Abstract
The spindle checkpoint inhibits anaphase until all kinetochores have attached properly to spindle microtubules. The protein kinase Bub1 is an essential checkpoint component that resides at kinetochores during mitosis. It is shown herein that Xenopus Bub1 becomes hyperphosphorylated and the kinase is activated on unattached chromosomes. MAP kinase (MAPK) contributes to this phosphorylation, as inhibiting MAPK or altering MAPK consensus sites in Bub1 to alanine or valine (Bub15AV) abolishes the phosphorylation and activation on chromosomes. Both Bub1 and Bub15AV support the checkpoint under an optimal condition for spindle checkpoint activation. However, Bub1, but not Bub15AV, supports the checkpoint at a relatively low concentration of nuclei or the microtubule inhibitor nocodazole. Similar to Bub15AV, Bub1 without the kinase domain (Bub1ΔKD) is also partially compromised in its checkpoint function and in its ability to recruit other checkpoint proteins to kinetochores. This study suggests that activation of Bub1 at kinetochores enhances the efficiency of the spindle checkpoint and is probably important in maintaining the checkpoint toward late prometaphase when the cell contains only a few or a single unattached kinetochore.
Keywords: Bub1, kinetochore, MAP kinase, phosphorylation, spindle checkpoint
Introduction
The metaphase-to-anaphase transition occurs only after all chromosomes have established bipolar attachment to the mitotic spindle. The spindle checkpoint delays anaphase onset when any kinetochore in the cell is not properly bound with the spindle microtubules or when kinetochores are not under tension normally produced by bipolar attachment (Li and Nicklas, 1995; Rieder et al, 1995). Activation of the checkpoint involves kinetochore localization of several spindle checkpoint proteins, including Mad1, Mad2, Bub1, BubR1 (Mad3 in yeast), Bub3, MAPK, and Mps1 (Cleveland et al, 2003), leading to the complex formation between Mad2, BubR1, Bub3, and Cdc20 (Hardwick et al, 2000; Fraschini et al, 2001; Zhang and Lees, 2001; Chen, 2002). When sequestered by the checkpoint proteins, Cdc20 is unable to activate the anaphase-promoting complex (APC), an E3 ubiquitin protein ligase that triggers degradation of several mitotic regulators, including the anaphase inhibitor Pds1 (also named securin) (Peters, 2002). Once the spindle checkpoint signal is terminated, Cdc20 is then able to direct APC toward Pds1. Degradation of Pds1 frees the protease Esp1 that in turn cleaves cohesin complex, allowing sister chromatids to separate (Peters, 2002). In this way, the spindle checkpoint delays anaphase onset until all kinetochores are properly attached to spindle microtubules.
Kinetochore localization of the spindle checkpoint proteins is regulated in different ways. Microtubules binding to kinetochores dissociate Mad1 and Mad2 from kinetochores (Chen et al, 1996, 1998; Li and Benezra, 1996; Waters et al, 1998), whereas Bub1, BubR1, Bub3, and Mps1 remain at kinetochores until early anaphase (Taylor and McKeon, 1997; Jablonski et al, 1998; Abrieu et al, 2001; Sharp-Baker and Chen, 2001). Both Bub1 and BubR1 bind to Bub3 throughout the cell cycle, and the interaction is important for kinetochore localization of all three proteins (Basu et al, 1998; Taylor et al, 1998; Sharp-Baker and Chen, 2001; Chen, 2002). Mad1 binds tightly to Mad2 during interphase and mitosis, and it recruits Mad2 to kinetochores (Chen et al, 1998; Chung and Chen, 2002). In vertebrate cells, a small fraction of MAPK is activated and enriched at kinetochores during mitosis, and the level of active MAPK at kinetochores decreases at and after metaphase (Shapiro et al, 1998; Zecevic et al, 1998). MAPK is important for the spindle checkpoint in egg extracts and in somatic cells (Minshull et al, 1994; Wang et al, 1997; Chung and Chen, 2003).
Recruitment of the checkpoint proteins to unattached kinetochores may stimulate the interaction between Cdc20 and the checkpoint proteins through their close proximity. Alternatively, checkpoint proteins may become activated upon binding to kinetochores, thus allowing their interaction with Cdc20. In either case, the unattached kinetochore may continuously generate and release the checkpoint complex that binds and inhibits Cdc20. Indeed, fluorescence recovery after photobleaching (FRAP) analysis shows that both Mad2 and Cdc20 bind kinetochores with a fast turnover rate (Howell et al, 2000; Kallio et al, 2002).
Despite the extensive studies on the order of kinetochore association of the checkpoint proteins, not much is known about the regulation and function of each checkpoint protein at kinetochores. Four of the proteins are protein kinases: MAPK, Mps1, Bub1, and BubR1. It has been shown that MAPK interacts with the kinetochore motor protein CENP-E and that MAPK phosphorylates CENP-E in vitro to create a tension-sensitive epitope that is recognized by the 3F3 monoclonal antibody (Zecevic et al, 1998). However, the physiological relevance of CENP-E phosphorylation remains to be determined. MAPK also contributes to Cdc20 phosphorylation, and this phosphorylation is required for the checkpoint proteins to bind and inhibit Cdc20 (Chung and Chen, 2003). Mps1 is known to be the upstream kinase for Mad1 in budding yeast (Hardwick et al, 1996), but a similar function has not been demonstrated in metazoans. Bub1 is a serine/threonine protein kinase and is required for recruitment of Mad1, Mad2, Bub3, and CENP-E to kinetochores (Sharp-Baker and Chen, 2001). BubR1, a protein kinase with some homology with Bub1, is involved in kinetochore localization of Mad1, Mad2, Bub1, Bub3, and CENP-E (Chen, 2002). Both Bub1 and BubR1 are phosphoproteins (Farr and Hoyt, 1998; Chan et al, 1999; Sharp-Baker and Chen, 2001; Chen, 2002; Yamaguchi et al, 2003). Immunoblot analysis of chromosomes isolated from Xenopus egg extracts shows that Bub1 and BubR1 are phosphorylated at unattached kinetochores to a much greater extent than their cytosolic counterparts (Chen, 2002). Interestingly, the hyperphosphorylation requires Mad1, even though Mad1 is dispensable for kinetochore binding of Bub1 and BubR1 (Chen, 2002). In this study, the role of Bub1 phosphorylation at kinetochores in the regulation of the kinase activity and the checkpoint function of Bub1 is studied.
Results
Bub1 is hyperphosphorylated and activated at unattached kinetochores
To analyze the biochemical regulation of spindle checkpoint proteins associated with kinetochores, chromosomes assembled in Xenopus egg extracts were isolated through a sucrose cushion. Immunoblot analysis showed higher levels of Bub1 and BubR1 in chromosomal fractions prepared from extracts treated with the microtubule-disrupting agent nocodazole than those from untreated extracts (Figure 1, lanes 7 and 8), consistent with previous immunofluorescence studies showing an increased amount of Bub1 and BubR1 at unattached kinetochores (Sharp-Baker and Chen, 2001; Chen, 2002). If chromosomes were isolated in the absence of the protein phosphatase inhibitor microcystin, the electrophoretic mobility of Bub1 and BubR1 in the chromosomal fraction was similar to that of their cytosolic counterparts regardless of whether the samples were treated with nocodazole or not (Figure 1, lanes 7 and 8). Interestingly, in the presence of microcystin, Bub1 and BubR1 from nocodazole-treated samples gave a marked mobility shift (Figure 1, lane 11), which was due to phosphorylation (Chen, 2002). Without nocodazole, chromosomal Bub1 and BubR1 also showed slower mobility, compared to the cytosolic proteins (Figure 1, compare lanes 4 and 10), but the mobility shift was much less extensive than that from nocodazole-treated samples (Figure 1, compare lanes 10 and 11). On the other hand, microcystin had no effect on the mobility of cytosolic Bub1 and BubR1 that remained on top of the sucrose cushion (Figure 1, lanes 1–6), indicating that the effect of microcystin was specific to chromosome-bound Bub1 and BubR1. These results suggest that Bub1 and BubR1 at kinetochores are very sensitive to protein phosphatase and they lose their phosphorylation even in the presence of the general phosphatase inhibitors β-glycerophosphate and sodium vanadate. When chromosomes were isolated from extracts treated with the microtubule-stabilizing drug taxol, Bub1 and BubR1 did not show the same mobility shift as that from the nocodazole-treated sample (Figure 1, lane 12). This result indicates that hyperphosphorylation of Bub1 and BubR1 is induced by a lack of microtubule occupancy at kinetochores.
Figure 1.
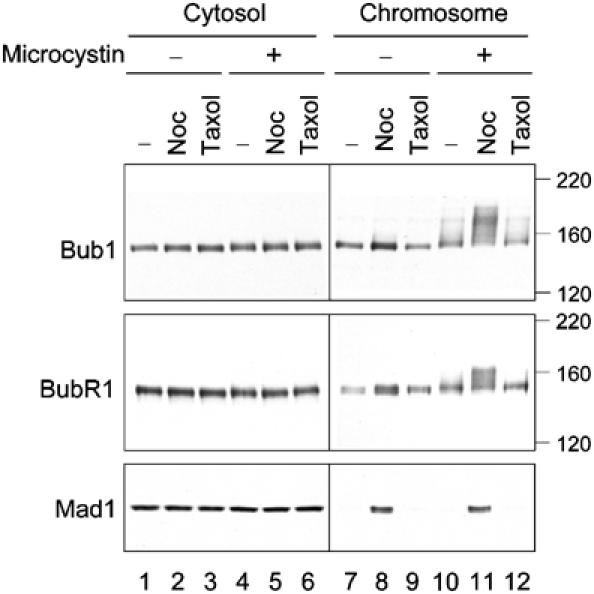
Bub1 and BubR1 are hyperphosphorylated at unattached kinetochores. Egg extracts containing mitotic chromosomes were left untreated (lanes 1, 4, 7, and 10), or treated with nocodazole (lanes 2, 5, 8, and 11) or taxol (lanes 3, 6, 9, and 12). The samples were separated into cytosolic (lanes 1–6) and chromosomal (lanes 7–12) fractions and immunoblotted for Bub1, BubR1, and Mad1 as indicated. Microcystin was omitted (lanes 1–3 and 7–9) or included (lanes 4–6 and 10–12) in the solutions during chromosome isolation. The migration of molecular size standards is indicated on the right.
Microcystin had no effect on the electrophoretic mobility of Mad1 (Figure 1, compare lanes 8 and 11), whose homolog in budding yeast is phosphorylated during mitosis and under checkpoint-active conditions (Hardwick and Murray, 1995). Immunoblot of Mad1 showed very little protein in metaphase chromosomes (Figure 1, lanes 7 and 10), consistent with a previous immunofluorescence study (Chen et al, 1998). Microcystin had no apparent effect on Mad1, Mad2, and Bub3 at a concentration of up to 2 μM (data not shown).
It was then examined whether hyperphosphorylation of Bub1 affects the kinase activity. There was little autophosphorylation in Bub1 immunoprecipitated from egg extracts or from chromosomal fractions that were prepared in the absence of microcystin (Figure 2, lanes 1–3). The phosphorylated protein had a mass of about 150 kDa, similar to the size of the protein detected by immunoblot (Figure 2, lanes 1–3). The autophosphorylation activity was enhanced if the chromosomes were isolated in the presence of microcystin (Figure 2, compare lanes 3 and 4), and was even more pronounced from the nocodazole-treated extract (Figure 2, compare lanes 4 and 5). The phosphorylated protein had a mass of about 190–200 kDa, which was indeed Bub1, rather than a Bub1-associated protein, because the denatured protein can be re-immunoprecipitated with anti-Bub1 antibodies (Figure 2, lanes 9 and 10). This result suggests that hyperphosphorylation of Bub1 correlates with the activation of its kinase activity.
Figure 2.
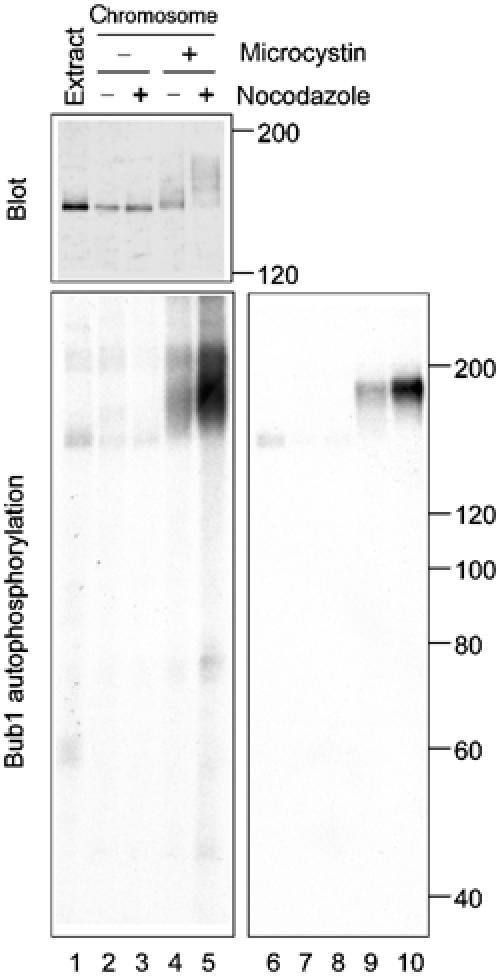
Hyperphosphorylation of Bub1 correlates with its activation at unattached kinetochores. Chromosomes were isolated under conditions indicated on the top and chromosomal proteins were eluted for immunoprecipitation with anti-Bub1 antibody. Lanes 1 and 6 are Bub1 immunoprecipitated from the CSF-arrested extract. Top panel, immunoblot of Bub1 in the immunoprecipitates; lower panel, an autoradiogram of in vitro phosphorylation reaction of Bub1 immunoprecipitates. Lanes 6–10, the denatured samples in lanes 1–5 were re-immunoprecipitated with anti-Bub1 antibody. The migration of molecular size standards is indicated on the right.
MAPK contributes to Bub1 phosphorylation
Bub1 contains several Ser/Thr-Pro sequences that are potential phosphorylation sites for MAPK or Cdc2. To determine whether MAPK is involved in hyperphosphorylation of Bub1 and BubR1 at kinetochores, the MAPK kinase (MEK) inhibitor U0126 is used to block the phosphorylation and activation of MAPK in egg extracts (Figure 3A, lanes 3 and 4). The bulk of Bub1 and BubR1 in the U0126-treated extract showed a slightly lower degree of phosphorylation compared to that in the untreated extract (Figure 3A, lanes 1 and 2). Immunoblot of the chromosomal fractions showed that U0126 almost completely abolished Bub1 and BubR1 phosphorylation (Figure 3A, compare lanes 6 and 8). To determine if MAPK or its downstream target Rsk was involved in the phosphorylation, the effect of MAPK and Rsk immunodepletion on the phosphorylation is studied. Figure 3B shows that depletion of MAPK diminished Bub1 and BubR1 phosphorylation (lanes 9 and 10), whereas immunodepletion of Rsk had no effect (lanes 11 and 12). These results indicate that MAPK, but not Rsk, contributes to Bub1 and BubR1 phosphorylation at kinetochores. Interestingly, Rsk was present in the chromosomal fractions in a nocodazole-independent manner (Figure 3B, lanes 7 and 8). Upon MAPK depletion, Rsk lost phosphorylation (Figure 3B, lanes 3 and 4) and was no longer detectable in the chromosomal fractions (Figure 3B, lanes 9 and 10), indicating that only the phosphorylated form of Rsk can associate with chromosomes. Furthermore, U0126 treatment or MAPK depletion resulted in a significant reduction of Mad1 and Bub3 associated with chromosomes (Figure 3A and B).
Figure 3.
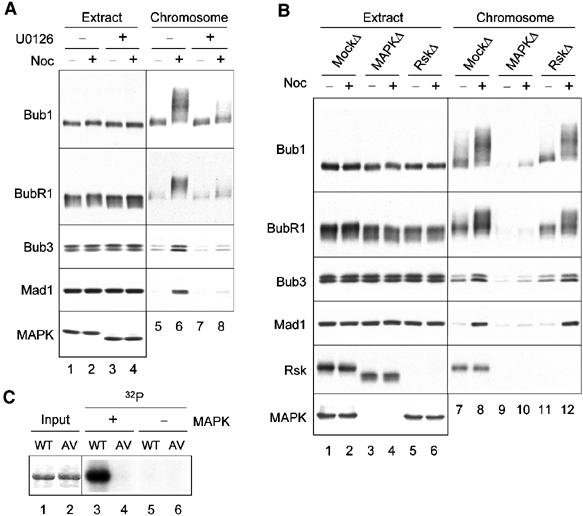
MAPK is involved in Bub1 and BubR1 phosphorylation at kinetochores. (A) Chromosomes were isolated from egg extracts that were treated with DMSO (lanes 1, 2, 5, and 6) or U0126 (lanes 3, 4, 7, and 8). The extracts (lanes 1–4) and the chromosomal fractions (lanes 5–8) were immunoblotted for proteins indicated on the left. (B) Egg extracts were mock-depleted (lanes 1, 2, 7, and 8), or depleted for MAPK (lanes 3, 4, 9, and 10) or Rsk (lanes 5, 6, 11, and 12), followed by incubation with sperm nuclei in the presence (even lanes) or absence (odd lanes) of nocodazole. The extracts (lanes 1–6) and chromosomal fractions (lanes 7–12) were immunoblotted for proteins indicated on the left. (C) Bub1 is a substrate for MAPK. MAPK (lanes 3 and 4) or mock (lanes 5 and 6) immunoprecipitates were used to phosphorylate recombinant Bub1 (odd lanes) or Bub15AV (even lanes) in vitro. Lanes 1 and 2 represent Coomassie blue staining of the recombinant proteins. An autoradiogram of the in vitro phosphorylation is shown (lanes 3–6).
Five of the Ser/Thr-Pro sequences in Xenopus Bub1 (T482, T493, S500, S634, T650), all within the amino-terminal noncatalytic region, are conserved in human and mouse homologs and likely to be functionally important. To test this possibility, these serine and threonine residues were altered to alanine and valine, respectively, and Bub15AV was generated. In vitro phosphorylation reaction showed that purified MAPK readily phosphorylated recombinant Bub1 but not Bub15AV (Figure 3C), indicating that Bub1 is a substrate for MAPK and that phosphorylation occurs within these five evolutionarily conserved residues.
To determine the kinase activity of Bub15AV at kinetochores, Bub1-depleted extracts were supplemented with wild-type Bub1, Bub15AV, or Bub1ΔKD, and in vitro autophosphorylation reaction was performed with these proteins eluted from chromosomes. A small amount of phosphorylation was present in Bub1ΔKD, which was probably from a co-immunoprecipitated kinase (Figure 4). This result suggests that the kinase activity measured in Bub1 immunoprecipitate was largely from Bub1, with a small contribution from a Bub1 upstream kinase. In all experiments, Bub15AV contained essentially no kinase activity (Figure 4), indicating that Bub15AV was not activated at unattached kinetochores.
Figure 4.
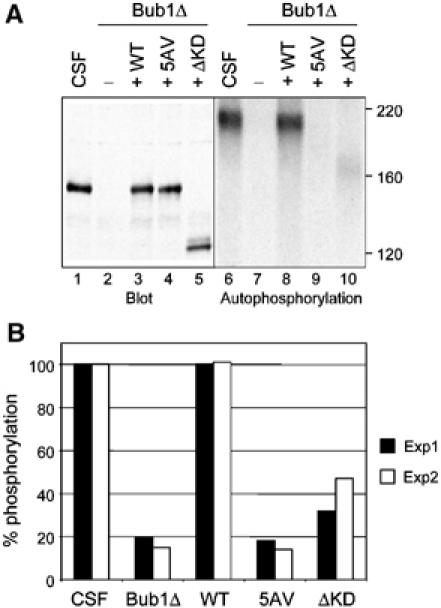
Bub15AV is not activated at unattached kinetochores. (A) Chromosomes were isolated from CSF-arrested extracts (lanes 1 and 6) or extracts depleted for endogenous Bub1 and then supplemented with mock translation (lanes 2 and 7), Bub1 (lanes 3 and 8), Bub15AV (lanes 4 and 9), or Bub1ΔKD (amino acids 1–838, lanes 5 and 10), followed by Bub1 immunoprecipitation and in vitro autophosphorylation reaction. Lanes 1–5, immunoblot of Bub1 in extracts; lanes 6–10, an autoradiogram of in vitro phosphorylated Bub1. (B) The results of two independent experiments of autophosphorylation reactions are plotted. The activity of Bub1 in CSF-arrested extracts was designated as 100% and used to normalize that of various Bub1 mutant proteins.
Bub15AV and Bub1ΔKD are partially functional
We previously reported that kinase-dead Bub1 was able to restore the spindle checkpoint in the Bub1-depleted extract (Sharp-Baker and Chen, 2001). We performed these experiments under an optimal condition for spindle checkpoint activation, using a high density of sperm nuclei (9000–15 000/μl extract) and 10 ng/μl nocodazole. However, any subtle difference in the checkpoint function between wild-type and kinase-dead Bub1 may be revealed only under a suboptimal condition for the spindle checkpoint activation. This possibility was tested by using various concentrations of nocodazole to disrupt kinetochore attachment to different degrees. When the spindle checkpoint is triggered in the cytostatic factor (CSF)-arrested extract with enough nuclei and nocodazole, the mitotic arrest is maintained even after calcium is added to overcome the CSF activity. In the presence of 1, 3, or 10 ng/μl nocodazole and 10 000 nuclei/μl, the Bub1-containing extract maintained the Cdc2-associated histone H1 kinase activity for at least 1 h after calcium addition and the chromosomes remained condensed at the end of the experiment (Figure 5A). This result shows that kinetochore attachment defect produced by even 1 ng/μl nocodazole is able to generate enough spindle checkpoint signal to block completely APC-Cdc20. However, extract containing Bub15AV and 1 ng/μl nocodazole started to lose Cdc2 activity at 15 min after calcium addition and the nuclei were at interphase at 65 min (Figure 5A), indicative of a defective spindle checkpoint. With 3 ng/μl nocodazole, Cdc2 activity slowly declined over time (Figure 5A) and the nuclei eventually entered interphase at 90 min (data not shown). With 10 ng/μl nocodazole, Cdc2 activity was maintained further. These results suggest that Bub15AV-containing kinetochores cannot efficiently generate the spindle checkpoint signal. Similarly, Bub1ΔKD failed to support the checkpoint in the presence of 1 ng/μl nocodazole, whereas the checkpoint was fully functional with 3 or 10 ng/μl nocodazole. This titration experiment shows that Bub1ΔKD and Bub15AV are partially functional.
Figure 5.
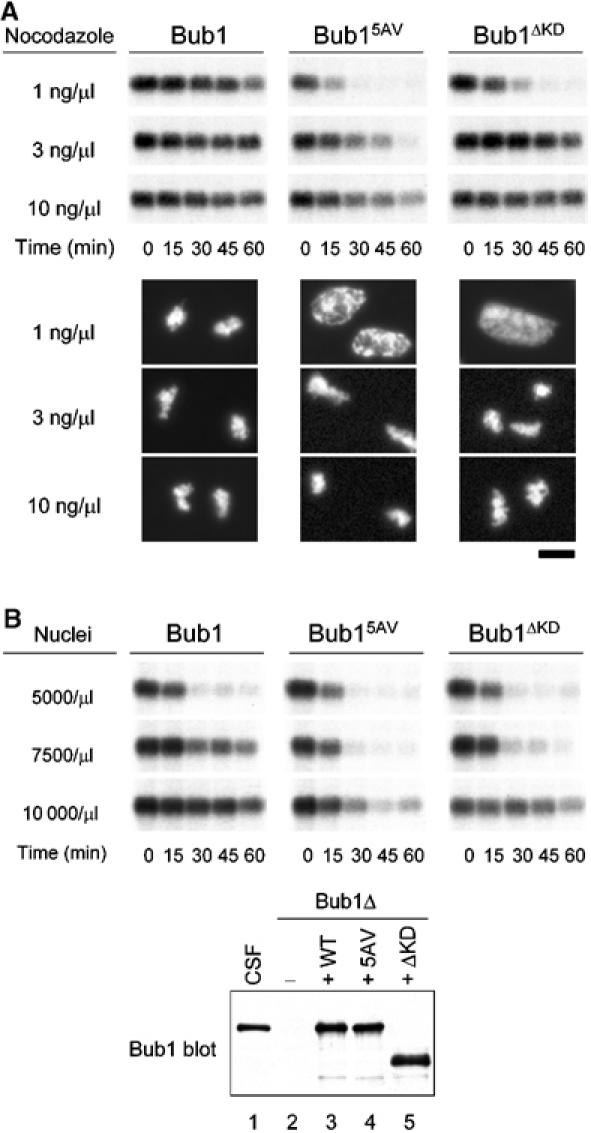
Kinase-deficient Bub1 mutants are partially functional in the spindle checkpoint. (A) Egg extracts containing wild-type Bub1 (left panels), Bub15AV (middle panels), or Bub1ΔKD (right panels) were incubated with 10 000 nuclei/μl and nocodazole at concentrations indicated on the left. Calcium chloride was then added and samples were taken for histone H1 kinase measurement at times indicated at the bottom. Photographs of the nuclei at 65 min are shown below the autoradiogram of H1 phosphorylation. Scale bar: 10 μm. The levels of various Bub1 proteins are similar to that shown in (B). (B) Extracts containing Bub1, Bub15AV, or Bub1ΔKD were incubated with sperm nuclei at densities indicated on the left, followed by incubation with 10 ng/μl nocodazole. Samples were taken for histone H1 kinase assay as described in (A). Immunoblot of various Bub1 proteins in the extracts is shown below the autoradiogram of H1 phosphorylation. Lane 1, CSF-arrested extract; lane 2, Bub1-depleted extract; lanes 3, 4, and 5, Bub1-depleted extracts supplemented with wild-type Bub1, Bub15AV, and Bub1ΔKD, respectively. The capability of activating the checkpoint varies slightly between different batches of extracts.
The checkpoint efficiency under various concentrations of nuclei in the presence of 10 ng/μl nocodazole was also examined. With 5000 nuclei/μl, extracts containing any of the Bub1 proteins failed to maintain Cdc2 activity after calcium addition, indicating that the checkpoint signal generated from these kinetochores was not strong enough to inhibit Cdc20 completely (Figure 5B). Wild-type Bub1 sustained Cdc2 activity in the presence of 7500 or 10 000 nuclei/μl, whereas Bub15AV and Bub1ΔKD failed to maintain Cdc2 activity after 30 min of calcium addition in the presence of 7500 nuclei/μl (Figure 5B). This result is consistent with the notion that Bub15AV and Bub1ΔKD are partially compromised in their checkpoint function.
As Bub1 is required for Mad1, Mad2, BubR1, and Bub3 to bind kinetochores, the possibility whether Bub15AV and Bub1ΔKD are defective in recruiting other checkpoint proteins to kinetochores was examined. Immunoblot analysis showed that there were lower levels of the checkpoint proteins in chromosomes prepared from extracts treated with 1 ng/μl nocodazole than those with 10 ng/μl nocodazole (Figure 6, compare lanes 7 and 8). In samples containing Bub15AV or Bub1ΔKD, there were significantly less Bub3, Mad1, and Mad2 in chromosomal fractions from extracts treated with 1 ng/μl nocodazole, compared to the Bub1-containing sample (Figure 6). I consistently found lower levels of the checkpoint proteins in chromosomes containing Bub1ΔKD than those with Bub15AV. These results indicate that Bub15AV and Bub1ΔKD are partially impaired in their ability to recruit other checkpoint proteins to kinetochores. Furthermore, immunoblot for Bub1 proteins in the chromosomal fractions shows that Bub15AV lacked the characteristic phosphorylation, whereas Bub1ΔKD was still phosphorylated (Figure 6), suggesting that Bub15AV indeed loses the major phosphorylation sites at kinetochores and that Bub1 phosphorylation occurs within the N-terminal noncatalytic region. Interestingly, BubR1 in the chromosomal fractions lost most, if not all, of the phosphorylation in extracts containing Bub15AV or Bub1ΔKD (Figure 6, lanes 9–12), indicating that phosphorylation of BubR1 is dependent on the kinase activity of Bub1.
Figure 6.
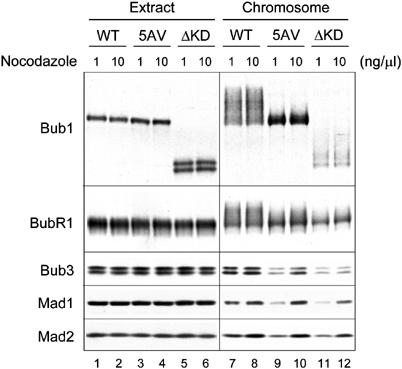
Kinase-deficient Bub1 mutants have reduced ability in recruiting other spindle checkpoint proteins to kinetochores. Egg extracts containing wild-type Bub1 (lanes 1, 2, 7, and 8), Bub15AV (lanes 3, 4, 9, and 10), or Bub1ΔKD (lanes 5, 6, 11, and 12) were incubated with sperm nuclei and 1 or 10 ng/μl nocodazole as indicated on the top. Immunoblots were performed for proteins in extracts (lanes 1–6) or in the chromosomal fractions (lanes 7–12) using antibodies indicated on the left.
To determine which of the five evolutionarily conserved MAPK consensus sites is the target for MAPK, these sites were individually mutated in Bub1. In vitro phosphorylation of recombinant proteins showed that wild-type Bub1 and T482V mutant were phosphorylated by MAPK equally well, whereas all other four single mutants (T493V, S500A, S634A, and T650V) were phosphorylated to a lesser degree (Figure 7A). This result indicates that T493, S500, S634, and T650 are the in vitro phosphorylation sites for MAPK.
Figure 7.
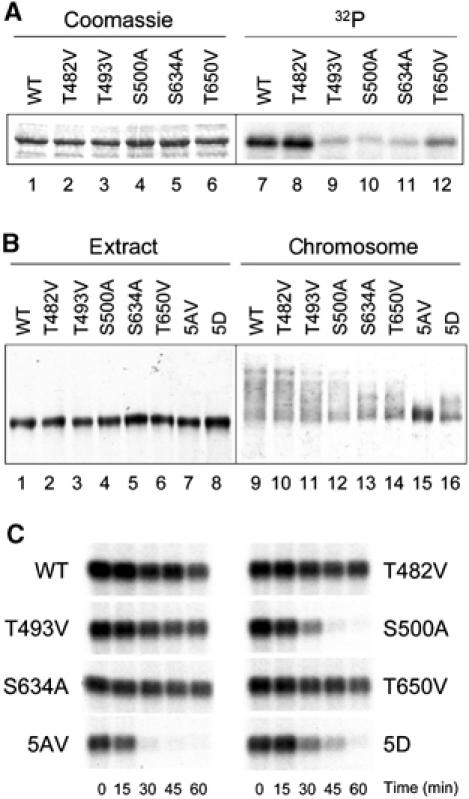
Analysis of individual phosphorylation site mutants. (A) In vitro phosphorylation of various recombinant Bub1 proteins by MAPK. Lanes 1–6, Coomassie staining of the substrate proteins; lanes 7–12, autoradiogram of the in vitro phosphorylation reaction. WT, wild-type Bub1. (B) Immunoblot of various Bub1 proteins associated with chromosomes. Mitotic chromosomes were isolated from extracts containing wild-type or various Bub1 mutants as indicated on the top. Extracts (lanes 1–8) or chromosomal fractions (lanes 9–16) were immunoblotted with anti-Bub1 antibody. (C) Spindle checkpoint function of various Bub1 proteins. Extracts containing various Bub1 proteins as indicated were incubated with 10 000 nuclei/μl and 1 ng/μl nocodazole. Samples were taken for histone H1 kinase measurement at times indicated at the bottom. An autoradiogram of the reaction is shown.
Then, these single site mutants on chromosomes were examined by immunoblot analysis. Wild-type Bub1 and T482V mutant showed similar mobility shift in the chromosomal fractions, whereas the mobility shifts of the other four single mutants were reduced to various degrees (Figure 7B). This result suggests that T482 is probably not the physiological phosphorylation site, consistent with the in vitro phosphorylation study (Figure 7A). Interestingly, Bub15D, with all five sites replaced with aspartic acid to mimic phosphorylation, showed some mobility shift in the chromosomal fraction (Figure 7B, lane 12). This suggests that Bub15D is still modified at other residues, unlike Bub15AV, which completely lost modification at kinetochores (Figure 7B, lane 11).
Next, the checkpoint function of these single mutants in egg extracts incubated with 10 000 nuclei/μl and 1 ng/μl nocodazole, a suboptimal condition for spindle checkpoint activation as shown in Figure 5A, was determined. Except S500A mutant, all other four single mutants were able to maintain Cdc2 activity under this condition (Figure 7C). The extract containing S500A sustained Cdc2 activity for a longer time than the Bub15AV-containing extract. These results indicate that S500 is the most critical phosphorylation site among all, even though other sites (T493, S634, and T650) are also important. Compared to Bub15AV, Bub15D maintained Cdc2 activity only slightly longer, indicating that aspartic acid substitutions did not completely mimic phosphorylation.
Discussion
This study reveals a regulatory mechanism of Bub1 at kinetochores. Bub1 becomes hyperphosphorylated and its kinase activity is induced specifically on unattached chromosomes. The phosphorylation is very sensitive to protein phosphatase and is preserved by the use of a potent phosphatase inhibitor microcystin. It is unlikely that the phosphorylation is an artifact produced by microcystin, because the effect is specific to Bub1 and BubR1 associated with unattached chromosomes, but not their cytosolic counterparts or other spindle checkpoint proteins. In addition, microcystin was added only to the ice-cold solutions during chromosome isolation and not to the egg extracts during the assembly of mitotic chromosomes. It is possible that microtubule attachment to kinetochores may either downregulate a Bub1 upstream kinase and/or upregulate a phosphatase, resulting in dephosphorylation of Bub1 at attached kinetochores.
Several lines of evidence indicate that MAPK contributes to Bub1 phosphorylation. First, blocking MAPK activation with U0126 or immunodepletion of MAPK abolishes the phosphorylation (Figure 3). Second, purified MAPK phosphorylates recombinant Bub1 in vitro (Figure 3C). Bub15AV, with five evolutionarily conserved MAPK consensus sites mutated to alanine or valine, is no longer a substrate for MAPK (Figure 3C). Importantly, Bub15AV also lacks phosphorylation at unattached kinetochores (Figure 6), indicating that some or all of these MAPK consensus sites are indeed the physiological phosphorylation sites. Analysis of single-site mutants shows that T482 is neither a MAPK phosphorylation site in vitro nor a phosphorylation site at kinetochores in egg extracts (Figure 7). It is possible that prior phosphorylation by MAPK primes Bub1 for further phosphorylation by other kinases, resulting in the large electrophoretic mobility shift. In fact, Bub15D shows some mobility shift in the chromosomal fractions, while Bub15AV does not (Figure 7B). This result suggests that aspartic acid substitution mimics some aspect of the phosphorylation and allows the protein to be further modified by other enzymes. It has been shown that MAPK downstream target Rsk activates cytosolic Bub1 during frog oocyte maturation (Schwab et al, 2001). Rsk is not involved in Bub1 phosphorylation at kinetochores, because immunodepletion of Rsk has no effect on the phosphorylation (Figure 3B). It appears that Bub1 is regulated by different kinases for different cellular processes.
It has been shown that fission yeast Bub1 is phosphorylated during mitosis and that the protein is a substrate for Cdc2 (Yamaguchi et al, 2003). Mutations at four putative Cdc2 sites abolish the checkpoint function (Yamaguchi et al, 2003). These four sites reside in a short stretch of 127 amino acids within the amino-terminal noncatalytic domain. Interestingly, the four phosphorylation sites of Xenopus Bub1 are similarly located within 158 amino acids in the amino-terminal noncatalytic region. Even though the regulatory region of fission yeast Bub1 is significantly different from that of Bub1 in metazoans, sequence alignment shows that Cdc2 phosphorylation site T423 in fission yeast Bub1 appears to correspond to S500 in Xenopus Bub1. Interestingly, S500 is the most critical phosphorylation site for the checkpoint function of Xenopus Bub1 (Figure 7C). Both Cdc2 and MAPK have similar consensus phosphorylation sites. In egg extracts, inhibition of MAPK abolishes Bub1 phosphorylation without an effect on Cdc2 activity, indicating that Cdc2 is not involved in Bub1 phosphorylation. This suggests that regulation of Bub1 by phosphorylation is an evolutionarily conserved process, even though the protein may be phosphorylated by different kinases in diverge species. In both budding and fission yeasts, the bulk of Bub1 is phosphorylated during mitosis (Farr and Hoyt, 1998; Yamaguchi et al, 2003). However, it is not clear whether there is differential phosphorylation of the protein in the cytosol and at kinetochores in yeast.
The present study shows that, in addition to Cdc20 (Chung and Chen, 2003) and CENP-E (Zecevic et al, 1998), Bub1 is another substrate of MAPK in the spindle checkpoint. Interestingly, U0126 treatment or MAPK depletion results in a significant reduction of the checkpoint proteins at kinetochores. This effect is not simply due to the loss of Bub1 phosphorylation or activity, because the unphosphorylated and inactive Bub15AV mutant is able to recruit other checkpoint proteins to kinetochores (Figure 6). It is likely that MAPK phosphorylates some other unknown proteins(s) in order that the checkpoint proteins localize to kinetochores.
Activation of Bub1 at unattached kinetochores correlates with the generation of spindle checkpoint signal. In the presence of kinase-deficient Bub15AV or Bub1ΔKD, the checkpoint is fully functional only under an optimal condition for spindle checkpoint activation, as indicated by the titration experiments using various concentrations of nuclei or nocodazole (Figure 5). These results suggest that the kinase activity of Bub1 does not function as an on–off switch of the checkpoint. Instead, it modulates the strength of the checkpoint signal generated from each kinetochore. Bub15AV and Bub1ΔKD are partially compromised in their checkpoint function, at least partly due to their reduced ability in supporting kinetochore binding of other checkpoint proteins (Figure 6). As a consequence, kinetochores containing Bub15AV or Bub1ΔKD are less efficient in generating the checkpoint signal, compared to wild-type Bub1. In early prometaphase cells or in cells treated with nocodazole, most or all of the kinetochores are not bound with microtubules and are able to stimulate the complex formation between Cdc20 and the checkpoint proteins Mad2, Bub3, and BubR1. Thus, Cdc20 may be efficiently inhibited even without the kinase activity of Bub1. On the other hand, in late prometaphase cells that contain only a few or even only one lagging chromosome, the kinase activity of Bub1 may become crucial for the few unattached kinetochores to assemble efficiently the checkpoint complex that binds and inhibits Cdc20. In this model, Bub1 protein functions as a scaffold at kinetochores to recruit physically other checkpoint proteins, and its kinase activity targets some kinetochore or checkpoint proteins to promote the kinetochore association. Unlike Mad1 and Mad2, which dissociate from kinetochores upon microtubule attachment, a fraction of Bub1 molecules remains at kinetochores until early anaphase. Regulation of the kinase activity of Bub1 by microtubule attachment may provide a way to quickly control Bub1 activity and the strength of the checkpoint signal. Before stable kinetochore–microtubule interaction is achieved, any microtubule detachment would quickly lead to phosphorylation and activation of Bub1 at kinetochores, thus sustaining the checkpoint signal.
The titration experiments consistently show that Bub15AV supports the spindle checkpoint less satisfactorily than Bub1ΔKD does (Figure 5). However, immunoblot analysis shows that Bub15AV recruits more checkpoint proteins to kinetochores than Bub1ΔKD does (Figure 6). These results suggest that dephosphorylation of Bub1 and deletion of the Bub1 kinase domain have different effects on the spindle checkpoint, even though both abolish the kinase activity of Bub1 at kinetochores. This indicates that phosphorylation and the kinase domain may be involved in separate aspects of the spindle checkpoint. Phosphorylation of Bub1 not only activates the kinase activity but may also stimulate the binding and inhibition of Cdc20 by the checkpoint proteins. The latter function may still be retained in Bub1ΔKD but is lost in Bub15AV. On the other hand, the kinase domain may physically interact with some other proteins that help to stabilize checkpoint proteins at kinetochores. This notion is consistent with the observation that Bub1ΔKD is less efficient in recruiting other checkpoint proteins to kinetochores than Bub15AV is.
Similar to Bub1, BubR1 is hyperphosphorylated at unattached kinetochores in a MAPK-dependent manner (Figures 1 and 3). BubR1 also contains several MAPK consensus sites that are conserved between human, mouse, and frog proteins. Mutations at these sites only partially reduce its phosphorylation at kinetochores (R-H Chen, unpublished data), indicating that other kinases are likely to be involved in the phosphorylation. As phosphorylation of BubR1 at kinetochores requires the kinase activity of Bub1, it is possible that Bub1 may directly or indirectly phosphorylate BubR1. It remains to be determined how BubR1 phosphorylation at kinetochores may regulate the spindle checkpoint.
Materials and methods
Isolation of mitotic chromosomes from egg extracts
Demembranated frog sperm nuclei and crude cytoplasmic extracts from mature eggs that were arrested at metaphase II by CSF were prepared as described (Murray, 1991). The derived CSF-arrested extracts were used in the experiments. Mitotic chromosomes were isolated from egg extracts as described (Chung and Chen, 2002). Briefly, 40 μl extracts were incubated with sperm nuclei at a density of 15 000/μl extract for 20 min at 23°C, followed by incubation with or without 10 ng/μl nocodazole for another 20 min. To isolate chromosomes, the samples were diluted with nine volumes (360 μl) of ice-cold lysis buffer (10 mM KPO4, pH 7.2, 1 mM EDTA, 5 mM EGTA, 1 mM MgCl2, 50 mM β-glycerophosphate, 1 mM sodium vanadate, 0.1% Triton X-100, and 10 μg/ml each of leupeptin, pepstatin, and chymostatin) with or without 200 nM microcystin-LR (Sigma, St Louis, MO). The diluted samples were then layered over 1 ml of 30% sucrose made in lysis buffer with or without microcystin and spun at 10 000 rpm for 15 min in an HB-6 rotor (Sorvall, Newtown, CT). Chromosomal pellets were washed in 500 μl of the sucrose solution and spun again for 5 min. The pellets were solubilized by heating at 95°C for 5 min in SDS–PAGE sample buffer and sonicated briefly to break the DNA before resolving the proteins by SDS–PAGE for immunoblot analysis. For inhibiting MAPK activity in Figure 3A, 400 μM U0126 (Upstate Biotechnology, Lake Placid, NY) or DMSO as control was incubated with CSF-arrested extracts at 23°C for 40 min before the addition of sperm nuclei. After Western transfer, the nitrocellulose membrane was stained with Ponceau S to verify the evenness of different chromosome preparations. The staining showed predominant proteins histones and condensin subunits XCAPs as described (Chung and Chen, 2002).
Immunoprecipitation of Bub1 from chromosomes and autophosphorylation reaction
Chromosomal pellets were resuspended and briefly sonicated in lysis buffer containing 0.5 M NaCl with or without 200 nM microcystin. Samples were then clarified by centrifugation at 13 000 rpm for 5 min, and the supernatants were subjected to immunoprecipitation with affinity-purified anti-Bub1 antibody. The immunoprecipitates were washed twice with lysis buffer plus 200 nM microcystin, twice with lysis buffer containing 0.5 M NaCl and 200 nM microcystin, and once with kinase buffer (20 mM Hepes, 10 mM MgCl2, 0.1 mg/ml BSA, and 3 mM β-mercaptoethanol). The immunoprecipitates were then resuspended in 10 μl of kinase buffer containing 100 μM ATP and 10 μCi [γ-32P]ATP, and incubated at 30°C for 10 min. The reactions were terminated by the addition of SDS–PAGE sample buffer and resolved by SDS–PAGE.
Immunodepletion and add-back
Immunodepletion of Bub1 was performed as described (Sharp-Baker and Chen, 2001). Immunodepletion of Rsk was performed with the antibody SC-231 (Santa Cruz Biotechnology, Santa Cruz, CA). Anti-MAPK antibodies were generated against hexahistidine-tagged MAPK expressed in and purified from bacteria. The antibodies were affinity-purified for immunodepletion. Mutations were made by the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) and verified by DNA sequencing. To add back various Bub1 proteins, the corresponding RNAs were synthesized in vitro using the mMESSAGE mMACHINE T7 transcription kit (Ambion) and then added to the Bub1-depleted extract to synthesize the proteins as described (Sharp-Baker and Chen, 2001).
Spindle checkpoint activation
CSF-arrested extracts were incubated for 10 min at 23°C with various concentrations of sperm nuclei as indicated, followed by another 20 min incubation with 1, 3, or 10 ng/μl nocodazole as indicated. Calcium chloride (0.5 mM) was then added to over-ride the CSF arrest. Samples were taken immediately before the addition of calcium chloride and every 15 min thereafter for histone H1 kinase assay as described (Chen et al, 1996). Nuclear morphology was examined by adding 1 μl of the sample to 4 μl of Fix solution (11.1% formaldehyde, 48% glycerol, 1 μg/ml Hoechst 33258 made in MMR (100 mM sodium chloride, 2 mM potassium chloride, 1 mM magnesium sulfate, 2 mM calcium chloride, 5 mM Hepes, and 0.1 mM EDTA)). Images were collected using a charge-coupled device camera (MicroMAX-5 MHz; Princeton Instruments, Princeton, NJ) attached to a fluorescence microscope (E800; Nikon, Melville, NY). Images were collected and processed with the MetaMorph Imaging System (version 4.0; Universal Imaging, Downingtown, PA) and converted to Photoshop format (Adobe Systems, Mountain View, CA).
Phosphorylation of recombinant Bub1 in vitro
Recombinant Bub1 and various Bub1 phosphorylation site mutants without the catalytic domain (amino acids 1–770) were expressed as hexahistidine-tagged proteins in bacteria from pQE9 vector. For in vitro phosphorylation, MAPK was immunoprecipitated from CSF-arrested extracts as described above for Bub1. The immunoprecipitates were then resuspended in 10 μl of kinase buffer containing 50 μM ATP, 1 μCi [γ-32P]ATP, and 2 μg of purified recombinant Bub1 proteins, and incubated at 30°C for 10 min. The reactions were terminated by the addition of SDS–PAGE sample buffer.
Acknowledgments
This work was supported by grants from the National Institutes of Health and the David and Lucile Packard Foundation.
References
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe J (2001) Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106: 83–93 [DOI] [PubMed] [Google Scholar]
- Basu J, Logarinho E, Herrmann S, Bousbaa H, Li Z, Chan GK, Yen TJ, Sunkel CE, Goldberg ML (1998) Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma 107: 376–385 [DOI] [PubMed] [Google Scholar]
- Chan GK, Jablonski SA, Sudakin V, Hittle JC, Yen TJ (1999) Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J Cell Biol 146: 941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH (2002) BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J Cell Biol 158: 487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Shevchenko A, Mann M, Murray AW (1998) Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol 143: 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Waters JC, Salmon ED, Murray AW (1996) Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science 274: 242–246 [DOI] [PubMed] [Google Scholar]
- Chung E, Chen RH (2002) Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol Biol Cell 13: 1501–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Chen RH (2003) Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol 5: 748–753 [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Mao Y, Sullivan KF (2003) Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112: 407–421 [DOI] [PubMed] [Google Scholar]
- Farr KA, Hoyt MA (1998) Bub1p kinase activates the Saccharomyces cerevisiae spindle assembly checkpoint. Mol Cell Biol 18: 2738–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, Beretta A, Sironi L, Musacchio A, Lucchini G, Piatti S (2001) Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J 20: 6648–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick K, Murray AW (1995) Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol 131: 709–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Johnston RC, Smith DL, Murray AW (2000) MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J Cell Biol 148: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW (1996) Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273: 953–956 [DOI] [PubMed] [Google Scholar]
- Howell BJ, Hoffman DB, Fang G, Murray AW, Salmon ED (2000) Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J Cell Biol 150: 1233–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Chan GK, Cooke CA, Earnshaw WC, Yen TJ (1998) The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107: 386–396 [DOI] [PubMed] [Google Scholar]
- Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ (2002) Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J Cell Biol 158: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Nicklas RB (1995) Mitotic forces control a cell cycle checkpoint. Nature 373: 630–632 [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R (1996) Identification of a human mitotic checkpoint gene: hsMAD2. Science 274: 246–248 [DOI] [PubMed] [Google Scholar]
- Minshull J, Sun H, Tonks NK, Murray AW (1994) MAP-kinase dependent mitotic feedback arrest in Xenopus egg extracts. Cell 79: 475–486 [DOI] [PubMed] [Google Scholar]
- Murray AW (1991) Cell cycle extracts. Methods Cell Biol 36: 573–597 [PubMed] [Google Scholar]
- Peters JM (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9: 931–943 [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G (1995) The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 130: 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab MS, Roberts BT, Gross SD, Tunquist BJ, Taieb FE, Lewellyn AL, Maller JL (2001) Bub1 is activated by the protein kinase p90(Rsk) during Xenopus oocyte maturation. Curr Biol 11: 141–150 [DOI] [PubMed] [Google Scholar]
- Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR, Ahn NG (1998) Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol 142: 1533–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp-Baker H, Chen RH (2001) Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J Cell Biol 153: 1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Ha E, McKeon F (1998) The human homologue of bub3 is required for kinetochore localization of bub1 and a Mad3/Bub1-related protein kinase. J Cell Biol 142: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, McKeon F (1997) Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89: 727–735 [DOI] [PubMed] [Google Scholar]
- Wang XM, Zhai Y, Ferrell JE (1997) A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J Cell Biol 137: 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Chen RH, Murray AW, Salmon ED (1998) Localization of mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol 141: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Decottignies A, Nurse P (2003) Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J 22: 1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic M, Catling AD, Eblen ST, Renzi L, Hittle JC, Yen TJ, Gorbsky GJ, Weber MJ (1998) Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J Cell Biol 142: 1547–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lees E (2001) Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation. Mol Cell Biol 21: 5190–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]


