Abstract
Behavioral sensitization following repeated amphetamine (AMPH) exposure is associated with changes in GABA function in the medial prefrontal cortex (mPFC). In rats exposed to AMPH during adolescence compared to adulthood, there are unique patterns of sensitization that may reflect age-dependent differences in drug effects on prefrontal GABAergic function. In the current study, we used a sensitizing regimen of repeated AMPH exposure in adolescent and adult rats to determine if a post-withdrawal AMPH challenge would alter inhibitory transmission in the mPFC in a manner that depends on age of exposure. Male Sprague-Dawley rats were treated with saline or 3 mg/kg AMPH (i.p.) during adolescence [postnatal day (P) 27 to P45] or adulthood (P85 to P103) and were sacrificed either at similar ages in adulthood (~P133; Experiment 1) or after similar withdrawal times (3-4 weeks; Experiment 2). Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in vitro from deep layer pyramidal cells in the mPFC using the whole-cell configuration. We found no effect of AMPH pre-exposure on baseline sIPSC frequency. Subsequent application of AMPH (25 μM) produced a stable increase in sIPSC frequency in controls, suggesting that AMPH increases inhibitory tone in the mPFC. However, AMPH failed to increase sIPSCs in adolescent- or adult-exposed rats. In Experiment 2, where withdrawal period was kept similar for both exposure groups, AMPH induced a suppression of sIPSC activity in adolescent-exposed rats. These results suggest that sensitizing treatment with AMPH during adolescence or adulthood dampens inhibitory influences on mPFC pyramidal cells, but potentially through different mechanisms.
Keywords: amphetamine, medial prefrontal cortex, adolescence, whole-cell recording, GABA
Repeated amphetamine (AMPH) exposure has been shown to to induce various cognitive abnormalities [1,2] that could be attributed to changes in the mesocortical dopamine circuit [3]. Because adolescents appear to have a heightened vulnerability for developing substance use and other mental disorders [4], many have suggested the adolescent brain is particularly sensitive to drug-induced plasticity. The mesocorticolimbic dopamine circuit may be especially vulnerable since it undergoes extensive developmental remodelling throughout adolescence and young adulthood [5].
To date, studies in laboratory animals have found evidence showing some of AMPH's effects on behavior are distinct if the exposure happens during adolescence compared to adulthood. For example, adolescent rats are more sensitive to AMPH-induced locomotor sensitization following repeated exposure to low doses (< 2 mg/kg) of the drug [6]. With higher doses, adolescent-exposed rats exhibit less AMPH-induced stereotypy and more ambulation when challenged following extended withdrawal [7–10]. In light of previous studies suggesting stereotypy following AMPH sensitization is mediated in part by GABAergic function in the prefrontal cortex (PFC) [11], we hypothesize that inhibitory tone in the PFC may be modulated differently by adolescent, compared to adult, AMPH exposure.
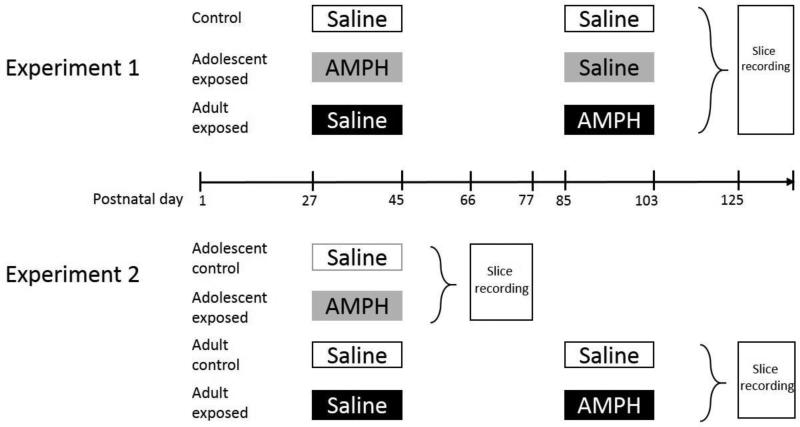
To test this hypothesis, we used an in vitro slice preparation to record from pyramidal cells in the medial PFC (mPFC) of rats exposed to AMPH during adolescence or adulthood. For these studies, which used procdures approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana-Champaign, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), we used male Sprague-Dawley rats that were offspring of rats bred in our facility. Rats were weaned on postnatal day (P) 22 and housed in groups of 2-3 on a 12-h light/dark cycle (lights on at 0800) with food and water available ad libitum. The treatment procedure for injections with saline and AMPH (d-amphetamine hemisulfate salt; Sigma-Aldrich, St. Louis, MO, USA) is summarized in Fig. 1. We have previously shown that this treatment procedure induces robust, long-lasting behavioral sensitization [7,8,10]. In Experiment 1, rats (n = 4/group) were sacrificed between P125 and P143 (mean = P133) to keep age at sacrifice approximately equal across groups. This resulted in different withdrawal duration (adult-exposed: 3-5 weeks; adolescent-exposed: 11-14 weeks). In Experiment 2 (n = 3- 4 rats/group), withdrawal duration was kept approximately equal (3-5 weeks) by sacrificing adult-exposed rats between P125 and P136 and adolescent-exposed rats between P66 and P77. For both experiments, spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded intracellularly from layer V/VI pyramidal cells in prelimbic and infralimbic mPFC using Cs-filled pipettes (containing in mM: 117.0 Cs-gluconate, 13.0 CsCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP and 0.3% biocytin) as previously described [12]. Briefly, coronal brain slices containing the mPFC (350 μm thickness) were incubated with physiological saline at room temperature for ≥ 1h before recording. This solution contained in mM: 126.0 NaCl, 2.5 KCl, 1.25 MgCl2, 2.0 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, and 10.0 glucose (gassed with 95% O2/5% CO2 to a final pH of 7.4). Spontaneous currents were recorded at 0 mV holding potential with glutamate receptor antagonists present (10 μM CPP and 20 μM DNQX; Tocris, St. Louis, MO) and were amplified with a Multiclamp 700 amplifier (Molecular Devices, Foster City, CA). At the end of recordings, cells were filled with biocytin and confirmed microscopically as pyramidal neurons by their soma shape and apical dendrite orientation. Each cell was recorded for 8 min of baseline sIPSC measurement and 25 min after the application of 25 μM AMPH. Time course data of sIPSC frequency and amplitude are presented in 1-min bins and normalized to the mean baseline values for individual cells. Analysis of sIPSC time course was done with two-way, mixed factor ANOVA. Peak response to AMPH was determined by using an adjacent-averaging data smoothing method as previously described [10, 12] and further analyzed with one-way ANOVA followed by Tukey's post-hoc analyses where appropriate.
Figure 1.
Schematic of the group design and timeline of treatment/recording schedule. Rats received 10 i.p. injections every other day from P27 to 45 and/or P85 to 103 and were sacrificed for recordings between P66 and P77 or between P125 and P143. To control for injection experience at the correspoinding developmental time period, rats in the adult-exposed group were given saline during the adolescent treatment period. For the rats in the adolescent-exposed group from Experiment 1, saline injections were given during the adult treatment period. The testing age was kept similar in Experiment 1, whereas withdrawal duration was kept similar for Experiment 2.
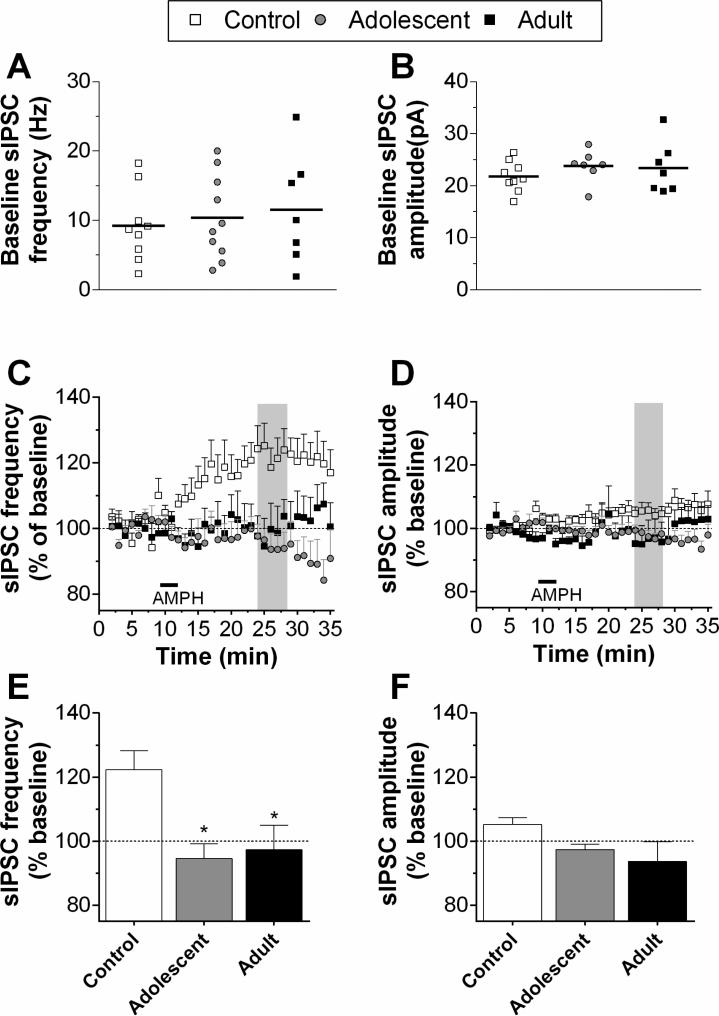
In experiment 1, we did not find any statistically significant group differences in baseline sIPSC amplitude or frequency (Fig. 2 A and B). However, due to the considerable variance in the baseline frequency between individual cells, time course data were normalized to their own baseline value for group comparison. Acute application of AMPH (25 μM for 4 min) increased the normalized sIPSC frequency in cells recorded from control rats, but this effect was not observed in either of the AMPH-treated groups (Fig. 2C). Statistical analysis of these data revealed a significant main effect of group (F2,689 = 6.56, p < 0.01), a group x time interaction (F68,689 = 3.15, p < 0.001) and a near significant main effect of time (F34,689=1.42, p =0.058). A one-way ANOVA of the peak response to AMPH revealed a significant group effect (F2,20 = 7.09, p < 0.01), with pairwise comparisons revealing both adolescent- and adult-exposed groups were significantly lower than controls (Fig. 2E). These results suggest that in pre-treated rats, regardless of age of exposure, the ability of AMPH to increase sIPSC activity is abolished. Statistical analysis of amplitude revealed a significant main effect of group (F2, 689 = 43.5, p < 0.001), with controls exhibiting a small increase in amplitude across time (Fig. 2D). However, the group differences in peak amplitude were not statistically significant (Fig. 2F).
Figure 2.
sIPSC activity recorded in pyramidal neurons in Layer V/VI of mPFC for Experiment 1. Recordings were obtained from 7-10 cells/group in slices from 4 rats/group. Baseline frequency and amplitude of sIPSCs are shown in A and B (average of 8-10 min baseline; horizontal bars indicate the mean of each group). After bath application of AMPH (25μM for 4 min; horizontal bar), sIPSC frequency increased in controls but was unchanged in rats exposed to AMPH during adolescence or adulthood, respectively (C). There was a slight increase of sIPSC amplitude in controls but not in exposed animals (D). Shaded regions in the time series indicate the areas of peak response. The mean responses during these periods are summarized in the bar graphs (E, F). *p < 0.05, vs. control.
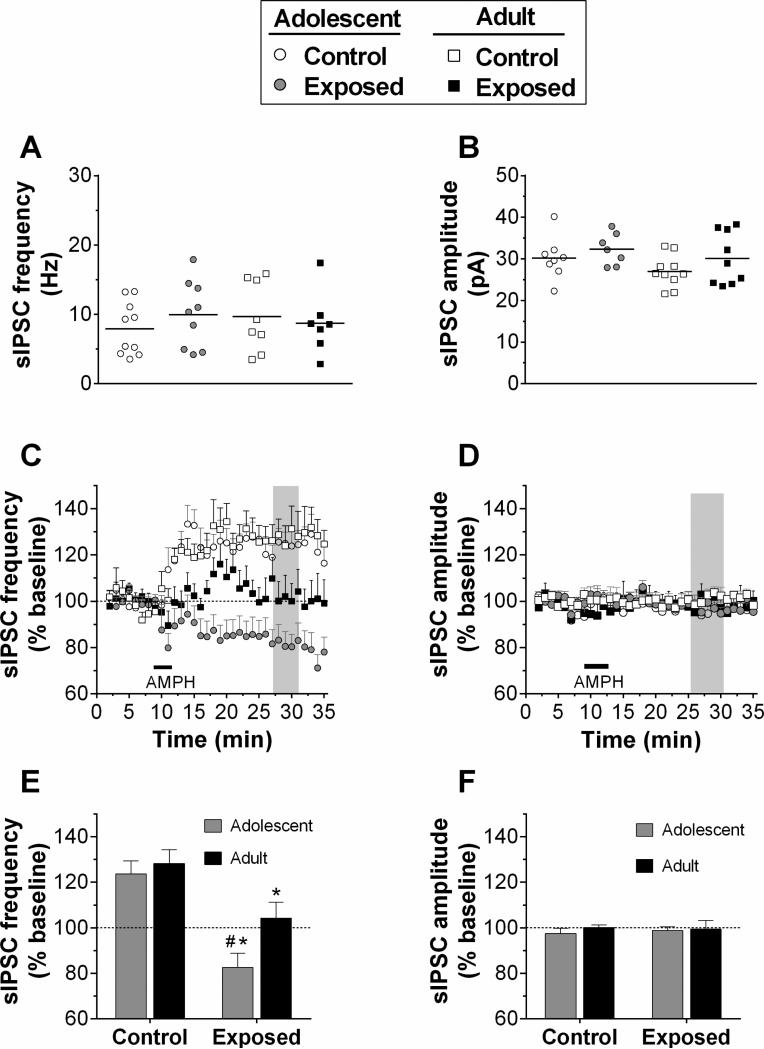
In experiment 2, where the withdrawal duration was similar in adolescent- and adult-exposed groups, no differences were found in the baseline sIPSCs (Fig. 3A and B). Analysis of the normalized data following AMPH application (25 μM for 4 min) revealed that sIPSC frequency was increased in both control groups, but not in either of the AMPH pre-treated groups (Fig. 3C). Two way ANOVA revealed significant main effects of time (F34,1088 = 3.87, p < 0.001), group (F3,1088=12.0, p < 0.001), as well as a significant interaction (F102,1088=3.78, p < 0.001). One way ANOVA of the peak changes in sIPSC frequency indicated a significant group effect (F3, 29 = 9.18, p < 0.001), with pairwise comparisons revealing both adolescent- and adult-exposed groups were significantly lower than their respective controls. Moreover, the adolescent-exposed group was significantly lower than the adult-exposed group (Fig. 3E). Thus, in rats pre-exposed to AMPH in adolescence, acute AMPH produced a suppression of sIPSC frequency that was below baseline. Rats pre-exposed during adulthood, in contrast, exhibited a reduction in AMPH-induced increases in sIPSC frequency. In all groups, there was no significant difference in sIPSC amplitude after acute AMPH application (Fig. 3D and F).
Figure 3.
sIPSC activity recorded in pyramidal neurons in Layer V/VI of mPFC for Experiment 2. Recordings were obtained from 7-10 cells/group in slices from 3 to 4 rats/group. Data are presented the same as Fig. 2. A and B, Baseline frequency and amplitude. C and D, time course of sIPSC frequency and amplitude in response to bath application of AMPH (25μM for 4 min; horizontal bar). Shaded regions indicate the areas of peak response, the means of which are summarized in E and F. *p < 0.05, vs. control; #p < 0.05, vs adult exposed group.
Taken together, our data show that following a history of chronic AMPH exposure, sIPSC activity in layer V/VI pyramidal cells became insensitive to AMPH-induced changes in inhibitory tone. Importantly, we found evidence that suggests the magnitude of this disruptive effect of AMPH depends on the developmental timing of drug exposure. AMPH is known to elevate extracellular dopamine in the mPFC [14] and dopamine has been shown to play a critical role in regulating inhibitory transmission in this brain region. In particular, activation of D1 or D2 receptors increases or decreases sIPSC frequency in pyramidal cells in adult rats, respectively [17]. Given the pre- and post-synaptic distribution of these receptors, dopamine's modulation could happen at both sites as evidence suggests that stimulation of D1 or D2 receptors influences the excitability of interneurons and also changes the post-synaptic mini-IPSC frequency and amplitude [12, 13, 17]. Previously, using the identical treatment schedule used here, we found that AMPH abolished the ability of D1 receptors to regulate sIPSC frequency [10]. In the current study, AMPH induced enduring and stable enhancement in the frequency of sIPSCs in controls but not in AMPH pre-exposed animals. Collectively, these results suggest that the loss of responsiveness to AMPH in pre-exposed animals could be due to impairments in D1 signaling. However, the specific mechanisms for the effects we observed here require further investigation. It is also unclear at this time if adaptive changes in other monoamine receptors contribute to our observed effects as the in vitro application of AMPH in the current study likely leads to release of 5-HT and norepinephrine as well dopamine. Both of these neurotransmitter systems have been shown to be involved in regulating sIPSC activity in mPFC pyramidal cells [15,16]. In Experiment 2, we found that AMPH actually suppressed sIPSC frequency in adolescent-exposed rats, which is an effect that is similar to that reported following D2 receptor activation on sIPSC frequency [17]. We speculate that adolescent, compared to adult, AMPH exposure might lead to a more enhanced D2–mediated regulation of inhibitory tone in the mPFC. This putative increase in D2 function may explain why stereotypy is expressed to a lesser degree following sensitization induced by AMPH exposure during adolescence because previous work has shown that AMPH-induced dopamine release leads to activation of cortical D2 receptors that leads to inhibition of drug-induced stereotyped behaviors [11,18]. Our results from experiment 1, which included a much longer withdrawal from adolescent AMPH exposure, suggest this putative enhancement of D2 signaling may reduce or dissipate over time as we found no evidence for supression of sIPSC frequency following a withdrawal period of > 11 weeks.
It has been hypothesized that dopamine hypofunction and altered GABA transmission in the mPFC may play a critical role in the development of behavioral sensitization to psychostimulants [19]. The current study, together with our recent finding [10], demonstrate that repeated AMPH induces a dysregulated dopamine-GABA interaction that persists after prolonged withdrawal. The impaired inhibition in the deep layers of mPFC may likely result in abnormal output into accumbens and dorsal striatum, and in turn generate an increased behavioral response to drug challenge [11]. Furthermore, our results suggest that repeated AMPH exposure during adolescence produced distinguishable changes in the dopamine-GABA system in the mPFC that might contribute to age-dependent differences in the expression of behavioral sensitization. Previous studies have shown that dopamine's modulation of inhibitory neurons in the mPFC features a protracted maturation, with dramatic functional changes occuring even in mid- to late-adolescence [20,21]. These delayed developmental processes may confer a period of vulnerability wherein drugs such as AMPH have the potential to alter the developmental trajectory of the PFC and its connectivity to other brain regions[22,23], which may in turn contribute to cognitive abnormalities that persist through adolescence and young adulthood. Indeed, using the same treatment schedule, we have found evidnece for enduring behavioral abnormalities that are specific to adolescent AMPH exposure [7,8,24].
In summary, our findings demonstrate that chronic AMPH exposure leads to a reduction in inhibitory transmission in the mPFC. If drug exposure occurred during adolescence, re-exposure to AMPH during young adulthood produced a unique disinhibition pattern in the mPFC. Increasing evidence suggests that dopamine-GABA interaction is a common target for drugs of abuse and that its dysfunction leads to cognitive impairments[17,25] that may bias individuals toward responses and actions that contribute to the cycle of addiction. The current study together with our recent work [10] hint that there may be unique changes in dopamine-GABA interplay in the mPFC following drug exposure during adolescence.
Highlights.
Inhibitory tone in prefrontal cortex pyramidal cells is increased by amphetamine
This effect is abolished in rats pre-exposed to amphetamine
With pre-exposure during adolescence, amphetamine has a disinhibiting effect
Acknowledgements
We thank Nikki Kofsky for technical assistance.
Funding
This work was supported by NIH grants DA029815 and EY014024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
disclosure
The authors declare no conflicts of interest.
References
- 1.McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug Alcohol Depend. 1997;48:235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- 2.Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 3.Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J. Neuropsychiatry Clin. Neurosci. 2003;15:317–25. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 4.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathews IZ, Kelly H, McCormick CM. Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacol. Biochem. Behav. 2011;97:640–646. doi: 10.1016/j.pbb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav. Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankosky ER, Kofsky NM, Gulley JM. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav. Brain Res. 2013;252:117–25. doi: 10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richetto J, Feldon J, Riva MA, Meyer U. Comparison of the long-term consequences of withdrawal from repeated amphetamine exposure in adolescence and adulthood on information processing and locomotor sensitization in mice. Eur. Neuropsychopharmacol. 2013;23:160–170. doi: 10.1016/j.euroneuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Kang S, Paul K, Hankosky ER, Cox CL, Gulley JM. D1 receptor-mediated inhibition of medial prefrontal cortex neurons is disrupted in adult rats exposed to amphetamine in adolescence. Neuroscience. 2016;324:40–49. doi: 10.1016/j.neuroscience.2016.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine in the mouse frontal cortex: a new hypothesis of behavioral sensitization to amphetamine and cocaine. Pharmacol. Biochem. Behav. 1998;61:435–43. doi: 10.1016/s0091-3057(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 12.Paul K, Cox CL. Age-dependent actions of dopamine on inhibitory synaptic transmission in superficial layers of mouse prefrontal cortex. J. Neurophysiol. 2013;109:1323–32. doi: 10.1152/jn.00756.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelova N, Seamans JK. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J. Neurophysiol. 2002;88:3150–66. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 14.Hedou G, Homberg J, Martin S, Wirth K, Feldon J, Heidbreder CA. Effect of amphetamine on extracellular acetylcholine and monoamine levels in subterritories of the rat medial prefrontal cortex. Eur. J. Pharmacol. 2000;390:127–36. doi: 10.1016/s0014-2999(00)00038-8. [DOI] [PubMed] [Google Scholar]
- 15.Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J. Neurosci. 2004;24:5000–8. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J. Neurosci. 1998;18:6963–76. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karler R, Bedingfield JB, Thai DK, Calder LD. The role of the frontal cortex in the mouse in behavioral sensitization to amphetamine. Brain Res. 1997;757:228–35. doi: 10.1016/s0006-8993(97)00221-7. [DOI] [PubMed] [Google Scholar]
- 19.Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Rev. 2003;41:203–28. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- 20.Tseng KK-YY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex. 2007;17:1235–40. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–50. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb. Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds LM, Makowski CS, V Yogendran S, Kiessling S, Cermakian N, Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology. 2015;40:1101–12. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav. Brain Res. 2014;263:22–33. doi: 10.1016/j.bbr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol. Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]