Abstract
Schwann cells are a regenerative cell type. Following nerve injury, a differentiated myelinating Schwann cell can dedifferentiate and regain the potential to proliferate. These cells then redifferentiate during the repair process. This behaviour is important for successful axonal repair, but the signalling pathways mediating the switch between the two differentiation states remain unclear. Sustained activation of the Ras/Raf/ERK cascade in primary cells results in a cell cycle arrest and has been implicated in the differentiation of certain cell types, in many cases acting to promote differentiation. We therefore investigated its effects on the differentiation state of Schwann cells. Surprisingly, we found that Ras/Raf/ERK signalling drives the dedifferentiation of Schwann cells even in the presence of normal axonal signalling. Furthermore, nerve wounding in vivo results in sustained ERK signalling in associated Schwann cells. Elevated Ras signalling is thought to be important in the development of Schwann cell-derived tumours in neurofibromatosis type 1 patients. Our results suggest that the effects of Ras signalling on the differentiation state of Schwann cells may be important in the pathogenesis of these tumours.
Keywords: dedifferentiation, ERK, NF1, Ras, Schwann cells
Introduction
The Ras/Raf/ERK pathway has a role in regulating many physiological processes (Barbacid, 1987; Bar-Sagi and Hall, 2000). Sustained signalling through this pathway often acts to promote cell differentiation (Crespo and Leon, 2000). In the PC12 cell line, for example, NGF addition results in prolonged and sustained ERK activity, leading to cell cycle exit and the development of a neuronal phenotype, while transient ERK activation is associated with proliferation (Marshall, 1995). In vivo, Ras/Raf/ERK signalling regulates vulval development in Caenorhabditis elegans and eye development in Drosophila (Freeman, 1998; Sternberg and Han, 1998). Recently, more detailed studies of Drosophila eye development have provided further support for the hypothesis that different thresholds of ERK activity result in different outcomes, with high levels of Ras/Raf/ERK activity associated with the promotion of differentiation (Halfar et al, 2001).
Schwann cells ensheath and myelinate axons in the peripheral nervous system in response to axon-derived signals (Mirsky and Jessen, 2001). They are a regenerative cell type, which, throughout the lifespan of an animal, can dedifferentiate in response to nerve damage as part of a process called Wallerian degeneration. The dedifferentiated Schwann cells can proliferate, then redifferentiate during the repair process (Scherer and Salzer, 2001). This regenerative response of Schwann cells to nerve injury is important for successful nerve repair, but the signals involved remain unclear.
Rat Schwann cells dissociated from peripheral nerves can be purified and expanded indefinitely in culture (Mathon et al, 2001). They can be induced to redifferentiate to the myelinating form in vitro, either by the addition of cAMP or by axonal contact (Lemke and Chao, 1988; Morgan et al, 1991; Fernandez-Valle et al, 1993). We previously showed that activation of the Ras/Raf/ERK pathway in these isolated cells results in a cell cycle arrest mediated by a p53-dependent induction of the cyclin-dependent kinase inhibitor (CDKI) p21Cip1 (Lloyd et al, 1997). Such an arrest has been proposed to act as a protective checkpoint mechanism to prevent unregulated proliferation in response to a single genetic alteration with further genetic changes required for Ras to drive the cell cycle positively (Lloyd et al, 1997; Serrano et al, 1997; Mitchell et al, 2003). Interestingly, in some primary cell types, for example keratinocytes, the Ras-induced cell cycle arrest is also associated with the promotion of a more differentiated phenotype (Lin et al, 1998; Roper et al, 2001). The effects of Ras signalling in Schwann cells are of particular interest as Ras is thought to be important in the development of tumours in patients with the genetic disorder neurofibromatosis type 1 (NF1). These patients are susceptible to developing benign and malignant Schwann cell-derived tumours (Cichowski and Jacks, 2001). The formation and maintenance of these tumours appear to be dependent on elevated Ras signalling that occurs as a result of loss of the Ras-GAP neurofibromin (Basu et al, 1992; DeClue et al, 1992).
In view of the evidence that the Ras/Raf/ERK pathway can regulate differentiation in a number of cell types, we decided to explore the effects of activating this pathway on the differentiation state of Schwann cells. Contrary to our expectations, we found that sustained Ras/Raf/ERK signalling acts as a dedifferentiation signal in these cells.
Results
Raf activation blocks Schwann cell differentiation
The differentiation of Schwann cells to a myelinating state can be induced in vitro, in the absence of neurons, by an elevation of cAMP levels. The addition of forskolin or dibutyryl cAMP (db-cAMP) results in the expression of myelination-associated proteins, including periaxin, the myelin sheath proteins P0, PMP22, and MBP and the transcription factors Oct-6 and Krox-20 (Lemke and Chao, 1988; Monuki et al, 1989; Morgan et al, 1991; Zorick et al, 1996). Importantly, the switch between these differentiation states can be studied independently from the cell cycle (Morgan et al, 1991). Schwann cells quiesce upon the removal of mitogens but do not differentiate without the addition of a differentiation signal, such as cAMP. This is in contrast to some cell types that can be studied in vitro, such as oligodendrocyte precursor cells (Raff et al, 1998), where withdrawal from the cell cycle is sufficient to trigger differentiation, thus making it difficult to distinguish differentiation signals from the effects on the cell cycle.
To address the effects of activation of the Ras/Raf/ERK pathway on Schwann cell differentiation, primary Schwann cells were infected with a retrovirus encoding an inducible Raf fusion protein (NSΔRafER), which contains the kinase domain of Raf-1 (ΔRaf) fused to the oestrogen receptor hormone-binding domain (ER). ΔRafER can be reversibly activated by the addition of tamoxifen (Tmx), resulting in rapid and sustained ERK activation (Samuels et al, 1993).
Addition of db-cAMP to NSΔRafER cells cultured in defined medium in the absence of mitogens resulted in a strong induction of P0, PMP22, MBP and periaxin, confirming that the differentiation programme could be induced in these cells by cAMP elevation (Figure 1A–C). In contrast, addition of Tmx (to induce Raf kinase activity) resulted in an elongated, refractile morphology typical of Ras/Raf-transformed cells, with elevated p21Cip1 and cyclin D1 levels, which we have previously shown to be induced in Schwann cells following Raf activation (Lloyd et al, 1997). Furthermore, activation of Raf signalling in these cells did not result in the induction of any of the differentiation markers. Thus, Raf activation in primary Schwann cells is insufficient to induce differentiation.
Figure 1.
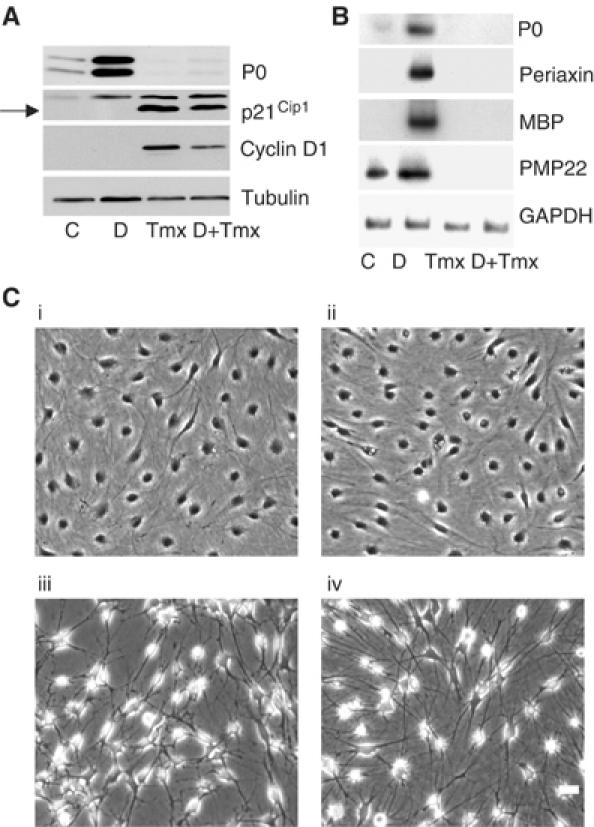
Raf activation blocks Schwann cell differentiation. (A) NSΔRafER cells in defined medium were treated with either control solvents (C), 1 mM db-cAMP (D), or db-cAMP and Tmx simultaneously (D+Tmx) for 72 h, or Tmx alone for 48 h. Protein lysates were analysed by Western blotting with the stated antibodies. (B) PO, MBP, PMP22 and periaxin were analysed by semiquantitative RT–PCR analysis. NSΔRafER cells were treated as described in (A) for 48 h. (C) Phase micrographs of NSΔRafER cells treated with control solvents (i), db-cAMP (ii), Tmx (iii) or db-cAMP and Tmx (iv) as in (A) for 48 h. Scale bar, 20 μm.
We noted that the levels of P0 protein in cells with activated Raf were lower than in the nondifferentiated cells (Figure 1A), suggesting that Raf may negatively regulate differentiation. To examine this further, we turned on Raf in the presence of the differentiating signal db-cAMP. Interestingly, we found that Raf activation completely blocked the ability of db-cAMP to induce differentiation (Figure 1). This effect was specific to Schwann cells expressing the ΔRafER construct since freshly isolated normal Schwann cells differentiated normally in response to db-cAMP in the presence of Tmx (data not shown). Thus, Raf activation can block Schwann cell differentiation.
Raf activation induces Schwann cell dedifferentiation
To examine the effects of Raf activation on differentiated Schwann cells, we first induced Schwann cell differentiation by the addition of db-cAMP for 3 days, then treated the cells with Tmx to activate Raf signalling. Remarkably, within 48 h, Raf activation resulted in the switching off of differentiation markers to basal levels or below (Figure 2A and B). These results demonstrate that activation of Raf signalling not only inhibits differentiation but can also reverse differentiation. The ability of Raf to dedifferentiate Schwann cells does not appear to be the result of simply blocking the differentiation signal as washing-out db-cAMP after 3 days does not result in a reduction of P0 levels over a similar time period suggesting that Raf actively drives the dedifferentiation process (Figure 2C).
Figure 2.
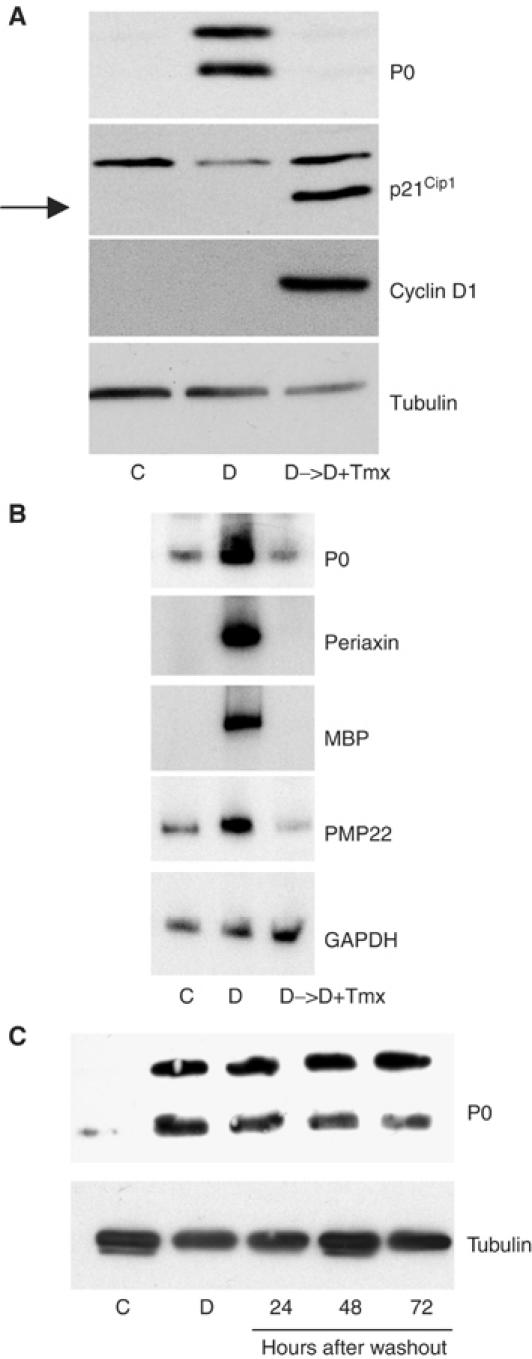
Raf activation induces Schwann cell dedifferentiation. (A) NSΔRafER cells were treated with db-cAMP (D) or control solvents (C) for 72 h, then Tmx (D->D+Tmx) was added where stated for 48 h. Protein lysates were analysed by Western blotting with the specified antibodies. (B) Semiquantitative RT–PCR analysis of the effects of Raf activation on the expression patterns of other myelination markers. NSΔRafER cells were treated as described in (A). (C) Schwann cells were maintained in defined medium for 3 days with db-cAMP or control solvents. After 3 days, the control cells and a db-cAMP-treated dish of cells were collected. The remaining db-cAMP-treated cells were washed into defined medium alone and harvested at the stated times. Lysates were analysed by Western blotting with the stated antibodies.
Schwann cell differentiation is regulated by a number of transcription factors, including Oct-6 and Krox-20 (Topilko and Meijer, 2001). Krox-20 appears to be fundamental in controlling Schwann cell differentiation, regulating the expression of a number of genes, including periaxin, P0, MBP and PMP22 (Zorick et al, 1999; Nagarajan et al, 2001). In the absence of Krox-20, Schwann cells are unable to differentiate fully, whereas decreased levels of Krox-20 are associated with the Schwann cell dedifferentiation that occurs following nerve injury (Zorick et al, 1999). Oct-6 has been implicated in the expression of Krox-20 and may positively regulate myelin gene expression (Bermingham et al, 2001; Topilko and Meijer, 2001). The expression patterns of Krox-20 and Oct-6 are summarised in Figure 3A with the appearance of P0 indicating the onset of myelination.
Figure 3.
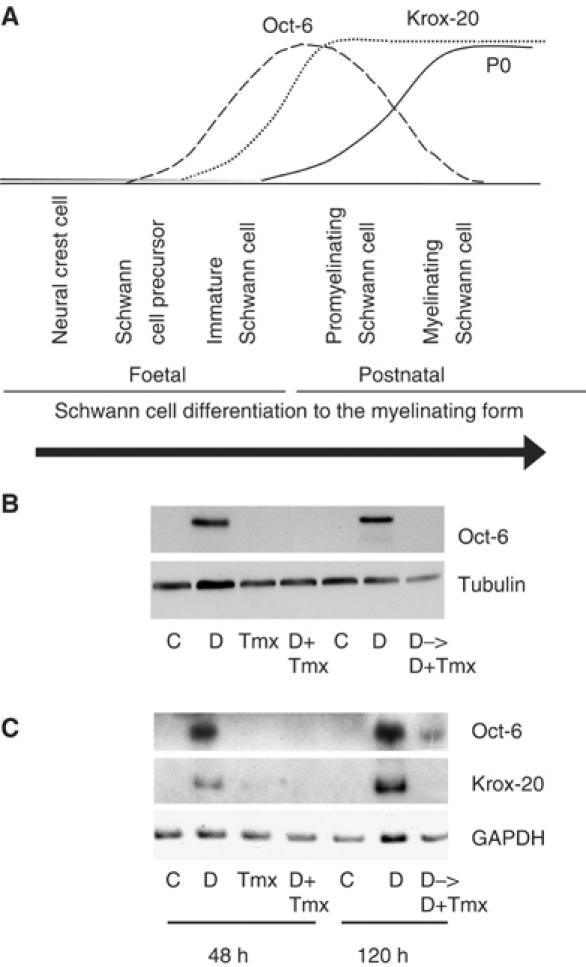
Raf activation can regulate the expression of transcription factors associated with Schwann cell differentiation. (A) Diagram summarising the expression patterns of the transcription factors Oct-6 and Krox-20, and the myelin sheath proteins, represented by P0, during Schwann cell differentiation. (B) Western blot analysis and (C) semiquantitative RT–PCR analysis of NSΔRafER cells treated with either control solvents (C), 1 mM db-cAMP (D), Tmx, or db-cAMP and Tmx simultaneously (D+Tmx) for 48 h. A second set of NSΔRafER cells were treated with db-cAMP or control solvents for 72 h, then Tmx (D->D+Tmx) was added, where stated, for 48 h while maintaining the samples C and D for 120 h in total. Samples were analysed for the effects of Raf activation on the expression patterns of Krox-20 and Oct-6.
The addition of db-cAMP to NSΔRafER cells induced the expression of Krox-20 and Oct-6 consistent with the induction of the myelinating state (Figure 3B and C). Tmx alone had no effect on the levels of either Oct-6 or Krox-20, but in the presence of db-cAMP it blocked both Oct-6 and Krox-20 induction. This suggests that Raf signalling acts upstream of the induction of Oct-6 and Krox-20 to regulate the Schwann cell differentiation state. In addition, the Raf-induced dedifferentiation of Schwann cells was associated with reduced levels of both Oct-6 and Krox-20 (Figure 3B and C), suggesting that downregulation of these transcription factors is part of the mechanism by which Raf signalling induces dedifferentiation.
Raf signals via the ERK pathway to block and reverse differentiation
To determine whether the effects of Raf activation on Schwann cell differentiation were mediated by the ERK pathway, we activated Raf signalling in NSΔRafER cells in the presence or absence of the MEK inhibitor U0126 (Figure 4Ai). Complete inhibition of the pathway was confirmed using a phospho-specific antibody to activated ERK in Western blots and by the observation that Raf was no longer able to induce p21Cip1 in the presence of the inhibitor (Figure 4Ai). Addition of the MEK inhibitor to Schwann cells under db-cAMP-induced differentiating conditions did not prevent P0 or Oct-6 induction, suggesting that ERK activity is not required for differentiation (Figure 4Ai, lane 6). This is in agreement with a previous report that inhibition of the ERK pathway had no effect on Schwann cell differentiation (Maurel and Salzer, 2000). We did, however, detect a slight inhibition of P0 expression (Figure 4Ai) and of other differentiation markers by RT–PCR analysis (data not shown) in the presence of U0126. However, in the presence of U0126, the ability of Raf to block the db-cAMP induction of P0 and Oct-6 was lost (Figure 4Ai). In addition, the downregulation of P0 and Oct-6 expression following Raf activation in differentiated cells was also blocked (Figure 4Aii). In order to confirm the specificity of the effects of the MEK inhibitor, we repeated the experiments with a second MEK inhibitor, PD184352, and obtained the same results (Figure 4B). In addition, at the concentrations used in our experiments, we found that the inhibitors had no effect on the activation of the related kinases ERK5 and JNK in response to sorbitol stimulation (data not shown). These results demonstrate that the effects of Raf signalling on the differentiation state of Schwann cells are mediated by the ERK pathway.
Figure 4.
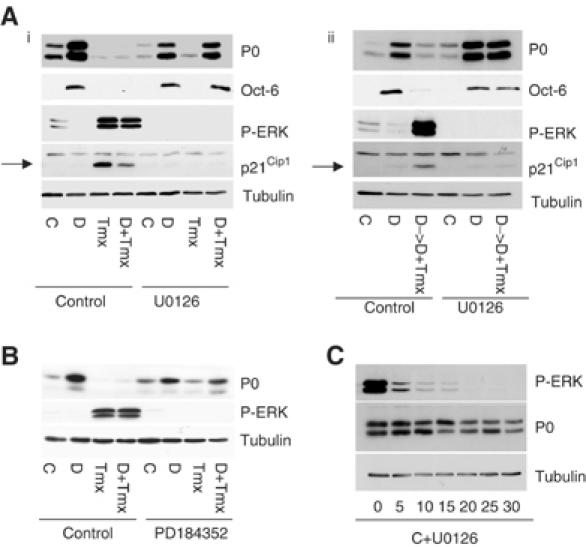
Raf signals via the ERK pathway to block or reverse Schwann cell differentiation. NSΔRafER cells in defined medium were treated with control solvents (C), db-cAMP (D), Tmx, or db-cAMP and Tmx (D+Tmx) for 48 h in the presence or absence of the MEK inhibitors U0126 (30 μM) (Ai) or PD184352 (1 μM) (B). Lysates were analysed by Western blotting with the specified antibodies. (Aii) NSΔRafER cells were treated with control solvents (C) or db-cAMP (D) for 72 h. U0126 was then added to one set of dishes with fresh control solvent or db-cAMP, and with Tmx (D->D+Tmx) where stated for 48 h. (C) NSΔRafER cells in defined medium were treated with the specified dose of U0126 (μM) for 48 h.
As in some other cell types, activation of cAMP signalling was associated with decreased ERK activity (Figure 4Ai, lane 2) (Cook and McCormick, 1993; Sevetson et al, 1993; Wu et al, 1993). This led us to consider whether cAMP signalling induces Schwann cell differentiation solely by inhibiting signalling through the ERK pathway. To test this, we analysed the effects of inhibiting the ERK pathway on Schwann cell differentiation. We added increasing concentrations of the MEK inhibitor U0126 to Schwann cells in defined medium and analysed P0 levels after 48 h. Inhibition of the ERK pathway, however, had little effect on P0 levels, except to cause a slight decrease at high concentrations (Figure 4C). Thus, although inhibition of the ERK pathway may be necessary for differentiation to take place, it is not sufficient to drive the differentiation of Schwann cells. Additional, as yet unknown, differentiation signals must be provided by cAMP signalling pathways.
Oncogenic Ras blocks Schwann cell differentiation
Elevated Ras signalling, due to loss of neurofibromin, is thought to be important in both the initiation and maintenance of the transformed phenotype of Schwann cell-derived tumours in patients with NF1 (Basu et al, 1992; DeClue et al, 1992). Ras signals, in part, through the ERK pathway, and so our findings would predict that Ras-stimulated ERK activation would lead to dedifferentiation of Schwann cells. This would have important implications for our understanding of the development of tumours in this cell type. However, Ras also activates other signalling pathways, and previous work has suggested that Ras signalling induces Schwann cell differentiation (Rosenbaum et al, 1999). We therefore investigated the effects of activated Ras expression on Schwann cell differentiation. Early-passage Schwann cells were infected, at high efficiency, with the retroviral vector LXSN constructed to express oncogenic Ras (Ras). In controls, cells were infected with the empty vector (LXSN). The infected cells were drug-selected for 3 days and then replated into defined medium in the absence or presence of db-cAMP. In the control LXSN cells, db-cAMP treatment resulted in the induction of P0 expression as expected (Figure 5Ai). In the Ras-expressing cells (Ras), however, the levels of P0 were undetectable, even in cells treated with db-cAMP. In addition, real-time PCR analysis demonstrated that Ras blocked the db-cAMP-induced induction of PMP22, periaxin, MBP, Krox-20 and Oct 6 (Figure 5 Aii). Thus, Ras expression is able to block Schwann cell differentiation. Interestingly, expression of oncogenic Ras in Schwann cells resulted in elevated levels of phospho-ERK and cyclin D1 levels comparable to those induced by activating ΔRafER (Figure 5Ai), demonstrating that Ras signals strongly through the Raf/ERK pathway in this cell type.
Figure 5.
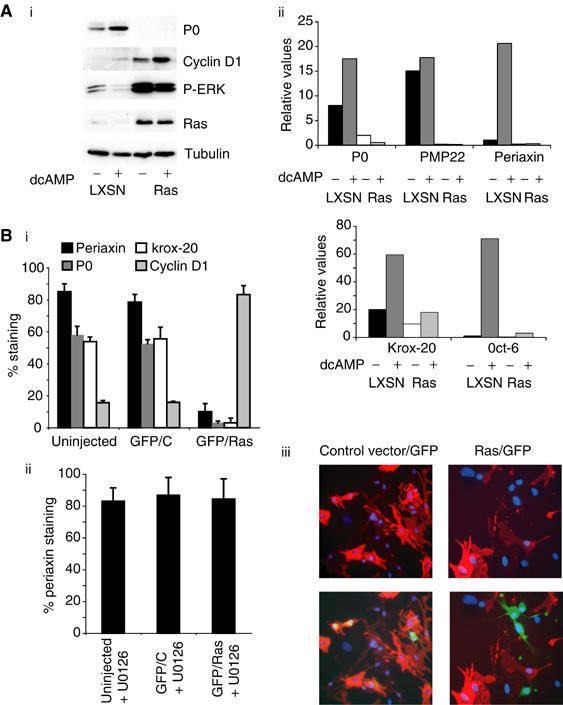
Oncogenic Ras blocks Schwann cell differentiation and can induce dedifferentiation. (A) NS cells infected with retroviruses expressing oncogenic Ras LXSN (Ras) or vector alone (LXSN) were treated with db-cAMP in defined medium for 3 days prior to analysis by Western blotting (i) or real-time PCR (ii). The real-time PCR data shown have been equalised for GAPDH levels. (B) Freshly prepared NS cells treated with db-cAMP for 2 days were microinjected with H-RasVal12 or empty vector control, together with pEGFP-C1. Cultures were fixed after 48 h and analysed by immunofluorescence. (i, ii) Quantification of results showing the percentage of GFP-labelled periaxin-, P0-, Krox-20- and cyclin D1-positive cells and s.d. for each experimental condition. (iii) An example of the results obtained using immunofluorescence against periaxin (red), GFP (green) and with Hoechst (blue) to visualise DNA. (The larger nuclei belong to fibroblasts contaminating the primary Schwann cell cultures.) The bottom panels show triple labelling with the same field shown in the top panels without the GFP channel, in order to enable clear identification of periaxin-positive cells.
To determine whether Ras can induce Schwann cell dedifferentiation, we microinjected a vector expressing oncogenic Ras together with a GFP-expressing construct into differentiated Schwann cells. At 2 days postinjection, we immunostained the cells for expression of the myelin-associated proteins periaxin and P0, the transcription factor, Krox-20 and cyclin D1. Control vector-expressing cells remained differentiated, as determined by the coexpression of GFP and periaxin, P0 and Krox-20; however, expression of oncogenic Ras resulted in the loss of expression of these markers (Figure 5Bi and iii). In contrast, Ras expression led to the induction of cyclin D1. The effects of Ras on periaxin expression were completely blocked by the MEK inhibitor U0126 (Figure 5Bii). Thus, oncogenic Ras expression induces Schwann cell dedifferentiation via the ERK signalling pathway.
Raf/ERK signalling can induce dedifferentiation in the presence of axons
In vivo, Schwann cell differentiation is regulated by axon-derived signals, although the nature of these signals remains unclear (Mirsky and Jessen, 1996). So, while elevated cAMP levels can induce Schwann cell differentiation in vitro, the role of cAMP in this process in vivo is controversial (Poduslo et al, 1995; Howe and McCarthy, 2000). We therefore decided to address the effects of Ras/Raf/ERK activation on Schwann cell differentiation in conditions where the differentiation state of the Schwann cells is under the control of axons. NSΔRafER cells were co-cultured with dissociated neurons isolated from neonatal rat dorsal root ganglia (DRG), using conditions that promote myelination (see Materials and methods). To confirm that the Schwann cells present in the co-cultures were exogenous in origin, we used NSΔRafER cells that had been infected with an EGFP-expressing retrovirus. We observed colocalisation of EGFP-positive cells and myelin proteins and saw similar levels of myelination in cultures containing NSΔRafER cells compared to those with endogenous Schwann cells, confirming that the added NSΔRafER cells were capable of efficient myelination (data not shown).
In the absence of Tmx, immunostaining for MBP revealed the formation of myelin sheaths surrounding the axons (Figure 6A). The sheaths were smooth in structure and had regular endings. In the cultures treated with Tmx for 72 h however, fragmentation of the myelin sheaths was observed, consistent with the initiation of myelin sheath breakdown (Figure 6A and Supplementary Figure S1A). In control cultures containing endogenous Schwann cells, treatment with Tmx had no effect on myelin sheath structure confirming that this effect was due to activation of the ΔRafER protein (data not shown). Myelin sheath breakdown was not associated with apoptosis of the axons or the Schwann cells—the axons associated with degenerating myelin sheaths were intact as indicated by continuous neurofilament staining (data not shown) and TUNEL staining detected no increase in apoptosis following Tmx treatment (Supplementary Figure S1B). The damage to the myelin sheath was indicative of Schwann cell dedifferentiation but, due to the relative stability of the myelin sheath (LeBlanc and Poduslo, 1990), we decided to assess the regulation of the differentiation markers at the mRNA level as we felt this would be a more accurate determinant of the differentiation state of the cells. mRNA was purified from untreated myelinating co-cultures and co-cultures treated with Tmx, and the levels of differentiation markers were determined by semiquantitative RT–PCR. Tmx treatment of myelinated Schwann cell–DRG co-cultures resulted in a strong downregulation of P0, MBP, PMP22 and periaxin mRNA levels (Figure 6B). This result indicated that Raf/ERK signalling causes dedifferentiation of Schwann cells that had been induced to undergo myelination in response to axonal signals.
Figure 6.
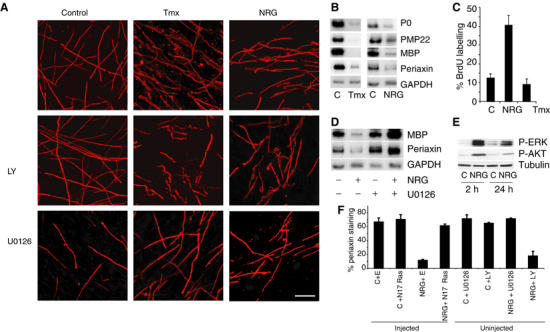
Activation of Raf/ERKK signalling in myelinated DRG–Schwann cell co-cultures induces demyelination. (A) DRG–Schwann cell co-cultures were treated with 50 μg/ml ascorbic acid for 3 weeks to induce myelin formation, prior to the addition of Tmx, 200 ng/ml NRG or control solvents for 72 h in the presence or absence of the inhibitors U0126 (30 μM) and LY294002 (20 μM). Cultures were fixed and stained with anti-MBP antibody (red) and Hoechst to visualise DNA (see Supplementary Figure S1). Scale bar, 50 μm. (B) Semiquantitative RT–PCR analysis of the control-, Tmx- and NRG-treated cultures in (A) was carried out on the pooled lysates of five coverslip cultures per condition. (C) 20 μM BrdU was added to the cultures for 48 h following the addition of Tmx or NRG. The percentage of cells that incorporated BrdU was determined by immunofluorescence. (D) Semiquantitative RT–PCR analysis of pooled myelinated DRG–Schwann cell co-cultures treated with control solvents or 200 ng/ml NRG for 3 days, with or without 30 μM U0126. (E) Western blot analysis of phospho-ERK and phospho-AKT activity in DRG–Schwann cell co-cultures following 2 or 24 h treatment with 200 ng/ml NRG. (F) Freshly purified Schwann cells were plated into defined medium and then microinjected with a dominant-negative form of Ras (N17Ras) or control vector (E) along with a vector expressing GFP. At 6 h following injection, the cells were treated with 200 ng/ml NRG or control solvent (LHS). In addition (RHS), uninjected cells were pretreated with 30 μM U0126 or 20 μM LY294002 prior to stimulating with 200 ng/ml NRG or control solvent (C). After 48 h, the cells were stained for periaxin, and GFP-positive cells were scored for periaxin staining.
A recent report has shown that high levels of the mitogen neuregulin (NRG) can lead to the breakdown of myelin sheaths in DRG–Schwann cell co-cultures and induce Schwann cell proliferation (Zanazzi et al, 2001). We observed that addition of NRG to our myelinated Schwann cell–DRG co-cultures resulted in breakdown of the myelin sheath to a similar extent to that induced by Raf activation (Figure 6A and Supplementary Figure S1A). In addition, NRG addition also led to a downregulation of differentiation markers (Figure 6B), demonstrating that treatment of the co-cultures with high levels of NRG can result in Schwann cell dedifferentiation. In contrast to NRG addition however, Raf activation is unable to stimulate proliferation (Figure 6C). This is likely to be due to the inability of NRG to induce p21Cip1, the CDKI that is responsible for the Raf-induced cell cycle arrest (Supplementary Figure S2) (Lloyd et al, 1997). However, it is clear that proliferation is a late event following extensive myelin degradation and so is not required for dedifferentiation (Zanazzi et al, 2001).
In these cultures, the addition of NRG results in sustained activation of the ERK pathway (Figure 6E). To test whether demyelination was mediated by the Raf/ERK pathway, we inhibited the ERK pathway using U0126, prior to the addition of NRG. U0126 blocked the effects of NRG treatment on MBP and periaxin mRNA levels and myelin sheath breakdown (Figure 6A and D), demonstrating that NRG signals via the ERK pathway to induce dedifferentiation. NRG also results in activation of the PI-3 kinase pathway (Figure 6E); however, inhibition of this pathway with LY294002 had no effect on the ability of NRG to induce myelin breakdown (Figure 6A).
The addition of 200 ng/ml NRG to quiescent Schwann cells results in activation of Raf kinase activity and sustained ERK activation (data not shown). In order to determine whether NRG-induced dedifferentiation requires Ras signalling pathways, we microinjected a dominant-negative form of Ras into differentiated Schwann cells freshly purified from sciatic nerve. Figure 6F clearly shows that the dominant-negative form of Ras blocks NRG-induced dedifferentiation of these cells, demonstrating that NRG drives Schwann cell dedifferentiation via Ras/ERK signalling pathways. In these cells, NRG-induced dedifferentiation was also blocked by the MEK inhibitor U0126 but not by the PI3 kinase inhibitor LY294002, further demonstrating the requirement for ERK signalling in inducing Schwann cell dedifferentiation.
Sciatic nerve transection induces increased ERK signalling
To assess the role of ERK signalling in Schwann cell dedifferentiation in vivo, we examined the effect of sciatic nerve transection on the levels of ERK activity in the degenerating distal nerve stump. We used Western blot analysis and immunocytochemistry to determine the levels of phospho-ERK as a measure of ERK activity. Sciatic nerve transection resulted in a large increase in the levels of phospho-ERK at 1 day post-transection compared to control nerves (Figure 7A). In addition, the levels of phospho-ERK remained elevated at 3 days post-transection, although the signal was less pronounced by this stage. Thus, sciatic nerve transection results in a sustained induction of ERK activity, similar to that seen with Raf activation in NSΔRafER cells. Following nerve transection, Schwann cells do not dedifferentiate in the proximal stump, apart from at the very tip (Carroll et al, 1997). We therefore do not expect to find sustained ERK activation in this section of nerve. To test this, we analysed phospho-ERK levels in sections of both stumps. We found, consistent with the findings of Sheu et al (2000), that although we could detect similar levels of ERK activation in the tips of the stumps, only the distal stump showed ERK activation along the nerve (Figure 7B).
Figure 7.
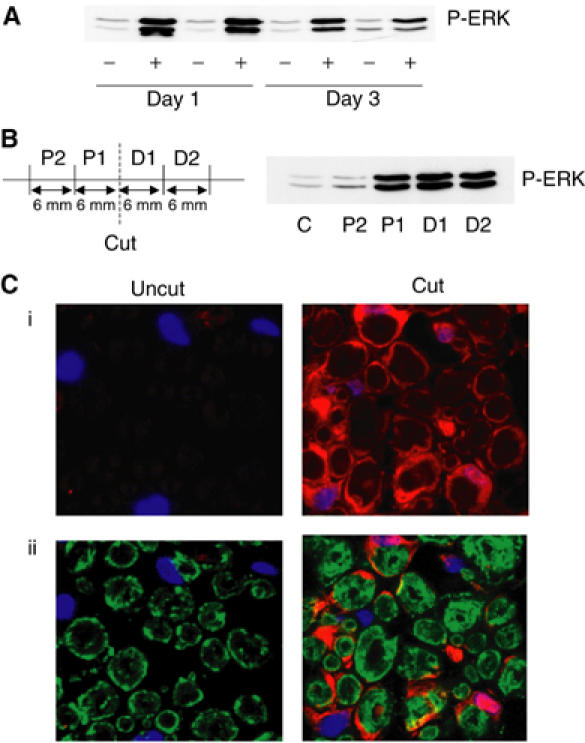
Sciatic nerve transection induces elevated ERK signalling. (A) Transected (+) or uncut control (−) nerves from 8-week-old Sprague–Dawley rats were analysed by Western blotting for ERK activity at 1 day or 3 days post-insult. Two animals were examined per time point. (B) At 24 h following transection, 6 mm sections were isolated from the distal and proximal stumps of the cut nerve and analysed by Western blotting for phospho-ERK. (C) Sections of transected (cut) and untransected (uncut) sciatic nerves were stained with antibodies recognising phospho-ERK (red) and P0 (green) at 1 day post-transection. Nuclei were stained with Hoechst (blue). (i) Projection of a series of confocal images showing elevated phospho-ERK levels in cut nerves. (ii) Single-layer confocal images of triple-labelled sections.
To assess whether the increase in ERK activity was associated with Schwann cells in the distal nerve stump, we compared the localisation of phospho-ERK with immunostaining for a myelin sheath protein (P0) and DNA (Hoechst) (Figure 7C). We observed, in transected nerves, that elevated levels of phospho-ERK were found in the cytoplasm and nucleus of cells that were P0 positive (Figure 7C) and S100 positive (data not shown), confirming that ERK was activated in myelinating Schwann cells. High levels of phospho-ERK staining were still detectable 3 days after the transection (data not shown), demonstrating that axonal damage results in the activation of a strong, sustained signal through the ERK pathway in the associated Schwann cells.
Discussion
The production of new cells in an adult organism requires strict controls. Many cell types that are produced continuously, such as some epithelial cells and cells of the haematopoietic system, arise from a slowly dividing population of stem cells, which give rise to rapidly dividing precursor cells that divide a limited number of times before undergoing terminal differentiation. In other cell types such as Schwann cells, liver cells and endothelial cells, which tend to divide infrequently and usually following specific demands such as injury, a different mechanism of producing new cells tends to be used. In these cell types, the differentiated cell can generate new cells by dedifferentiating and re-entering the cell cycle. This regenerative capacity of mature cells also needs to be under tight regulatory controls, both to stop inappropriate proliferation and to supply fresh cells when required. The regenerative capacity of Schwann cells is important for successful nerve repair throughout the lifespan of the adult, but the signalling pathways controlling their dedifferentiation are poorly understood. Here, we demonstrate that sustained signalling through the Ras/Raf/ERK pathway is able to drive the dedifferentiation of Schwann cells. These findings have important implications for our understanding of the regenerative process and also have intriguing implications for our understanding of tumour formation in this cell type.
Nerve injury leads to degeneration of damaged axons and myelin sheaths in a process known as Wallerian degeneration (Scherer and Salzer, 2001). During this degeneration process, the Schwann cells dedifferentiate and proliferate, then redifferentiate and remyelinate regenerated axons as part of the repair process. The switch between the two states can be separated from the effects on the cell cycle as Schwann cells quiesce in the absence of mitogen but require an additional signal, such as elevated cAMP signalling, to drive the differentiation process. We show that constitutive activation of Ras/Raf/ERK signalling is sufficient to induce Schwann cell dedifferentiation. This effect is also independent of the effects on the cell cycle as activation of Ras/Raf/ERK under these conditions does not drive re-entry into the cell cycle. Indeed activation of this pathway induces a cell cycle arrest in Schwann cells (Ridley et al, 1988). The effects of Ras/Raf/ERK signalling are dominant over the differentiation signal as Ras/Raf/ERK drives the dedifferentiation of Schwann cells in the continual presence of a differentiation signal, whether this be cAMP or the presence of axons.
The kinetics of Schwann cell dedifferentiation is also consistent with Ras/Raf/ERK actively driving the dedifferentiation process rather than simply blocking the differentiation signal. We found that removal of cAMP from differentiated cells results in only a very slow decrease in the expression levels of P0. In addition, previous work showed that Schwann cells purified and plated in defined medium in the absence of mitogens maintain P0 expression for many days, even in the absence of axonal signals (Cheng and Mudge, 1996). In contrast, activation of Ras/Raf/ERK signalling results in the complete loss of differentiation markers within 48 h, with a decrease in expression detectable at much earlier time points. Our results in vitro are also consistent with in vivo observations of Schwann cell dedifferentiation following nerve transection. It has previously been reported that the mRNA levels of myelin sheath proteins such as MBP and P0 are substantially reduced 1–2 days postinjury, again suggesting that active signalling is driving the dedifferentiation of the Schwann cells in vivo (Trapp et al, 1988; LeBlanc and Poduslo, 1990). The sustained elevation of phospho-ERK levels following nerve injury that we see in the present study, which are similar to levels we see with oncogenic Ras expression, is consistent with this pathway being responsible for actively driving the dedifferentiation process.
The signal that drives the dedifferentiation process in response to axonal damage is unknown. Most studies to date have focused on the role of NRG signalling, which is important in several stages of Schwann cell development (Garratt et al, 2000). However, high concentrations of NRG have been shown to both block myelination in Schwann cell–DRG co-cultures and induce the breakdown of myelin sheaths in myelinating cultures (Zanazzi et al, 2001). In addition, we now show that treatment of myelinated Schwann cell–DRG co-cultures with high concentrations of NRG induces downregulation of MBP and periaxin mRNAs and that this is blocked by a MEK inhibitor. This could lead to speculation that NRG is the signal responsible for triggering dedifferentiation in vivo. However, there are problems with this suggestion: NRG expression in vivo during Wallerian degeneration is detected at 3 days postinjury, which coincides with the peak of Schwann cell proliferation but is too late to account for dedifferentiation (Carroll et al, 1997). Moreover, erBB2/3 receptor upregulation and sustained erbB2 phosphorylation occur only 5 days postaxotomy (Carroll et al, 1997; Kwon et al, 1997). The timing of NRG expression therefore suggests that NRG signalling is more likely to play a role in regulating Schwann cell proliferation rather than affecting the differentiation state. Another possible contender is fibrin, which has recently been shown to be an important regulator of remyelination following nerve injury (Akassoglou et al, 2002). This report associated fibrin accumulation with Schwann cell proliferation and an inhibitory effect on Schwann cell remyelination. Interestingly, culture of Schwann cells on fibrin results in a sustained increase in ERK activity. However, fibrin appeared to have only a small effect on P0 expression, which would argue against fibrin being the sole dedifferentiation signal. It will be interesting to determine the signal(s) responsible for driving Schwann cell dedifferentiation and to establish how sustained signalling through the ERK pathway is maintained.
Ras/Raf/ERK signalling induces dedifferentiation of myelinating Schwann cells, but it also results in a cell cycle arrest. In vivo, however, Schwann cell dedifferentiation is accompanied by proliferation. How can we account for this disparity? Interestingly, although Schwann cell dedifferentiation, as determined by a decrease in mRNA levels of myelin markers, occurs within the first 48 h following axonal damage, Schwann cell proliferation occurs later, 3 days after injury (Carroll et al, 1997). Analysis of the levels of phospho-ERK in the damaged nerve shows that by 3 days the levels of phospho-ERK, although still elevated, are much lower than at 1 day. This may suggest that initial high levels of phospho-ERK may inhibit proliferation at the same time as they induce dedifferentiation and that proliferation is only triggered once ERK activity returns to lower levels.
We have shown that Ras/Raf/ERK activation can drive the dedifferentiation of Schwann cells and that NRG-driven dedifferentiation signals through Ras/Raf/ERK signalling pathways. However, we have been unable to measure Raf activation following nerve transection in vivo (E Perez-Nadales, unpublished data, 2004). This result, however, is difficult to interpret. Mitogen activation of synchronous, quiescent cultures results in a transient, measurable increase in Raf activity that is sufficient however to trigger sustained activation of ERK. It is therefore likely that Raf activation following nerve transection would be very difficult to detect. We cannot rule out however that other signalling pathways are responsible for triggering sustained ERK activation following nerve transection.
NF1 patients characteristically develop benign neurofibromas, which consist primarily of Schwann cells and have an increased risk of developing malignant Schwann cell tumours (Cichowski and Jacks, 2001). Elevated Ras signalling, as a result of loss of expression of the Ras-GAP neurofibromin, has been shown to be involved in the development and maintenance of these tumours. It is therefore of interest how Ras signalling affects Schwann cell behaviour. We find that elevated Ras signalling reverses Schwann cell differentiation. Our results, however, are in contrast to those reported by Rosenbaum et al (1999), who found that oncogenic Ras expression leads to the constitutive upregulation of P0 expression and concluded that Ras signalling acts to promote differentiation of Schwann cells. We are unable to explain the differences in these results. In our hands, constitutive Ras expression, even at low levels, abolishes P0 expression. Moreover, the use of an inducible expression system and the microinjection experiments of Ras into passage 1 Schwann cells would argue against the possibility that our cells were a selected subpopulation of Schwann cells. Our finding that Ras/Raf/ERK signalling pushes Schwann cells towards a less differentiated state has possible implications for our understanding of the development of tumours in NF1 patients. It would seem likely that dedifferentiated cells would be more susceptible to proliferative signals. However, both Ras-expressing and NF1−/− Schwann cells are cell cycle inhibited. This has led to the speculation that either a second genetic event or a specific cellular environment is required for the Ras signal to become proliferative (Cichowski and Jacks, 2001). We showed previously that p53 loss is sufficient for the Ras signal to be proliferative in the absence of further mitogenic stimulation (Lloyd et al, 1997; Mitchell et al, 2003). Intriguingly, it has also been shown that cAMP signalling is able to convert the Ras signal into a proliferative signal (Kim et al, 1997). We show here that cAMP signalling inhibits ERK signalling, and this may be sufficient to block the cell cycle inhibitory signal. It could be envisaged that either p53 mutation or the presence of a cAMP-inducing signal would be sufficient to promote the dedifferentiated Schwann cell to proliferate.
It is not clear why some differentiated cell types are able to dedifferentiate and proliferate whereas others cannot. An increase in our understanding of the mechanisms involved in dedifferentiation may enable us to control and possibly alter the plasticity of the differentiated state.
Materials and methods
Cell culture and in vitro differentiation assays
Primary Schwann cells derived from postnatal day 7 Sprague–Dawley rat sciatic nerves were purified by sequential immunopanning (Cheng et al, 1995). Cells were cultured as described by Mathon et al (2001). NSΔRafER cells are as previously described (Lloyd et al, 1997). NSLXSN or NSRasLXSN cells were generated using producer cells expressing LXSN or RasLXSN. For the duration of the differentiation assays, NSΔRafER, NSLXSN or NSRasLXSN cells were cultured in serum-free defined medium with B/S supplement (Mitchell et al, 2003). Cells were induced to differentiate by the addition of 1 mM db-cAMP.
DRG and Schwann cell co-cultures
DRG were dissected from E15 Sprague–Dawley rats and plated on poly-D-lysine and laminin precoated coverslips in defined medium supplemented with 50 ng/ml NGF (Alomone labs). Cultures were treated with 10−5 M cytosine β-D-arabinofuranoside for 1 day, then pulsed twice with 10−5 M 5-fluoro deoxyuridine. A total of 2 × 105 Schwann cells were plated per neuronal culture and, after 4 days, treated with 50 μg/ml ascorbic acid to induce basal lamina formation and myelination. Cultures were fed with fresh medium every 3 days.
Microinjection
Passage 1 Schwann cells were plated on PDL/laminin precoated coverslips in defined medium and when indicated were induced to differentiate for 2 days with db-cAMP. The Schwann cells were microinjected in the nucleus with H-RasVal12 or N17H-RasVal12 in the pRK5myc vector (Lamarche et al, 1996) or empty vector together with pEGFP-C1 (Clontech). Cells were fixed and stained for periaxin, P0, Krox-20 or cyclin D1 expression 48 h later.
Immunofluorescence
DRG–Schwann cell co-cultures were fixed with 4% paraformaldehyde, permeabilised with ice-cold methanol, blocked in 3% BSA and incubated with 1:500 anti-MBP antiserum (Boehringer Mannheim) in 3% BSA in PBS. Microinjected Schwann cell cultures were fixed as above, blocked and permeabilised in antibody-diluting solution (ADS; 10% calf serum with 0.1 M lysine, 0.2% Triton X-100 in PBS). The periaxin antibody was a gift from P Brophy, P0 (P07) from J Archelos, cyclin D1 (sc450) from Santa Cruz and Krox-20 from Berkeley Antibody Company. The nuclei were stained using Hoechst (Sigma). BrdU antibody was used according to the manufacturer's instructions (Roche).
Western blots
Protein extraction and Western blotting were carried out as previously described by Lloyd et al (1997). Blots were analysed using anti-P0 (P07 gift from J Archelos), cyclin D1 (sc450, Santa Cruz), p21Cip1 (sc397g, Santa Cruz), anti-Ras antibody (259-Y13, gift from A Hall), anti-Oct-6 (gift from D Miejer), anti-α-tubulin antibody (Sigma), anti-phospho-AKT (Cell Signalling Technology) and anti-phospho-ERK (Sigma). Proteins were visualised using ECL normal or plus chemiluminescent solutions (Amersham).
Semiquantitative RT–PCR
RNA was isolated using RNAgents total RNA isolation system (Promega). Samples of RNA were treated with 1 μl DNase (10 U/μl, Amersham) prior to cDNA synthesis using the Superscript preamplification system for first-strand synthesis (Gibco BRL). Samples were equalised for GAPDH before semiquantitative PCR reactions were carried out over a range of cycles. Reactions were separated on a 4% nondenaturing acrylamide gel and signals were quantified using an FX phosphoimager and Quantity-One quantification software (both Bio-Rad). Primers used were as follows: Krox-20 sense ATAGCCGGCTTGGGG, antisense TCCGAGTAGCTTCGC; periaxin sense CCTGAATTCACCTTCTTCCC, antisense ACGTCACCAGTGAGTAGCCACGCGGTC; MBP sense ATGTGATGGCATCACAGAAGAGAC, antisense GTCTGCTCTAACTAGCTATTTG; Oct-6, P0 and PMP22 (Blanchard et al, 1996). Real-time PCR was carried out using an Opticon 2 DNA engine (MJ Research) with a Sybr green qPCR kit (GRI). The real-time PCR data were equalised for differences in GAPDH levels during analysis by the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Sciatic nerve transection and analysis
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary methods
Acknowledgments
We thank M Raftopoulou for help with microinjection, R Marais, G Zanazzi and P Cohen for helpful discussions and reagents, K Jessen for help with the in vivo studies, and M Raff and A Hall for critical reading of the manuscript. ACL is a Cancer Research UK Senior Cancer Research Fellow. EP-N and DSM were funded by Cancer Research UK. MCH was funded by an MRC studentship and is currently funded by the Wellcome Trust grant 066061. DP is funded by the Wellcome Trust grant 042257. AWM is funded by the MRC.
References
- Akassoglou K, Yu WM, Akpinar P, Strickland S (2002) Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron 33: 861–875 [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A (2000) Ras and Rho GTPases: a family reunion. Cell 103: 227–238 [DOI] [PubMed] [Google Scholar]
- Barbacid M (1987) ras genes. Annu Rev Biochem 56: 779–827 [DOI] [PubMed] [Google Scholar]
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J (1992) Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356: 713–715 [DOI] [PubMed] [Google Scholar]
- Bermingham JR Jr, Shumas S, Whisenhunt T, Rosenfeld MG, Scherer SS (2001) Modification of representational difference analysis applied to the isolation of forskolin-regulated genes from Schwann cells. J Neurosci Res 63: 516–524 [DOI] [PubMed] [Google Scholar]
- Blanchard AD, Sinanan A, Parmantier E, Zwart R, Broos L, Meijer D, Meier C, Jessen KR, Mirsky R (1996) Oct-6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: comparison with Oct-1, Krox-20, and Pax-3. J Neurosci Res 46: 630–640 [DOI] [PubMed] [Google Scholar]
- Carroll SL, Miller ML, Frohnert PW, Kim SS, Corbett JA (1997) Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci 17: 1642–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Khan M, Mudge AW (1995) Calcitonin gene-related peptide promotes Schwann cell proliferation. J Cell Biol 129: 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Mudge AW (1996) Cultured Schwann cells constitutively express the myelin protein P0. Neuron 16: 309–319 [DOI] [PubMed] [Google Scholar]
- Cichowski K, Jacks T (2001) NF1 tumor suppressor gene function: narrowing the GAP. Cell 104: 593–604 [DOI] [PubMed] [Google Scholar]
- Cook SJ, McCormick F (1993) Inhibition by cAMP of Ras-dependent activation of Raf. Science 262: 1069–1072 [DOI] [PubMed] [Google Scholar]
- Crespo P, Leon J (2000) Ras proteins in the control of the cell cycle and cell differentiation. Cell Mol Life Sci 57: 1613–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue JE, Papageorge AG, Fletcher JA, Diehl SR, Ratner N, Vass WC, Lowy DR (1992) Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69: 265–273 [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Fregien N, Wood PM, Bunge MB (1993) Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development 119: 867–880 [DOI] [PubMed] [Google Scholar]
- Freeman M (1998) Complexity of EGF receptor signalling revealed in Drosophila. Curr Opin Genet Dev 8: 407–411 [DOI] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C (2000) Neuregulin, a factor with many functions in the life of a Schwann cell. BioEssays 22: 987–996 [DOI] [PubMed] [Google Scholar]
- Halfar K, Rommel C, Stocker H, Hafen E (2001) Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development 128: 1687–1696 [DOI] [PubMed] [Google Scholar]
- Howe DG, McCarthy KD (2000) Retroviral inhibition of cAMP-dependent protein kinase inhibits myelination but not Schwann cell mitosis stimulated by interaction with neurons. J Neurosci 20: 3513–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, DeClue JE, Ratner N (1997) cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res 49: 236–247 [PubMed] [Google Scholar]
- Kwon YK, Bhattacharyya A, Alberta JA, Giannobile WV, Cheon K, Stiles CD, Pomeroy SL (1997) Activation of ErbB2 during wallerian degeneration of sciatic nerve. J Neurosci 17: 8293–8299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A (1996) Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87: 519–529 [DOI] [PubMed] [Google Scholar]
- LeBlanc AC, Poduslo JF (1990) Axonal modulation of myelin gene expression in the peripheral nerve. J Neurosci Res 26: 317–326 [DOI] [PubMed] [Google Scholar]
- Lemke G, Chao M (1988) Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development 102: 499–504 [DOI] [PubMed] [Google Scholar]
- Lin BM, Stone JC, van Aelst L, Serrano M, Lowe SW (1998) Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev 12: 3008–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lloyd AC, Obermuller F, Staddon S, Barth CF, McMahon M, Land H (1997) Cooperating oncogenes converge to regulate cyclin/cdk complexes. Genes Dev 11: 663–677 [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185 [DOI] [PubMed] [Google Scholar]
- Mathon NF, Malcolm DS, Harrisingh MC, Cheng L, Lloyd AC (2001) Lack of replicative senescence in normal rodent glia. Science 291: 872–875 [DOI] [PubMed] [Google Scholar]
- Maurel P, Salzer JL (2000) Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci 20: 4635–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR (1996) Schwann cell development, differentiation and myelination. Curr Opin Neurobiol 6: 89–96 [DOI] [PubMed] [Google Scholar]
- Mirsky R, Jessen KR (2001) Embryonic and early postnatal development of Schwann cells. In Glial Cell Development, Jessen KR, Richardson WD (eds) Oxford: Oxford University Press [Google Scholar]
- Mitchell PJ, Perez-Nadales E, Malcolm DS, Lloyd AC (2003) Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype. Mol Cell Biol 23: 2530–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Weinmaster G, Kuhn R, Lemke G (1989) SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron 3: 783–793 [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R (1991) The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol 112: 457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J (2001) Egr2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 30: 355–368 [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Walikonis RS, Domec MC, Berg CT, Holtz-Heppelmann CJ (1995) The second messenger, cyclic AMP, is not sufficient for myelin gene induction in the peripheral nervous system. J Neurochem 65: 149–159 [DOI] [PubMed] [Google Scholar]
- Raff MC, Durand B, Gao FB (1998) Cell number control and timing in animal development: the oligodendrocyte cell lineage. Int J Dev Biol 42: 263–267 [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Noble M, Land H (1988) Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J 7: 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper E, Weinberg W, Watt FM, Land H (2001) p19ARF-independent induction of p53 and cell cycle arrest by Raf in murine keratinocytes. EMBO Rep 2: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum KH, Boissy YL, Ling B, Ratner N (1999) Neurofibromin, the neurofibromatosis type 1 Ras-GAP, is required for appropriate P0 expression and myelination. Ann NY Acad Sci 883: 203–214 [PubMed] [Google Scholar]
- Samuels ML, Weber MJ, Bishop JM, McMahon M (1993) Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol 13: 6241–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer Ss, Salzer JL (2001) Axon–Schwann cell interactions during peripheral nerve degeneration and regeneration. In Glial Cell Development, Jessen KR, Richardson WD (eds) Oxford: Oxford University Press [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602 [DOI] [PubMed] [Google Scholar]
- Sevetson BR, Kong X, Lawrence JC Jr (1993) Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA 90: 10305–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JY, Kulhanek DJ, Eckenstein FP (2000) Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol 166: 392–402 [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Han M (1998) Genetics of RAS signaling in C. elegans. Trends Genet 14: 466–472 [DOI] [PubMed] [Google Scholar]
- Topilko P, Meijer D (2001) Transcription factors that control Schwann cell develoment and myelination. In Glial Cell Development, Jessen KR, Richardson WD (eds) Oxford: Oxford University Press [Google Scholar]
- Trapp BD, Hauer P, Lemke G (1988) Axonal regulation of myelin protein mRNA levels in actively myelinating Schwann cells. J Neurosci 8: 3515–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW (1993) Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262: 1065–1069 [DOI] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL (2001) Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol 152: 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick SD, Brown A, Gridley T, Lemke G (1999) Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 126: 1397–1406 [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G (1996) The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci 8: 129–145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary methods


