (Selkoe and Kopan, 2003). Activation of Notch leads to its proteolytic processing by γ-secretase, a high-molecular-weight membrane protein complex that cleaves its substrates within their transmembrane domain (Haass, 2004). This complex also mediates the final step in the proteolytic processing of the amyloid precursor protein (APP) leading to the generation of the amyloid β-peptide (Aβ), which is deposited in the brains of Alzheimer's disease (AD) patients (Hardy and Selkoe, 2002; Sisodia and St George-Hyslop, 2002). Genetic and biochemical approaches led to the identification of four components of this complex, the Presenilins, Nicastrin, APH-1 and PEN-2 (Francis et al, 2002; Haass, 2004). Presenilin 1 and 2 are polytopic membrane proteins mutated in the majority of patients with early-onset familiar Alzheimer's disease (FAD) (Selkoe, 2001). They are believed to represent aspartyl proteases and provide the active site of the γ-secretase complex (Wolfe et al, 1999). Nicastrin, APH-1 and PEN-2 are required for complex assembly (Edbauer et al, 2002; Hu et al, 2002; Kimberly et al, 2002; Leem et al, 2002; Edbauer et al, 2003), but their precise role is unknown. γ-Secretase activity has been reconstituted in yeast by expressing all four components demonstrating that they represent the core proteins of the complex (Edbauer et al, 2003).
Nicastrin is a type 1 transmembrane protein (Yu et al, 2000) with a large extracytosolic domain containing an ∼200-amino-acid region, which is predicted to adopt a fold similar to the aminopeptidase (AP) domain found in several active aminopeptidases and the transferrin receptor (TfR) (Fagan et al, 2001). We have now identified Nicalin, a novel Nicastrin-related AP domain protein, and its binding partner Nomo (Nodal modulator, pM5) as components of a novel protein complex involved in Nodal signaling. In zebrafish embryos, Nicalin and Nomo modulate mesendodermal patterning and interfere with Lefty function. In a cell-based assay, they inhibit Nodal- as well as Activin-dependent signaling. We, therefore, suggest a role of the Nicalin/Nomo complex in antagonizing Nodal signaling during mesendodermal patterning in zebrafish.
Results
Nicalin is distantly related to Nicastrin and present in a high-molecular-weight complex
We searched current protein and genome databases for proteins homologous to the γ-secretase complex component Nicastrin. By using standard database comparison methods like BLAST, we found significant similarity only to Nicastrin orthologs from various organisms. In order to find more distantly related sequences, we searched the databases with generalized profiles (Bucher et al, 1996) constructed from the ectodomain of the Nicastrin family. This search yielded two highly significant matches (P<1E-5), the open reading frame (ORF) T05F1.1 from Caenorhabditis elegans and an unnamed human ORF with the SwissProt accession number Q96H48. These two sequences encode orthologs of a novel protein with the same phyletic distribution as Nicastrin (Figure 1A). Since this protein is predicted to represent a type I transmembrane protein and shares the same overall architecture with Nicastrin, we refer to it as Nicalin (Nicastrin-like protein). Significant sequence similarity is confined to a region of 180 residues (see Supplementary Figure), which roughly corresponds to the previously described aminopeptidase domain (Fagan et al, 2001). The dendrogram analysis shown in Figure 1A confirms that Nicastrin is the closest relative of Nicalin. Similar to Nicastrin, Nicalin lacks the amino-acid conservation required for catalytically active aminopeptidases.
Figure 1.

Nicalin is distantly related to Nicastrin and part of a high-molecular-weight complex unrelated to γ-secretase. (A) Phylogenetic tree showing that the Nicalins are more closely related to Nicastrins than to transferrin receptors and active aminopeptidases. NAALADL: N-acetyl-alpha-linked acidic dipeptidase-like protein; PSMA: prostate-specific membrane antigen; TFR: transferrin receptor; NCL: Nicalin; NCT: Nicastrin; HS: Homo sapiens; RN: Rattus novegicus; MM: Mus musculus; DM: Drosophila melanogaster; CE: C. elegans; AT: Arabidopsis thaliana. (B) Western blot of postnuclear (pns) and membrane (mem) protein fractions of cells expressing endogenous Nicalin (Sw and SH-SY5Y) or myc-tagged Nicalin (Sw/Ncl). Immunodetection with α-myc or α-Nicalin antibodies reveals a 60 kDa protein enriched in membrane fractions. (C) Immunoblot of Blue-Native gels of Sw and Sw/Ncl cell membrane protein extracts. Nicalin immunoreactivity is seen in a high-molecular-weight complex with a size similar to γ-secretase, which is visualized using an α-Presenilin 1 antibody (α-PS1). (D) Nicastrin and Nicalin were immunoprecipitated from Sw/Ncl cells and the bound material was analyzed by Western blotting. In Nicastrin immunoprecipitates (IP Nct), Presenilin N-terminal fragments (PS1-NT) and APH-1a were present, but Nicalin could not be detected. In Nicalin immunoprecipitates (IP Ncl), neither Nicastrin nor PS1-NT or APH-1a could be detected (band denoted with * is unspecific).
To analyze a putative role of Nicalin in the processing of APP and the generation of amyloid β-peptide (Aβ), we used human embryonic kidney 293 (HEK293)/APPSw (Sw) cells expressing the Swedish APP mutant (Citron et al, 1992) to generate a stable Nicalin-expressing cell line (Sw/Ncl). Immunoblotting of total protein extracts using an anti-myc or anti-Nicalin antibody resulted in the detection of a single band of about 60 kDa (Figure 1B), consistent with the calculated molecular weight of human Nicalin (63.7 kDa). Endogenous Nicalin levels were barely detectable in these lysates, but enriched in membrane preparations of HEK293 cells and SH-SY5Y human neuroblastoma cells (Figure 1B), suggesting that Nicalin is indeed a transmembrane protein expressed in neuronal and non-neuronal cells. Analysis of the levels of APP C-terminal fragments and of Aβ in Sw and Sw/Ncl cells did not reveal significant differences (data not shown), indicating that Nicalin is not involved in γ-secretase-mediated processing of APP (see below).
The γ-secretase complex can be isolated in its native form in DDM-solubilized membranes and detected as a high-molecular-weight band in Blue-Native polyacrylamide gel electrophoresis (BN-PAGE) by immunostaining with anti-bodies against complex components (Steiner et al, 2002). We used this method to analyze if Nicalin is present in a similar complex. Whereas in Sw cells endogenous Nicalin levels were too low to be detected in BN-PAGE, in Sw/Ncl cells a band of 500–550 kDa was visible, indicating the presence of Nicalin in a high-molecular-weight complex (Figure 1C). Similar to γ-secretase, the Nicalin complex is disrupted upon treatment with Triton X-100 (data not shown). We next examined if Nicalin interacts with known γ-secretase complex components by co-immunoprecipitation experiments using detergents that allow the isolation of the intact complex (Steiner et al, 2002). Whereas the well-established γ-secretase complex components Presenilin 1 and APH1a co-precipitated with Nicastrin as expected, they could not be detected in Nicalin immunoprecipitates (Figure 1D). Likewise, Nicalin did not co-precipitate with Nicastrin. In addition, overexpression of Nicalin failed to rescue the assembly of a functional γ-secretase complex in Nicastrin-RNAi cells (Edbauer et al, 2002) (data not shown). Taken together, these data show that Nicalin forms a complex distinct from γ-secretase.
Nicalin interacts with the 130 kDa protein Nomo (pM5)
To identify binding partners of Nicalin that may contribute to its native molecular weight, we immunoprecipitated Nicalin from DDM-solubilized membranes and separated the bound material by SDS–PAGE. Coomassie blue staining identified two bands of 60 and 130 kDa that were specifically enriched in material from Sw/Ncl cells (Figure 2A). The two bands were isolated, digested with trypsin and peptide mass fingerprints were generated by MALDI analysis. Whereas the 60 kDa band, as expected, corresponded to Nicalin, the 130 kDa band was identified as pM5, an uncharacterized protein that had emerged in a PCR-based screen for new members of the collagenase family, a subfamily of metalloproteases (Templeton et al, 1992). However, pM5 does not contain the HEXXH motif conserved in all metalloproteases and does not show any homology to collagenases. Instead, it appears to represent a type I transmembrane protein with no recognizable functional motif. Based on our data on pM5 function (see below), we refer to it as Nomo (Nodal modulator). To verify the interaction between Nicalin and Nomo, FLAG-tagged Nomo expressed in HEK293 cells was immunoprecipitated, and analysis of the bound material revealed co-precipitation of Nicalin (Figure 2B). These data suggest that Nomo is a major binding partner of Nicalin.
Figure 2.

Nomo (pM5) is a major binding partner of Nicalin. (A) Myc-tagged Nicalin was immunoprecipitated from Sw/Ncl cell membrane preparations and the bound proteins were visualized by Coomassie staining. Bands of molecular weight 60 and 130 kDa were specifically enriched in precipitates from Sw/Ncl cells. Peptide mass fingerprints generated by MALDI analysis identified these bands as Nicalin and pM5, which we refer to as Nomo (bands denoted with * represent immunoglobulin heavy and light chains). (B) FLAG-tagged Nomo was transfected into Sw/Ncl cells and immunoprecipitated. Western blot analysis of bound (b) and unbound (s) material revealed co-precipitation of Nicalin in Nomo-overexpressing cells (+), but not in control cells (−). (C) Northern blot analysis using full-length Nicalin and Nomo probes and 2 μg each of mRNA from various human tissues. Molecular weight markers in kilobases are shown on the left.
To examine the tissue distribution of Nicalin and Nomo, we performed Northern blot analysis with mRNA from various human tissues. Both proteins show an almost identical expression pattern, with highest mRNA levels in pancreas, skeletal muscle and, at somewhat lower levels, in heart (Figure 2C). After longer exposure, transcripts of both proteins were found in all tissues examined (data not shown). Whereas only one Nomo transcript of about 4.5 kb was detected, three different Nicalin mRNAs of 4.6, 4.0 and 2.3 kb exist, the sizes of which correspond roughly to cDNA sequences present in the databases. Since these sequences differ only in the length of their 3′ untranslated regions, the various Nicalin transcripts are most likely generated through alternative polyadenylation. In addition to the overlapping tissue distribution of Nicalin and Nomo, we also found colocalization of both proteins on the subcellular level. In iodixanol density gradients, endogenous Nicalin as well as Nomo cofractionized with endoplasmic reticulum (ER) membranes (data not shown). Taken together, these data supported a possible functional relationship between Nicalin and Nomo.
Ectopic expression of Nicalin and Nomo in zebrafish embryos causes cyclopia
The γ-secretase-mediated cleavage of Notch is a highly conserved process required for normal embryonic development (Selkoe and Kopan, 2003). The evolutionary conservation of Nicalin and Nomo in metazoans and plants indicated that the Nomo/Nicalin complex could have a similarly important role. To analyze its function in development, we chose the zebrafish system, which has been successfully used to recapitulate the defects in somite formation associated with the inhibition of Notch signaling (Geling et al, 2002). Database analysis revealed that the zebrafish genome contains two Nicalin homologs with 72 and 47% identity to human Nicalin and one Nomo homolog with 70% identity to human Nomo (named ncl1, ncl2 and nomo, respectively, according to the Zebrafish Nomenclature Committee). In situ hybridization demonstrated maternal expression as well as ubiquitous early zygotic expression of ncl1 and nomo, while ncl2 was not transcribed at appreciable levels during early embryogenesis (not shown). ncl1 is strongly and ubiquitously expressed at early stages of somitogenesis (Figures 3A and B). Later, prominent sites of expression include the developing retina, optic tectum and somites (Figure 3C) as well as endoderm (Figure 3C, arrowhead). nomo expression follows a very similar pattern at the 20-somite stage (Figure 3F). At earlier stages, nomo is most abundantly transcribed in the endoderm (Figures 3E and F, arrowheads) and anterior mesendoderm (hatching gland) (Figures 3D and E, arrows).
Figure 3.
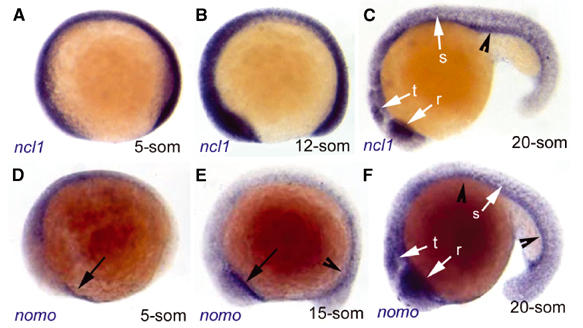
Zebrafish ncl1 and nomo are expressed in largely overlapping domains at somitogenesis stages. Expression of ncl1 (A–C) and nomo (D–F) revealed by whole-mount in situ hybridization (blue staining) at the stages indicated (anterior left, dorsal up). ncl1 expression is ubiquitous, with higher levels in the presumptive tectum (t), retina (r) and somites (s) (white arrows) and in the endoderm (black arrowhead) at 20 somites. nomo is transcribed at lower levels during earlier stages, with the most prominent expression in the anterior mesendoderm (black arrows) and endoderm (black arrowhead). At the 20-somite stage, nomo is expressed in a pattern very similar to ncl1.
The expression patterns of ncl1 and nomo are compatible with a function in embryonic development and we, therefore, analyzed the effects of ectopic expression of both proteins. Whereas injection of ncl1 or nomo capped RNA alone produced no discernible phenotype (not shown), simultaneous injection of both RNAs led to cyclopic embryos (27% of cases, n=26) (Figures 4B and B′), a phenotype known to arise through developmental failure of the basal forebrain (Blader and Strahle, 1998; Varga et al, 1999; Chow and Lang, 2001). Thus, when overexpressed, Nicalin and Nomo can collaborate in a process that impairs forebrain patterning, providing evidence for the existence of a functional Nomo/Nicalin complex.
Figure 4.
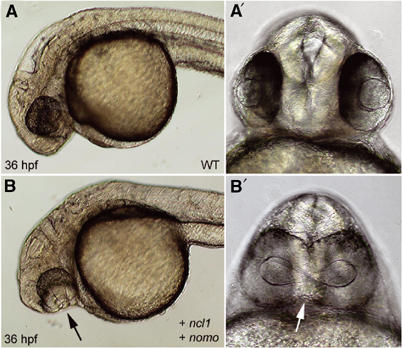
Coexpression of ncl1 and nomo impairs forebrain patterning leading to cyclopia. 36 hpf morphology of wild-type embryos (A, A′) and embryos coinjected with ncl1 and nomo capped RNAs (150 ng/μl each) at the one-cell stage (B, B′). Note the prominent cyclopia of the injected embryo (arrows).
Nomo is required to restrict the formation of anterior axial mesendoderm
Basal forebrain identity itself is induced by the underlying anterior axial mesendoderm (Beddington and Robertson, 1998; Rubenstein et al, 1998; Knoetgen et al, 1999; Kiecker and Niehrs, 2001). Thus, we next investigated whether endogenous Ncl1 and/or Nomo are required for mesendoderm patterning. Blocking ncl1 activity by the injection of antisense oligonucleotides (ncl1-grip) into wild-type zebrafish oocytes did not trigger a specific phenotype (data not shown). Blocking the production of Nomo protein using a nomo-grip, however, resulted in the development of massively enlarged hatching glands (Figures 5A–B′, arrows) (68% of cases, n=33). At the molecular level, nomo knockdown embryos display an increased number of gsc-, hgg1- and hlx1-positive cells, which mark the anterior axial mesendoderm (Figures 5C–H, arrows). Correlatively, the amount of immediately posterior axial mesendoderm (notochordal tissue), as revealed by znot expression, is reduced (81% of cases, n=26) (Figures 5I and J). The effect of blocking Nomo was dose dependent, as the amount of hgg1-positive cells was in inverse proportion to the concentration of the injected nomo-grip (data not shown). Importantly, the coinjection of a nomo* capped RNA that carries point mutations preventing the nomo-grip binding but encodes a wild-type Nomo protein rescues this phenotype (66% of cases, n=31) (Figures 5K and L), arguing for its specificity. Staining with the endoderm marker sox17 did not reveal differences between wild-type and nomo-grip-injected embryos, indicating that endoderm formation is not affected by Nomo (Figures 5M and N). The effect of nomo-grip injection on mesendoderm patterning was quantified by counting hgg1-positive cells of flat-mounted tail-bud-stage embryos (n=5): whereas in nomo-grip-injected embryos, the cell number (714±90) was threefold higher compared to the wild-type embryos (240±48), in embryos coinjected with nomo-grip and nomo* capped RNA, the number of hgg1-positive cells (279±60) was not increased. Thus, Nomo is required to limit the amount of anterior axial mesendoderm and allow proper axial mesendoderm patterning. Together with the above gain-of-function data, this suggests that Nomo and Ncl1 cooperate to regulate mesendoderm patterning in vivo. The failure to get a phenotype in ncl1 knockdown embryos might result from an incomplete downregulation of ncl1 expression or the presence of an ncl1-redundant factor.
Figure 5.
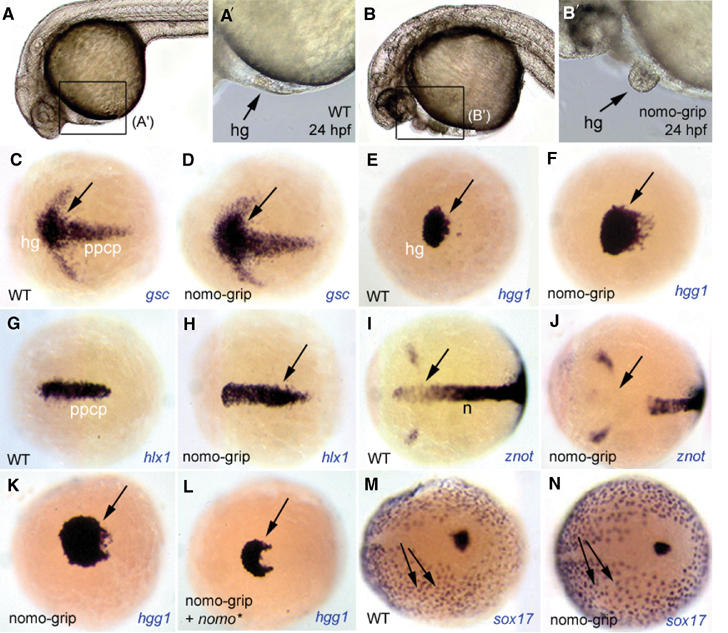
Nomo activity controls the extent of anterior mesendoderm. (A–B′) Morphology at 24 hpf of wild-type embryos (A, A′) and embryos injected with an antisense gripNA oligonucleotide blocking translation of endogenous nomo (nomo-grip) (B, B′). All embryos are anterior left, dorsal up; (A′) and (B′) are higher magnifications of the territories boxed in (A) and (B), respectively. Blocking Nomo activity leads to the formation of enlarged hatching glands, the most anterior mesendodermal derivative (arrows). (C–J) Expression of the markers indicated (bottom right of each panel) at the end of gastrulation (tail-bud stage) in wild-type (C, E, G, I) or nomo-grip-injected embryos (D, F, H, J). All panels are dorsal views, anterior left. The expression of anterior mesendodermal markers (gsc: presumptive hatching gland+prechordal plate; hgg1: presumptive hatching gland; hlx1: prechordal plate) is enlarged in injected embryos (C–H, arrows to the enlarged domains), while znot expression in the anterior notochordal domain is absent (I, J, arrows). (K, L) The number of hatching gland precursors, labeled with hgg1, is restored when nomo-grip is coinjected with a capped RNA encoding Nomo (nomo*) (L, compare with K and E, F). (M, N) Expression of the endodermal marker sox17 is similar in wild-type (M) and nomo-grip-injected embryos (N).
Nomo and Nicalin attenuate Nodal signaling
Patterning of the mes- and endoderm during vertebrate gastrulation is primarily controlled by Nodal signaling (Schier, 2001; Whitman, 2001). Specifically, increasing levels of Nodal account for the formation of mes- and endodermal derivatives of progressively more anterior character. Thus, ectopic expression of the Nodal inhibitor Lefty leads to the sequential disappearance of the hatching gland, posterior prechordal plate derivatives and notochord in a dose-dependent manner (Cheng et al, 2000; Thisse et al, 2000; Branford and Yost, 2002; Chen and Schier, 2002; Feldman et al, 2002). Therefore, we hypothesized that Nomo, like Lefty, might inhibit Nodal signaling. To test this hypothesis, we activated lefty and blocked nomo by the coinjection of lefty capped RNA and the nomo-grip. While expression of lefty alone severely reduced the number of hatching gland precursor cells (Figure 6A) (70% of cases, n=28), the concomitant inhibition of nomo function restored the cell number to a level similar to or higher than in wild-type controls (Figure 6B) (100% of cases, n=41). We, therefore, conclude that nomo controls mesendoderm patterning in vivo by interfering with Nodal activity (Figures 6C–F).
Figure 6.
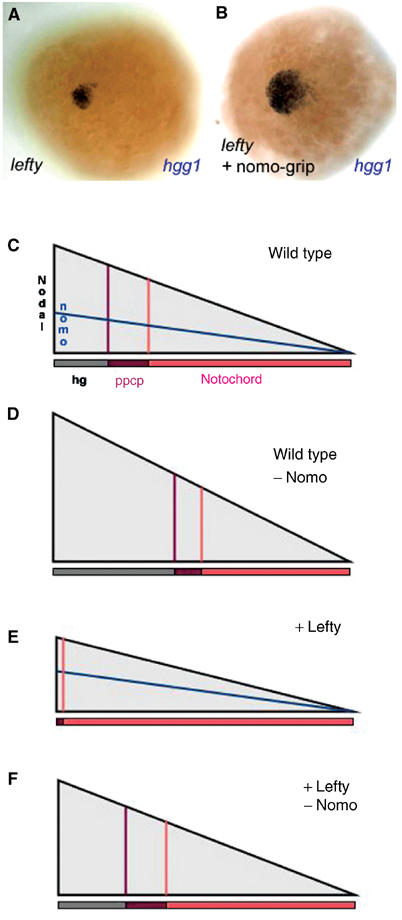
Nomo antagonizes Nodal signaling. Expression of the anterior mesendodermal marker hgg1 in embryos injected with 1 ng capped lefty RNA (A) or with lefty RNA and nomo-grip (B). Activation of Nodal signaling by Lefty reduces the number of hgg1-positive cells dramatically (compare to Figure 5E). Coinjection of the nomo-grip rescues this effect. (C–F) Model of the effects of lefty and nomo on Nodal signaling. In wild-type embryos, increasing levels of Nodal account for the generation of mesendodermal derivatives of progressively more anterior character (ppcp and hg) (C). Their formation is enhanced upon downregulation of nomo, indicating upregulated Nodal signaling (D). The endoderm, which requires highest Nodal activity, is not affected (not represented). Ectopic expression of lefty leads to inhibition of Nodal signaling and the formation of primarily posterior structures (n) (E). This effect can be reversed by simultaneously downregulating nomo (F). hg: presumptive hatching gland; n: notochord; ppcp: posterior part of the prechordal plate.
To demonstrate that the effect of Nomo (and possibly Nicalin) on Nodal signaling is direct, we used a reporter assay based on the A3-lux reporter construct that contains three tandem copies of the Nodal- and activin-responsive element from the Xenopus Mix2 promoter (Chen et al, 1996). This construct has been successfully used as a reporter of Nodal signaling in HEK293T cells (Yan et al, 2002). To reconstitute the Nodal pathway in these cells, we transfected Nodal, the EGF-CFC coreceptor Cripto and the transcription factor FAST2, all of which are not expressed endogenously. As shown before (Yan et al, 2002), this led to a strong increase in reporter activity (Figure 7A). Addition of increasing amounts of Nicalin or Nomo expression plasmids to the transfection mix resulted in a dose-dependent inhibition of Nodal signaling, whereas expression of Nicastrin had no effect. The reduction in reporter activity corresponded with Nicalin and Nomo expression levels as determined by immunoblotting (Figure 7A). Nicalin was about 10-fold more efficient than Nomo in this assay. This may reflect interaction of ectopic Nicalin with excess amounts of endogenous Nomo (see below). To exclude a possible effect of Nicalin and Nomo on the expression, processing or secretion of Nodal, we directly activated the pathway using a purified ligand. Activin is known to use the same receptors and intracellular signal transducers as Nodals except that they do not require a coreceptor of the EGF-CFC family. Accordingly, we observed reporter activation in 293T cells transfected with FAST2 only and treated with purified Activin (Figure 7B). Similar to Nodal signaling, Nicalin and Nomo were able to inhibit Activin signaling in a dose-dependent manner. The dose–response curves were almost identical in both assays, strongly suggesting that the mechanism of inhibition is the same. In cells transfected with both Nomo and Nicalin, we observed a more potent inhibition compared to cells expressing only one of the proteins (Figure 7C), similar to the situation in zebrafish embryos (see Figure 4). In summary, these data demonstrate a direct involvement of the Nicalin/Nomo complex in Nodal and Activin signaling.
Figure 7.

Nicalin and Nomo inhibit Nodal- and Activin-induced signaling in 293T cells. Transient transfection assays were performed with the A3-lux luciferase reporter plasmid containing three tandem copies of a Nodal-responsive element. Luciferase activity is shown in arbitrary units with each bar representing four measurements from two independent transfections. (A) Strong induction of luciferase activity was measured in cells transfected with expression plasmids encoding Nodal, Cripto and FAST2. Adding increasing amounts of plasmids encoding Nicalin or Nomo led to a dose-dependent reduction of the luciferase signal, which corresponded to Nicalin and Nomo protein levels as demonstrated by immunoblotting. Transfection of Nicastrin (Nct) had no effect. (B, C) Luciferase activity was induced by transfection of FAST2 and treatment of the cells with 20 ng/ml recombinant, purified Activin for 18–22 h. Again, Nicalin and Nomo cause a dose-dependent reduction of luciferase activity (B). The reduction of Activin-induced luciferase activity is enhanced in cells transfected with both Nicalin and Nomo, suggesting a synergistic effect (C).
Discussion
Nicalin is a component of a high-molecular-weight complex unrelated to γ-secretase
We have identified Nicalin, a novel transmembrane protein of the aminopeptidase/transferrin receptor (TfR) superfamily, distantly related to the γ-secretase component Nicastrin. Both proteins have a similar topology, are conserved in higher eukaryotes and can be found in high-molecular-weight membrane protein complexes. However, we did not detect interactions between Nicalin and the γ-secretase complex components Nicastrin, Presenilin 1 and APH-1a by co-immunoprecipitation. Moreover, we were unable to rescue the assembly of a functional γ-secretase complex in Nicastrin knockdown cells (Edbauer et al, 2002) by overexpressing Nicalin (data not shown). Finally, Nomo (pM5), a protein unrelated to γ-secretase, was identified as major Nicalin-binding partner and the Nicalin/Nomo complex was found to have a function different from γ-secretase (see below). Taken together, these data demonstrate that Nicalin is part of a novel membrane protein complex.
Nomo is a major Nicalin-binding protein
Our search for potential Nicalin complex components resulted in the detection of a 130 kDa protein in Nicalin immunoprecipitates from kidney cell membranes. Using mass spectrometry, this protein was identified as pM5, the cDNA of which had originally been cloned by PCR and suggested to represent a new member of the collagenase family (Templeton et al, 1992). However, no data were provided to support this assumption. Instead, pM5 does not show homology to any class of protein nor does it contain a known functional domain. Based on our analysis of pM5 function in vivo (see below), we, therefore, we refer to it as Nomo (Nodal modulator). The significance of the Nicalin/Nomo interaction is underscored by the following observations: both proteins are highly conserved from C. elegans to mammals and in plants, and their tissue distribution in humans is almost identical. In addition, Nicalin and Nomo show overlapping expression patterns during zebrafish embryogenesis. These data indicated that they interact functionally.
Nomo/Nicalin complex regulates mesendodermal patterning in the zebrafish by attenuating Nodal signaling
A possible functional relationship between Nicalin and Nomo was examined in the zebrafish model system, which is highly suitable for transient functional tests at embryonic stages. Interfering with Nomo and/or Nicalin 1 (Ncl1) expression levels failed to produce phenotypes related to Notch signaling deficiencies, pointing to the distinct functions of the γ-secretase and the Nomo/Nicalin complex in vivo. In contrast, we found that Nomo and Ncl1 collaborate to modulate the activity of another crucial signaling pathway driven by the TGFβ factor Nodal. Our arguments are threefold: (i) ectopic expression of Nomo and Ncl1, but not of each factor alone, interferes with midline development, a known Nodal target, (ii) blocking Nomo function increases in a dose-dependent manner the amount of anterior mesendodermal derivatives at the expense of more posterior ones, thus mimicking enhanced Nodal activity, and (iii) blocking Nomo function counteracts the effect of the Nodal inhibitor Lefty. Thus, we propose that the Nomo/Ncl1 complex is a new modulator of the Nodal pathway that acts dose-dependently to attenuate Nodal signaling and refine patterning of the mesendoderm (Figures 6C–F). This hypothesis was confirmed in a well-defined reporter assay system. Using a reporter specific for the Nodal/Activin family of TGFβ ligands, we could show that (i) the antagonistic effect of Nicalin and Nomo on Nodal signaling is direct and is not based on crosstalk with another pathway, (ii) the Nicalin/Nomo complex acts in a cell-auto-nomous manner, that is, it interferes with the signal transduction in the signal-receiving cell, and (iii) Nicalin and Nomo also block Activin signaling, suggesting that they do not affect the function of EGF-CFC coreceptors.
To date, and in spite of the involvement of Nodal signaling in multiple developmental processes, only a few down-modulators of this pathway have been identified. These can act as long-range secreted antagonists such as Lefty (Chen and Schier, 2002; Sakuma et al, 2002; Cheng et al, 2004), as membrane-bound competitors for binding to the Nodal coreceptor Cripto/One-eye-pinhead (Oep) (Harms and Chang, 2003) or as intracellular transcriptional repressors of Nodal targets (Yamamoto et al, 2001; Iratni et al, 2002; Bell et al, 2003). The ER localization of Nomo and Nicalin may suggest that this new inhibitory complex could act by yet another mechanism, possibly by modifying and/or trapping Nodal pathway components that route through the ER. The trafficking of various plasma membrane proteins has been shown to be regulated at the level of the ER (Ma and Jan, 2002). Recently, the ER-resident membrane proteins BAP29 and BAP31 were shown to be involved in the ER retention of membrane-bound IgD (Schamel et al, 2003), and it is conceivable that the Nomo/Nicalin complex might have a comparable function. It remains, however, important to determine the exact biochemical action of Nomo and Nicalin, as well as whether its activity extends to other developmental processes involving Nodal. The necessity for additional local cofactors is suggested by the nonresponse of endodermal precursors to Nomo/Nicalin.
Materials and methods
Cloning of Nicalin and Nomo cDNAs
Database searches for Nicastrin homologs were performed with a nonredundant data set constructed from current releases of SwissProt, TrEMBL and GenPept. Generalized profiles from the ectodomain of the Nicastrin family were constructed using the BLOSUM45 substitution matrix (Bucher et al, 1996) and default penalties of 2.1 for gap opening and 0.2 for gap extension. The searches were run locally using the pftools package, version 2.1 (program available from the URL ftp://ftp.isrec.isb-sib.ch/sib-isrec/pftools/). Human Nomo and zebrafish sequences were identified using BLAST searches against the GenBank database or the zebrafish EST database at the NCBI, respectively. All cDNA clones described were obtained from the German Resource Center for Genome Research (RZPD). For expression studies, full-length human Nicalin (acc. no. BG422523) and human Nomo (acc. no. AL832855) were subcloned into the pCDNA4/TO/myc-His vector (Invitrogen).
Antibodies
The polyclonal anti-Nicalin antibody 2221 was generated by Eurogentech (Seraing, Belgium) by immunizing rabbits with a mixture of two peptides (LESHRDGQRSSIMDV, NQPRAAQLVDK DSTF) of the ectodomain of human Nicalin. The following other antibodies were used: monoclonal α-myc (9E10, ATCC), monoclonal α-FLAG (Kodak), polyclonal α-Nicastrin (N1660, Sigma), monoclonal α-Presenilin 1 (Capell et al, 1997), polyclonal α-calnexin (Stressgen) and polyclonal α-APH1a (K Shirotani and C Haass, unpublished).
Cell lines and transfection
HEK293 cells and SH-SY5Y neuroblastoma were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 1% penicillin/streptomycin and 2 mM L-glutamine. HEK293 cells overexpressing the APPSw mutant (Sw) (Citron et al, 1992) and HEK293/TR cells (Invitrogen) were transfected using Lipofectamine 2000 (Invitrogen) and selected with the appropriate antibiotics.
Blue-Native PAGE and immunoprecipitation
Membrane proteins for Blue-Native PAGE were extracted as described (Edbauer et al, 2002) and subjected to immunoprecipitation or analyzed on 4–12.5 or 4–16% Blue-Native gels as described (Schagger and von Jagow, 1991). Immunoprecipitation was performed as described (Edbauer et al, 2002).
MALDI analysis
Coomassie-stained gel bands were excised from the gel, washed with water, shrinked twice with 50% acetonitrile and air dried for 5 min. Proteins were trypsinized for 3 min with 0.2 μg/μl modified trypsin solution (Promega) and trypsin fragments were eluted overnight with 40 mM ammonium bicarbonate solution (pH 7.8), concentrated using ZipTips (Millipore), bound onto the reversed phase material, washed and eluted with a saturated matrix (α-cinnamic acid) solution (0.3% TFA, 50% acetonitrile) directly onto the stainless steel target plate. Mass spectra were recorded on a Voyager DE STR (Applied Biosystems) according to the manufacturer's instructions. Mass spectra were calibrated internally with the autolysis products of porcine trypsin with a mass of 842.51 and 2211.1046 Da. Mass spectra were processed, trypsin peaks was removed and the peak list was submitted to Mascot (Matrixscience) for database searching and protein identification. Database searches were performed with a mass tolerance of 50 ppm or less after internal calibration.
Northern blot analysis
Nicalin and Nomo probes were generated using [32P]dCTP (Amersham Biosciences), the Random Primers DNA Labeling System (Invitrogen) and human cDNAs as templates. A human multiple tissue Northern blot (Clontech) was hybridized to the probes according to the manufacturer's directions and exposed to Super RX film (Fuji) for 4–16 h.
Fish strains
Wild-type embryos were obtained from natural spawning of AB adults, and raised and staged according to Kimmel et al (1995).
Antisense experiments
The antisense oligonucleotides (gripNAs) for ncl1 (5′-ACCAGCCTCCTCGAACAT-3′) and nomo (5′-TTTAATTCCACCCATCGT-3′) were purchased from Active Motifs Europe (Brussels, Belgium), dissolved to a stock concentration of 1 mM in H2O and pressure-injected into 1-cell-stage embryos at 0.5 or 1 mM using an Eppendorf microinjector.
Capped RNA injections
For in vitro transcription experiments, a full-length zebrafish ncl1 clone (ncl1) was generated by PCR (forward primer: 5′-GCGAATTCTCCAGCATGTTTGAAGAAGCGGGCGAG GTGTTGGAG-3′;reverse primer: 5′-GCGTCGACTCAGTGCTGTTTGACC-3′) using ncl1 (acc. no. BI472741) as template and subcloned into the pCS2+ vector. A full-length nomo clone was generated by subcloning human nomo (pM5) (acc. no. AL832855) into the pCS2+ vector. For the rescue experiment, a gripNA-resistant version of zebrafish nomo (nomo*) was constructed in the pXT7 vector using an N-terminal fragment (acc. no. BM859276) modified by PCR (forward primer: 5′-GCGGTACCGCCACCATGGGAGGTATAAAGGAGCTA GCAATTCTT-3′; reverse primer: 5′-GCGAATTCGATATCCTCCTTCGTTAC-3′), a central fragment obtained by PCR (forward primer: 5′-AGTGTCTTCACGCATGCTGGAG-3′; reverse primer: 5′-GCCCAATAGGAGAATTCATAGT-3′) from tail-bud-stage cDNA and a C-terminal fragment (acc. no. BI887091). lefty encodes a soluble antagonist of Nodal signaling and was described previously (Thisse and Thisse, 1999). All capped RNAs were synthesized using the mMessage mMachine kit (Ambion) following the manufacturer's instructions, resuspended in H2O and injected at the following concentrations: ncl1*, nomo and nomo* RNA at 100–150 ng/μl and lefty RNA at 1 ng/μl.
Whole-mount in situ hybridization
In situ hybridization was carried out on embryos according to standard protocols (Hammerschmidt et al, 1996). The following marker probes were used: gsc, hgg1 (Thisse et al, 1994), hlx1 (Seo et al, 1999), znot (Melby et al, 1996) and sox17 (Alexander and Stainier, 1999).
Nodal reporter assay
The luciferase reporter assay in 293T cells was performed essentially as described (Yan et al, 2002). A total of 2.5 × 105 cells were plated on polylysine-coated 24-well plates, grown overnight and transfected with 200 ng each of A3-lux (Chen et al, 1996), pcDNA3-Nodal, pcDNA3/FLAG-Cripto, pcDNA3/myc-FAST2 (Yan et al, 2002) and varying amounts of pcDNA4/myc-Nicalin or pcDNA4/myc-Nomo using Lipofectamine 2000 (Invitrogen). After 22–24 h, cells were washed once with PBS and lysed in 25 mM glycyl-glycine, pH 7.8, 10 mM MgSO4, 10 mM dithiothreitol and 0.2% N-P40. After removal of the insoluble material, 20 μl cell lysate was mixed with 100 μl assay buffer (25 mM glycyl-glycine, pH 7.8, 10 mM MgSO4, 10 mM dithiothreitol, 1 mM ATP, 35 μM D-luciferin) and luminescence was measured in a Wallac Victor 1420 luminometer.
Supplementary Material
Supplementary Figure
Acknowledgments
We thank F Rosa for the lefty and Sox17 cDNAs, M Shen for the Nodal, Cripto and FAST2 cDNAs, M Whitman for the A3-lux reporter plasmid, B and C Thisse for hgg1 and gsc cDNA and C Kimmel for znot cDNA. We are grateful to M Rex-Haffner and T Weiler for technical support, B Schmid for help with the analysis of the injection experiments and F Rosa, R Rupp, C Kaether and M Willem for comments on the manuscript. This work was supported by Deutsche Forschungsgemeinschaft (Leibniz-preis, SFB596 and Priority Program ‘Cellular Mechanisms of Alzheimer's Disease' to C Haass and grant BA2024/2-1 to L Bally-Cuif) and by VolkswagenStiftung (‘junior research grant' to L Bally-Cuif).
References
- Alexander J, Stainier DY (1999) A molecular pathway leading to endoderm formation in zebrafish. Curr Biol 9: 1147–1157 [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W (2002) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250: 231–250 [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ (1998) Anterior patterning in mouse. Trends Genet 14: 277–284 [DOI] [PubMed] [Google Scholar]
- Bell E, Munoz-Sanjuan I, Altmann CR, Vonica A, Brivanlou AH (2003) Cell fate specification and competence by Coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development 130: 1381–1389 [DOI] [PubMed] [Google Scholar]
- Blader P, Strahle U (1998) Casting an eye over cyclopia. Nature 395: 112–113 [DOI] [PubMed] [Google Scholar]
- Branford WW, Yost HJ (2002) Lefty-dependent inhibition of Nodal- and Wnt-responsive organizer gene expression is essential for normal gastrulation. Curr Biol 12: 2136–2141 [DOI] [PubMed] [Google Scholar]
- Bucher P, Karplus K, Moeri N, Hofmann K (1996) A flexible motif search technique based on generalized profiles. Comput Chem 20: 3–23 [DOI] [PubMed] [Google Scholar]
- Capell A, Saffrich R, Olivo JC, Meyn L, Walter J, Grünberg J, Mathews P, Nixon R, Dotti C, Haass C (1997) Cellular expression and proteolytic processing of presenilin proteins is developmentally regulated during neuronal differentiation. J Neurochem 69: 2432–2440 [DOI] [PubMed] [Google Scholar]
- Carmany-Rampey A, Schier AF (2001) Single-cell internalization during zebrafish gastrulation. Curr Biol 11: 1261–1265 [DOI] [PubMed] [Google Scholar]
- Chen C, Shen MM (2004) Two modes by which lefty proteins inhibit nodal signaling. Curr Biol 14: 618–624 [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M (1996) A transcriptional partner for MAD proteins in TGF-beta signalling. Nature 383: 691–696 [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF (2002) Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol 12: 2124–2128 [DOI] [PubMed] [Google Scholar]
- Cheng AM, Thisse B, Thisse C, Wright CV (2000) The lefty-related factor Xatv acts as a feedback inhibitor of nodal signaling in mesoderm induction and L–R axis development in Xenopus. Development 127: 1049–1061 [DOI] [PubMed] [Google Scholar]
- Cheng SK, Olale F, Brivanlou AH, Schier AF (2004) Lefty blocks a subset of TGFbeta signals by antagonizing EGF-CFC coreceptors. PLoS Biol 2: E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA (2001) Early eye development in vertebrates. Annu Rev Cell Dev Biol 17: 255–296 [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672–674 [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Haass C, Steiner H (2002) Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc Natl Acad Sci USA 99: 8666–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C (2003) Reconstitution of γ-secretase activity. Nat Cell Biol 5: 486–488 [DOI] [PubMed] [Google Scholar]
- Fagan R, Swindells M, Overington J, Weir M (2001) Nicastrin, a presenilin-interacting protein, contains an aminopeptidase/transferrin receptor superfamily domain. Trends Biochem Sci 26: 213–214 [DOI] [PubMed] [Google Scholar]
- Feldman B, Concha ML, Saude L, Parsons MJ, Adams RJ, Wilson SW, Stemple DL (2002) Lefty antagonism of squint is essential for normal gastrulation. Curr Biol 12: 2129–2135 [DOI] [PubMed] [Google Scholar]
- Feldman B, Dougan ST, Schier AF, Talbot WS (2000) Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr Biol 10: 531–534 [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS (1998) Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395: 181–185 [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch RD, Ruble C, Nye JS, Curtis D (2002) aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev Cell 3: 85–97 [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C (2002) A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep 3: 688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C (2004) Take five-BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C (1996) dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123: 95–102 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- Harms PW, Chang C (2003) Tomoregulin-1 (TMEFF1) inhibits nodal signaling through direct binding to the nodal coreceptor Cripto. Genes Dev 17: 2624–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T (2004) The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left–right patterning in zebrafish. Development 131: 1741–1753 [DOI] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME (2002) Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev Cell 2: 69–78 [DOI] [PubMed] [Google Scholar]
- Iratni R, Yan YT, Chen C, Ding J, Zhang Y, Price SM, Reinberg D, Shen MM (2002) Inhibition of excess nodal signaling during mouse gastrulation by the transcriptional corepressor DRAP1. Science 298: 1996–1999 [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) The role of prechordal mesendoderm in neural patterning. Curr Opin Neurobiol 11: 27–33 [DOI] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ (2002) Complex N-linked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J Biol Chem 277: 35113–35117 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310 [DOI] [PubMed] [Google Scholar]
- Knoetgen H, Teichmann U, Kessel M (1999) Head-organizing activities of endodermal tissues in vertebrates. Cell Mol Biol (Noisy-le-grand) 45: 481–492 [PubMed] [Google Scholar]
- Leem JY, Vijayan S, Han P, Cai D, Machura M, Lopes KO, Veselits ML, Xu H, Thinakaran G (2002) Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J Biol Chem 277: 19236–19240 [DOI] [PubMed] [Google Scholar]
- Ma D, Jan LY (2002) ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol 12: 287–292 [DOI] [PubMed] [Google Scholar]
- Massague J (1998) TGF-beta signal transduction. Annu Rev Biochem 67: 753–791 [DOI] [PubMed] [Google Scholar]
- Melby AE, Warga RM, Kimmel CB (1996) Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 122: 2225–2237 [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L (1998) Regionalization of the prosencephalic neural plate. Annu Rev Neurosci 21: 445–477 [DOI] [PubMed] [Google Scholar]
- Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H (2002) Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells 7: 401–412 [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199: 223–231 [DOI] [PubMed] [Google Scholar]
- Schamel WW, Kuppig S, Becker B, Gimborn K, Hauri HP, Reth M (2003) A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum. Proc Natl Acad Sci USA 100: 9861–9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF (2001) Axis formation and patterning in zebrafish. Curr Opin Genet Dev 11: 393–404 [DOI] [PubMed] [Google Scholar]
- Schier AF (2003) Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol 19: 589–621 [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM (2000) Nodal signalling in vertebrate development. Nature 403: 385–389 [DOI] [PubMed] [Google Scholar]
- Selkoe D, Kopan R (2003) Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26: 565–597 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741–766 [DOI] [PubMed] [Google Scholar]
- Seo HC, Nilsen F, Fjose A (1999) Three structurally and functionally conserved Hlx genes in zebrafish. Biochim Biophys Acta 1489: 323–335 [DOI] [PubMed] [Google Scholar]
- Shen MM, Schier AF (2000) The EGF-CFC gene family in vertebrate development. Trends Genet 16: 303–309 [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700 [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH (2002) gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci 3: 281–290 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L (2003) Vertebrate development: taming the nodal waves. Curr Biol 13: R7–R9 [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C (2002) PEN-2 is an integral component of the γ-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem 277: 39062–39065 [DOI] [PubMed] [Google Scholar]
- Templeton NS, Rodgers LA, Levy AT, Ting KL, Krutzsch HC, Liotta LA, Stetler-Stevenson WG (1992) Cloning and characterization of a novel human cDNA that has DNA similarity to the conserved region of the collagenase gene family. Genomics 12: 175–176 [DOI] [PubMed] [Google Scholar]
- Thisse B, Wright CV, Thisse C (2000) Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature 403: 425–428 [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B (1999) Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development 126: 229–240 [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Halpern ME, Postlethwait JH (1994) Goosecoid expression in neurectoderm and mesendoderm is disrupted in zebrafish cyclops gastrulas. Dev Biol 164: 420–429 [DOI] [PubMed] [Google Scholar]
- Varga ZM, Wegner J, Westerfield M (1999) Anterior movement of ventral diencephalic precursors separates the primordial eye field in the neural plate and requires cyclops. Development 126: 5533–5546 [DOI] [PubMed] [Google Scholar]
- Whitman M (2001) Nodal signaling in early vertebrate embryos: themes and variations. Dev Cell 1: 605–617 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ (1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398: 513–517 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Meno C, Sakai Y, Shiratori H, Mochida K, Ikawa Y, Saijoh Y, Hamada H (2001) The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior–posterior patterning and node formation in the mouse. Genes Dev 15: 1242–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YT, Liu JJ, Luo Y, Chaosu E, Haltiwanger RS, Abate-Shen C, Shen MM (2002) Dual roles of Cripto as a ligand and coreceptor in the nodal signaling pathway. Mol Cell Biol 22: 4439–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407: 48–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure


