Abstract
Resolution of inflammation is a finely regulated process mediated by specialized pro-resolving lipid mediators (SPMs) including docosahexaenoic acid (DHA)-derived resolvins and maresins. The immunomodulatory role of SPMs in adaptive immune cells is of interest. Here, we report that the D-series resolvins Resolvin D1 and Resolvin D2 and Maresin 1 modulate adaptive immune responses in human peripheral blood lymphocytes. These lipid mediators reduce cytokine production by activated CD8 T cells and CD4 T-helper (TH) 1 and TH17 cells, but do not modulate T cell inhibitory receptors or abrogate their capacity to proliferate. Moreover, these SPMs prevented naïve CD4 T-cell differentiation into TH1 and TH17 by downregulating their signature transcription factors, T-bet and Rorc, in a mechanism mediated by the GPR32 and ALX/FPR2 receptors; they concomitantly enhanced de novo generation and function of Foxp3+ regulatory T (Treg) cells via the GPR32 receptor. These results were also supported in vivo in a mouse deficient for DHA synthesis (Elovl2−/−) that showed an increase in TH1/TH17 cells and a decrease in Treg cells compared to wild type mice. Additionally, either DHA supplementation in Elovl2−/− mice or in vivo administration of Resolvin D1 significantly reduced cytokine production upon specific stimulation of T cells. These findings demonstrate actions of specific SPMs on adaptive immunity and provide a new avenue for SPM-based approaches to modulate chronic inflammation.
SINGLE SENTENCE SUMMARY
Specialized pro-resolving lipid mediators resolvin D1, resolvin D2 and maresin 1 reduce CD8 and CD4 cell activation as well as prevent Th1 and Th17 cell differentiation from naïve T cells via GPR32 and ALX/FPR receptors while promoting de novo Treg induction via GPR32 receptor.
INTRODUCTION
Acute inflammation is generally protective for the host and is mediated by a plethora of well-known chemical messengers including cytokines, chemokines and lipid-derived mediators (mostly produced from the essential fatty acid arachidonic acid) released by innate immune cells (1–3). Resolution of inflammation is a finely orchestrated active process governed by temporally and spatially regulated synthesis of local mediators that act in concert to reestablish tissue homeostasis after injury and phlogistic processes (for recent review see reference 4). The resolvins (Rvs), protectins (PDs), maresins (MaRs) and lipoxins (LXs), often referred together as specialized pro-resolving lipid mediators (SPMs) given their functions (4), are novel families of autacoids with a central role in resolving processes, which act as local mediators controlling the magnitude and extent of inflammatory events.
SPMs are produced mainly by macrophages and neutrophils via separate pathways from omega-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – the former yielding E-series Rvs and the latter D-series Rvs, MaRs and PDs – as well as from omega-6 PUFA arachidonic acid (AA), which gives rise to LXs, via the action of lipoxygenases ALOX-5, ALOX-12, ALOX-15 and cyclooxygenase COX-2. (5–8). These SPMs have received considerable attention in recent years because of their ability to stereoselectively regulate and reduce inflammatory conditions in animal disease models (4). Thus, SPMs prevent inflammation from spreading and halt the transition from acute to chronic. Yet, published studies focus almost exclusively on acute conditions and innate immunity and, little is currently known about their possible action on the cellular components of adaptive immunity. This includes the finding that Resolvin E1 induces apoptosis of activated T cells and Protectin D1 reduces T-cell migration (9, 10). The present study investigated the selective actions of D-Series resolvins and Maresin 1, major SPMs that were recently found in human lymphoid tissues including human spleen and lymph nodes (11). Hence, we focused either on circulating CD8+ and CD4+ T lymphocytes and on CD4+ subsets, which include highly pathogenic T helper (TH) TH1 and TH17 cells, as well as regulatory T (Treg) lymphocytes. These results document the pivotal role(s) for specific SPMs in the control of adaptive immunity, thus providing a better understanding of the impact of these potent new bioactive lipid mediators on the spectrum of immune cells, and ultimately setting the standard for the potential development of new treatments for chronic inflammatory diseases.
RESULTS
Pro-resolving lipid mediators modulate CD8+ and CD4+ T cell responses
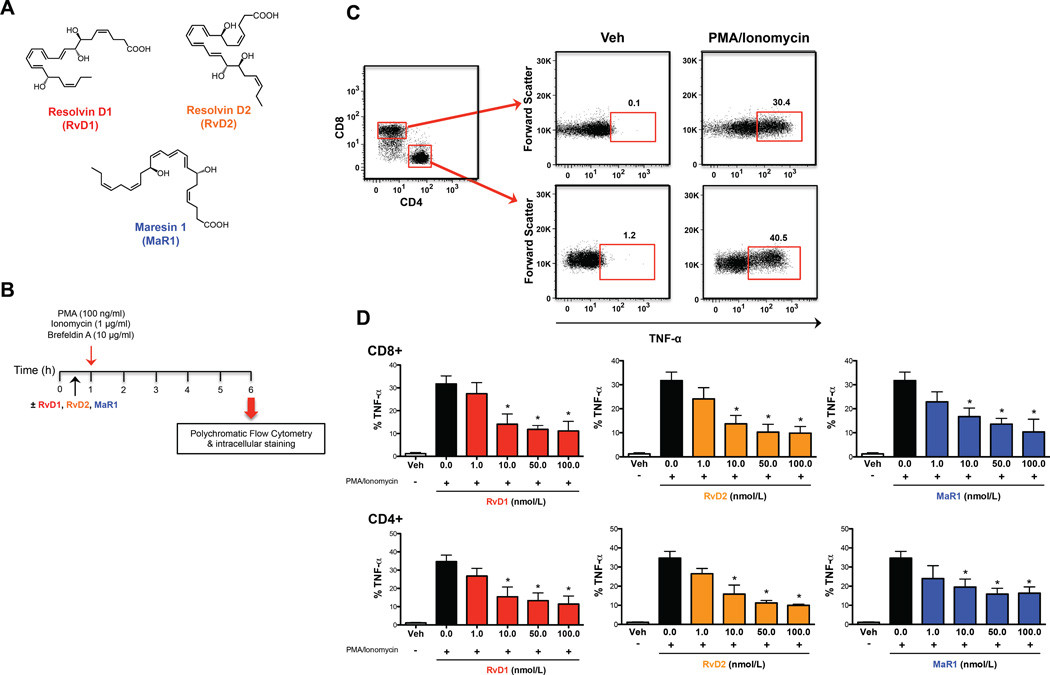
Although data on SPMs mostly focuses on innate immune cells involved in the resolution of acute inflammation (4), we hypothesized that the several SPMs, specifically RvD1, RvD2, and MaR1 (Fig. 1A) could also affect the immune responses of adaptive immune cells. To test this hypothesis, initial studies were performed to assess whether increasing concentrations of RvD1, RvD2, and MaR1 (in the 1–100 nmol/L physiological range, 12) could affect the production of TNF-α from human CD8+ and CD4+ T lymphocytes (Fig. 1B). Both T cell subsets when activated with PMA/Ionomycin produced high amounts of intracellular TNF-α (Fig. 1C and 1D), which was reduced upon pretreatment with all SPMs tested (Fig. 1D). Each of the specific mediators inhibited TNF-α production in a dose-dependent manner, and substantially reduced cytokine production at doses as low as 10.0 nmol/L. The lowest dose tested (1.0 nmol/L) only showed a slight and non-significant reduction of TNF-α production from both T cell subsets (Fig. 1D). The same result was also observed with an epimer of another newly discovered resolvin, the aspirin-triggered resolvin D3 (AT-RvD3), which dose-dependently reduced TNF-α production from both CD8+ and CD4+ T cells and was significant (p < 0.05) as low as 10 nmol/L (Fig. S1A). For this reason, in all further experiments, SPMs were used at the lowest effective concentration of 10.0 nmol/L. These initial results suggested that SPMs might indeed be effective in modulating adaptive immune responses.
Fig. 1.
Pro-resolving lipid mediators dose-dependently reduce TNF-α from CD8+ and CD4+ T cells. (A) Chemical structures of the SPMs Resolvin D1, Resolvin D2 and Maresin 1. (B) PBMCs (1×106 cells/well) were left untreated (Veh) or treated with different concentrations (1–100 nmol/L) of SPMs (RvD1, RvD2, and MaR1) for 30 min. Cells were then stimulated with PMA/ionomycin for 6 hours, stained at cell surface and intracellularly, and analyzed by flow cytometry (C) A representative cytofluorimetric plot of the gating strategy for TNF-α evaluation from CD8+ and CD4+ T cells. PBMCs were appropriately gated according to physical parameters. (D) Percentages of intracellular cytokine production in both CD8+ and CD4+ T cells. Data are shown as means ± SEM of four independent experiments. *p <0.05 (one-way Anova).
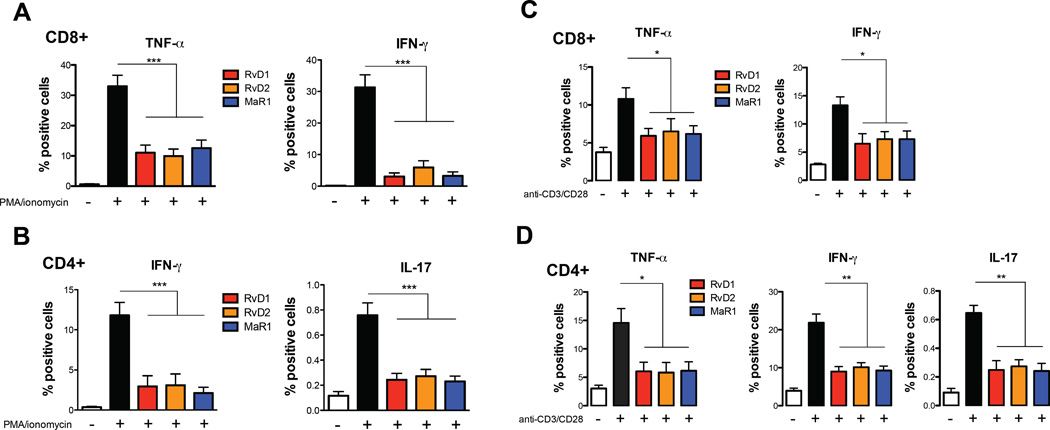
We next ascertained the possible impact of these SPMs (at 10 nmol/L) on the production of the specific cytokines that characterize the main proinflammatory T cell subsets, i.e. cytotoxic CD8+ T cells and CD4+ T helper-1 (TH1) and T helper-17 (TH17) cells. Indeed, PMA/Ionomycin-activated CD8+ T cells produced high amounts of TNF-α and IFN-γ, which were strongly reduced by each of these SPMs (Fig. 2A). Furthermore, the production of both IFN-γ and IL-17 from PMA/Ionomycin-activated CD4+ T cells was also strongly reduced by incubation with these SPMs (Fig. 2B). Interestingly, the ability of the different SPMs to suppress cytokine production from CD8+ T cells and CD4+ T cells was independent of the chemical class, suggesting that distinct lipid mediators are able to similarly modulate adaptive immune cells. Since the cytokine profile of human T cells may be differently determined depending on the assay and conditions used, we further investigated the immunomodulatory role of RvD1, RvD2 and MaR1 using a more specific and physiological stimulus for activating T cells, i.e. polyclonal activation of the T-cell receptor with anti-CD3 and anti-CD28. Cytokine production after stimulation of T cells with anti-CD3/CD28 was almost identical to that of PMA/ionomycin stimulation, even if intracellular levels of cytokines were lower, as expected (13) (Fig, 2C and 2D). In particular, RvD1, RvD2 and MaR1 significantly (p < 0.05) reduced the capability of CD8+ T cells to produce TNF-α and IFN-γ (Fig. 2C) and that of CD4+ T cells to produce TNF-α (p < 0.05), IFN-γ (p < 0.01) and IL-17 (p < 0.01) (Fig. 2D), overall suggesting that SPMs might regulate TH1 and TH17 responses.
Fig. 2.
Pro-resolving lipid mediators reduce CD8+ and CD4+ T cell responses. PBMCs (1×106 cells/well) were left untreated or treated with 10 nmol/L SPMs (RvD1, RvD2, and MaR1) for 30 min. Cells were then stimulated with PMA/ionomycin for 6 hours (A–B) or with anti-CD3/CD28 beads (C–D), stained at cell surface and intracellularly, and analyzed by flow cytometry, as detailed in Materials and Methods. Percentages of intracellular production of TNF-α and IFN-γ from CD8+ and of TNF-α, IFN-γ and IL-17 from CD4+ T cells are shown as means ± SEM of eight independent experiments. *p <0.05, **p <0.01, ***p <0.001 (one-way Anova).
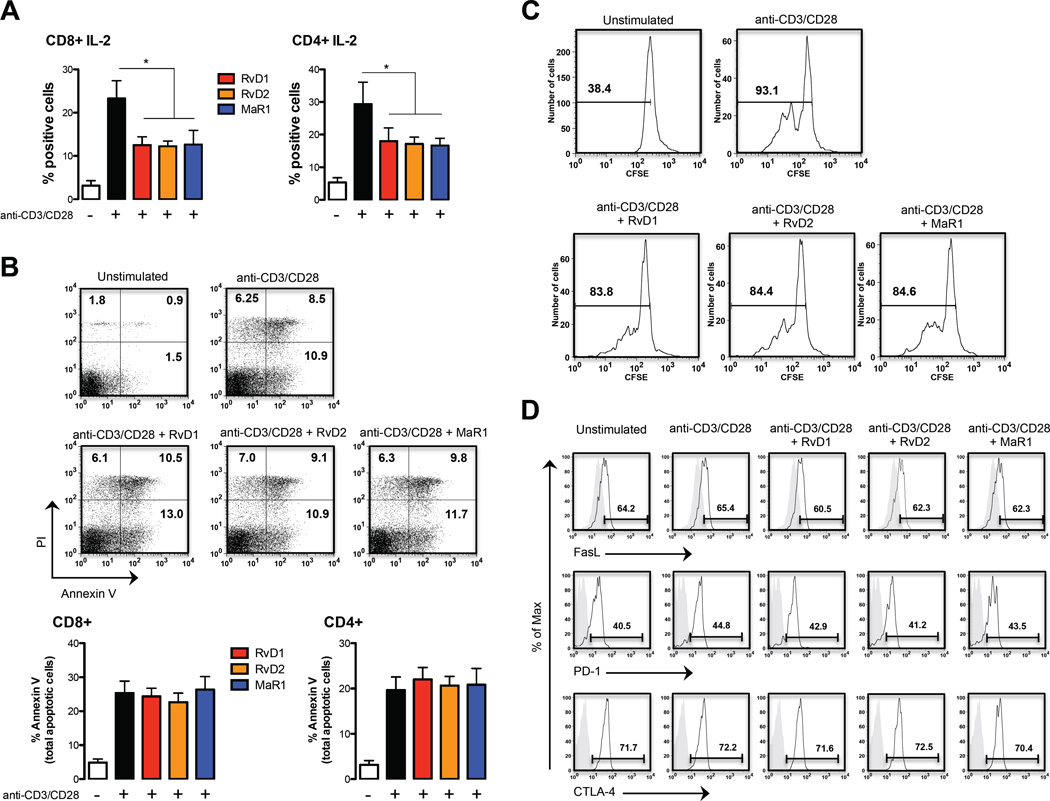
SPMs regulate IL-2 production from T cells without affecting their viability
The immunomodulatory activity of D-series resolvins and MaR1 on T cell responses was also demonstrated by reduction of the crucial growth factor IL-2 compared with IL-2-producing anti-CD3/anti-CD28-activated CD8+ and CD4+ T cells (Fig. 3A). This effect was not due to an induction of cell death, as assessed by annexin-V staining that was used as a marker for apoptosis in combination with PI, in order to distinguish between apoptotic and necrotic cells. As expected, resting T cells were all annexin-V and PI negative, whereas ~20% of anti-CD3/CD28-activated T cells were apoptotic (annexin-V positive) (Fig. 3B). Almost no variation in live cells (annexin-V and PI negative), early apoptosis (annexin-V positive and PI negative), late apoptosis (annexin-V and PI positive), or necrosis (PI positive and annexin-V negative) could be detected in RvD1-, RvD2- and MaR1-treated activated T cells, either on the total CD3+ T cell population (Fig. 3B upper panel) or in CD8+ or CD4+ T cells (Fig. 3B lower panel). Even after 24h of treatment, no significant increase in the proportion of total apoptotic cells was observed in both CD8+ and CD4+ T cells treated with the different SPMs compared to activated cells, although general cell viability showed a ~50% decrease (Fig. S1B). SPMs treatment of resting T cells did not induce apoptosis, ruling out a potential cytotoxic role of these SPMs (Fig. S1C). Interestingly, the decrease in IL-2 did not result in a significant decrease in T cell proliferation, (Fig. 3C) nor was it associated with an altered cell-surface expression of several inhibitory receptors, including FasL, PD-1 and CTLA4 (Fig. 3D).
Fig. 3.
Pro-resolving lipid mediators inhibit IL-2 production from TCR-activated CD8+ and CD4+ T cell without affecting their viability. PBMCs (1×106 cells/well) were left untreated or treated with 10 nmol/L RvD1, RvD2 and MaR1 for 30 min. Cells were then stimulated with anti-CD3/anti-CD28 for 8 hours, stained at cell surface and intracellularly, and analyzed by flow cytometry. (A) Percentages of intracellular production of IL-2 from CD8+ and CD4+ T cells are shown as means ± SEM of six independent experiments. *p <0.05 (one-way Anova). (B) Cell death of CD8+ and CD4+ T cells after stimulation with anti-CD3/CD28 beads through staining for Annexin V and PI flow cytometry analysis. The percentage of Annexin V+/PI-cells (early apoptotic cells) and Annexin V+ cells (total apoptotic cells) is reported in the cumulative graph. Data are shown as means ± SEM of four independent experiments. (C) Cell proliferation of CD3+ T cells after stimulation with anti-CD3/CD28 beads (day 4) shown by CSFE dilution (D) Surface expression of FASL, PD-1 and CTLA-4 in CD3+ T cells after stimulation with anti-CD3/CD28. A representative experiment (of four independent experiments) of receptor expression is shown (in grey the isotype is shown).
SPMs critically affect TH1/TH17 and Treg differentiation
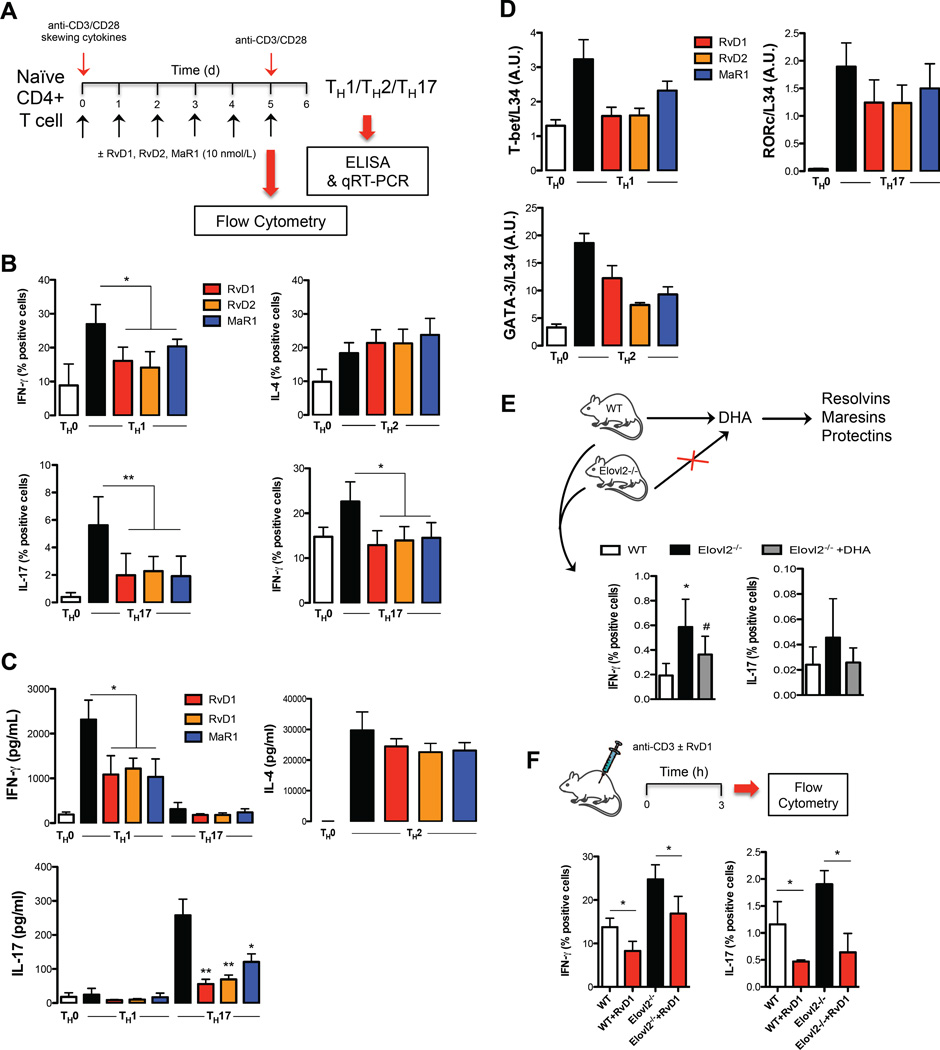
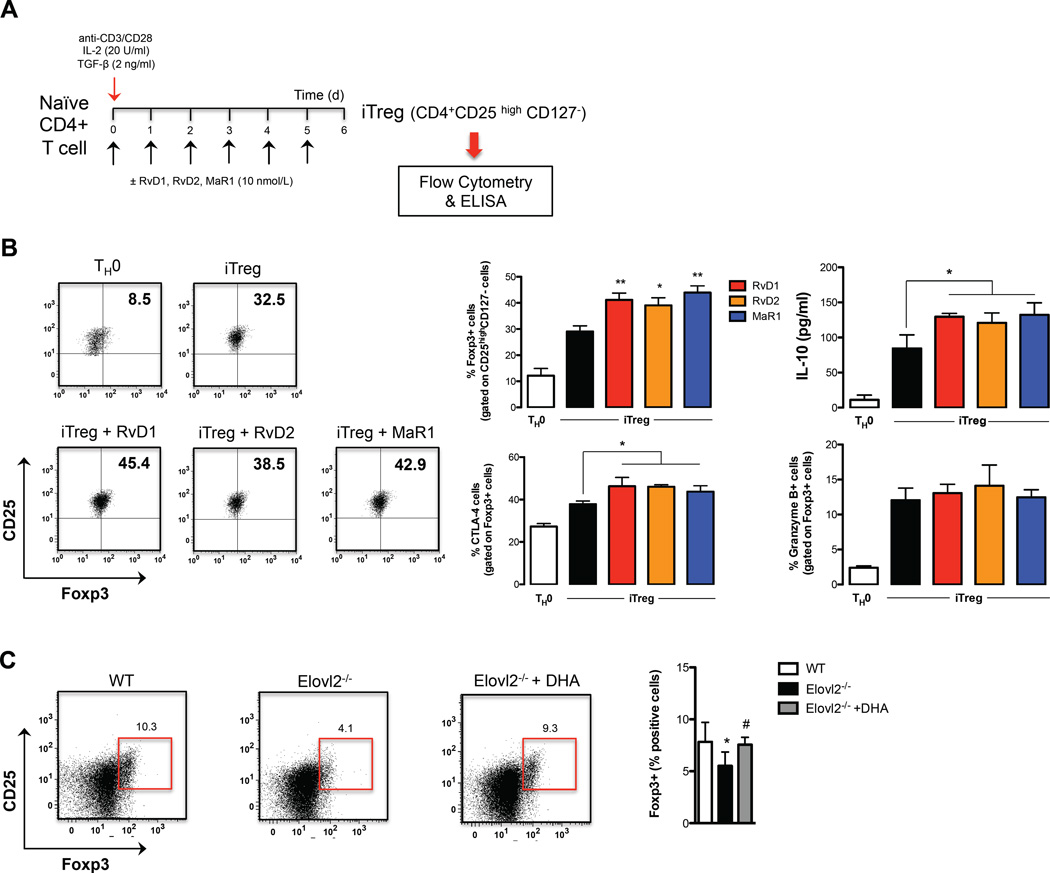
TH1 and TH17 subsets in peripheral blood are both derived from naïve CD4+ T cells upon antigen stimulation and specific skewing cytokines (14). Since both classes of SPMs dampened the inflammatory response of TNF-α- and IFN-γ-producing TH1 cells, and of IL-17-producing TH17 cells from peripheral blood mononuclear cells, we next investigated whether RvD1, RvD2 or MaR1 were able to directly affect their differentiation from naïve CD4+ T cells into TH1 and TH17 lineages. To this aim, a standard naïve CD4+ T cells differentiation assay was performed by polyclonal stimulation with anti-CD3/CD28, and specific polarizing cytokines in the presence of RvD1, RvD2 or MaR1 (Fig. 4A).
Fig. 4.
Pro-resolving lipid mediators affect T-helper cells polarization. (A) Schematic representation of TH1, TH17 and TH2 generation. (B) Percentages of intracellular cytokine production from polarized TH1, TH17 and TH2 cells in presence or absence of RvD1, RvD2 or MaR1 10 nmol/L, assessed after 6 h of restimulation with anti-CD3/CD28. Data are shown as means ± SEM of six independent experiments. *p <0.05, **p <0.01 (one-way Anova). (C) ELISA of IFN-γ, IL-17 and IL-4 in supernatants of TH1, TH17 and TH2 cells polarized in presence or absence of RvD1, RvD2 or MaR1 nmol/L, measured after 24 h of restimulation with anti-CD3/CD28. Data are shown as means ± SEM of six independent experiments. *p <0.05, **p <0.01 (one-way Anova). (D) qRT-PCR analysis of the expression of T-bet, RORc and GATA-3 in TH1, TH17 and TH2 cells. Cycling threshold values are normalized to those of mRNA encoding ribosomal protein L34, and data are normalized to the maximum value obtained for each donor. Data are expressed as arbitrary units (A.U.) and are shown as means ± SEM of four independent experiments. (E) Percentages of intracellular production of IFN-γ and IL-17 from CD4+ T cells of splenocytes obtained from wild type (WT), Elovl2 knock out (Elovl2−/−) and Elovl2−/− + DHA mice. Data are shown as means ± SEM of 4 different mice per experimental group and performed in duplicate. *p <0.05 versus WT, #p <0.05 versus Elovl2−/− (one-way Anova). (F) Percentages of intracellular production of IFN-γ and IL-17 from peripheral blood CD4+ T cells obtained from wild type (WT) and Elovl2 knock out (Elovl2−/−) mice injected intraperitoneal with 100 ng RvD1 for 15 min and then with 50 µg of anti-CD3 for 3h. Data are shown as means ± SEM of 4 different mice. *p <0.05 versus WT or Elovl2−/− (t-test).
Under specific polarizing conditions, highly purified naïve CD4+ T cells displayed significantly higher amounts of intracellularly produced and extracellularly released IFN-γ (TH1) (p < 0.05) and IL-17 (TH17) (p < 0.01), as compared to non-polarized (TH0) cells (Fig. 4B and 4C). Furthermore, TH17 cells produced less IFN-γ, while TH1 cells produced very low levels of IL-17, confirming previous data showing that the TH17 cytokine profile overlaps with TH1 cells (15). RvD1, RvD2 and MaR1 all affected TH1 and TH17 polarization (Fig. 4B and 4C). In particular, in non-skewed TH0 cells, which produce very low levels of both IFN-γ and IL-17, RvD1, RvD2 and MaR1 induced a slight and not significant decrease in both cytokines (Fig. S2), whereas they significantly reduced TH1 and TH17 generation, acting on both intracellular production (Fig. 4B) and extracellular release (Fig. 4C) of IFN-γ (p < 0.05) from TH1 cells and that of IL-17 (p < 0.01) and IFN-γ (p < 0.05) from TH17 cells. Next, to address whether TH1 and TH17 polarization was associated with the acquisition of their typical features, we also measured the mRNA encoding for the transcription factors known to be critical for their differentiation, T-bet and RORc, respectively. As expected, TH1 and TH17 conditions induced the highest expression of their specific transcription factors. The presence of RvD1, RvD2 or MaR1 during TH1/TH17 polarization led to decreased T-bet in TH1 cells and RORc in TH17 cells, with D-series resolvins being more effective than MaR1 (Fig. 4D). These findings support a pivotal role for SPMs also in hindering de novo TH1/TH17 differentiation. Of note, these SPMs also slightly increased intracellular IL-4 production (Fig. 4B) but not its extracellular release (Fig. 4C) while they decreased the signature TH2 transcription factor GATA-3 (Fig. 4D).
In order to obtain some in vivo evidence for the role of SPMs in reducing TH1 and TH17 responses, we analyzed the ability of these cells to express IFN-γ and IL-17 in mice deficient for elongase 2 (Elovl2−/−), the key enzyme involved in the synthesis of DHA (the precursor of D-series resolvins and maresins) from EPA. Elovl2−/− mice have significantly reduced DHA levels and increased levels of DHA precursors, including EPA (16). As shown in Fig. 4E, splenic T cells produce higher amounts of IFN-γ and IL-17 in Elovl2−/− mice compared to wild type (WT) control mice and this was reversed following DHA supplementation, suggesting that in absence of the precursor of D-series resolvins and maresins, TH1 and TH17 responses are exacerbated. Furthermore, in order to corroborate the in vivo role of SPMs in reducing T cell activation, intraperitoneal administration of RvD1 (100 ng per mouse) together with anti-CD3 (50 µg per mouse) significantly reduced the percentage of IFN-γ and IL-17 production (p < 0.05) from peripheral blood CD4+ T cells (Fig. 4F) in both WT and Elovl2−/− mice, whereby the latter showed increased cytokine production. Together these results suggest that the anti-inflammatory actions of SPMs at physiological doses on TH1 and TH17 cells are also demonstrable in vivo.
In light of the role of SPMs in resolving inflammation and since regulatory T (Treg) cells are an important cell subset involved in modulating and maintaining self-regulation of the immune system, we also investigated if SPMs could affect the generation of induced Treg (iTreg) cells. This cell subset develops from naïve CD4+ T cells upon antigen stimulation and TGF-β exposure (17). To this aim, highly purified naïve CD4+ T cells were cultured under Treg-inducing conditions in the presence of RvD1, RvD2, and MaR1 (all at 10 nmol/L) (Figure 5A). We found that each of these SPMs potentiated iTreg differentiation, with the lipids significantly enhancing Foxp3 expression compared to control iTreg cells (Fig. 5B) (p < 0.05 for RvD2 and p < 0.01 for RvD1 and MaR1). SPM-induced de novo generation of Treg cells was also paralleled by their capacity to increase their suppressive marker CTLA-4 and IL-10 release (p < 0.05), although they were incapable of modulating granzyme B production (Fig. 5B), suggesting that SPMs not only affect Treg induction but also impact specific functional properties. This action was further supported by in vivo evidence that Elovl2−/− mice have significantly lower levels (p < 0.05) of Foxp3+ Treg cells (identified as shown in Fig. S3) compared to WT mice, levels that were restored in DHA-supplemented mice (Fig. 5C).
Fig. 5.
Pro-resolving lipid mediators promote de novo generation of Foxp3-expressing Treg cells. (A) Schematic representation of iTreg generation. (B) Flow cytometry analysis of iTreg cells gated on CD4+CD25high and CD127- cells and expressing Foxp3, CTLA-4 and Granzyme B, and ELISA of IL-10 in supernatants of iTreg cells. Data are shown as means ± SEM of four independent experiments. *p <0.05 versus iTreg, **p <0.01 versus iTreg (one-way Anova). (C) Percentages of intracellular expression of Foxp3 in CD25high CD4+ T cells of splenocytes obtained from wild type (WT), Elovl2 knock out (Elovl2−/−) and Elovl2−/− + DHA mice. Data are shown as means ± SEM of 5 different mice. *p <0.05 versus WT, #p <0.05 versus Elovl2−/−(one-way Anova).
The actions of SPMs on T cells is mediated by GPR32 and ALX/FPR2 receptors
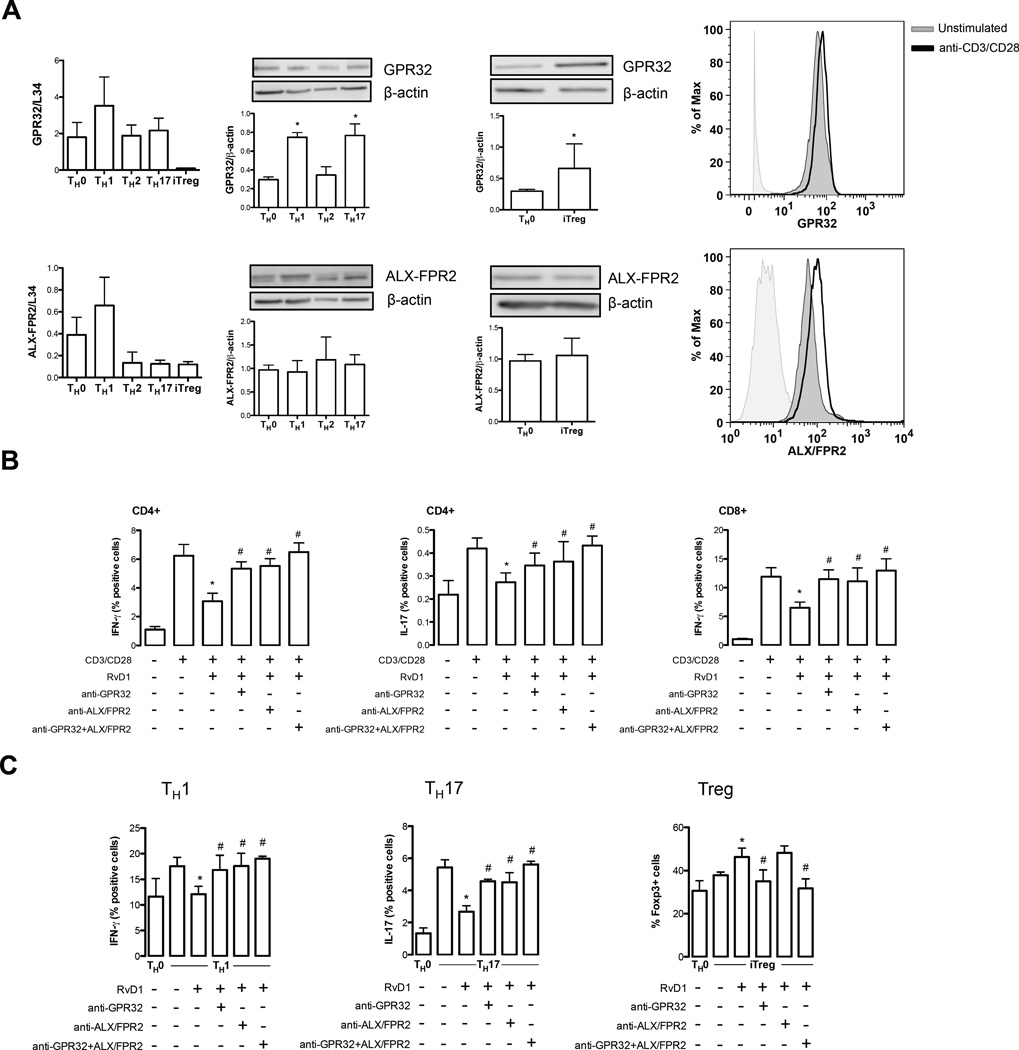
In order to verify whether SPMs-induced effects were associated with a higher CD4+ T cell response and to ascertain the molecular mechanism behind the immunomodulatory role of these lipid mediators on T cells, we sought to investigate the involvement of SPMs receptors in the effects we observed on TH1, TH17 and iTreg cells. Since we still possess limited information regarding the full spectrum of receptors engaged by the different classes of SPMs, we focused on the known receptors for D-series resolvins, GPR32 and ALX/FPR2 (18, 19). In Fig. 6 (left panels) we show that TH1 cells displayed the highest mRNA expression of both GPR32 and ALX/FPR2 compared to TH0 cells, whereas TH17 cells and TH2 showed similar levels of GPR32 and lower levels of ALX/FPR2. Furthermore, iTreg cells expressed very low levels of both receptors. Interestingly, the levels of GPR32 were higher in all the T cell subsets compared to ALX/FPR2, whereas iTreg cells showed a similar expression to TH0 cells. Immunoblotting analysis demonstrated that all T helper subsets express both GPR32 and ALX/FPR2, showing SPMs receptors expression on T cells. In particular TH1, TH17 and iTregs showed significantly higher (p < 0.05) levels of GPR32 with respect to TH0, whereas the expression of this receptor in TH2 cells was similar to that of TH0 control group (Fig. 6A, middle panels). Conversely, ALX/FPR2 showed no differential expression and remained unchanged among all T cell subsets. Flow cytometry analysis also revealed increased expression of both receptors on CD3/CD28-activated total peripheral CD3 T cells (Figure 6A, right panels).
Fig. 6.
The effects of Pro-resolving lipid mediators on T cells are mediated by GPR32 and ALX/FPR2 receptors. (A) qRT-PCR analysis, immunoblotting of GPR32 and ALX/FPR2 in polarized TH0, TH1, TH2, TH17 and iTreg, as detailed in Materials and Methods. Data are shown as means ± SEM of four independent experiments. *p <0.05 versus TH0 (one-way Anova for TH1, TH2, TH17 and t-test for iTreg). A flow cytometry representative analysis of GPR32 and ALX/FPR2 is shown in resting and anti-CD3/CD28-activated total peripheral CD3+ T cells (B–C) Intracellular cytokine production from CD4+ and CD8+ T cells as well as from polarized TH1, TH17 and iTreg pre-treated with neutralizing antibodies against GPR32 or ALX/FPR2 (alone or in combination, 2 µg/ml) in presence of RvD1 and following anti-CD3/CD28 stimulation for 8 hours. Data are shown as means ± SEM of four independent experiments. *p <0.05 versus anti-CD3/CD28-activated cells; #p <0.05 versus RvD1-treated cells (one-way Anova).
Since these two G protein-coupled receptors are currently the only known receptors for RvD1, we next sought to verify their possible role as mediators of the observed effect of this pro-resolving lipid on T cells. Pre-incubation with anti-GPR32 or anti-ALX/FPR2 neutralizing antibodies alone or in combination abrogated the suppressive activity of RvD1 on TH1 and TH17, as well as its enhancing activity on iTreg lymphocytes. In particular, the single neutralization of either GPR32 or ALX/FPR2 significantly counteracted the inhibitory action of RvD1 on both PBMC-derived (Figure 6B) and de novo-generated TH1 and TH17 cells (Figure 6C) (p < 0.05), whereas the inactivation of both receptors was more potent, completely restoring the intracellular levels of IFN-γ and IL-17 as to those of activated T cells (Figures 6B and C), suggesting that the role of these two receptors might be additive. Conversely, the RvD1-induced de novo generation of Foxp3+ iTregs was specifically and significantly counteracted when neutralizing only GPR32 (p < 0.05) and not ALX/FPR2 (Figure 6C), also confirmed by the evidence that the neutralization of both receptors was not additive.
DISCUSSION
Since their first identification 15 years ago, SPMs, which include resolvins, protectins and maresins, have proven to act as initiators of resolution programs of acute inflammation, thereby reducing granulocyte trafficking and the production of cytokines and extracellular reactive oxygen species, as well as by lowering the magnitude of the overall inflammatory response by enhancing macrophage-mediated clearance of cellular debris and invading microbes (4). Although the role of each of the SPMs is highly associated with the resolution of acute inflammation operated by cells of innate immunity, it is becoming increasingly clear that these bioactive lipids might take part also in the control of chronic inflammation, possibly via acting on cells of adaptive immunity (20–23). In this regard, little systematic evidence for a direct role of these resolving mediators on the distinct adaptive cell populations has been assessed (24, 25).
In the present study, we interrogated the adaptive immune responses to specific SPMs that have not been systematically addressed earlier. Cytotoxic CD8+ T cells eliminate neoplastic, infected or damaged cells mainly through the release of cytotoxins (perforin, granzymes and granulysins), and potentiate innate immune responses (macrophages and NK cells) through the release of cytokines such as IFN-γ and TNF-α (26). The observed ability of SPMs to dampen cytokines from CD8+ cells suggest that their role in resolving inflammation is exerted not only directly by clearing and blunting the responses of those innate immune cells during acute inflammation, but also indirectly by avoiding further recruitment or activation of innate cells, thus avoiding the onset of chronic inflammation or immune-mediated damage. Furthermore, CD8+ cells are also able to prime naïve and restimulate experienced CD4+ T cells to release high levels of helper cytokines (27). TH cells develop from naïve CD4+ T cells and differentiate into specialized TH subsets after encountering foreign or auto-antigens (14, 28). However, persistent or uncontrolled TH cell responses are often associated with pathological states and tissue damage. In particular, excessive and/or abnormal TH1 and TH17 cell responses are involved in chronic inflammation and mediate several autoimmune diseases, including multiple sclerosis, rheumatoid arthritis, and psoriasis (29).
Our present results reveal that specific SPMs, namely RvD1, RvD2 and MaR1, not only can directly modulate the inflammatory responses of already existing and activated TH1 and TH17 cells, but can also critically prevent their generation from naïve CD4+ T cells acting on their transcription factor-induced activation programs. Additionally, SPMs are able to enhance the differentiation of CD4+ T cells into Treg cells. Since Treg cells typically serve to dampen excessive immune responses, these cells play an important role in preventing the over-activation of TH1 and TH17 cells. This result is in line with the very recent report of MaR1 in engaging Treg cells in mice to promote resolution of lung inflammation (30). Although further studies are needed to demonstrate the existence of an indirect modulation of TH1 and TH17 cells by a SPM-mediated sustained induction of Treg cells, these findings suggest that SPMs might modulate inflammatory responses via several selective mechanisms on specific adaptive immune cells. This hypothesis is conceivable, also in the light of the recent discovery that TH17 cells transdifferentiate into Treg cells during resolution of inflammation (31), where SPMs might be possible key players of such TH17 instability and plasticity.
TH2 lymphocytes were relatively unresponsive to SPMs treatment under the conditions tested, in that none of the pro-resolving mediators tested regulated the generation of mature effector cells or cytokine release. These results are aligned with recent papers reporting that, in TH2-driven pathologies and mouse models, DHA-derived SPMs like RvD1 and PD1 do not affect IL-4 release and might ameliorate clinical outcome by acting on different targets than TH2 cells (32–35). Conversely, RvE1, which is derived from EPA, facilitates resolution of TH2-mediated asthmatic airway inflammation and atopic dermatitis by reducing TH2 cytokines (29, 36, 37). Our findings are also supported by the in vivo evidence that mice incapable of producing DHA-derived SPMs have higher numbers of hyperactive TH1 and TH17 cells and concomitantly reduced levels of Treg cells. In this genetic background, either DHA supplementation or in vivo injection of RvD1 in anti-CD3 treated mice reduced the activation of TH1 and TH17 cells and restored Treg cell numbers. In line with this, it has also been shown that deletion or pharmacological inhibition of SPM-generating 12/15-lipoxygenase regulated murine and human dendritic cell maturation and activation, favoring TH17 differentiation of CD4+ T cells (38), thus highlighting the critical role for SPMs in modulating T helper responses. The recent identification of SPMs in human secondary lymphoid organs (11), where most naïve-to-effector or iTreg differentiation happens, provides in vivo relevance of our findings. Of note, the expression of GPR32 and ALX/FPR2, the RvD1 receptors, by all T-cell subsets suggests that these receptors are involved in DHA-derived SPMs-induced effects on cell-mediated adaptive immunity. Interestingly, both receptors seemed to be involved in RvD1 signaling on both TH1 and TH17 cells independently of their relative expression, while GPR32 appeared to specifically mediate the effects of RvD1 on Treg cells. On the other hand, TH2 cells, which were not modulated by SPMs, was the only T cell subset to display no variation in the expression of any of these receptors compared to their TH0 precursors. Furthermore, the finding that SPMs preserve cell viability and proliferation while modulating pro-inflammatory responses is of note, because it rules out the possibility that the observed immunomodulation of T cells is caused by SPM-induced cell death.
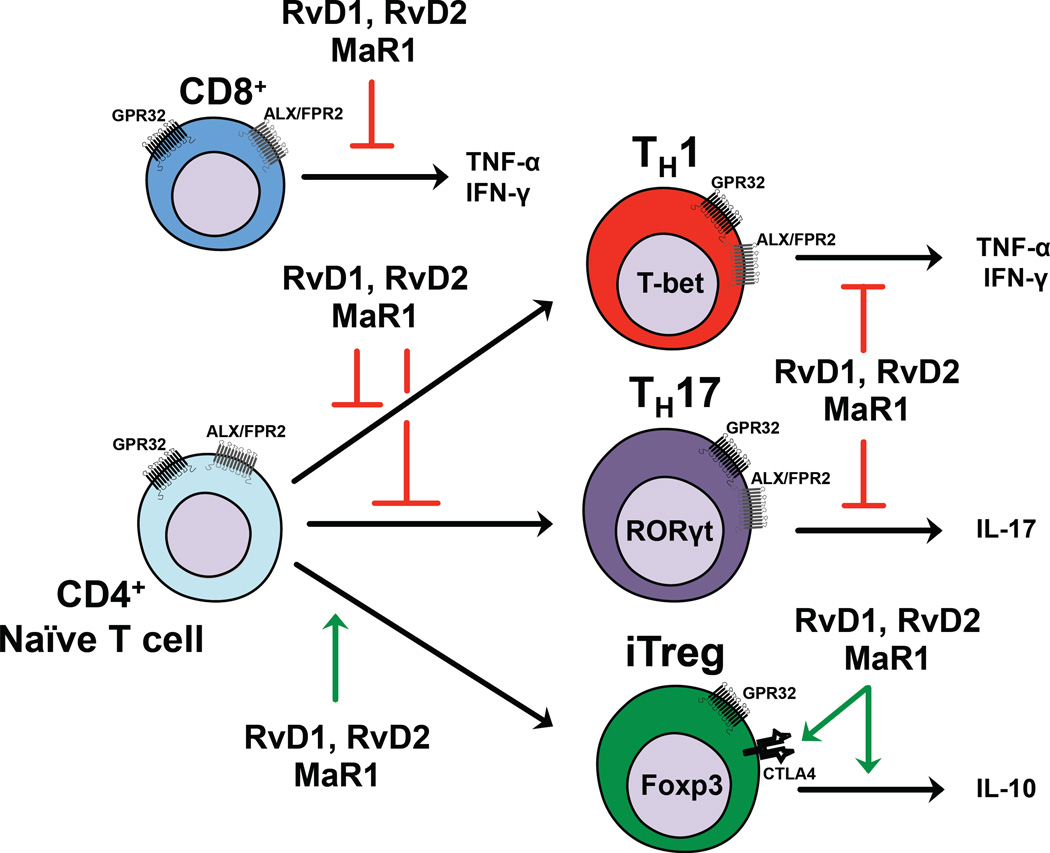
Current clinical research is increasingly directed to the possibility of interfering with the functions of TH cells. Thus the finding that natural endogenous mediators like SPMs (i.e. RvD1, RvD2 and MaR1) exert a non-cytotoxic regulatory role on cells central to induction of autoimmunity represents a promising beginning for a new avenue of research. These results suggest that SPMs might possibly act on the balance between pathogenic TH1/TH17 and tolerogenic Treg cells, which is typically altered during chronic inflammatory and autoimmune diseases. This study, schematically summarized in Fig 7, extends the original paradigm whereby the SPMs found in the resolving exudates not only stimulate signs of resolution – terminating acute inflammation and restoring homeostasis (4) - but as reported herein can also modulate adaptive immunity.
Fig. 7.
Schematic representation of SPMs checkpoints on the regulation of acute and chronic inflammatory processes. During the acute inflammation, the site of infection features a massive infiltration of innate immune cells (e.g. neutrophils and monocytes) that trigger inflammatory processes through the production of cytokines, chemokines and omega-6 derivates, (e.g. prostaglandins, prostacyclins, leukotrienes and thromboxanes) and reactive species, that promote vasodilatation, recruitment of other neutrophils and monocytes as well as elimination of pathogens. In the advanced phases of acute inflammation, neutrophils and resolving macrophages undergo a molecular reconfiguration of their lipid mediators’ profile, and start producing SPMs (including Rvs and MaRs) that promote the resolution of inflammation, which is characterized by the induction of apoptosis in neutrophils and clearance of cellular debris. Here we demonstrate that Rvs and MaR1 can also act on adaptive immune cells that play a central role later, during chronic inflammation, significantly reducing the release of pro-inflammatory cytokines in activated CD8+ T cells as well as in TH1 and TH17 CD4+ cell subsets engaging both GPR32 ALX/FPR2 receptors. Furthermore, Rvs and MaR1 prevent de novo generation of TH1 and TH17 from CD4+ naïve T cells via both receptors, while favoring that of iTreg cells through only GPR32. Hence, in the event that the acute inflammation is not properly resolved, the SPMs that reside in the resolving exudate, might be also involved in limiting the chronic inflammatory responses acting on the balance between highly pathogenic TH1/TH17 and regulatory iTreg cells. Of note, adaptive immunity can also regulate several functions of innate immunity, thus establishing a complex network, typical of several pathophysiological conditions (such as autoimmunity). Foxp3, forkhead box p3; IFN-γ, interferon-γ; IL-17, interleukin-17; MaR, maresin; PMN, polymorphonucleate cells; PUFA, polyunsaturated fatty acids; RORγt, RAR-related orphan receptor γ; Rsv, resolvin; SPMs, specialized pro-resolving lipid mediators; T-bet, T-box expressed in T-cells; TH, T-helper; TNF-α, tumor necrosis factor-α.
MATERIALS AND METHODS
Study Design
This is an experimental laboratory study performed with human blood samples (n= 40) and animals (n=26). The objective was to study the role of specific specialized pro-resolving lipid mediators (Resolvin D1, Resolvin D2 and Maresin 1) on activation and differentiation of T cell subsets with the translational perspective to gain insights into the possibility that such bioactive lipids could regulate adaptive immunity and thus control T-cell mediated chronic inflammatory processes. All the healthy donors gave their written informed consent to the study. In vivo studies were carried out with ethical permission from the Animal Ethics Committee of the North Stockholm region, Sweden. The number of replicates is indicated for each experiment in the respective figure legends. Mechanistic studies on cells from healthy blood donors were performed with in vitro assays without blinding or randomization.
Materials
RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid), RvD2 (7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid), AT-RvD3 (4S,11,17R-trihydroxydocosa-5Z,7,9,13,15E,19Z-hexaenoic acid), MaR1 (7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) were prepared by total organic synthesis as essentially described (39–42) or purchased from Cayman Chemical.
Peripheral blood cells isolation and purification of naïve CD4+ T lymphocytes
Peripheral blood mononuclear cells (PBMCs) were isolated after venous puncture from healthy donors, and were separated by density gradient over Ficoll-Hypaque (Amersham Biosciences). CD4+ T lymphocytes were purified by immuno-magnetic depletion with the human CD4+ T Cell Isolation Kit II (Miltenyi Biotec), and were purified by means of negative selection through AutoMACS Pro Separator (Miltenyi Biotec). Briefly, effector and memory T cells, NK cells, B cells, dendritic cells, and granulocytes were indirectly labeled using a cocktail of biotin-conjugated antibodies and anti-biotin magnetic microbeads. Highly purified unlabeled naïve CD4+ T cells (CD4+CD45RA+CD27+CD45RO-) were obtained by depletion of the magnetically labeled cells and had a purity of over 95%, which was confirmed by flow cytometry.
Flow cytometry
In order to measure the intracellular cytokine levels, secretion was inhibited by adding 1 µg/ml brefeldin A (Sigma-Aldrich), 5 hours before the end of stimulation with either PMA/Ionomycin or Dynabeads CD3/CD28 T Cell Expander (one bead per cell; Invitrogen) (16). At the end of the incubation, cells were stained at cell surface with e780-conjugated anti-CD3 (eBiosciences), PerCP5.5-conjugated anti-CD4 (eBiosciences), Brilliant Violet-conjugated anti-CD8 (Biolegend), made permeable with Cytofix/Cytoperm reagents (BD Biosciences), and then stained intracellularly with Phycoerythrin-Cy7-conjugated anti-TNF-α (eBiosciences), Allophycocyanin (APC)-conjugated anti-IFN-γ (eBiosciences), Phycoerythrin (PE)-conjugated anti-IL-17 (eBiosciences), anti-PercP5.5-conjugated anti-IL-2 (Biolegend), Brilliant Violet 421-conjugated IL-4 (eBioscience) and Fluorescein isothiocyanate (FITC)-conjugated anti-Granzyme B in 0.5% saponin, at RT for 30 min. In some experiments, cells were also stained at cell surface with PE-conjugated anti-FasL (Miltenyi Biotec), APC-conjugated anti-PD-1 (eBioscience) and PE-conjugated anti-CTLA-4 (Miltenyi Biotec). Intracellular cytokines were analyzed by flow cytometry in a FACS-Cyan ADP (Beckman Coulter). For each analysis, at least 300,000 events were acquired gating on Pacific Orange-conjugated Live/Dead negative cells. In some experiments PBMCs were pre-incubated for 30 min with anti-GPR32 (2 µg/ml; clone GTX71225, GeneTex) and/or anti-FPR2/ALX (2 µg/ml, clone FN-1D6-A1, Genovac, Freiburg, Germany) prior to incubation with vehicle or 10 nmol/L RvD1 and to stimulation with Dynabeads CD3/CD28 T Cell Expander (one bead per cell; Invitrogen). The list of antibodies used for flow cytometry and their dilution is detailed in Table S1.
Detection of apoptotic and necrotic cells
Apoptotic and necrotic cells were detected using Annexin-V-FITC and propidium iodide (PI) staining (eBioscience), respectively. Cells were washed twice in in PBS followed by re-suspension in Binding Buffer (Annexin-V Kit, eBioscience) and then incubated with 5µl of Annexin-V-FITC for 15 min at RT. Cell were then extensively washed with Binding Buffer and 5µl of PI was added and cells analyzed within 2 hours on a FACS-Cyan ADP (Beckman Coulter).
Proliferation Assay
CD3+ T cells were isolated through positive selection with AutoMACS Pro Separator and 10×106 cells were labeled with CFSE at a final concentration of 5 µM for 10 min at 37 °C in agitation. Then, cells were washed twice with PBS/10% FBS, suspended in culture media and analyzed immediately on a FACS-Cyan ADP (Beckman Coulter). Analysis of cells immediately after CFSE labeling indicated a labeling efficiency higher than 99%. Cell proliferation was followed by flow cytometry at day 2 and 4 upon stimulation with Dynabeads CD3/CD28 T Cell Expander in presence or absence of 10 nmol/L RvD1, RvD2 and MaR1.
T helper cell differentiation assay
For TH1 and TH17 polarization of T cells, highly purified naïve CD4+ T cells were cultured in round bottom 96-well plates (Falcon) at a density of 5×104 cells at 37°C in 200µl final volumes of X-VIVO 15 medium with Dynabeads CD3/CD28 T Cell Expander and under TH1-, TH2- and TH17-polarizing conditions in presence or absence of RvD1, RvD2 or MaR1 (10 nmol/L). The following human recombinant cytokines were used: for TH1 polarization, 10ng/ml IL-12 (Miltenyi Biotech); and for TH17 polarization, 10ng/ml IL-1β, 20ng/ml IL-6, 100ng/ml IL-23, and 1ng/ml TGF-β (Miltenyi Biotech). After 5 days, cells were collected and washed extensively and their viability was determined by trypan blue exclusion. Cells (1×106 cells/ml) were re-stimulated for 6h (for flow cytometry) or 24h (for ELISA and qRT-PCR) with Dynabeads CD3/CD28 T Cell Expander (one bead per cell). Cultures were supplemented with RvD1, RvD2 or MaR1 every other day for 5d for flow cytometry analysis and then were re-stimulated with CD3/CD28 beads for 24h for ELISA and qRT-PCR. For TH2 polarization, naïve CD4+ T cells were kept under polarizing conditions using 25ng/ml IL-4 (Miltenyi Biotec) and Dynabeads CD3/CD28. Cells were extensively washed and re-stimulated with CD3/CD28 beads every 3 days with fresh medium plus IL-4 and 20ng/ml IL-2 (Miltenyi Biotec) up to day 12. After 12 days cells were collected for ELISA or qRT-PCR or re-stimulated for 6h with 100nmol/l PMA/Ionomycin for intracellular staining. In some experiments TH1, TH17 and iTreg cells were incubated for 30 min with anti-GPR32 (2 µg/ml) and anti-FPR2/ALX (2 µg/ml) antibodies, alone or in combination, washed with complete medium, subsequently treated with vehicle or 10 nmol/L RvD1 and then stimulated with CD3/CD28 beads.
Generation of induced Treg cells
Highly purified naïve CD4+ T cells were cultured in round bottom 96-well plates (Falcon) at a density of 5×104 cells at 37°C in 200µl final volumes of X-VIVO 15 medium in presence of Dynabeads CD3/CD28 T Cell Expander (one bead per cell; Invitrogen), TGF-β (2 ng/ml; Miltenyi Biotec) and IL-2 (20 U/ml), in the presence or absence of RvD1, RvD2, or MaR1 (10 nmol/L). Cultures were supplemented with SPMs every other day for 5 d.
ELISA
Cytokine content was determined by standard 2-site sandwich enzyme-linked immunosorbent assays (ELISA), using available commercial kits for IFN-γ and IL-17 (eBioscience), as previously reported (43) and through Multiplex Bead-based Luminex Assay for measurement of IL-4 and IL-10 (R&D Systems).
qRT-PCR
Total RNA was extracted with an RNeasy Micro kit (Qiagen). A mixture containing random hexamers, oligo(dT)15 (Promega) and SuperScript II Reverse Transcriptase (Invitrogen) was used for cDNA synthesis. Transcripts were quantified by real-time quantitative PCR on an ABI PRISM 7900 sequence detector (Applied Biosystems) with Applied Biosystems predesigned TaqMan Gene Expression Assays and Absolute QPCR ROX mix (Thermo Fisher Scientific). The following probes were used (Applied Biosystems, assay identification numbers in parentheses): T-bet (Hs00203436_m1), RORc (Hs01076112_m1), GATA-3 (Hs00231122), GPR32 (Hs01102536_s1) and FPR2 (Hs02759175_s1). For each sample, mRNA abundance was normalized to the amount of ribosomal protein L34 (Hs00241560_m1).
Immunoblotting
Purified and polarized TH0, TH1, TH2, TH17 and iTreg cells were lysed with lysis buffer and cell homogenates were subjected to 10% SDS–PAGE (50 µg/lane) under reducing conditions. Gels were then electroblotted onto 0.45-µm nitrocellulose filters (Bio-Rad, Hercules, CA, USA) and were incubated with primary anti-GPR32 polyclonal mouse antibody (1:500, clone GTX71225, GeneTex), anti-ALX/FPR2 monoclonal rabbit antibody (1:500, clone FN-1D6-A1, Genovac) or with anti-β-actin monoclonal mouse antibody (1:10000, Bio-Rad), and then with secondary goat-anti-rabbit polyclonal antibody (1:2000, Santa Cruz Biotechnologies) for GPR32 and goat-anti-mouse polyclonal antibody (1:2000 for ALX and 1:10000 for β-actin).
Elovl2 knock out animals and in vivo experiments
Elovl2−/− mice were generated as described previously (16). All animals were housed at room temperature and maintained on a 12 h light/ dark cycle. Adult mice were fed standard chow DHA-free diet (10% kcal fat, D12450H, Research Diets, New Brunswick, NJ, USA) or DHA-enriched (10% kcal fat, 14% DHA, D13021002, Research Diets, New Brunswick, NJ, USA), according to the experimental groups. All animals were fed ad libitum and had free access to water. At the end of the study, animals were euthanized with CO2 and cervical dislocation. For in vivo experiments, mice were pretreated intraperitoneally (ip) with 100 ng RvD1 for 15 min and then injected with 50 µg of anti-CD3 (Biolegend, Armenian hamster IgG, clone 145-2C11). Blood samples were recovered 3 h after antibody injection and a cell suspension was prepared for flow cytometry analysis.
Statistical analysis
All data were expressed as means ± SEM. Differences between groups were compared using Student’s t-test (two groups) or one-way ANOVA (multiple groups) followed by a post hoc Bonferroni test. The criterion for statistical significance was p < 0.05 or less. All statistical analyses were performed with GraphPad Prism. FACS analysis was performed using the FlowJo analysis program (Treestar, Ashland, OR).
Supplementary Material
Acknowledgments
The authors kindly thank Dr. Romain Colas for expert help with validation of the SPM physical properties via LC-MS-MS profiling with authentic SPMs and Dr. Giovanna Borsellino for helpful discussions.
Funding.
This work was supported by Fondazione Italiana Sclerosi Multipla (FISM) to V.C. (grant 2015/R/8) and in part by National Institutes of Health (P01095467 and GM38765) to C.N.S, by Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN grant 2010–2011) to M.M., and by Ministero della Salute (RF-2011-02346771) and FISM (grant 2013/R/2) to L.B.
Footnotes
The published article is available at: http://stm.sciencemag.org/cgi/content/full/8/353/353ra111?ijkey=Ts2ilETnwGkdI&keytype=ref&siteid=scitransmed
SUPPLEMENTAL MATERIAL
Fig. S1. AT-RvD3 dose-dependently reduces TNF-α and Resolvin D1, D2 and Maresin 1 do not affect long-term cell death in both CD8+ and CD4+ T cells.
Fig. S2. Resolvin D1, D2 and Maresin 1 do not affect TH0 cells.
Fig. S3. Gating strategy for Treg identification.
Table S1. Antibodies used for T cell surface staining and for analysis of intracellular cytokine production.
Author contributions
V.C. conceived, designed and supervised the study, performed flow cytometry and ELISA experiments, analyzed all data and wrote the manuscript. A.L. performed cell-culture and polarization experiments, qRT-PCR, immunoblotting, in vivo experiments and analyzed data. J.D. prepared and validated SPMs, checked their integrity, contributed to experimental design and helped with manuscript preparation. A.J. provided WT and Elovl2 knock out mice. L.B. provided the use of flow cytometry unit and scientific suggestions. M.M. supervised the study, provided reagents and revised the manuscript. C.N.S. designed and supervised experiments, provided reagents, and contributed to the manuscript preparation.
Competing Interests
C.N.S. is an inventor on several patents assigned to Brigham and Women's Hospital and licensed to Resolvyx Pharmaceuticals [WO2004014835 A3, Resolvins: biotemplates for therapeutic interventions; WO2005089744 A3, Use of docosatrienes, resolvins and their stable analogs in the treatment of airway diseases and asthma; EP 2344441 A2, 14-hydroxy-docosahexaenoic acid compounds]. C.N.S. was also the scientific founder of Resolvyx Pharmaceuticals with equity ownership in the company; and has interests reviewed and managed by the Brigham and Women's Hospital and Partners Health Care in accordance with their conflict of interest policies. L.B. received honoraria for speaking or consultation fees from TEVA, Baxter, and Genzyme. He also received grants from TEVA. The other authors declare no commercial or financial conflict of interest.
REFERENCES
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual review of immunology. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 3.Serhan N, Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Serhan N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of experimental medicine. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews. Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli P, Levy BD. Resolvins and protectins: mediating solutions to inflammation. British journal of pharmacology. 2009;158:960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. Journal of immunology. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 10.Ariel, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. The Journal of biological chemistry. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 11.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology. Cell physiology. 2014;307:C39–C54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal immunology. 2016;9:757–766. doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC. Polyfunctional responses by human T cells result from sequential release of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1607–1612. doi: 10.1073/pnas.1117194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raphael, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 16.Pauter M, Olsson P, Asadi A, Herslof B, Csikasz RI, Zadravec D, Jacobsson A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. Journal of lipid research. 2014;55:718–728. doi: 10.1194/jlr.M046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. Journal of immunology. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 18.Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1970–1978. doi: 10.1161/ATVBAHA.112.249508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacology & therapeutics. 2014;141:272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ. Resolvin D1 Reduces Emphysema and Chronic Inflammation. The American journal of pathology. 2015;185:3189–3201. doi: 10.1016/j.ajpath.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwanke RC, Marcon R, Bento AF, Calixto JB. EPA- and DHA-derived resolvins' actions in inflammatory bowel disease. European journal of pharmacology. 2015 doi: 10.1016/j.ejphar.2015.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Poisson LM, Suhail H, Singh J, Datta I, Denic A, Labuzek K, Hoda MN, Shankar A, Kumar A, Cerghet M, Elias S, Mohney RP, Rodriguez M, Rattan R, Mangalam AK, Giri S. Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Drug-like Properties in Chronic Animal Model of Multiple Sclerosis. The Journal of biological chemistry. 2015;290:30697–30712. doi: 10.1074/jbc.M115.679068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. Journal of immunology. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TH, Kim GD, Jin YH, Park YS, Park CS. Omega-3 fatty acid-derived mediator, Resolvin E1,ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. International immunopharmacology. 2012;14:384–391. doi: 10.1016/j.intimp.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nature reviews. Immunology. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 27.Romagnoli PA, Premenko-Lanier MF, Loria GD, Altman JD. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PloS one. 2013;8:e56999. doi: 10.1371/journal.pone.0056999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman L. A brief history of T(H)17,the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature medicine. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 29.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. Journal of immunology. 2015;194:863–867. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature reviews. Immunology. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. Journal of immunology. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogerio P, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. Journal of immunology. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochemical and biophysical research communications. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, Sunaga N, Nakakura T, Okajima F, Dobashi K, Mori M. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochemical and biophysical research communications. 2010;400:128–133. doi: 10.1016/j.bbrc.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Rothe T, Gruber F, Uderhardt S, Ipseiz N, Rossner S, Oskolkova O, Bluml S, Leitinger N, Bicker W, Bochkov VN, Yamamoto M, Steinkasserer A, Schett G, Zinser E, Kronke G. 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. The Journal of clinical investigation. 2015;125:1944–1954. doi: 10.1172/JCI78490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 40.Spite, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler JW, Uddin J, Serhan CN, Petasis NA. Stereocontrolled total synthesis of the potent anti-inflammatory and pro-resolving lipid mediator resolvin D3 and its aspirin-triggered 17R–epimer. Organic letters. 2013;15:1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PloS one. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.