Abstract
Neuronal output typically involves neurotransmitter release via axonal terminals; however, a subpopulation of neurons can also release neurotransmitters through vesicle-containing presynaptic dendrites. In the thalamus, local circuit inhibitory interneurons are a class of cells that can release γ-aminobutyric acid (GABA) via both axon terminals (termed F1 terminals) as well as presynaptic, vesicle-containing dendrites (termed F2 terminals). For example, in the visual thalamus, these F2 terminals are tightly coupled to the primary sensory afferents (axons of retinogeniculate neurons) that synapse onto thalamocortical relay neurons. The F2 terminals are primarily localized to distal dendrites of the interneurons, and in certain situations the excitation/output of F2 terminals can occur independent of somatic activity within the interneuron thereby allowing these F2 terminals to serve as independent input/output components giving rise to focal inhibition. On the other hand, somatically evoked Na+-dependent action potentials can backpropagate throughout the dendritic arbor of the interneuron. The transient depolarizations, or stronger somatically initiated events (e.g., activation of low threshold calcium transients) can initiate a backpropagating Ca2+-mediated potential that invades the dendritic arbor activating F2 terminals and leading to a global form of inhibition. These distinct types of output (focal versus global) could play an important role in the temporal as well as spatial roles of inhibition that in turn impacts thalamocortical information processing.
Introduction
Classic synaptic transmission within neural networks typically consists of suprathreshold excitation of the presynaptic neuron, action potential propagation through the axonal arbor, and subsequent neurotransmitter release from axonal terminals, which ultimately influences postsynaptic targets. Dendrites characteristically have the responsibility of integrating afferent synaptic activity which can be a dynamic process considering these structures may contain a wide variety of voltage dependent conductances that can influence their integrative properties [1]. In a subpopulation of neurons, there is an added complication in that the dendrites may also contain synaptic vesicles and serve to release neurotransmitters. Therefore, these local areas of integration/synaptic release may bypass the complex somatic integration and axonal output. Dendritic transmitter release has been associated with neurons found in multiple brain areas (for review, see [2]. Furthermore, there is increasing evidence that dendritic release of neurotransmitters may be regulated by a variety of modulatory systems given the convergence of multiple transmitter systems upon dendritic arbors.
The thalamus serves as the gateway through which sensory information passes prior to entering the neocortex. It is now widely accepted that information processing through the thalamus is a dynamic process rather than a mere passive relay of information to various neocortical areas. Primary sensory afferents entering the thalamus typically synapse onto two subtypes of neurons: thalamocortical relay neurons that subsequently project to primary sensory cortex and inhibitory local circuit neurons with axonal arbors that remain within the thalamus (1A). It is worth noting that the proportion of local interneurons within thalamic nuclei varies across species. In rodents, these local interneurons are predominantly found in visual related thalamic nuclei and are absent in many other thalamic nuclei; however in higher order mammals including cats, nonhuman primates and primates, these local interneurons are localized within most thalamic nuclei [3].
For this review, we will focus on the visual system, in which retinogeniculate neurons innervate thalamocortical neurons and local circuit interneurons within the dorsal lateral geniculate nucleus (dLGN). In addition to this bottom up circuitry, there is strong feedback circuitry originating from layer VI corticothalamic neurons that innervate: 1) thalamocortical neurons, 2) local circuit interneurons, and 3) inhibitory thalamic reticular nucleus (TRN) neurons that in turn project back into dLGN. The local circuit interneurons serve as a feedforward inhibitory circuit predominantly driven by primary sensory afferents, and functionally are thought to enhance stimulus selectivity, refine receptive fields of thalamocortical neurons, improve sensory coding, and ensure temporal precision of spiking [4–7]. To complicate matters, afferents from corticothalamic neurons also innervate the local interneurons providing a cortically driven feedback on inhibitory activity within the thalamus, and these inputs likely influence how subsequent retinogeniculate information is processed [8–10].
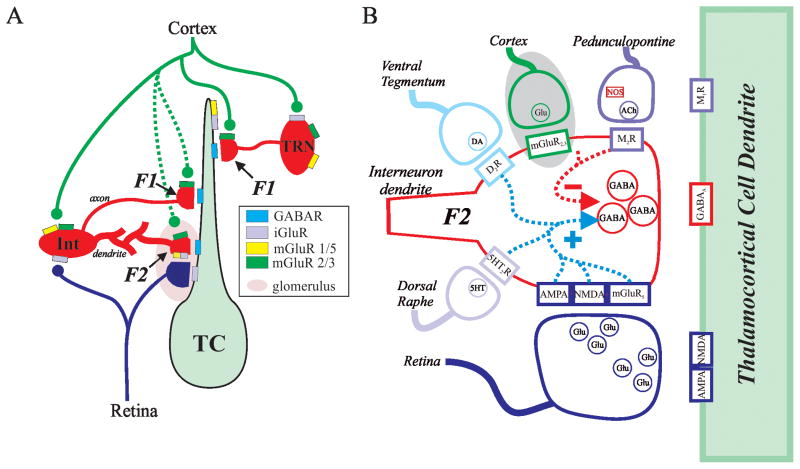
These local circuit inhibitory interneurons provide two types of GABAergic output: axonal and dendritic (Figure 1A). Similar to nearly all other neurons, the axonal arbor consists of vesicle containing terminals that form both axodendritic and axosomatic synaptic contacts onto thalamocortical relay neurons (F1 terminals). An additional feature of these neurons is that they form dendrodendritic synapses onto thalamocortical neurons, which have been named F2 terminals [11–17]. These F2 terminals are part of a triadic arrangement that includes three components: retinogeniculate axon terminal, distal presynaptic dendrite of the interneuron, and the proximal dendrite of the thalamocortical neuron (Figure 1). The retinogeniculate axon innervates the proximal dendrite of the thalamocortical neuron forming a monosynaptic excitatory synapse. In addition, the same retinogeniculate axon forms a synapse on the distal dendrite of the interneuron, which in turn is presynaptic to the dendrite of the thalamocortical neuron providing a disynaptic inhibitory pathway to the local region of the dendrite (Figure 1B). The compact localization of these multiple synaptic contacts suggests that there could be a tight regulation of retinogeniculate excitation of the thalamocortical neurons.
Figure 1.
A. Simplified schematic diagram of visual system thalamic circuitry depicting multiple types in inhibitory inputs. Inhibitory axonal outputs (F1 terminals) arise from both local circuit neurons in dLGN as well thalamic reticular nucleus (TRN) neurons. Presynaptic dendrites (F2 terminals) of interneurons (Int) are innervated by retinogeniculate axons, which also innervate the dendrite of thalamocortical neuron (TC). Adapted from [22]. B. Schematic illustrating multiple transmitter systems that have been shown to alter the output of putative F2 terminals. The cortical pathway engaging mGluR2/3 (gray circle) clearly involves F1 terminals but unclear if engages F2 terminals [22].
Regulation of dendritic outputs via multiple neuromodulators
Despite anatomical evidence depicting the presence of F2 terminals within the thalamus, their function remained hypothetical for many years [12,15]. During this time, numerous studies focused on the role of inhibition within thalamocortical processing; however, the potential contribution of F2 terminals to this inhibitory activity remained speculative. Prior studies on local interneurons have focused on changes in excitability (i.e., postsynaptic actions) produced by various neuromodulators. Over the last decade, a number of studies focusing on these dendrodendritic synapses (F2 terminals) have demonstrated that output of these terminals is complicated and can be regulated by numerous different neurotransmitter/modulators including glutamate, acetylcholine, serotonin, and dopamine (Figure 1B). The complex regulation of F2 terminal output likely involves the concerted convergence of multiple modulatory systems.
Glutamate
There is considerable evidence suggesting GABA release from F2 terminals is regulated by the activation of ionotropic glutamate receptors (iGluRs) including AMPA and NMDA receptors [18–21] as well as metabotropic glutamate receptors (mGluRs)[18,22–25]. The mGluRs are differentially distributed within the dLGN [26]: mGluR1 are localized to thalamocortical relay neurons, whereas mGluR5 are selectively localized on local circuit interneurons. Taking advantage of this distinct localization of the mGluRs, pharmacological activation of mGluR5 produced a robust increase in inhibitory activity within the thalamocortical neuron [18,24]. Through a series of pharmacological manipulations, this increase appeared to be due to activation of F2 terminals. Key features regarding the mGluR-dependent activation of F2 terminals are that 1) GABAergic release did not require suprathreshold excitation of the interneuron (i.e. activation of F2 terminals persisted in tetrodotoxin (TTX)), 2) F2 terminal output was Ca2+-dependent similar to neurotransmitter release from axonal terminals, and 3) synaptic activation of F2 terminal output via mGluRs required tetanic stimulation [21,23,24,27]. The original interpretation of these results were that activation of F2 terminals, which are localized to the distal dendrites of dLGN interneurons, could provide local inhibitory outputs onto the thalamocortical neurons, and occur independent of somatic alterations in interneuron activity. This interpretation was further supported by the observation that the general mGluR agonist, ACPD, produced a very small membrane depolarization in somatic recordings from local interneurons [24,28]. However, the actions of mGluRs is actually more complicated: activation of group I mGluRs (mGluR1, mGluR5) can produce a depolarization, whereas activation of group II mGluRs (mGluR2, mGluR3) produces a hyperpolarization in interneurons [22,29]. Whether these specific subtypes can be selectively activated by synaptic afferents remains unclear, but could have important consequences on our understanding of mGluR-dependent actions on thalamocortical processing. This is an relatively important issue consider that over the last several years, the independence between the distal events and somatic activity of the dLGN interneurons has been questioned based on studies indicating that somatic events can backpropagate into the dendritic arbor of the interneurons [19,29,30]. The functional significance of these differences is further discussed below (Local vs. Global influences of interneuron outputs).
In addition to mGluR activation, F2 terminal output can also be regulated by ionotropic glutamate receptor (iGluR) activation, including both NMDA and AMPA receptors. Several different approaches including electrical stimulation of synaptic inputs, local pressure application of pharmacological agonists, and photo-uncaging of caged glutamate have demonstrated that iGluR activation produces a short-lasting robust increase in potential F2 output. The iGluR-dependent response has a short latency and relatively short duration (typically <100ms) [19]. In contrast, the mGluR-dependent response has a longer latency and can have a duration of tens of seconds [21]. In addition, the iGluR-dependent response can be readily evoked by single stimuli whereas the mGluR-dependent response requires relatively high frequency tetanic stimulation. Focal uncaging of glutamate on the distal dendrites of interneurons revealed two subgroups of F2 terminals [31]. One type of F2 terminals can be activated by both iGluRs and mGluRs in an activity dependent manner; at relatively low stimulation frequencies, iGluRs are engaged resulting in short-lasting inhibition, whereas at higher frequencies the mGluRs are also engaged leading to longer lasting inhibitory action. The other, more frequent type of F2 terminal is only activated by iGluR activation, not mGluR activation, and thus produce a short-lasting inhibition.
The relationship of synaptic stimulation with F2 terminal output is a bit more difficult to unravel. In an early study by Govindaiah and Cox (2004), under conditions to pharmacologically isolate the mGluR-dependent activation of F2 terminals, the authors found that tetanic stimulation of the optic tract lead to a frequency dependent increase in mGluR5-dependent activation of F2 terminals and a subsequent lasting increase in inhibition in the postsynaptic thalamocortical neuron. In that study, when recording directly from rat dLGN interneurons, the same tetanic stimulation produced a short-lasting depolarization in the somatic recording that was completely blocked by iGluR antagonists [22,23]. In a follow-up study, it was found that tetanic stimulation of corticothalamic inputs would reduce inhibitory activity via activation of group II mGluRs, but whether this is due to direct action on the F2 terminals is unclear [22]. Overall, these results were interpreted that tetanic stimulation of retinogeniculate axons evoked GABA release following mGluR5 activation from F2 terminals on distal dendrites of the interneurons, and this could occur independent of somatic events in the interneurons.
Recently, it has been shown that optic tract stimulation can evoke an EPSP with a lasting depolarizing plateau (~100 ms duration) that is dependent on activation of L-type calcium channels in mouse dLGN interneurons [19]. In a subsequent study, strong tetanic stimulation could produce long-lasting action potential discharge in these interneurons, which could be associated with a prolonged depolarizing plateau potential (>10 s duration) and associated increase in inhibitory output [29]. Under these strong intensity stimulus conditions (0.5–1.0 mA), the initiation of the plateau potential appeared dependent on activation of mGluR5. The authors hypothesized that the prolonged increase in inhibitory output from such stimulation resulted from the backpropagation of a Ca2+-dependent current into the dendritic arbor that in turn could stimulate GABA release from F2 terminals.
Acetylcholine
Acetylcholine is a neuromodulator that is typically associated with arousal or attentive mechanisms, and this transmitter has also been shown to have a complex role in regulating inhibitory output from these interneurons. Cholinergic fibers arising from the parabrachial region innervate retinal recipient areas of thalamocortical neurons, and these terminals have been found to be associated with F2 terminals [32,33]. Acetylcholine, via the activation of muscarinic M2 subtype receptors (M2Rs) strongly hyperpolarizes local circuit interneurons, which can in turn result in decreased inhibitory activity in thalamocortical neurons [28,34]. However, in the presence of TTX, the significant decrease in inhibitory activity produced by M2R activation persisted [18,35]. These results were interpreted to demonstrate that activation of M2Rs on distal dendrites of interneurons could decrease F2 terminal activity thereby decreasing inhibitory activity in cat dLGN thalamocortical neurons, which is the opposite effect observed via activation of mGluR5.
In mouse dLGN, pharmacological activation of M2Rs was shown to regulate synaptically evoked feedforward inhibition via interneurons in a stimulus intensity dependent manner [36]. At relatively low stimulus intensities, M2R activation blocked feedforward inhibition evoked by retinogeniculate axon (RGC) stimulation; however, at stronger stimulus intensities, M2R activation led to a long-lasting increase in inhibition in the thalamocortical neuron. The authors propose that M2R-mediated postsynaptic hyperpolarization of the interneuron decreases suprathreshold synaptic activation with weaker RGC stimulation. At higher intensities synaptic stimulation can overcome the hyperpolarization, and evoke action potentials as well as activate a prolonged, voltage-dependent, long-lasting depolarization in the interneuron (10–20s duration) leading to lasting inhibitory output. The activation of M2Rs, either by strong somatic hyperpolarization or by presumed hyperpolarization of distal dendrites, can dampen inhibitory activity on the thalamocortical neuron that in turn would enhance the excitatory drive onto these cells. It is currently unclear if activation of intrinsic cholinergic pathways can produce focal effects on the interneurons such as selective hyperpolarization of a single dendritic branch, or if such actions would impact the entire dendritic arbor.
Monoamines
Thalamic nuclei are innervated by serotonergic neurons arising from the dorsal raphe nucleus, which are thought to be involved with the regulation/modulation of thalamocortical processing associated with sleep/wake cycles [37]. Serotonin, via activation of 5HT2 receptors, can increase F2 terminal output via a Ca2+-dependent manner similar to that observed by mGluR5 activation [35]. The underlying mechanisms of these different metabotropic receptors (5HT2 vs. mGluR5) appear to involve distinct intracellular pathways. Activation of the 5HT2 receptors leads to Ca2+ influx via transient receptor potential protein (TRP4), whereas the mGluR-mediated increases in F2 terminal output does not require TRP4 activity indicating that two different signaling pathways can result in increased F2 terminal release [35]. Dopamine, via activation of D2 subtype receptors, also increases the inhibitory activity recorded from thalamocortical neurons [38]. D2 receptor agonists have been found to selectively depolarize dLGN interneurons, which could account for the increased GABAergic output of the interneurons; however, this increase persisted following blockade of action potential output by TTX, consistent with the hypothesis that such inhibition resulted from output of the F2 terminals.
As summarized in Figure 1B, there are multiple transmitter systems that can modify the output of the F2 terminals of thalamic inhibitory interneurons. Currently, our understanding of how different neuromodulators regulate F2 terminal output is predominantly based on pharmacological activation. Future studies should focus on the endogenous actions of these different modulatory pathways and how these regulate F2 terminal activity. In addition, at the intracellular level, at what point do these different modulatory systems converge and possibly interact?
Local vs. Global influences of interneuron outputs
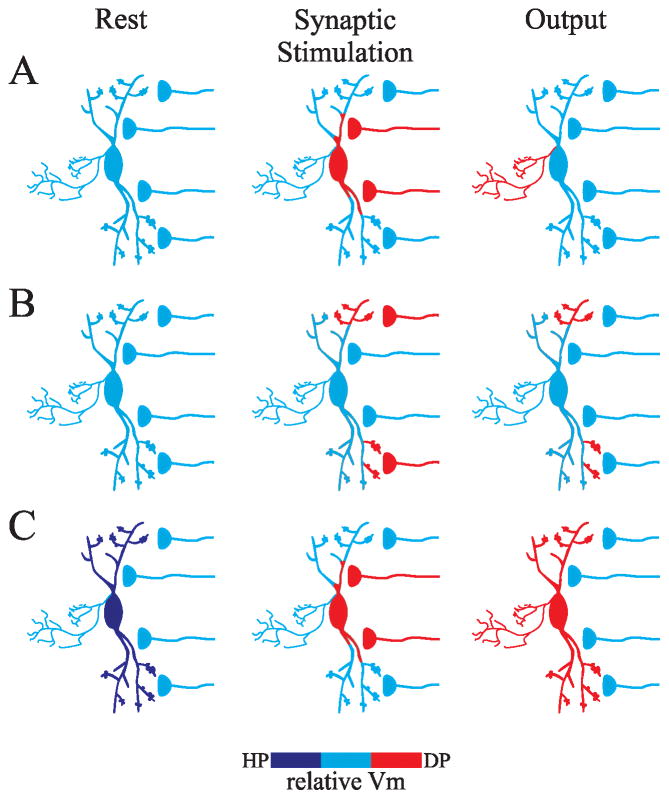
Typically, dendrites contribute to neural output by collecting, integrating, and transmitting afferent information to the soma/axon, where action potentials can be initiated and communication continues to downstream neurons via the axonal arbor (Figure 2A)[39]. However, with respect to the F2 terminals, afferent synaptic inputs are in very close proximity to release sites, making it possible to couple local input with proximal output. Early hypotheses regarding the function of F2 terminals were based on anatomical data that the F2 terminals typically have a knob-like appearance and are located on distal dendrites of the interneurons [11,14,40]. Subsequent modeling approaches that assumed passive properties of the dendrites led to the hypothesis that these terminals could integrate local subthreshold synaptic information independent of other F2 terminals as well as the axon [27,41]. In essence, a single interneuron could have multiple, independent operating input/output devices. However, recent studies indicate that these dendrites are not merely passive cables, but have the ability to propagate somatic generated potentials back into the dendritic arbor via activation of voltage dependent conductances supporting the hypothesis that certain events could in fact produce widespread activation of F2 terminals throughout the dendritic arbor [19,29,30]. Experimental findings indicate that there may be multiple types of inhibitory output from these interneurons (Figure 2), and these different output types could have significant influence on the spatial and/or temporal influence of inhibition on thalamocortical processing.
Figure 2.
Multiple output modes of dLGN interneurons. A. Axonal output of interneuron. Strong synaptic stimulation near soma/proximal dendrites produce suprathreshold activation of interneuron leading to action potential discharge and subsequent release of neurotransmitter from axonal arbor. B. Local output of F2 terminals. Local synaptic activation of distal dendrites (F2 terminals) leads to focal depolarizations that do not influence the soma/axonal region of the interneuron; however, the focal depolarization allows for GABA release from F2 terminals. Under these conditions, the neuron acts as a multiplexor. C. Global output of F2 terminals. When the interneuron is slightly hyperpolarized, strong synaptic stimulation near soma/proximal dendrites produce suprathreshold activation of interneuron leading to action potential discharge and axonal output. In addition, this can evoke activation of a lasting Ca2+ potential that backpropagates into the dendrites producing a lasting activation of F2 terminals throughout the dendritic arbor.
Local influence
Our previous results have supported the hypothesis of compartmentalization of local signaling in the inhibitory interneurons. We found that activity-dependent, mGluR-mediated increases in inhibition (i.e., increased F2 output) within thalamic relay neurons produced by mGluR agonists could occur independent of significant changes in membrane potentials of interneurons at the somatic level [18,24]. Furthermore, tetanic electrical stimulation that evoked lasting mGluR-dependent increases in F2 terminal output produced only a transient, iGluR-dependent depolarization of the interneuron at the soma that was not sufficient to account for the longer duration of inhibitory output [22,23]. Focal photouncaging of glutamate at distal sites of interneurons was sufficient to produce the increased inhibitory activity via F2 terminals, but produced little or no alterations of the membrane potential at the somatic level in the interneuron. These results indicate that events generated at distal dendrites can occur without influencing the somatic/axonal level, nor adjacent dendritic branches (Figure 2B)[21]. Furthermore, these localized sites can be differentially regulated by iGluR and/or mGluR leading to different features of inhibitory influence. These data reveal that the output of distal dendrites (F2 terminals) by glutamatergic activation can occur independent of somatic activity and subsequently provide focal inhibitory influence on retinogeniculate integration, which could allow the interneuron to serve as a multiplexor integrating multiple independent dendritic sites simultaneously.
Global influence
Recent studies suggest that activation of F2 terminals may not occur independent of each other. Suprathreshold activation of interneurons at the somatic level can produce action potentials as well as longer lasting Ca2+ potentials that can backpropagate into the dendritic arbor [19,30]. While the backpropagating action potential could produce a short, synchronous transient depolarization throughout the dendritic arbor, local synaptic inputs did not initiate spikes within the dendritic arbor [30]. In essence, the backpropagating action potential could serve as a synchronous precise “global” output if this transient potential is sufficient to active F2 terminals, which is currently unclear.
On the other hand, backpropagating Ca2+ potentials are dependent on the resting state of the interneuron. At relatively hyperpolarized membrane potentials, synaptic activation can lead to initial excitation followed by a relatively short calcium plateau potential (~100 ms) that can propagate through the dendritic arbor leading to transient increases in F2 terminal output (Figure 2C)[19]. More recently, tetanic stimulation of retinogeniculate axons using relatively high intensity stimulation (0.5–1 mA) can produce prolonged action potential discharge in mouse dLGN interneurons [29]. In a subpopulation of interneurons, this strong stimulus produces a robust, large amplitude, long duration (>10s) Ca2+-dependent plateau potential that can in turn backpropagate into the dendritic arbor and leads to a robust increase in GABA output from these cells. The backpropagation of these Ca2+ potentials could produce relatively synchronous, lasting activation of F2 terminals, and in this case the dendritic arbor would provide a global output of inhibition. This would be analogous to the widespread output that typically occurs when the axonal arbor is excited. It is unclear whether single interneurons can give rise to both local and global F2 output or if this may be due to intrinsic characteristics of distinct interneuron subtypes.
As we continue to investigate the distinctive conditions that engage these different types of inhibitory outputs and the underlying cellular pathways, it will be imperative to determine if these findings can be generalized across other thalamic nuclei that contain interneurons and F2 terminals (e.g., [42]). As an example, when recording from rat dLGN interneurons, strong tetanic stimulation of retinogeniculate afferents produces a short-lasting synaptic depolarization that is completely blocked by iGluR antagonists [22,23]. In contrast, similar tetanic stimulation in mouse (C57/Bl6: GAD-GFP) dLGN interneurons can produce long duration calcium potential (duration ~100–200 ms), and at higher intensities a robust prolonged depolarization (>10s) accompanied by sustained action potential discharge [19,29]. To date, studies supporting local influence of F2 function have been conducted in cat and rat dLGN, whereas the global actions of F2 terminals and backpropagation have been carried out in mouse dLGN. The implications of the focal versus global inhibitory output of these presynaptic dendrites could significantly impact information transfer via thalamocortical pathway. While current studies have focused on this issue with regards to glutamatergic regulation of interneuron output, it is unclear if other neuromodulators (e.g., acetylcholine, dopamine, serotonin) can have distinct types of spatial and/or temporal regulation. Future studies should also address if the qualitative differences that have been reported (e.g., prolonged Ca2+-dependent plateaus) are due to distinct subtypes of interneurons, and thus perhaps a specific subtype of interneuron have backpropagating Ca2+ potentials whereas other interneurons do not posses such characteristics. Clearly our understanding of interneuron function, but more specifically presynaptic dendrites, is in its infancy, and how these F2 outputs shape and regulate thalamocortical processing is a work in progress.
Highlights.
dLGN interneurons release neurotransmitters via axonal outputs as well as presynaptic dendrites.
Neurotransmitter release from presynaptic dendrites is regulated by multiple neuromodulators.
Local activation of presynaptic dendrites can occur independent of somatic activity.
Backpropagating activity into dendritic arbor can provide synchronous release throughout arbor
Acknowledgments
This work was supported by the National Institutes of Health grants EY014024 and MH085324. I thank Dr. J. Beatty for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnston D, Narayanan R. Active dendrites: colorful wings of the mysterious butterflies. Trends in Neurosciences. 2008;31:309–316. doi: 10.1016/j.tins.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Research Bulletin. 1997;42:27–37. doi: 10.1016/s0361-9230(96)00107-4. [DOI] [PubMed] [Google Scholar]
- 4.Sillito AM, Kemp JA. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN) Brain Research. 1983;277:63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- 5.Holdefer RN, Norton TT, Godwin DW. Effects of bicuculline on signal detectability in lateral geniculate nucleus relay cells. Brain Research. 1989;488:341–347. doi: 10.1016/0006-8993(89)90727-0. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wei Y, Vaingankar V, Wang Q, Koepsell K, Sommer FT, Hirsch JA. Feedforward excitation and inhibition evoke dual modes of firing in the cat’s visual thalamus during naturalistic viewing. Neuron. 2007;55:465–478. doi: 10.1016/j.neuron.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Vaingankar V, Sanchez CS, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nature Neuroscience. 2011;14:224–231. doi: 10.1038/nn.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin MW, Cleland BG. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. Journal of Neurophysiology. 1977;40:410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- 9.Jurgens CW, Bell KA, McQuiston AR, Guido W. Optogenetic stimulation of the corticothalamic pathway affects relay cells and GABAergic neurons differently in the mouse visual thalamus. PloS One. 2012;7:e45717. doi: 10.1371/journal.pone.0045717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang S, Bickford ME. Comparison of the ultrastructure of cortical and retinal terminals in the rat dorsal lateral geniculate and lateral posterior nuclei. The Journal of Comparative Neurology. 2003;460:394–409. doi: 10.1002/cne.10646. [DOI] [PubMed] [Google Scholar]
- 11.Famiglietti EV, Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. Journal of Comparative Neurology. 1972;144:285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- 12.Ralston HJ. Evidence for presynaptic dendrites and a proposal for their mechanism of action. Nature. 1971;230:585–587. doi: 10.1038/230585a0. [DOI] [PubMed] [Google Scholar]
- 13.Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, Sherman SM. Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature. 1985;317:618–621. doi: 10.1038/317618a0. [DOI] [PubMed] [Google Scholar]
- 14.Montero VM. Localization of gamma-aminobutyric acid (GABA) in type 3 cells and demonstration of their source to F2 terminals in the cat lateral geniculate nucleus: a Golgi-electron-microscopic GABA-immunocytochemical study. Journal of Comparative Neurology. 1986;254:228–245. doi: 10.1002/cne.902540207. [DOI] [PubMed] [Google Scholar]
- 15.Guillery RW. The organization of synaptic interconnections in the laminae of the dorsal lateral geniculate nucleus of the cat. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1969;96:1–38. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- 16.Ohara PT, Lieberman AR. Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. Journal of Neurocytology. 1993;22:815–825. doi: 10.1007/BF01181326. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman AR. Neurons with presynaptic perikarya and presynaptic dendrites in the rat lateral geniculate nucleus. Brain Research. 1973;59:35–59. doi: 10.1016/0006-8993(73)90252-7. [DOI] [PubMed] [Google Scholar]
- 18.Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. doi: 10.1016/s0896-6273(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 19*.Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron. 2008;57:420–431. doi: 10.1016/j.neuron.2007.12.022. The authors provide initial evidence of active conductances within dLGN interneurons that backpropagate into the dendritic arbor leading to activation of F2 terminals. [DOI] [PubMed] [Google Scholar]
- 20.Blitz DM, Regehr WG. Timing and specificity of feed-forward inhibition within the LGN. Neuron. 2005;45:917–928. doi: 10.1016/j.neuron.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Crandall SR, Cox CL. Local dendrodendritic inhibition regulates fast synaptic transmission in visual thalamus. Journal of Neuroscience. 2012;32:2513–2522. doi: 10.1523/JNEUROSCI.4402-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govindaiah G, Cox CL. Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. Journal of Neuroscience. 2006;26:13443–13453. doi: 10.1523/JNEUROSCI.3578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Govindaiah, Cox CL. Synaptic activation of metabotropic glutamate receptors regulates dendritic outputs of thalamic interneurons. Neuron. 2004;41:611–623. doi: 10.1016/s0896-6273(04)00013-3. The authors demonstrate that tetanic syanptic activation of the optic tract can engage the mGluR-dependent activation of F2 terminals. [DOI] [PubMed] [Google Scholar]
- 24*.Cox CL, Zhou Q, Sherman SM. Glutamate locally activates dendritic outputs of thalamic interneurons. Nature. 1998;394:478–482. doi: 10.1038/28855. This is initial paper indicating that mGluR activation can increase F2 terminal output from interneurons that could occur independent of somatic activation of the interneurons. [DOI] [PubMed] [Google Scholar]
- 25.Errington AC, Di Giovanni G, Crunelli V, Cope DW. mGluR control of interneuron output regulates feedforward tonic GABAA inhibition in the visual thalamus. Journal of Neuroscience. 2011;31:8669–8680. doi: 10.1523/JNEUROSCI.0317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godwin DW, Van Horn SC, Erisir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. Journal of Neuroscience. 1996;16:8181–8192. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloomfield SA, Sherman SM. Dendritic current flow in relay cells and interneurons of the cat’s lateral geniculate nucleus. Proceedings of the National Academy of Sciences, USA. 1989;86:3911–3914. doi: 10.1073/pnas.86.10.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pape HC, McCormick DA. Electrophysiological and pharmacological properties of interneurons in the cat dorsal lateral geniculate nucleus. Neuroscience. 1995;68:1105–1125. doi: 10.1016/0306-4522(95)00205-w. [DOI] [PubMed] [Google Scholar]
- 29.Pressler RT, Regehr WG. Metabotropic glutamate receptors drive global persistent inhibition in the visual thalamus. Journal of Neuroscience. 2013;33:2494–2506. doi: 10.1523/JNEUROSCI.3458-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Casale AE, McCormick DA. Active action potential propagation but not initiation in thalamic interneuron dendrites. Journal of Neuroscience. 2011;31:18289–18302. doi: 10.1523/JNEUROSCI.4417-11.2011. Authors demonstrate that action potentials evoked in dLGN interneurons can backpropagate thoughout the dendritic arbor, which was shown utilizing voltage sensitive dyes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Crandall SR, Cox CL. Thalamic microcircuits: presynaptic dendrites form two feedforward inhibitory pathways in thalamus. Journal of Neurophysiology. 2013;110:470–480. doi: 10.1152/jn.00559.2012. The authors demonstrate that focal activation of F2 terminals can occur independent of somatic activity within the dLGN interneuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carden WB, Bickford ME. Location of muscarinic type 2 receptors within the synaptic circuitry of the cat visual thalamus. Journal of Comparative Neurology. 1999;410:431–443. [PubMed] [Google Scholar]
- 33.Erisir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: A comparison with corticogeniculate terminals. Journal of Comparative Neurology. 1997;377:535–549. [PubMed] [Google Scholar]
- 34.McCormick DA, Pape HC. Acetylcholine inhibits identified interneurons in the cat lateral geniculate nucleus. Nature. 1988;344:246–248. doi: 10.1038/334246a0. [DOI] [PubMed] [Google Scholar]
- 35.Munsch T, Freichel M, Flockerzi V, Pape HC. Contribution of transient receptor potential channels to the control of GABA release from dendrites. Proceedings of the National Academy of Sciences, USA. 2003;100:16065–16070. doi: 10.1073/pnas.2535311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antal M, Acuna-Goycolea C, Pressler RT, Blitz DM, Regehr WG. Cholinergic activation of M2 receptors leads to context-dependent modulation of feedforward inhibition in the visual thalamus. PLoS Biology. 2010;8:e1000348. doi: 10.1371/journal.pbio.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 38.Munsch T, Yanagawa Y, Obata K, Pape HC. Dopaminergic control of local interneuron activity in the thalamus. European Journal of Neuroscience. 2005;21:290–294. doi: 10.1111/j.1460-9568.2004.03842.x. [DOI] [PubMed] [Google Scholar]
- 39.Spruston N, Stuart G, Hausser M. Dendritic integration. In: Stuart G, Spruston N, Hausser M, editors. Dendrites. Oxford UP; 2008. [Google Scholar]
- 40.Rafols JA, Valverde F. The structure of the dorsal lateral geniculate nucleus in the mouse. A Golgi and electron microscopic study The Journal of Comparative Neurology. 1973;150:303–332. doi: 10.1002/cne.901500305. [DOI] [PubMed] [Google Scholar]
- 41.Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends in Neuroscience. 2004;27:670–675. doi: 10.1016/j.tins.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Govindaiah G, Cox CL. Distinct roles of metabotropic glutamate receptor activation on inhibitory signaling in the ventral lateral geniculate nucleus. Journal of Neurophysiology. 2009;101 :1761–1773. doi: 10.1152/jn.91107.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]