Abstract
von-Willebrand factor (vWF) and tissue-type plasminogen activator (tPA) are products of endothelial cells acutely released into the vasculature following cell activation. Both factors are secreted after intraendothelial Ca2+ mobilization, but exhibit opposing physiological effects with vWF inducing coagulation and tPA triggering fibrinolysis. To identify components that could regulate differentially the release of pro- and antithrombogenic factors, we analyzed the contribution of Rab3D and the annexin A2/S100A10 complex, proteins implicated in exocytotic events in other systems. We show that mutant Rab3D proteins interfere with the formation of bona fide Weibel–Palade bodies (WPbs), the principal storage granules of multimeric vWF, and consequently the acute, histamine-induced release of vWF. In contrast, neither appearance nor exocytosis of tPA storage granules is affected. siRNA-mediated downregulation of annexin A2/S100A10 and disruption of the complex by microinjection of peptide competitors result in a marked reduction in vWF but not tPA secretion, without affecting the appearance of WPbs. This indicates that distinct mechanisms underlie the acute secretion of vWF and tPA, enabling endothelial cells to fine-regulate the release of thrombogenic and fibrinolytic factors.
Keywords: calcium, coagulation, exocytosis, fibrinolysis, Weibel–Palade bodies
Introduction
Endothelial cells supply the blood with factors regulating fibrinolysis and thrombogenesis, thereby preventing coagulation under physiological conditions but promoting thrombus formation at sites of tissue and blood vessel damage. These factors include procoagulant and proinflammatory proteins like von-Willebrand factor (vWF), Factor VIII and P-selectin, as well as anticoagulant factors like tissue-type plasminogen activator (tPA) and protein S (for reviews, see Holvoet and Collen, 1997; Michiels, 2003). Typically, these factors are released into the blood stream (soluble factors) or exposed on the endothelial cell surface (membrane proteins) by constitutive and regulated exocytosis. The latter follows endothelial cell activation, for example, by stimuli like histamine, platelet-activating factor or purine nucleotides, and requires an intraendothelial increase in free Ca2+ concentration (for reviews, see Datta and Ewenstein, 2001; Nilius and Droogmans, 2001). Some endothelial factors participating in Ca2+-dependent exocytosis and probably acting downstream of the Ca2+ signal have been described. They include the Ca2+-binding protein calmodulin, the small GTPase RalA and possibly other small and heterotrimeric G proteins (Datta and Ewenstein, 2001). It has also been shown that at least the regulated secretion of vWF requires Rho signaling and a participation of the microfilament system (Vischer et al, 2000; Klarenbach et al, 2003). Nonetheless, the molecular mechanisms underlying regulated exocytosis in endothelial cells, in particular possible differences in the machineries controlling the acute release of pro- and anticoagulant factors, are to a large extent unknown.
Most thrombogenic factors released from endothelial cells in an acute manner, in particular vWF and P-selectin, are stored in specialized granules, the Weibel–Palade bodies (WPbs) (Weibel and Palade, 1964; Wagner, 1993). WPbs are thought to belong to a group of secretion-competent lysosome-related organelles that exhibit a complex maturation process (Hannah et al, 2003). They are large in size (up to 3 μm in length and 0.2 μm in diameter) and acquire multimeric forms of vWF. High-resolution capacitance measurements have revealed that WPb exocytosis following an intraendothelial rise in Ca2+ only occurs after some delay (Zupancic et al, 2002), whereas the secretion of small, non-WPb granules, for example, those containing antithrombogenic tPA, is observed immediately in response to intracellular Ca2+ mobilization (Kooistra et al, 1994; Lupu et al, 1995). However, the site of intraendothelial tPA storage has not been assigned unambiguously. Whereas a unique storage granule for tPA that is small and morphologically distinct from WPbs has been described (Emeis et al, 1997), other groups have reported a colocalization of tPA with WPbs (Datta et al, 1999; Rosnoblet et al, 1999).
Storage of vWF and tPA in the same granule would result in the simultaneous release of both factors following intraendothelial Ca2+ rise, whereas the use of distinct granules could allow the cell to control the acute release of pro- (vWF) and antithrombogenic (tPA) factors in a differential manner. To obtain evidence for the existence of separate mechanisms regulating the secretion of vWF and tPA, we elucidated whether factors implicated in regulated secretion in other cell systems selectively participate in the acute, histamine-evoked exocytosis of vWF or tPA. On the one hand, we concentrated on the Rab3 isoform Rab3D known, for example, to regulate ACTH secretion in AtT-20 cells and Ca2+-triggered exocytosis in stimulated PC12 cells (Baldini et al, 1998; Schlüter et al, 2002). On the other hand, we examined the contribution of the annexin A2/S100A10 complex previously implicated in mediating Ca2+-regulated chromaffin granule exocytosis and a Ca2+-induced membrane capacitance increase in cultured endothelial cells (Ali et al, 1989; Sarafian et al, 1991; König et al, 1998). We show that Rab3D is recruited to vWF-positive WPbs but not tPA-containing granules, whereas annexin A2/S100A10 resides in the cytoplasm and on the plasma membrane. Functionally interfering with Rab3D and annexin A2/S100A10 by expression of dominant-negative mutants and siRNA-mediated downregulation, respectively, results in an inhibition of histamine-evoked vWF but not tPA secretion. This identifies endothelial cell components selectively regulating the Ca2+-induced exocytosis of WPbs but not tPA granules, and thus provides evidence for the existence of distinct mechanisms underlying the acute release of pro- and antithrombogenic factors from the vascular endothelium.
Results
Rab3D localizes to WPbs but not to tPA-containing granules
We first analyzed whether Rab3D is localized to certain secretory granules of cultured human umbilical vein endothelial cells (HUVECs). Rab3D, whose expression in HUVECs was confirmed by RT–PCR (data not shown), was expressed as a YFP-tagged fusion protein and its localization was compared to that of endothelial granule markers. Figure 1 shows that Rab3D colocalizes with vWF and P-selectin, whereas no colabeling with tPA-containing vesicles is observed. Interestingly, the morphological appearance of the Rab3D-positive granules differs with the level of Rab3D expression. Cells expressing low levels of YFP–Rab3D show the characteristic cigar-shaped WPbs positive for vWF, P-selectin and Rab3D, whereas cells with high Rab3D expression levels contain bigger and more spherical WPbs (Figure 1). While some Rab3D also resides on Golgi structures (as revealed by costaining with antibodies against GM 130; not shown), storage granules for another secreted factor produced by endothelial cells, endothelin-1, are negative for Rab3D (not shown). The recruitment of Rab3D to WPbs also shows specificity among Rab proteins, since YFP-tagged Rab4, Rab11b and Rab18 are not localized to WPbs and the morphology of WPbs is not altered in cells expressing these Rabs (not shown). Moreover, the specificity of YFP–Rab3D targeting to WPbs is not a consequence of the YFP fusion, since myc-tagged Rab3D shows the identical recruitment to vWF- and P-selectin-positive WPbs (not shown).
Figure 1.

Localization of YFP-tagged Rab3D in HUVECs. HUVECs expressing YFP–Rab3Dwt were processed for immunofluorescence using anti-human vWF (A), anti-human P-selectin (B) or anti-human tPA antibodies (C) followed by Texas red-conjugated secondary antibodies. Comparison of the YFP fluorescence with the respective antibody stainings reveals the colocalization of Rab3D with vWF- (filled arrowheads) and P-selectin- but not tPA-containing (empty arrowheads) granules. The morphological appearance of the vWF-positive granules differs in cells expressing low levels of Rab3D (cigar-shaped structures, see inset showing a four-fold magnification) and high levels of Rab3D (bigger, more spherical granules, see four-fold magnification inset). In addition to the labeling of the respective granules, some Golgi staining most likely reflecting newly synthesized protein is observed, particularly in the case of tPA. Bars represent 10 μm.
Rab proteins cycle between an active, membrane-bound GTP conformation and an inactive, cytosolic GDP conformation, with the cycle being controlled by accessory factors. To elucidate whether Rab3D recruitment to vWF- and P-selectin-positive granules requires the active GTP-bound conformation, we recorded the distribution of different YFP-tagged Rab3D mutant proteins. The mutants introduced were YFP–Rab3DQ81L, which has suffered a loss in intrinsic GTPase activity, resulting in a permanently active, GTP-bound protein, and two mutants deficient in GTP binding, YFP–Rab3DT36N and YFP–Rab3DN135I, previously shown to act in a dominant-negative manner (Baldini et al, 1998; Chen et al, 2002). Rab3DQ81L continues to colocalize with vWF and P-selectin (Figure 2), but shows no staining of tPA-positive granules. As already seen in cells expressing high levels of wild-type (wt) Rab3D (Figure 1), WPbs are bigger and more spherical in YFP–Rab3DQ81L-expressing cells (Figure 2A). In contrast to Rab3DQ81L, the mutants exhibiting impaired GTP binding, Rab3DT36N and Rab3DN135I (Figure 2B), show a general cytosolic/nuclear distribution and no colocalization with any of the endothelial storage granules.
Figure 2.

Intracellular distribution of constitutively active and dominant-negative Rab3D mutants. HUVECs expressing YFP–Rab3DQ81L (A), YFP–Rab3DN135I or YFP–Rab3DT36N (B) were processed for vWF, P-selectin- and tPA immunofluorescence (see Figure 1) and the patterns obtained were compared to the distribution of the fluorescently labeled Rab3D mutants. Note that YFP–Rab3DQ81L colocalizes with vWF- and P-selectin but not tPA-positive granules, and that the vWF/P-selectin granules appear larger and more spherical in the YFP–Rab3DQ81L-expressing cells as compared to typical WPbs (inset showing four-fold magnification in the vWF labeling). The YFP–Rab3DN135I and T36N mutants show a general cytosolic plus nuclear distribution, and are not present on any of the granules. Note that the number of vWF-positive structures is greatly reduced in YFP–Rab3DN135I- and YFP–Rab3DT36N- expressing cells. Bars represent 10 μm.
Importantly, the number of WPbs positive for vWF (Figure 2B) and P-selectin (not shown) is greatly reduced in cells expressing Rab3DT36N and N135I, whereas the number and appearance of tPA-containing vesicles are unaltered (Figure 2B). On average, the Rab3DN135I-expressing cells showed no, or less than 10%, of the vWF-positive WPbs found in mock-transfected or nontransfected HUVECs. A similar reduction in the number of vWF- and P-selectin-positive granules is also seen in cells expressing myc-tagged Rab3DN135I (not shown).
Histamine-induced secretion of vWF but not tPA is affected by overexpression of Rab3D wt and mutant proteins
Given the effect of Rab3D mutant expression on the morphological appearance and number of WPbs, we next analyzed whether the Ca2+-triggered exocytosis of vWF is influenced by the overexpression of Rab3Dwt or mutant proteins. Histamine was employed as an established agonist known to evoke a transient increase in intraendothelial Ca2+ concentration (Datta and Ewenstein, 2001) and the acute release of vWF was first visualized microscopically using an antibody internalization assay. In this assay, anti-vWF antibodies are present in the culture medium when the cells are stimulated for secretion. Concomitant with vWF release, the antibodies get access to WPbs, which have fused with the plasma membrane most likely without complete flattening into the cell surface (Schneider, 2001). The antibodies are then trapped intracellularly upon a recapture of the granules, with the postexocytotic granules differing in morphology from the nonexocytosed WPbs (Knop and Gerke, 2002) (Figure 3A). The assay is highly specific since other antibodies are not found in the re-endocytosed granules, and most likely reflects the fact that partially emptied secretory granules are recaptured largely intact following stimulated exocytosis, a phenomenon recently observed in cultured endocrine cells (Taraska et al, 2003). Figure 3B reveals that cells overexpressing YFP–Rab3Dwt show a significantly decreased anti-vWF antibody internalization following histamine stimulation. The number of antibody-containing granules is typically reduced by approximately 80% in cells ectopically expressing YFP–Rab3Dwt, with the extent of reduction correlating with the level of YFP–Rab3Dwt expression (not shown). A similar effect, that is, an almost complete inhibition of antibody internalization in histamine-stimulated HUVECs, is also observed upon expression of the permanently active Rab3D mutant, Rab3DQ81L (not shown).
Figure 3.

Rab3Dwt inhibits histamine-evoked internalization of anti-vWF antibodies into postexocytotic granules. (A) Nontransfected HUVECs were cultivated for 30 min in control medium (left panels) or histamine-containing stimulation medium (right panels). In both cases, the medium also contained polyclonal rabbit anti-vWF antibodies. Subsequently, cells were fixed and permeabilized, and the internalized anti-vWF antibodies were visualized with Texas red-conjugated goat anti-rabbit antibodies (anti-vWF). vWF present in nonexocytosed WPbs was labeled in fixed and permeabilized cells using mouse monoclonal anti-human vWF antibodies and AMCA-conjugated goat anti-mouse IgGs (WPbs). Note that the monoclonal anti-vWF antibody does not react with the vWF-anti-vWF complexes present in the re-internalized vesicles, thereby allowing a distinction between the nonsecreted vWF (blue in the merge image) and the internalized anti-vWF antibodies in postexocytotic vesicles (red in the merge image; see also higher magnification inset). The antibody internalization is only observed following histamine stimulation, and thus is an indicator of previous histamine-triggered exocytosis. (B) The antibody internalization assay shown in (A) was carried out with HUVECs transiently transfected with YFP–Rab3Dwt. A YFP–Rab3Dwt-expressing cell (left) next to a nontransfected control cell (right) is shown following 30 min of histamine stimulation in anti-vWF antibody-containing medium. Note that the anti-vWF antibody internalization indicative of histamine-stimulated secretion is significantly reduced in the YFP–Rab3Dwt-expressing cell. Bars represent 10 μm.
The inhibition of granule recapture in histamine-triggered cells expressing Rab3D or Rab3DQ81L is suggestive of Rab3D functioning in the acute WPb exocytosis. However, it could also reflect an inhibitory effect of Rab3D overexpression on general endocytic membrane uptake. To elucidate this, we visualized receptor-mediated and fluid-phase endocytosis in YFP–Rab3D-expressing HUVECs. Figure 4 reveals that neither of these is affected by overexpression of Rab3Dwt. Likewise, expression of Rab3DQ81L has no effect on receptor-mediated (transferrin) and fluid-phase (dextran) endocytosis (not shown). Together, these data indicate that increasing the amount of Rab3Dwt (or Rab3DQ81L) on WPbs interferes with efficient Ca2+-evoked exocytosis of the granules, thus resulting in a reduced number of postexocytotic granules. Expression of the dominant-negative mutant Rab3DN135I also abolished anti-vWF antibody uptake in histamine-stimulated HUVECs (not shown). In this case, however, the inhibition reflects the marked reduction in the number of WPbs in Rab3DN135I-expressing cells (see Figure 2).
Figure 4.

Receptor-mediated and fluid-phase endocytosis is not affected in YFP–Rab3D-expressing cells. HUVECs transiently expressing YFP–Rab3Dwt were incubated for 15 min in basal medium (without serum and antibiotics) containing either Texas red-conjugated transferrin (TxTf, A) or Alexa568-labeled dextran (AlxDex, B). Cells were then fixed and inspected by fluorescence microscopy. Note that the uptake and distribution of endocytic tracers internalized by receptor-mediated (TxTf) or fluid-phase (AlxDex) endocytosis are not affected by YFP–Rab3D expression (compare the nontransfected and transfected cells shown in the same image). Bars represent 10 μm.
To show unambiguously that overexpression of Rab3Dwt or mutant proteins affects the acute release of vWF, and to evaluate a possible effect on the secretion of tPA, we measured directly the Ca2+-triggered release of both factors. Cell culture media harvested following HUVEC stimulation with histamine were subjected to specific ELISAs for vWF and tPA. The values obtained were compared to those of endothelial cells kept under stimulant-free culture conditions for the respective duration, thus showing only constitutive secretion (Figure 5A). In the case of vWF, the histamine-induced secretion observed in control cells (nontransfected or mock-transfected) is significantly reduced in cells overexpressing YFP–Rab3Dwt or YFP–Rab3DQ81L, respectively (Figure 5B). Similar inhibition of acute vWF secretion is observed in HUVECs overexpressing myc-Rab3Dwt (not shown). Cells expressing YFP–Rab3DN135I could not be analyzed reliably in this assay since they showed a greatly reduced number of WPbs (see Figure 2) and thus only a minimal histamine-induced release of vWF (not shown). The inhibitory effect of overexpressing wt or mutant Rab3D does not result from a displacement of Rab3A from secretory sites previously observed in Rab3DN135I-expressing PC12 cells (Martelli et al, 2000), as RT–PCR did not reveal Rab3A expression in HUVECs (not shown). In contrast to vWF, the histamine-triggered secretion of tPA is not affected to a significant extent by the overexpression of either YFP–Rab3Dwt, YFP–Rab3DQ81L (Figure 5B) or YFP–Rab3DN135I (not shown). Similar results, that is, a decrease in acute vWF but not tPA release in cells overexpressing Rab3Dwt or its mutants, were obtained when the Ca2+-dependent secretion of vWF and tPA was triggered directly by applying the Ca2+-ionophore A23187 (not shown). Thus, increasing the amount of Rab3D in HUVECs specifically interferes with the Ca2+-dependent secretion of vWF but not that of tPA.
Figure 5.

Histamine-induced secretion of vWF but not tPA is inhibited by YFP–Rab3Dwt or YFP–Rab3DQ81L expression. (A) HUVECs grown to confluency in 24-well plates were subjected to histamine stimulation for 5 and 30 min. The acute release of vWF (left panel) and tPA (right panel) into the cell culture supernatant was then quantified by specific vWF- and tPA-ELISAs. In control experiments, cells were kept for the same time in histamine-free medium to reveal the constitutive secretion of both factors. This was set to 100%, and the values obtained with histamine-treated cells are given as the sum of constitutive and regulated secretion. (B) Experiments were carried out as described in (A), using HUVECs expressing YFP–Rab3Dwt or YFP–Rab3DQ81L. Mean values (±s.e.m.) of histamine-triggered release are given as percent increase over constitutive secretion and compared to those obtained for nontransfected control cells (values obtained for mock-transfected cells are equivalent to those of the nontransfected controls, not shown). In the case of tPA, only 5 min values are shown because the 30 min values are only marginally increased over constitutive secretion (see (A)). The effect on secretion was determined in sets of independent experiments (number of experiments shown at the base of each column) and statistical significance (P<0.005 marked with *) was calculated by unpaired Student's t-test.
Downregulation of the annexin A2/S100A10 complex affects the acute secretion of vWF
Previous work had implicated the Ca2+- and membrane-binding protein annexin A2, which resides in a tight heterotetrameric complex with the S100A10 protein, in Ca2+-regulated exocytosis in endothelial cells. By employing membrane capacitance measurements and synthetic annexin A2 peptides capable of disrupting the annexin A2/S100A10 complex, it was shown that the Ca2+-induced increase in whole-cell membrane capacitance resulting from exocytotic fusion events was reduced by approximately 50% in cells treated with complex-disrupting peptides (König et al, 1998). However, the capacitance measurements did not allow a distinction between the exocytotic fusion of different types of granules. Moreover, the only partial inhibition of Ca2+-induced capacitance increase indicated that the annexin A2/S100A10 complex might be involved in only a subset of the exocytotic events occurring in response to Ca2+ elevation. To distinguish between these events and to target directly the function of annexin A2/S100A10, we attempted to specifically downregulate annexin A2 and S100A10 in HUVECs and to analyze the effect of such downregulation on vWF and tPA secretion.
In HUVECs, annexin A2 (Figure 6) and S100A10 (not shown) are present in the cytoplasm and at the plasma membrane. No significant association with either WPbs or tPA-containing granules is observed (Figure 6). Downregulation of both subunits was achieved by specific siRNAs, which efficiently depleted HUVECs of their endogenous annexin A2 and S100A10. The extent of downregulation was similar to that previously observed in HeLa cells transfected with annexin A2/S100A10 siRNAs (Zobiack et al, 2003). Typically, transfection rates of 60–80% were achieved, with the transfected cells showing very little, if any, residual annexin A2 or S100A10. This translates into an overall reduction of annexin A2 and S100A10 levels in HUVEC cultures (transfected plus non-transfected cells) by approximately 70–80% (not shown). In line with the lack of colocalization between annexin A2/S100A10 and WPbs or tPA granules, downregulation of annexin A2 does not affect the number and morphological appearance of WPbs (Figure 7) and tPA granules (not shown).
Figure 6.

Localization of annexin A2 in comparison to that of vWF and tPA in cultivated HUVECs. HUVECs were analyzed by immunofluorescence, employing rabbit polyclonal anti-annexin A2 as well as mouse monoclonal anti-vWF (A) or sheep polyclonal anti-tPA (B) antibodies and appropriate fluorescently labeled secondary antibodies in double-labeling experiments. Note that annexin A2 shows a general cytosolic distribution and some plasma membrane staining (arrowheads). No colocalization with vWF- or tPA-containing granules is observed. Bars represent 10 μm.
Figure 7.
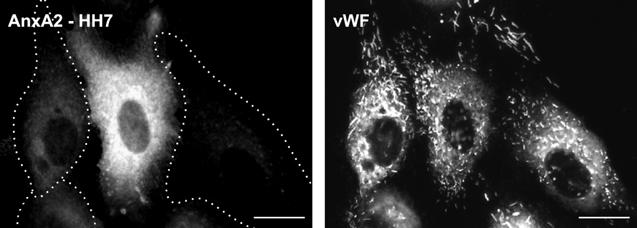
siRNA-mediated downregulation of annexin A2 and S100A10 does not affect the number or appearance of WPbs. HUVECs were transfected with siRNA duplexes targeted at annexin A2 and downregulation of the protein was revealed by immunofluorescence employing monoclonal anti-annexin A2 antibodies. WPb distribution in the same cells was visualized by colabeling with polyclonal anti-human anti-vWF antibodies. The circumferences of transfected cells showing reduced levels of annexin A2 are depicted as dashed lines (the cells were identified in phase-contrast images, not shown). Note that the anti-vWF staining is not affected by annexin A2 depletion. Bar represents 10 μm.
The effect of annexin A2/S100A10 downregulation on secretion was first analyzed by employing the anti-vWF antibody internalization assay. Here, cells with significantly reduced annexin A2 levels hardly show any antibody uptake, indicative of a block in WPb secretion (Figure 8A). Since annexin A2 has been implicated in endocytosis (Gerke and Moss, 2002), we also analyzed whether the inhibition of anti-vWF antibody capture observed in annexin A2/S100A10-downregulated cells could be due to a block or reduction of general endocytic membrane uptake. Figure 8B reveals that receptor-mediated endocytosis of transferrin is not affected in the downregulated cells. Likewise, the uptake of fluid-phase tracers occurs unaltered in HUVECs transfected with annexin A2 or S100A10 siRNAs (not shown). Thus, the inhibitory effect of annexin A2/S100A10 downregulation on the recapture of post-exocytotic vWF granules is not due to an inhibition of endocytic membrane uptake, but most likely results from an inhibition of the histamine-triggered exocytosis of WPbs.
Figure 8.

Downregulation of annexin A2 interferes with histamine-triggered internalization of anti-vWF antibodies into postexocytotic granules, but does not affect transferrin uptake. (A) HUVECs transiently transfected with annexin A2-specific siRNA duplexes were subjected to a 30 min histamine treatment in medium containing anti-vWF antibodies. Internalized antibody, indicative of previous histamine-triggered exocytosis of WPbs, was stained using Cy2-labeled secondary antibodies (anti-vWF). Annexin A2 was visualized by immunofluorescence using the mouse monoclonal HH7 antibody and Texas red-conjugated secondary antibodies (AnxA2-HH7). Note that efficient anti-vWF antibody internalization into postexocytotic granules is only seen in cells expressing annexin A2. The annexin A2-depleted cells, marked by dashed lines around the circumferences of the respective cells, identified in phase-contrast images (not shown), contain very little anti-vWF antibody captured following histamin-triggered exocytosis. (B) HUVECs transiently transfected with annexin A2 siRNA were incubated for 15 min in medium containing Texas red-conjugated transferrin (TxTf). Annexin A2 downregulation was visualized as before (A) and annexin A2-depleted cells were marked by a dashed line around the cell circumference. Note that TxTf is efficiently internalized in both nontransfected and annexin A2-depleted cells. Bars represent 10 μm.
To show this more directly, we quantified the amount of vWF and tPA released from mock-transfected control and annexin A2/S100A10-depleted cells using cell culture supernatant ELISAs. Figure 9 reveals that the histamine-evoked release of vWF is reduced significantly in cells depleted of their endogenous annexin A2 or S100A10. A similar inhibitory effect of annexin A2/S100A10 depletion on vWF secretion was observed when the release was triggered by direct intracellular Ca2+ mobilization through the ionophore A23187 (not shown). In contrast to the effect on vWF secretion, the histamine-induced release of tPA is not affected significantly by downregulation of annexin A2 or S100A10 (Figure 9).
Figure 9.

Histamine-induced secretion of vWF but not tPA is inhibited in annexin A2 and S100A10 downregulated cells. At 48 h following transfection with siRNA duplexes specific for annexin A2 (AnxA2-siRNA) or S100A10 (S100A10-siRNA) or an inactive annexin A2 siRNA duplex (Mock) (Zobiack et al, 2003), HUVECs were subjected to histamine treatment for 5 and 30 min. The amount of vWF and tPA released into the cell culture supernatant was then determined by specific ELISAs. The relative histamine-triggered increase of vWF and tPA secretion exceeding the constitutive levels (calculated as described in Materials and methods) is given in percentages as mean values±s.e.m. The effect on secretion was determined in sets of independent experiments (number of experiments shown at the base of each column) and statistical significance (P<0.005 marked with *) was calculated for the absolute as well as relative values by unpaired Student's t-test.
Due to a limited stability of noncomplexed S100A10, downregulation of annexin A2 by siRNA also leads to a depletion of S100A10 in transfected HeLa (Zobiack et al, 2003) as well as endothelial cells (not shown). Thus, the above experiments do not allow the conclusion that the entire annexin A2/S100A10 complex is required for efficient vWF secretion. Since S100A10 is downregulated in both S100A10 siRNA- and annexin A2 siRNA-transfected cells, it remains possible that S100A10 alone is the factor required for efficient WPb exocytosis. To elucidate whether the entire annexin A2/S100A10 complex is involved, we performed anti-vWF antibody internalization assays with HUVECs microinjected with a synthetic, complex-disrupting annexin A2 peptide. The peptide corresponds to residues 1–14 of the annexin A2 sequence and contains an N-terminal acetyl group (Ac1–14) also found in endogenous annexin A2 (Johnsson et al, 1988). It harbors the entire S100A10-binding site and is capable of disrupting preformed annexin A2/S100A10 complexes, most likely by competing with annexin A2 for the S100A10-binding site (König et al, 1998). In contrast, a peptide of the same sequence but lacking the N-terminal acetyl group (N1–14) is not able to bind to S100A10 (Johnsson et al, 1988). It is thus incapable of disrupting the complex and was employed as a control in the microinjection experiments. Figure 10 shows that microinjection of this nonacetylated peptide had no effect on the recapture of postexocytotic granules containing internalized anti-vWF antibodies. However, the acetylated variant, which disrupts the annexin A2/S100A10 complex, significantly reduces the number of anti-vWF antibody-containing postexocytotic granules in histamine-triggered HUVECs.
Figure 10.

Microinjection of N-terminal annexin A2 peptides affects anti-vWF antibody internalization into postexocytotic granules. HUVECs were microinjected with peptides corresponding to the N-terminal 14 residues of annexin A2 either as acetylated (Ac1–14) or nonacetylated (N1–14) derivatives. This sequence, when present in the acetylated form, represents the S100A10-binding site and efficiently disrupts annexin A2/S100A10 complexes (König et al, 1998). The nonacetylated variant is ineffective in complex disruption and thus serves as a negative control. Microinjected cells were visualized by dextran–FITC, which was added as a tracer to the peptide solutions. Following injection, cells were stimulated with histamine for 30 min in medium containing rabbit polyclonal anti-vWF antibodies. Antibodies internalized after histamine-triggered exocytosis into postexocytotic granules were visualized by Texas red-conjugated anti-rabbit IgGs (red, anti-vWF). (A) shows cells injected with the nonacetylated peptide, whereas panels of Ac1–14-injected cells are depicted in (B). Noninjected cells, which can be identified by the high number of postexocytotic granules containing internalized anti-vWF antibody (red), are also shown in the same frames. The number of anti-vWF-containing granules was counted in eight N1–14- and Ac1–14-injected cells, and is given as mean values±s.e.m. (C). Bars represent 10 μm.
Discussion
Using different approaches, we have shown here that the histamine-evoked and Ca2+-dependent secretion of vWF from cultured endothelial cells is affected by interfering with Rab3D and by depleting the cells of annexin A2 and its ligand S100A10. In contrast, the acute secretion of tPA, which can be induced by the same histamine treatment and also occurs in a Ca2+-regulated manner, is not altered by the Rab3D and annexin A2/S100A10 manipulations. In line with the intracellular localization of both factors to morphologically distinct granules, these findings provide strong evidence for a separate regulation of the acute vWF and tPA release from activated endothelial cells. While Rab3D and annexin A2/S100A10 participate in the former (most likely at different stages, see below), the latter has at least in part different, yet to be described, requirements. Differences in the machineries mediating acute exocytosis of different granules could allow individual endothelial cells to respond to a given stimulus in a finely tuned manner with respect to the secretion of thrombogenic (vWF, P-selectin) and fibrinolytic (tPA) factors. In each case, however, the increase in intracellular Ca2+ concentration, elicited, for example, by histamine receptor activation, is the common trigger for exocytosis. Thus, a differential secretion of vWF and tPA following endothelial activation can only occur if the machineries mediating vWF and tPA exocytosis are regulated differently by the Ca2+ signals elicited. This could be achieved when the extent and/or duration of the Ca2+ signals differ between those triggering vWF and tPA secretion. Indeed, in endothelial cells, a hormonally evoked Ca2+ mobilization, which is transient and only elevates intracellular Ca2+ to about 10 μM, is capable of triggering tPA release (Kooistra et al, 1994; Carter et al, 1998), whereas a prolonged elevation of intracellular Ca2+ to higher levels (above 10 μM) or repeated Ca2+ spikes are required for vWF secretion (Birch et al, 1994; Zupancic et al, 2002). The histamine treatment employed in this study is capable of initiating both vWF and tPA release, most likely because cytosolic Ca2+ levels become sufficiently high. While this enabled us to differentiate between different downstream effectors being involved in WPb as compared to tPA granule exocytosis, it seems plausible that endothelial activation under physiological conditions can induce the different Ca2+ signals required to trigger either the vWF or the tPA secretion machinery.
Although Rab3D and annexin A2/S100A10 both participate in Ca2+-regulated WPb exocytosis, it is at present premature to link mechanistically their functions to one another. Two alternatives remain: Rab3D and annexin A2/S100A10 could function either sequentially in the same pathway or in parallel yet distinct pathways leading to vWF secretion. Our antibody internalization assays argue for the former alternative, since Rab3D overexpression (Figure 3) or annexin A2 downregulation (Figure 8) reduces the antibody capture into postexocytotic granules to almost background levels. Thus, if parallel pathways of vWF secretion would exist, the one still operating in cells with compromised Rab3D or annexin A2 function only contributes marginally to Ca2+-regulated WPb exocytosis. Regardless of a possible mechanistic link, Rab3D and annexin A2 are likely to act at different stages. Rab3D is targeted to WPbs and expression of the dominant-negative Rab3D mutant severely reduces the number of WPbs (Figures 1 and 2). Annexin A2, on the other hand, is present in the cytoplasm and on the endothelial plasma membrane, showing no colocalization with WPbs, and its downregulation does not affect the number of WPbs (Figures 6 and 7). Thus, it appears plausible that already the generation of mature WPbs or a compound WPb fusion occurring prior to exocytosis (Zupancic et al, 2002) is affected by the Rab3Dwt and mutant expression, whereas the downregulation of annexin A2/S100A10 affects a later step in Ca2+-regulated WPb exocytosis. In line with a role for Rab3D in WPb maturation and/or maintenance is the observation that the number of secretory granules in neuroendocrine PC12 cells is reduced upon ectopic expression of dominant-negative mutants of Rab3D or Rab3A (Martelli et al, 2000). Our data also show that the presence of increased amounts of Rab3D on the WPb membrane results in a marked inhibition of the Ca2+-regulated exocytosis of these granules (Figures 3 and 5). This could be due to a direct inhibitory effect of the Rab3D coating in later steps in exocytosis, or an increased recruitment of Rab3D effectors that are required for WPb fusion/maturation but adversely affect exocytotic fusion with the plasma membrane.
A role of Rab3D in the maturation and/or maintenance of secretory granules has been deduced from the analysis of Rab3D knockout mice. Pancreatic and parotid acinar cells of these mice show an increased size of their secretory granules, consistent with Rab3D acting as an inhibitor of granule–granule fusion in exocrine glands (Riedel et al, 2002). While our data also point towards a function of Rab3D at a stage prior to the actual exocytotic membrane fusion (see above), it appears that the protein does not act as an inhibitor of homotypic WPb fusion. In fact, WPbs are even enlarged in cells overexpressing Rab3Dwt or Rab3DQ81L, and overexpression of a dominant-negative mutant leads to a marked reduction in the number of WPbs in HUVECs (Figures 1 and 2). Moreover, the enlargement of secretory granules in exocrine glands of the knockout mice does not affect basal and evoked secretion, whereas overexpression of Rab3D or Rab3DQ81L inhibits Ca2+-triggered vWF release. The reason for the apparently different phenotype in exocrine glands of the knockout mice and in HUVECs expressing Rab3D mutants is not known. It could be related to the method employed for interfering with Rab function (gene ablation versus mutant expression), and/or could reflect the fact that the WPb is a specialized granule showing features of lysosome-related organelles and thus has a complex maturation that differs from that of secretory granules in exocrine glands (Blagoveshchenskaya et al, 2002; Hannah et al, 2003). Moreover, compensatory mechanisms guaranteeing proper WPb maturation and exocytosis could have become upregulated differently in the two systems and/or could differ between HUVECs and cells of exocrine glands.
Our previous work employing membrane capacitance measurements had implicated the annexin A2/S100A10 complex in Ca2+-regulated exocytosis in endothelial cells, but had also revealed that not every Ca2+-induced secretion event was affected by disrupting the endothelial annexin A2/S100A10 complex (König et al, 1998). Indeed, we now show that annexin A2/S100A10 is specifically required for WPb but not tPA granule exocytosis (Figures 8, 9 and 10). Thus, the complex is most likely not involved in general aspects of Ca2+-regulated exocytosis, but only participates in certain specific secretion events. This could explain that previous work using permeabilized cell systems had implicated annexin A2 in Ca2+-triggered exocytosis in chromaffin but not PC12 cells (Ali et al, 1989; Sarafian et al, 1991; Graham et al, 1997). Given the types of granules that require annexin A2 for exocytosis (WPbs, chromaffin granules), as compared to those showing no dependence on annexin A2 (tPA storage granules), it is noteworthy that the former are much larger in size and can secrete their contents by compound exocytosis. Based on recent findings linking annexin A2/S100A10 to the formation of actin-rich membrane domains (Merrifield et al, 2001; Zobiack et al, 2002), our data indicate that the complex could play a structural role in organizing plasma membrane sites for efficient WPb exocytosis.
In conclusion, we show here that Rab3D and annexin A2/S100A10 are involved in regulating the acute release of thrombogenic vWF but not fibrinolytic tPA. This reveals for the first time that the Ca2+-regulated exocytosis of different secretory granules in the nonexcitable endothelial cells can follow different mechanisms enabling the individual cell to react to a given stimulus in a fine-tuned manner with the release of either pro- or antithrombogenic factors.
Materials and methods
Cell culture, histamine/ionophore stimulation and antibodies
Primary HUVECs were either isolated from 3–5 different donors as described previously (Jaffe et al, 1973) or purchased as cryoconserved cells (Cambrex Bio Science). HUVECs were maintained in endothelial growth medium (EGM-BulletKit, Cambrex) and cultivated on collagen type 1-coated surfaces at 37°C in 5% CO2. To stimulate regulated secretion, cells were treated with 100 μM histamine (Sigma) or 1 μM Ca2+-ionophore A23187 (Calbiochem) in basal medium.
WPbs were stained with mouse monoclonal antibodies (clone F8/86) directed against human vWF (Dako), rabbit polyclonal anti-human vWF antibodies (Dako) and purified rabbit polyclonal anti-human P-selectin antibodies (Pharmingen), all diluted 1:100 in 2% BSA/PBS. Sheep polyclonal antibodies against human tPA (Biotrend) were used at 1:100 dilution in 2% BSA/PBS. Antibodies employed to detect annexin A2 and S100A10 in the siRNA-treated cells have been described (Zobiack et al, 2003).
Plasmids, siRNA and transfections
The full coding sequence of human Rab3D was obtained by Pfu-based PCR using a human placenta cDNA library (Clontech) as template and oligonucleotide primers based on the human Rab3D nucleotide sequence. Rab3D mutants (Rab3DT36N, Rab3DQ81L, Rab3DN135I) were generated using the respective mutant oligonucleotides and the quickchange site-directed mutagenesis system (Stratagene). cDNAs encoding the different Rab3D constructs were subcloned into peYFP-C1 (Clontech) to generate YFP–Rab3D fusion proteins. Sequences of all constructs were verified by automated sequencing (Seqlab).
RNA interference using small interfering RNA (siRNA) duplexes (Dharmacon) targeted at annexin A2 and S100A10 has been described (Zobiack et al, 2003). HUVECs were transfected with plasmids or annealed siRNA duplexes using the Nucleofection kit (Amaxa) according to the manufacturer's instructions. Following transfection, cells were cultured on coverslips or in 24-well plastic dishes for 48–72 h.
Anti-vWF antibody internalization
Anti-vWF antibody internalization following histamine stimulation of endothelial cells was visualized essentially as described (Knop and Gerke, 2002). Briefly, HUVECs grown on coverslips were stimulated with 100 μM histamine in basal medium containing 2% BSA and 0.1 mg/ml polyclonal rabbit anti-human vWF antibodies. Following incubation in 5% CO2 at 37°C for 30 min, cells were washed, fixed in 4% paraformaldehyde/PBS for 10 min and permeabilized with 0.2% Triton X-100/PBS for 2 min at room temperature. Subsequently, vWF present in nonexternalized WPbs was detected using the mouse monoclonal anti-vWF. Staining with appropriate secondary antibodies, Cy2- or Texas red-conjugated goat anti-rabbit and anti-mouse IgGs, enabled a distinction between the internalized anti-vWF antibody and the vWF found in nonexternalized WPbs, since the monoclonal anti-vWF antibody did not stain the vWF–anti-vWF complexes in postexocytotic granules (Knop and Gerke, 2002).
Fluorescence microscopy and endocytosis assays
HUVECs seeded on coverslips were fixed for 10 min using 4% paraformaldehyde/PBS, permeabilized with 0.2% Triton X-100/PBS for 2 min at room temperature and stained with primary and the appropriate secondary antibodies, each applied for 45 min at 37°C. All secondary antibodies (Dianova), goat anti-mouse, goat anti-rabbit and donkey anti-sheep IgGs used as Cy2, Texas red or AMCA conjugates, were diluted 1:200 in 2% BSA/PBS. Cells were analyzed by epifluorescence (DM RXA microscope, Leica) and confocal microscopy (LSM510, Zeiss).
Analysis of fluid-phase and receptor-mediated endocytosis in Rab3D-expressing or annexin A2-downregulated cells was carried out as described (Zobiack et al, 2003) using Alexa568-labeled dextran and Texas red-labeled transferrin (Molecular Probes) as respective markers.
Quantification of vWF and tPA secretion
Ca2+-regulated exocytosis was induced in HUVECs grown to confluency in 24-well plates by treatment with histamine- or ionophore A23187-containing stimulation medium (300 μl per well) for 5–30 min. Control experiments employed basal medium without any addition to measure the levels of constitutive exocytosis. The cell culture supernatant was then transferred into tubes containing a final concentration of 4 μg/ml aprotinin and 0.5 μg/ml leupeptin to inhibit proteases. vWF and tPA present in the collected supernatants were quantified using specific ELISAs (Affinity Biologicals) described previously (Knop and Gerke, 2002). Typically, the constitutive basal secretion, that is, secretion from cells kept in stimulant-free medium, amounted to 1.5±0.4 ng vWF and 0.3±0.1 ng tPA per well in 30 min. These values are in line with previous reports on constitutive and regulated secretion from HUVECs (Vischer and Wollheim, 1998), but varied between different HUVEC batches. To exclude such variations, we always used the same batch of HUVECs when comparing histamine-induced and constitutive secretion. Results obtained with different batches of cells were then compared with one another by calculating relative values for the stimulated secretion. Therefore, the constitutive secretion occurring in a given time interval (5 or 30 min) was set to 100% and the values obtained with histamine-treated cells of the same batch were expressed in percent as the sum of constitutive and regulated secretion (100+X%, with X representing the regulated secretion). Mean values±s.e.m. were always calculated and the statistical significance was evaluated by unpaired Student's t-test.
Microinjection
Microinjection was performed on the stage of an Axiovert 135M (Zeiss) inverted microscope using a pressure injector model 5246 (Eppendorf) and a micromanipulator (Luigs&Neumann, Ratingen, Germany). N-terminal annexin A2 peptides corresponding to residues 1–14 of human annexin A2 (STVHEILCKLSLEG) were synthesized as nonacetylated or acetylated forms as described (König et al, 1998) and employed as 0.1 mM solutions in PBS containing 0.1 mM EGTA and 5 mg/ml 70 kDa FITC-conjugated dextran (Molecular Probes) as a tracer to identify injected cells. Prior to injection, the peptide solutions were centrifuged and sterile filtered. Peptides were injected under constant pressure (70–90 hPa) for 0.2 s into the cytosol of HUVECs seeded on coverslips and grown to subconfluency (50–70%). During microinjection, cells were kept in basal medium containing 2% BSA and 25 mM Hepes in a CO2 atmosphere at 37°C. Cells were then stimulated with histamine and subjected to anti-vWF antibody internalization (see above).
Acknowledgments
We thank Ursula Rescher, Nicole Zobiack (Institute of Medical Biochemistry, Muenster) and Marino Zerial (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden) for helpful comments and for providing us with Rab5 and Rab11 expression plasmids used in control experiments. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 629) and the Interdisciplinary Center for Clinical Research (IZKF) of the University of Muenster Medical School.
References
- Ali SM, Geisow MJ, Burgoyne RD (1989) A role for calpactin in calcium dependent exocytosis in adrenal chromaffin cells. Nature 340: 313–315 [DOI] [PubMed] [Google Scholar]
- Baldini G, Wang G, Weber M, Zweyer M, Bareggi R, Witkin JW, Martelli AM (1998) Expression of Rab3D N135I inhibits regulated secretion of ACTH in AtT-20 cells. J Cell Biol 140: 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch KA, Ewenstein BM, Golan DE, Pober JS (1994) Prolonged peak elevations in cytoplasmic free calcium ions, derived from intracellular stores, correlate with the extent of thrombin-stimulated exocytosis in single human umbilical vein endothelial cells. J Cell Physiol 160: 545–554 [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hannah MJ, Allan S, Cutler DF (2002) Selective and signal-dependent recruitment of membrane proteins to secretory granules formed by heterologously expressed von Willebrand factor. Mol Biol Cell 13: 1582–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TD, Zupancic G, Smith SM, Wheeler-Jones C, Ogden D (1998) Membrane capacitance changes induced by thrombin and calcium in single endothelial cells cultured from human umbilical vein. J Physiol 513: 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Edwards JAS, Logsdon CD, Ernst SA, Williams JA (2002) Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J Biol Chem 277: 18002–18009 [DOI] [PubMed] [Google Scholar]
- Datta YH, Ewenstein BM (2001) Regulated secretion in endothelial cells: biology and clinical implications. Thromb Haemost 86: 1148–1155 [PubMed] [Google Scholar]
- Datta YH, Youssoufian H, Marks PW, Ewenstein BM (1999) Targeting of a heterologous protein to a regulated secretion pathway in cultured endothelial cells. Blood 94: 2696–2703 [PubMed] [Google Scholar]
- Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogan CM, de Priester W, Westmuckett A, Lupu F (1997) An endothelial storage granule for tissue-type plasminogen activator. J Cell Biol 139: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82: 331–371 [DOI] [PubMed] [Google Scholar]
- Graham ME, Gerke V, Burgoyne RD (1997) Modification of annexin II expression in PC12 cell line does not affect Ca(2+)-dependent exocytosis. Mol Biol Cell 8: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MJ, Hume AN, Arribas M, Williams R, Hewlett LJ, Seabra MC, Cutler DF (2003) Weibel–Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J Cell Sci 116: 3939–3948 [DOI] [PubMed] [Google Scholar]
- Holvoet P, Collen D (1997) Thrombosis and atherosclerosis. Curr Opin Lipidol 8: 320–328 [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52: 2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Marriott G, Weber K (1988) p36, the major cytoplasmic substrate of src tyrosine protein kinase, binds to its p11 subunit via a short amino-terminal amphiphatic helix. EMBO J 7: 2435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbach SW, Chipiuk A, Nelson RC, Hollenberg MD, Murray AG (2003) Differential actions of PAR2 and PAR1 in stimulating human endothelial cell exocytosis and permeability: the role of Rho-GTPases. Circ Res 92: 272–278 [DOI] [PubMed] [Google Scholar]
- Knop M, Gerke V (2002) Ca(2+)-regulated secretion of tissue-type plasminogen activator and von Willebrand factor in human endothelial cells. Biochim Biophys Acta 1600: 162–167 [DOI] [PubMed] [Google Scholar]
- König J, Prenen J, Nilius B, Gerke V (1998) The annexin II–p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J Biol Chem 273: 19679–19684 [DOI] [PubMed] [Google Scholar]
- Kooistra T, Schrauwen Y, Arts J, Emeis JJ (1994) Regulation of endothelial tPA synthesis and release. Int J Hematol 59: 233–255 [PubMed] [Google Scholar]
- Lupu C, Lupu F, Dennehy U, Kakkar VV, Scully MF (1995) Thrombin induces the redistribution and acute release of tissue factor pathway inhibitor from specific granules within human endothelial cells in culture. Art Thromb Vasc Biol 15: 2055–2062 [DOI] [PubMed] [Google Scholar]
- Martelli AM, Baldini G, Tabellini G, Koticha D, Bareggi R, Baldini G (2000) Rab3A and Rab3D control the total granule number and the fraction of granules docked at the plasma membrane in PC12 cells. Traffic 1: 976–986 [PubMed] [Google Scholar]
- Merrifield CJ, Rescher U, Almers W, Proust J, Gerke V, Sechi AS, Moss SE (2001) Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr Biol 11: 1136–1141 [DOI] [PubMed] [Google Scholar]
- Michiels C (2003) Endothelium cell function. J Cell Physiol 196: 430–443 [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G (2001) Ion channels and their functional role in vascular endothelial cells. Physiol Rev 81: 1415–1459 [DOI] [PubMed] [Google Scholar]
- Riedel D, Antonin W, Fernandez-Chacon R, Alvarez de Toledo G, Jo T, Geppert M, Valentijn JA, Valentijn K, Jamieson JD, Südhof TC, Jahn R (2002) Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol 22: 6487–6497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnoblet C, Vischer UM, Gerard RD, Irminger JC, Halban PA, Kruithof EK (1999) Storage of tissue-type plasminogen activator in Weibel–Palade bodies of human endothelial cells. Arterioscler Thromb Vasc Biol 19: 1796–1803 [DOI] [PubMed] [Google Scholar]
- Sarafian T, Pradel L-A, Henry J-P, Aunis D, Bader M-F (1991) The participation of annexin II (Calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol 114: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter OM, Khvotchev M, Jahn R, Südhof TC (2002) Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J Biol Chem 277: 40919–40929 [DOI] [PubMed] [Google Scholar]
- Schneider SW (2001) Kiss and run mechanism in exocytosis. J Membr Biol 181: 67–76 [PubMed] [Google Scholar]
- Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W (2003) Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA 100: 2070–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer UM, Barth H, Wollheim CB (2000) Regulated von Willebrand factor secretion is associated with agonist-specific pattern of cytoskeletal remodeling in cultured endothelial cells. Arterioscler Thromb Vasc Biol 20: 883–891 [DOI] [PubMed] [Google Scholar]
- Vischer UM, Wollheim CB (1998) Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca(2+) and cyclic adenosine monophosphate-dependent signaling in endothelial exocytosis. Blood 91: 118–127 [PubMed] [Google Scholar]
- Wagner DD (1993) The Weibel–Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemostasis 70: 105–110 [PubMed] [Google Scholar]
- Weibel ER, Palade GF (1964) New cytoplasmic components in arterial endothelia. J Cell Biol 23: 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobiack N, Rescher U, Laarmann S, Michgehl S, Schmidt MA, Gerke V (2002) Cell surface attachment of pedestal forming enteropathogenic E. coli induces a clustering of raft components and a recruitment of annexin 2. J Cell Sci 115: 91–98 [DOI] [PubMed] [Google Scholar]
- Zobiack N, Rescher U, Ludwig C, Zeuschner D, Gerke V (2003) The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell 14: 4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupancic G, Ogden D, Magnus CJ, Wheeler-Jones C, Carter TD (2002) Differential exocytosis from human endothelial cells evoked by high intracellular Ca(2+) concentration. J Physiol 544.3: 741–755 [DOI] [PMC free article] [PubMed] [Google Scholar]


