SUMMARY
Here we demonstrate that the RNase E-based degradosome is required for poly(A) polymerase I (PAP I)-dependent polyadenylation after Rho-independent transcription terminators for both mono- and polycistronic transcripts. Disruption of degradosome assembly in mutants lacking the polynucleotide phosphorylase (PNPase) binding domain led to a significant increase in the level of PNPase synthesized polynucleotide tails in the rpsJ and rpsM polycistronic transcripts and the lpp monocistronic transcript. The polynucleotide tails were mostly located within the coding sequences in the degradosome mutants compared to the wild type control where the majority of the PAP I synthesized poly(A) tails were after the Rho-independent transcription terminators. For the Rho terminated metNIQ operon, the tails for all three ORFs were predominately polynucleotide and were located within the coding sequences in both wild type and degradosome mutant strains. Furthermore, by employing a pnp-R100D point mutant that encodes a catalytically inactive PNPase protein that still forms intact degradosomes, we show that a catalytically active PNPase is required for normal mRNA polyadenylation by PAP I. Our data suggest that polyadenylation requires a functional degradosome to maintain an equilibrium between free PNPase and the PAP I polyadenylation complex.
Keywords: poly(A) polymerase I, polynucleotide phosphorylase, RhlB RNA helicase, enolase, Hfq
INTRODUCTION
Polyadenylation at the 3′ termini of mRNAs is an important post-transcriptional event in both eukaryotes and prokaryotes. In Escherichia coli, poly(A) polymerase I (PAP I) encoded by the pcnB gene (Cao & Sarkar, 1992) is responsible for ~90% of the poly(A) tails that exclusively contain adenosine residues (homopolymers) and are up to ~40 nt in length (O’Hara et al., 1995, Mohanty & Kushner, 1999, Mohanty et al., 2012). PAP I adds poly(A) tails to both full-length transcripts and decay products (Mohanty & Kushner, 1999, Mohanty & Kushner, 2000, Mohanty et al., 2004, Mohanty & Kushner, 2006). For full-length transcripts the poly(A) tails are usually added after Rho-independent transcription terminators (Mohanty et al., 2004, Mohanty & Kushner, 2006). While transcripts from a majority of ORFs are polyadenylated by PAP I, only a small fraction of these transcripts are polyadenylated at any given time (Mohanty & Kushner, 2006). The addition of poly(A) tails usually leads to more rapid transcript degradation (Hajnsdorf et al., 1995, O’Hara et al., 1995, Xu & Cohen, 1995, Coburn & Mackie, 1996, Li et al., 1998, Mohanty & Kushner, 2010). Recently, polyadenylation has been shown to help regulate functional tRNA levels in E. coli (Mohanty et al., 2012, Mohanty & Kushner, 2013).
Polynucleotide phosphorylase (PNPase) is responsible for the remaining 10% of the post-transcriptionally added tails in E. coli (Mohanty & Kushner, 2000, Mohanty & Kushner, 2006). This enzyme catalyzes a reversible reaction, with an equilibrium constant of 1, in which it functions as a 3′ → 5′ exonuclease in the presence of inorganic phosphate to degrade RNA using a phosphorolytic mechanism releasing nucleoside diphosphates (Grunberg-Manago, 1963) or as a polynucleotide polymerase that biosynthetically adds long polynucleotide tails containing all four nucleotides (heteropolymers) (Mohanty & Kushner, 2000). The biological function of polynucleotide tails is predicted to be similar to poly(A) tails based on their unstructured sequences (Blum et al., 1999, Mohanty & Kushner, 2010), but this hypothesis remains unproven. PNPase, unlike PAP I, normally does not add tails after secondary structures such as Rho-independent transcription terminators (Mohanty & Kushner, 2000, Mohanty et al., 2004, Mohanty & Kushner, 2006), although the reasons for this distinction are currently unclear. It is the major polyadenylating enzyme in a number of bacteria lacking a PAP I homolog (Rott et al., 2003, Sohlberg et al., 2003, Bralley et al., 2006).
Pull-down and immunoprecipitation experiments have demonstrated protein-protein interactions among PAP I, PNPase and the RNA binding protein Hfq independent of any initial RNA association, suggesting the existence of a multiprotein complex that is required for the polyadenylation of mRNAs containing Rho-independent transcription terminators (Mohanty et al., 2004). Disruption of the polyadenylation complex by the complete absence of either Hfq or/and PNPase dramatically alters the intracellular polyadenylation profile (Mohanty et al., 2004). While the absence of Hfq leads to an increase in the addition of PNPase dependent polynucleotide tails within the coding sequences, the loss of both Hfq and PNPase results in a shift of the PAP I dependent poly(A) tails from after the Rho-independent transcription terminator to within the coding sequences. Although the specific role of PNPase in the complex is not known, Hfq is believed to be responsible for recruiting the Rho-independent transcription terminator (Mohanty et al., 2004, Otaka et al., 2011, Sauer & Weichenrieder, 2011) and stimulating the processivity of PAP I (Hajnsdorf & Régnier, 2000). In fact, macroarray analysis of more than 240 ORFs, associated with either mono- or polycistronic transcripts, suggests that Rho-independent transcription terminators serve as preferred polyadenylation sites for the PAP I polyadenylation complex (Mohanty & Kushner, 2006).
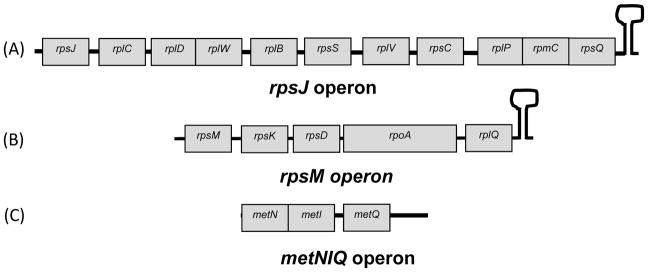
Previously, the study of the Rho-independent transcription terminator as the site for polyadenylation has been limited to monocistronic transcripts (Mohanty et al., 2004). Interestingly, only ~6% of the polyadenylated ORFs identified in the macroarray experiments were monocistronic (Mohanty & Kushner, 2006). The majority of the polyadenylated ORFs (~94%) resided within 21 polycistronic operons (Mohanty & Kushner, 2006). For example, many ORFs in the large rpsJ (~5,200 nt in length containing 11 ORFs) and rpsM (~3000 nt in length containing 5 ORFs) polycistronic mRNAs containing a Rho-independent transcription terminator (Fig. 1) were predicted to be polyadenylated based on higher levels of hybridization with oligo(dT)-dependent cDNAs (Mohanty & Kushner, 2006). However, the mechanism of polyadenylation of the ORFs upstream of the terminal Rho-independent transcription terminator in a polycistronic transcript is not clear.
Figure 1.
Graphical presentation of the rpsJ (also known as the S10 operon) (A), rpsM (B) and metN operons (C). The lack of spacers indicate translational overlap between specific ORFs.
Related experiments using microarray and high density tiling arrays suggest that ~60% of the E. coli ORFs, including many polycistronic transcripts, are affected by RNase E (Bernstein et al., 2002, Stead et al., 2010), which forms a multiprotein degradosome complex containing PNPase, the RhlB RNA helicase and enolase (Carpousis et al., 1994, Py et al., 1994, Py et al., 1996). Since PNPase is part of the RNase E-based degradosome as well as the PAP I polyadenylation complex, we hypothesized that PAP I-mediated polyadenylation might depend in part on the presence of a functional RNase E-based degradosome. In fact, the results presented in this report support this hypothesis. Furthermore, the data also suggest an active role of functional PNPase in normal PAP I-dependent polyadenylation.
RESULTS
The transcripts in polycistronic operons containing a Rho-independent transcription terminator are differentially polyadenylated by PAP I
Previous experiments have shown that Rho-independent transcription terminators on monocistronic transcripts serve as polyadenylation signals in E. coli (Mohanty et al., 2004, Mohanty & Kushner, 2006). The oligo(dT)-dependent cDNA hybridization observed in a macroarray analysis of the E. coli transcriptome suggested that multiple ORFs within many polycistronic operons containing a Rho-independent transcription terminator were also polyadenylated (Mohanty & Kushner, 2006), although the exact nature of the tails associated with each ORF was unknown. Accordingly, we were interested in determining both the polyadenylation sites and the composition of the poly(A) tails for such transcripts. We examined five representative transcripts from the rpsJ and rpsM operons (Fig. 1) for the presence of poly(A) tails that were most likely added by PAP I.
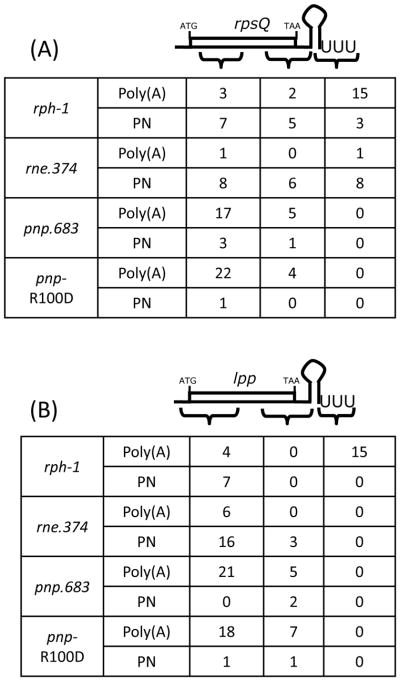
As shown in Table 1, for the rpsQ transcript (immediately upstream of the Rho-independent transcription terminator) poly(A) tails accounted for 57% (20/35) of the sequenced species in the rph-1 strain. Similarly, ~63% (10/16) of the tails associated with the rplQ transcript from the rpsM operon were poly(A) (Table 1). More importantly, 75% (15/20) of rpsQ and 70% (7/10) of rplQ poly(A) tails were added after the Rho-independent transcription terminator (Fig. 2A, data not shown). These results were in good agreement with previously reported data for the monocistronic lpp mRNA with the exception that for lpp no polynucleotide tails were found in the terminator region (Fig. 2B) (Mohanty & Kushner, 1999, Mohanty & Kushner, 2000). Furthermore, ~ 41% (13/32) of the rpsJ, ~20% (5/25) of the rplV and ~75% (9/12) of rpsK transcripts had poly(A) tails (Table 1). However, the sites of poly(A) tails for these ORFs were distributed throughout the coding sequences rather than being concentrated towards the end of the ORFs (data not shown), suggesting the lack of a specific targeting sequence for PAP I on these transcripts compared to rpsQ and rplQ transcripts that were immediately upstream of the Rho-independent transcription terminator.
Table 1.
Poly(A) tail analysis of specific transcripts in various genetic backgrounds.
| mRNA | Genotype | % of total tails | No. of clones analyzed2 | |
|---|---|---|---|---|
| Poly(A)1 | Polynucleotide1 | |||
| rpsQ | rph-1 | 57 | 43 | 35 |
| rneΔ374 | 8 | 92 | 24 | |
| rneΔ10 | 32 | 68 | 22 | |
| hfq-1 | 12 | 88 | 26 | |
| pnpΔ683 | 85 | 15 | 26 | |
| pnp-R100D | 96 | 4 | 27 | |
| rpsJ | rph-1 | 41 | 59 | 32 |
| rneΔ374 | 15 | 85 | 18 | |
| rneΔ10 | 30 | 70 | 23 | |
| hfq-1 | 7 | 93 | 15 | |
| pnpΔ683 | 91 | 9 | 22 | |
| pnp-R100D | 85 | 15 | 20 | |
| rplV | rph-1 | 20 | 80 | 25 |
| rneΔ374 | 21 | 79 | 24 | |
| rplQ | rph-1 | 63 | 37 | 16 |
| rneΔ374 | 25 | 75 | 12 | |
| rpsK | rph-1 | 75 | 25 | 12 |
| rneΔ374 | 0 | 100 | 12 | |
| lpp | rph-13 | 70 | 30 | 27 |
| rneΔ374 | 28 | 72 | 25 | |
| rneΔ10 | 27 | 73 | 22 | |
| hfq-1 | 30 | 70 | 26 | |
| pnpΔ683 | 93 | 7 | 28 | |
| pnp-R100D | 93 | 7 | 27 | |
| metN | rph-1 | 0 | 100 | 12 |
| rneΔ374 | 8 | 92 | 12 | |
| metI | rph-1 | 12 | 88 | 17 |
| rneΔ374 | NA | NA | NA | |
| metQ | rph-1 | 26 | 74 | 19 |
| rneΔ374 | 33 | 67 | 12 | |
p < 0.0002
Total number of independent isolates sequenced from each genetic background.
Data for the lpp transcript in the rph-1 genetic background was taken from Mohanty et al. (Mohanty et al., 2004).
NA- Not analyzed
All strains were constructed in the MG1693 (rph-1) genetic background.
Figure 2.
Graphical presentation of poly(A) and polynucleotide tails found on (A) rpsQ and (B) lpp transcripts in various genetic backgrounds. Poly(A): poly(A) tail, Poly(N): polynucleotide tail. The numbers in each row of the table represent how many poly(A) or polynucleotide (PN) tails were obtained in that particular region from the corresponding genetic background. Independent isolates were sequenced as described in the text.
The transcripts from polycistronic operons terminated with a Rho-dependent transcription terminator have a majority of polynucleotide tails
The higher percentage of poly(A) tails downstream of the Rho-independent transcription terminators on the rpsQ and rplQ transcripts led us to examine the polyadenylation profiles of the three genes from the metNIQ polycistronic transcript, which is terminated in a Rho-dependent fashion (Fig. 1C). As expected, only 26% (5/19) of the metQ transcripts (the last gene in the operon) had poly(A) tails (Table 1) compared to 57–63% for rpsQ and rplQ transcripts (Table 1). Furthermore, only 5% (1/19) of the metQ tails were present downstream of its translation stop codon, compared to 70–75% for the rplQ and rpsQ transcripts (Fig. 2A, data not shown). In addition, >88% of the metN and metI tails were polynucleotide (Table 1). The cloning and sequencing data from the metNIQ operon were in agreement with the macroarray data (Mohanty & Kushner, 2006) and suggested that a polycistronic operon terminated with a Rho-dependent transcription terminator was not an effective substrate for PAP I, a result similar to what was found for the trxA monocistronic transcript (Mohanty et al., 2004).
Irrespective of which transcript was examined, the poly(A) tails varied in length from 14–28 A residues, while the polynucleotide tails ranged from 17–>150 nt (data not shown). Since all the tails were different in composition as well as at different physical locations, the data represented independent isolates (data not shown). Taken together, these results were consistent with previous reports that Rho-independent transcription terminators play a significant role in PAP I mediated polyadenylation (Mohanty et al., 2004, Mohanty & Kushner, 2006).
A functional RNase E based degradosome is required for normal polyadenylation by PAP I
While the higher percentage of poly(A) tails downstream of the Rho-independent transcription terminators associated with rpsQ and rplQ transcripts was predicted (Mohanty et al., 2004), with the exception of rpsK, 2/3 of the upstream ORFs (rpsJ and rplV) had low poly(A) levels. We hypothesized that initial endonucleolytic processing of these operons generated species that were with and without the Rho-independent transcription terminator and were thus differentially modified either by PAP I or PNPase. In fact, analysis of mutants in RNase E, RNase III, RNase Z, RNase G, and RNase LS showed that RNase E was primarily responsible for the initial processing of ~5.2 Kb rpsJ polycistronic transcript (Fig. 3, data not shown). There was over a 6-fold increase in the steady-state level of the full-length rpsJ polycistronic transcript in the temperature sensitive rne-1 strain at the nonpermissive temperature compared to the control strain (lanes 1, 3). Similar results were observed with the rneΔ1018 mutant (data not shown). Absence of either RNase G (lane 2) or RNase Z (lane 4) had no significant effect on the steady-state levels of the full-length rpsJ transcript.
Figure 3.
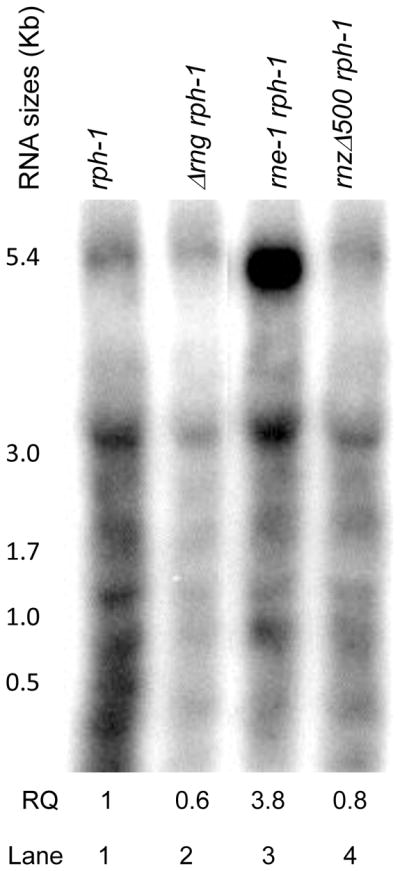
Northern analysis of the rpsJ primary transcript. Total RNA was isolated and separated on a 1.5% glyoxal agarose gel as described in the Experimental Procedures. Lane 1, MG1693; lane 2, SK2538; lane 3, SK5665; lane 4, SK9795. The sizes of the various transcripts are indicated on the left of the blot and are based on a RNA sizing ladder (Riboruler™, Fermentas). Lanes that were not relevant were removed from the blot. The blot was scanned using a STORM 840 PhosphorImager (GE Healthcare) and the intensities of the bands were quantified using ImageQuant TL 5.2 software. The relative quantity (RQ) of the full-length transcript compared to the MG163 control is shown below each lane and represents the average of three independent determinations.
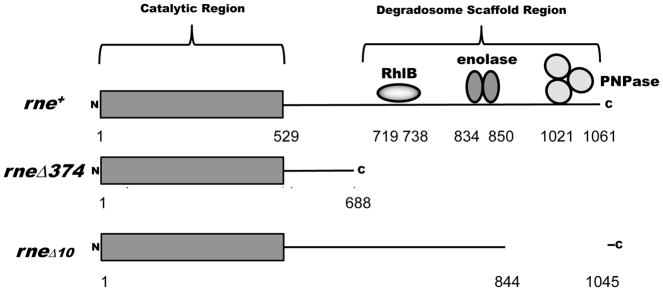
Since RNase E was primarily responsible for the initial processing/degradation of the full-length rpsJ transcript, we tested if the RNase E-based degradosome (Carpousis et al., 1994, Py et al., 1996) was required for PAP I-dependent polyadenylation. Experiments using deletion mutants have identified association regions for the RhlB RNA helicase, enolase and PNPase to be at amino acids 719-731, 834-850 and 1021-1061 of the RNase E scaffold, respectively (Fig. 4) (Vanzo et al., 1998, Ait-Bara et al., 2015). Accordingly, we determined the polyadenylation profiles of the rpsQ and rplQ mRNAs in an rneΔ374 mutant, which is missing amino acids 689-1060 that contain the binding domains for RhlB, enolase, and PNPase (Fig. 4) and cannot form a functional degradosome (Ow et al., 2000). The RneΔ374 protein retains the wild-type catalytic region of RNase E, which functions normally with regard to mRNA decay and tRNA maturation (Ow et al., 2000, Leroy et al., 2002) and has a generation time similar to an rne+ control (data not shown).
Figure 4.
Graphical representation of the RNase E degradosome mutants used in this study. The catalytic region of amino acids 1-529 and the degradosome scaffold region of 530-1061 amino acids are shown. The degradosome scaffold region includes the binding sites for the RhlB RNA helicase, enolase, Hfq and PNPase (Vanzo et al., 1998, Ikeda et al., 2011, Ait-Bara et al., 2015).
The percentage of poly(A) tails associated with the rpsQ and rplQ transcripts decreased dramatically in the rneΔ374 strain compared to the rne+ control (Table 1). Since the polyadenylation profile of the lpp mRNA has previously been well characterized in an rph-1 strain (Mohanty & Kushner, 1999, Mohanty & Kushner, 2000, Mohanty et al., 2004), we carried out a similar analysis of the lpp mRNA in the rneΔ374 strain. There was also a large reduction in the level of lpp poly(A) tails in the rneΔ374 strain compared to the rne+ control (Table 1). In addition, the majority of the tails were added within the coding sequences rather than after the transcription terminator for the rpsQ, rplQ and lpp mRNAs in the absence of degradosome assembly (Figs. 2A–B, data not shown). In the case of the lpp mRNA, the absence of a functional degradosome led to the majority of the tails [both poly(A) and polynucleotide] being added very close to the start of transcription (Fig. 2B). A similar cloning and sequencing analysis of metN and metQ transcripts of metNIQ operon (Fig. 1C) derived from the rneΔ374 mutant showed no significant difference in the polyadenylation profile compared to the rph-1 strain (Table 1).
The data presented above suggested that the uncoupling of PNPase from the degradosome might be responsible for the increase in polynucleotide tails in rneΔ374 mutant. In order to prove this hypothesis, we tested a partial degradosome scaffold mutant, in which only the PNPase binding region [rneΔ10 (Leroy et al., 2002), amino acids 845-1045] was missing (Fig. 4). As expected, significant reductions in poly(A) tails were also observed for both the rpsQ and lpp mRNAs in the rneΔ10 mutant (Table 1).
The level of polynucleotide tails on the rpsJ and rpsQ mRNAs increased in the absence of Hfq but decreased significantly in the absence of PNPase
The distribution of rpsQ, rplQ, and lpp tails in the degradosome mutants was reminiscent of what was observed for lpp tails in an hfq mutant (Mohanty et al., 2004). In fact, the percentage of polynucleotide tails increased significantly for both the rpsQ and rpsJ mRNAs in the hfq-1 mutant and their levels were very similar to what was observed in the rneΔ374 strain (Table 1). The percentage of the polynucleotide tails on the rpsQ and rpsJ mRNAs was even greater than previously observed for the monocistronic lpp mRNA in the hfq-1 genetic background (Table 1) (Mohanty & Kushner, 1999, Mohanty et al., 2004). As expected, the percentage of the polynucleotide tails decreased significantly in the pnpΔ683 mutant in rpsQ, rpsJ and lpp transcripts (Table 1) indicating that these tails were added by PNPase in both the hfq-1 and rneΔ374 mutants. The few polynucleotide tails found on either the rpsQ or lpp mRNAs had only 1–3 nt non-A residues (data not shown), which may have arisen from the rare addition of non-A residues by PAP I (Yehudai-Resheff & Schuster, 2000). In addition, the location of the tails [both poly(A) and polynucleotide] shifted dramatically towards the 5′ end of each transcript in both thehfq-1 (data not shown) and pnpΔ683 (Fig. 2A–B) mutants.
A catalytically active PNPase protein is required for PAP I-dependent polyadenylation of mRNAs after Rho-independent transcription terminators
It has been hypothesized that PAP I adds poly(A) tails after a Rho-independent transcription terminator through its interaction with both Hfq and PNPase in such a way that in their absence poly(A) tails are added mostly within the coding sequences (Mohanty et al., 2004). However, the significant increase in the level of polynucleotide tails in the degradosome mutant missing only the PNPase binding domain (rneΔ10) was unexpected and suggested that an association of RNase E and PNPase was required for PAP I-dependent polyadenylation. Furthermore, the fact that PNPase is also part of the PAP I polyadenylation complex (Mohanty et al., 2004) raised an important question of whether the PNPase protein was playing a structural and/or catalytic role in the polyadenylation pathway.
In order to distinguish between these two possibilities, we took advantage of a specific PNPase point mutation (pnp-R100D) that was isolated by Jarriage et al. (Jarrige et al., 2002) in which a single amino acid change led to no detectable 3′ → 5′ exonuclease or polymerization activities, but did not disrupt the protein’s normal trimeric structure in vivo. We constructed a low-copy number plasmid carrying the pnp-R100D allele under the control of the native rpsO pnp promoter (pKMK13, Table 2) and transformed it into a pnpΔ683 deletion strain. Immunoprecipitation analysis confirmed the interaction between the PNPase R100D and RNase E proteins (Fig. S1, Supplementary Material).
Table 2.
Bacterial strains and plasmids used in this study.
| Strains | Genotype | Reference or Source |
|---|---|---|
| AC-24 | MC1061 rneΔ10 (aaΔ 844-1045) zce-726::Tn10 TcR | (Leroy et al., 2002) |
| MG1693 | rph-1 thyA715 | E. coli Genetic Stock Center |
| NEB5α |
fhuA2 Δ(argF-lacZ)U169 phoA glnV44 Φ80 Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 |
New England Biolabs |
| SK2538 | Δrng::cat rph-1 thyA715 | (Ow et al., 2003) |
| SK2683 |
rneΔ1018::bla rph-1 thyA715 recA56 srlD300::Tn10 pDHK3*(Sp/SmR rneΔ374) pWSK129 (KmR) |
This Study |
| SK4433 | rneΔ10(aaΔ 844-1045) zce-726:: Tn10 rph-1 thyA715 TcR | This Study |
| SK4436 |
pnpΔ683::spc/str rph-1 thyA715 Sp/SmR pKMK12** (rpsO+pnp+, ApR) |
This Study |
| SK4437 |
pnpΔ683::spc/str rph-1 thyA715 Sp/SmR pKMK13** (rpsO+ pnp-R100D, ApR) |
This Study |
| SK4443 |
pnpΔ683::spc/str rph-1 thyA715 Sp/SmR pWSK29** (empty vector, ApR) |
This Study |
| SK5665 | rne-1 rph-1 thyA715 | (Arraiano et al., 1988) |
| SK7988 | ΔpcnB::kan rph-1 thyA715 KmR | (O’Hara et al., 1995) |
| SK9714 |
rneΔ1018::bla thyA715 rph-1 pSBK1** (rne+, CmR) |
(Ow et al., 2000) |
| SK9795 | rnzΔ500::kan rph-1 thyA715 | (Perwez & Kushner, 2006) |
| SK10019 | pnpΔ683::spc/str rph-1 thyA715 Sp/SmR | (Mohanty & Kushner, 2003) |
| SK10023 | hfq-1::Ω rph-1 thyA715 KmR | (Mohanty et al., 2004) |
pDHK3 and pDHK30 are single copy plasmids.
pKMK12, pKMK13, pSBK1, pWSK29, and pWSK129 are 6–8 copy number plasmids.
We hypothesized that if the presence of a trimeric PNPase protein in the degradosome and/or polyadenylation complex was sufficient for normal polyadenylation of mRNAs, then an E. coli strain carrying the pnp-R100D allele would have a polyadenylation profile comparable to what was seen in a pnp+ control. However, the bulk of the tails (85–96%) associated with the rpsJ, rpsQ and lpp transcripts obtained from a pnp-R100D mutant were poly(A) tails (Table 1) and were mostly added within the coding sequences instead of downstream of the Rho-independent transcription terminator (Fig. 2A–B). These results were comparable to what was obtained with the pnpΔ683 strain (Table 1 and Fig. 2A–B).
Degradosome regulation of polyadenylation is not specific to the rpsJ and rpsM operons and lpp mRNA
Although the data obtained from transcripts of the rpsJ, rpsM operons and the lpp mRNA demonstrated an important role for a functional degradosome in the polyadenylation of specific mRNAs (Table 1), we wanted to determine if there were an impact on global poly(A) levels in the cell. Accordingly, we compared the total polyadenylation profile in strains defective in functional degradosome assembly with a wild type control, using a poly(A) sizing assay. The rph-1 control strain showed poly(A) tails ranging from 4 to 40 nt (Fig. 5, lane 2). Inactivation of PAP I led to the loss of almost all poly(A) tails longer than 10 nt (Fig. 5, lane 3), in agreement with previously reported results (Mohanty & Kushner, 1999). The degradosome mutants (rneΔ374 and rneΔ10) as well as the hfq-1 strain showed significant reductions in both total poly(A) levels and the amount of tails longer than 10 nt (Fig. 5, lanes 4–6). The reduction in tail length was consistent with the digestion of polynucleotide tails obtained from the degradosome mutants by RNase A and RNase T2 following G, C and U residues (Mohanty et al., 2004). It should be noted that most poly(A) tails <10 nt are associated with tRNAs (Mohanty et al., 2012) and their levels were not affected in the degradosome mutants (Fig. 5, lanes 5,6).
Figure 5.
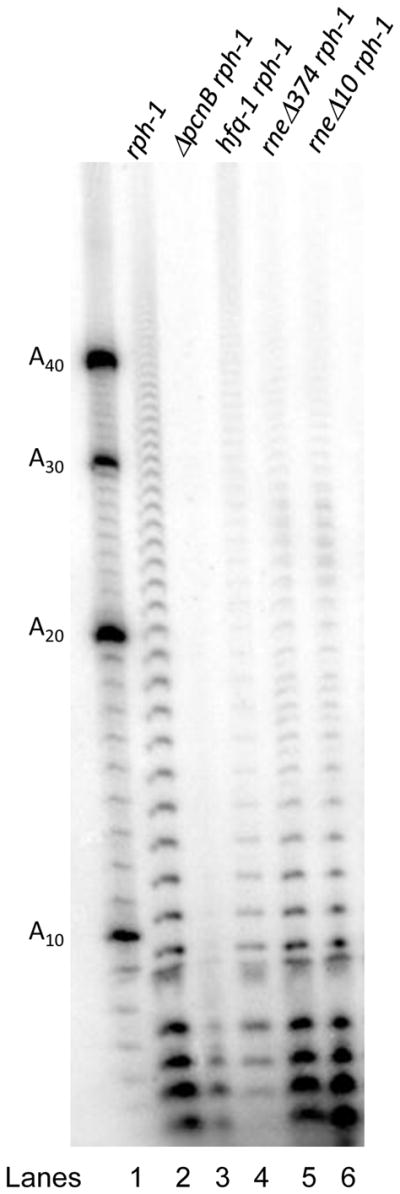
Poly(A) sizing assay for various mutants. Total RNA from various strains was 3′-end-labeled with [32P]-pCp, digested, and then separated on a 20% polyacrylamide gel as described in the Experimental Procedures. The genotype of each strain is listed above each lane. Lane 1, A 5′-[32P]-end-labeled oligo(A) ladder (nt).
DISCUSSION
The data presented here demonstrate for the first time that in E. coli the RNase E-based degradosome helps regulate the polyadenylation of mRNAs containing Rho-independent transcription terminators. Specifically, in the absence of either the complete degradosome scaffold region (rneΔ374) or the PNPase binding domain (rneΔ10), the profiles of post-transcriptionally added tails on mRNAs derived from either polycistronic or monocistronic transcripts changed from being mostly PAP I-mediated to being carried out primarily by PNPase (Table 1). In the absence of degradosome assembly in the rneΔ374 mutant, not only was there a dramatic shift in the ratio of poly(A)/polynucleotide tails (Table 1), but the physical locations of the tails were significantly changed. For example, the majority of the PAP I added tails for the rpsQ, rplQ and lpp mRNAs in an rph-1 strain were located after the Rho-independent transcription terminator (Fig. 2A–B, data not shown). In contrast, in the absence of an intact degradosome, the majority of the tails on these transcrripts were very close to the 5′ ends of the transcripts (Fig. 2A–B, data not shown). In fact, none of the tails on thelpp and rplQ mRNAs, either poly(A) or polynucleotide, were found after the Rho-independent transcription terminator (Fig. 2B, data not shown) in the rneΔ374 strain. The polynucleotide tails associated with the rpsQ transcripts were localized to three major regions of the transcript, with some being added after the Rho-independent transcription terminator (Fig. 2A).
The amount of PAP I-dependent polyadenylation on ORFs upstream of the Rho-independent transcription terminator (rpsJ, rplV and rpsK) in the polycistronic transcripts varied considerably. Although the results were in contrast to the macroarray analysis (Mohanty et al, 2006), which showed them all to be polyadenylated, it was possible that the increase in oligo(dT) hybridization observed for these transcripts was misleading, since both poly(A) and polynucleotide tails could hybridize to this primer. However, the fraction of poly(A) tails associated with the rpsJ, rpsQ, rpsK and rplQ transcripts was significantly higher than what was observed for the three ORFs of the polycistronic metNIQ transcript, which lacks a Rho-independent transcription terminator (Table 1). Thus, a Rho-independent transcription terminator on a polycistronic transcript may play a role in PAP I dependent polyadenylation of some ORFs within the operon.
Furthermore, in vivo analysis of total polyadenylation levels from various degradosome mutants revealed that the loss of degradosome assembly altered PAP I-dependent polyadenylation at the transcriptome-wide level and was not specific to the lpp, rpsQ, and rpsM transcripts (Fig. 5). Thus, the reduction in the level of longer poly(A) tails observed in the degradosome mutants compared to the MG1693 control demonstrates an important role for a functional RNase E-based degradosome in the overall polyadenylation of mRNAs, but not tRNAs, since the absence of a functional degradosome did not alter the levels of short poly(A) that are associated primarily with tRNAs (Fig. 5) (Mohanty et al., 2012).
The significant change in the polyadenylation profile in the degradosome mutants suggested the possibility of some type of direct interaction between the RNase E-based degradosome and the polyadenylation complex, most likely via their common partner PNPase. However, since replacing the wild type PNPase with a functionally inactive Pnp-R100D protein that still interacted with RNase E (Fig. S1, Supplementary Material) did not restore the polyadenylation profiles of mRNAs to those seen in a wild type control and were identical to a what was obtained from a pnpΔ683 mutant (Table 1, Fig. 2A–B), it does not seem likely that there is a direct interaction of the degradosome with the PAP I polyadenylation complex via PNPase.
Rather, it is important to remember that the proteins associated with either the degradosome or the PAP I polyadenylation complex are not present in equimolar concentrations within the cell. Quantitative analysis of the E. coli proteome has demonstrated that on average there are 437, 911, 3387, and 45 molecules/per cell of RNase E, PNPase, Hfq and PAP I, respectively (Soreq & Littauer, 1977, Mohanty et al., 2004, Wisniewski & Rakus, 2014). Although there will undoubtedly be some variation in the absolute numbers of each protein from determination to determination, these data suggest that all of the PAP I molecules are probably associated with Hfq in vivo. Since PNPase normally exists as a homotrimer, there are fewer PNPase trimeric complexes compared to the combined number of RNase E and PAP I molecules. Furthermore, that data also indicate that in all likelihood there will be far fewer PAP I complexes compared to functional degradosomes. It should be noted that PAP I complexes have been shown to form in the absence of an intact degradosome (Mohanty et al., 2004).
Accordingly, it is very likely that the failure of PNPase to bind to RNase E will lead to excess free PNPase, which may actively compete with the PAP I polyadenylation complex for specific substrates, either by binding directly to a 3′ terminus or by degrading poly(A) tails that have already been synthesized. In fact, the results observed with an rneΔ374 scaffold mutant were experimentally identical to those obtained with an hfq-1 strain (Table 1) where PNPase has been shown to be the primary post-transcriptional modifying enzyme (Mohanty et al., 2004). These data are also in agreement with previously published data for the lpp mRNA isolated from an hfq-1 mutant (Mohanty et al., 2004).
We have also shown that deletion of the pnp gene leads to a dramatic alteration in the ratio of poly(A) to polynucleotide tails for the rpsQ and lpp mRNAs (Table 1). In addition, the locations of the poly(A) tails changed from being after the Rho-independent transcription terminator to being within the coding sequences and mostly near the 5′ end of each specific transcript (Figs. 2A–B). One possibility for this observed difference is that the PNPase protein somehow alters the substrate specificity of PAP I, but does not actively participate in the actual polyadenylation reaction. However, the experiments using Pnp-R100D protein showed that the polyadenylation profiles for the rpsJ, rpsQ and lpp mRNAs were experimentally equivalent to what was observed with a pnpΔ683 deletion strain (Table 1). Not only were the bulk of the tails homopolymeric, but the shift in the locations of the tails for both the rpsQ and lpp mRNAs were almost identical in the pnpΔ683 and pnp-R100D mutants (Fig. 2A–B) compared to an rph-1 control. These data ruled out the possibility that PNPase only plays a structural role in the polyadenylation of mRNAs.
Instead, they strongly suggest that the PNPase protein has to be catalytically active for normal polyadenylation to occur after Rho-independent transcription terminators. One possibility is that a catalytically active PNPase is involved in the degradation of the RNA decay intermediates, effectively reducing their concentration and thereby facilitating the polyadenylation of more full-length transcripts by PAP I. In the absence of PNPase the RNA decay intermediates become the primary substrates for PAP I polyadenylation (Fig. 2A–B). It is also possible that a catalytically active PNPase improves the binding efficiency of PNPase-Hfq-PAP I complex to its preferred substrate, the Rho-independent transcription terminator, explaining why poly(A) tails are added at places other than after the Rho-independent transcription terminator in the absence of either Hfq (Mohanty et al., 2004) or PNPase (Fig. 2A–B). A similar type of function for PNPase has recently been shown for a PNPase-Hfq-small RNA complex (Bandyra et al., 2016).
Finally, it should be noted that there are a wide variety of RNA substrates that can be post-transcriptionally modified including full-length transcripts, RNA decay intermediates resulting from endonucleolytic cleavages or the stalling of 3′ → 5′ exonucleolytic degradation, RNA species arising from premature transcription termination, and small regulatory RNAs (sRNAs). It is possible that the mechanism of addition of poly(A) tail to a full-length transcript following the Rho-independent transcription terminator is actually distinct from the addition of poly(A) tails to other substrates, where a PAP I polyadenylation complex may not be necessary. Taken together, out data indicate that polyadenylation in E. coli is more highly regulated than previously thought.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Plasmids
The strains and plasmids used in this study are listed in Table 2. All the bacterial strains were derived from MG1693 (thyA715 rph-1). This strain has no RNase PH activity, a 3′ → 5′ exoribonuclease involved in tRNA maturation, due to a naturally occurring single nucleotide frame shift mutation in the rph gene (Jensen, 1993). The loss of RNase PH activity has a minor effect on total polyadenylation (Mohanty & Kushner, 2013). SK2683 [rneΔ1018::bla rph-1 thyA715/pDHK3 (rneΔ374/Sp/SmR)] missing the entire RNase E scaffold region was constructed using a plasmid displacement method (Ow et al., 2000). The rneΔ374 DNA fragment from the 6–8 copy plasmid pMOK16 (Ow et al., 2000) was transferred into the single copy plasmid pMOK40 (SmR/SpR) (Ow et al., 2002) at the EcoRI-NotI sites and subsequently transformed into SK9714 [rneΔ1018::bla rph-1 thyA715/pSBK1 (rne/CmR)] (Ow et al., 2000). The wild-type RNase E covering plasmid (pSBK1/rne+) was displaced with pWSK129 (Wang & Kushner, 1991), which has the same origin of DNA replication. SK4433 (rneΔ10 rph-1 thyA715 TcR) was constructed by P1-mediated transduction using AC24 (rneΔ10 zce-726::Tn10 TcR) (Leroy et al., 2002) as donor strains. The rneΔ10 allele is missing only the PNPase binding domain (Fig. 4). SK4433 was sequenced to confirm the presence of the rneΔ10 allele.
pKMK12 (rpsO pnp/ApR) encoding wild type PNPase was constructed by transferring a HindIII-KpnI fragment of pKAK7 (Donovan & Kushner, 1986) containing the native rpsO pnp operon into the HindIII-KpnI sites of a 6–8 copy vector pWSK29 (Wang & Kushner, 1991). Subsequently, pKMK12 was used to create the Pnp-R100D (pKMK13/rpsO pnp-R100D/ApR) by site-directed mutagenesis using the Q5 Site-Directed Mutagenesis Kit (NEB) as follows. The rpsO pnp operon was amplified using the primer R100Dpnp_Fwd2 (CCGCCCG ATTGACCCGCTGTTC) and R100Dpnp_Rev2 (TCAATCAGACGCGCGATC). The PCR product was digested with a KLD enzyme mixture as directed and transformed into NEB5α. The resulting plasmid (pKMK13/rpsO pnp-R100D/ApR) was sequenced for confirmation of the presence of pnp-R100D. SK10019 (pnpΔ683), missing the entire PNPase coding sequence (Mohanty & Kushner, 2003), was transformed with either pKMK12 (rpsO pnp/ApR), pKMK13 (rpsO pnp-R100D/ApR), or pWSK29 (ApR) to create SK4436, SK4437, and SK4443, respectively.
The pnp-R100D allele lacks both the biosynthetic and degradative activity of PNPase, but still generates the normal trimeric form of PNPase in vivo (Jarrige et al., 2002). The rne-1 allele encodes a temperature-sensitive mutation that functionally inactivates RNase E at 44°C (Babitzke & Kushner, 1991). The hfq-1 mutation is a deletion/insertion mutation that produces no Hfq protein (Mohanty et al., 2004).
Bacterial growth and doubling times
Bacterial strains were grown at 37°C with shaking at 255 rpm in Luria broth supplemented with thymine (50 μg/mL) and streptomycin (20 mg/mL) or ampicillin (200 mg/mL), where appropriate. Optical densities were measured every 30 minutes using a Klett-Summerson colorimeter with a green filter (No. 42). Cells were diluted with pre-warmed growth medium as needed to maintain the cultures in exponential growth. The generation times of MG1693 (rph-1), SK2683 (rneΔ374 rph-1) and SK4433 (rneΔ10 rph-1) were comparable (data not shown). The generation times of SK4436 (pnp+) and SK4437 (pnp R100D) were 29.5 min and 52.5 min, respectively.
Characterization of post-transcriptionally added poly(A) and polynucleotide tails
Total RNA was isolated from exponentially growing strains as described previously (O’Hara et al., 1995, Mohanty et al., 2008) and treated with DNase I to remove any residual DNA present. All RNA samples were quantified using a Nanodrop® ND-2000c spectrophotometer and the quality of each RNA sample was checked by agarose gel electrophoresis. Poly(A) and polynucleotide tailed RNA from from various strains was reverse transcribed by an adapter primer containing 17-dT residues [AP, (Mohanty et al., 2008)] from total RNA using Superscript III reverse transcriptase (Nitrogen). The oligo(dT17) primer can efficiently hybridize to either A or AG tracts ≥10 nt which are frequently found in polynucleotide tails (Mohanty et al., 2004). Subsequently, oligo(dT)-dependent cDNAs were amplified for rpsJ, rplV, rpsQ, metN, metQ, metI, rpsK, rplQ (Fig. 1) and lpp mRNAs using 5′ gene-specific primers (GSP) and 3′ AAP (abridged adapter primer homologous to the multiple cloning sites of [AP, Mohanty et al, 2008)](Mohanty et al., 2008). The 5′ GSPs were as follows: rpsJ 5′ GS (GTCTGAT CGATCAAGCAACC); rpsQ 5′ GS (TAATGATCGATAAAATCCGTAC TCTGC); rplV 5′ GS (AAACTATCGATAAACATCGCCATGCTC); metN 5′ (GGGTTATCGATTCTGTTGATCACCCAC); metQ 5′ (GTTTAATCGATTGCTGGTTACTCCAAG); metI 5′ (GGTACATCGATTGGTTTGCA); RPSK-693 (AATATCGATGGCAAAGGCACCAATTCG); RPLQ-2792 (TAAATCGATGCGCCATCGTAAGAGTGG) and LPP366 (GCTACATGGAGATTAACTCAAGCTTGAGGG). All GSPs contained a ClaI site, except for the lpp gene-specific primer which contained a HindIII site. The GSPs were designed to amplify the entire coding sequences of lpp and rpsQ. The GSPs for all other genes were at 260–480 nt upstream of the putative 3′ ends. All cDNAs were cloned into pWSK29 (Wang & Kushner, 1991), as previously described (Mohanty & Kushner, 2000, Mohanty et al., 2008) and sequenced by Macrogen USA. Sequence results with tails starting less than three nucleotides downstream of the 5′ gene-specific primer sequence or within A rich regions within coding sequences were not included to eliminate possible mispriming events. A chi-square test of the data for all the cloned sequences was performed to determine the experimental variability between the biological replications and the significant differences between wild type and mutant strains.
Northern Analysis
For the Northern analysis described here bacterial cultures were initially grown in L broth at 30°C until they reached a cell density of ~1 × 108/ml. Subsequently, the cultures were shifted to 44°C for one hour to inactivate the temperature sensitive RNase E protein encoded by the rne-1 allele. Total RNA was isolated as described previously (O’Hara et al., 1995, Mohanty et al., 2008). All RNA samples were quantitated initially using a Nanodrop® ND-2000c spectrophotometer, followed by running 500 ng of each sample in a mini agarose gel to confirm equal loading (Mohanty & Kushner, 2007). Glyoxyl agarose northern analysis was conducted using samples prepared with NorthernMax®-Gly sample loading dye (Ambion) and electrophoresis in a 1.5% agarose gel in BPTE buffer (Burnett, 1997) run at 80 volts for approximately 4.5 hours. Gel transfer and subsequent steps were conducted as previously described. The gel was probed with a 32P-labeled oligonucleotide specific for the rpsJ 5′ UTR (5′-CTCCTCAGACCCATTACGATTG-3′).
Poly(A) Sizing Assay
Total steady-state poly(A) levels were compared by using a poly(A) sizing assay as described previously (O’Hara et al., 1995, Mohanty et al., 2008).
Supplementary Material
Acknowledgments
Special thanks to Dr. A.J. Carpousis for his gift of AC-24 (rneΔ10) and PNPase rabbit antibodies. This work was supported in part by NIH grants GM57220 and GM81554 to S.R.K.
References
- Ait-Bara S, Carpousis AJ, Quentin Y. RNase E in the gamma-Proteobacteria: conservation of intrinsically disordered noncatalytic region and molecular evolution of microdomains. Mol Genet Genomics. 2015;290:847–862. doi: 10.1007/s00438-014-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arraiano CM, Yancey SD, Kushner SR. Stabilization of discrete mRNA breakdown products in ams pnp rnb multiple mutants of Escherichia coli K-12. J Bacteriol. 1988;170:4625–4633. doi: 10.1128/jb.170.10.4625-4633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitzke P, Kushner SR. The Ams (altered mRNA stability) protein and ribonuclease E are encoded by the same structural gene of Escherichia coli. Proc Natl Acad Sci U S A. 1991;88:1–5. doi: 10.1073/pnas.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyra KJ, Sinha D, Syrjanen J, Luisi BF, De Lay NR. The ribonuclease polynucleotide phosphorylase can interact with small regulatory RNAs in both protective and degradative modes. RNA. 2016 doi: 10.1261/rna.052886.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JA, Khodursky AB, Lin P-H, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci USA. 2002;99:9697–9702. doi: 10.1073/pnas.112318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum E, Carpousis AJ, Higgins CF. Polyadenylation promotes degradation of 3′-structured RNA by the Escherichia coli mRNA degradosome in vitro. J Biol Chem. 1999;274:4009–4016. doi: 10.1074/jbc.274.7.4009. [DOI] [PubMed] [Google Scholar]
- Bralley P, Gust B, Chang SA, Chater KF, Jones GH. RNA 3′-tail synthesis in Streptomyces: in vitro and in vivo activities of RNase PH, the SCO3896 gene product and polynucleotide phosphorylase. Microbiol-SGM. 2006;152:627–636. doi: 10.1099/mic.0.28363-0. [DOI] [PubMed] [Google Scholar]
- Burnett WV. Northern blotting of RNA denatured in glyoxal without buffer recirculation. Biotechniques. 1997;22:668–671. doi: 10.2144/97224st01. [DOI] [PubMed] [Google Scholar]
- Cao G-J, Sarkar N. Identification of the gene for an Escherichia coli poly(A) polymerase. Proc Natl Acad Sci USA. 1992;89:10380–10384. doi: 10.1073/pnas.89.21.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis AJ, Van Houwe G, Ehretsmann C, Krisch HM. Copurification of E. coli RNAase E and PNPase: evidence for a specific association between two enzymes important in RNA processing and degradation. Cell. 1994;76:889–900. doi: 10.1016/0092-8674(94)90363-8. [DOI] [PubMed] [Google Scholar]
- Coburn GA, Mackie GA. Differential sensitivities of portions of the mRNA for ribosomal protein S20 to 3′-exonucleases is dependent on oligoadenylation and RNA secondary structure. J Biol Chem. 1996;271:15776–15781. doi: 10.1074/jbc.271.26.15776. [DOI] [PubMed] [Google Scholar]
- Donovan WP, Kushner SR. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986;83:120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M. Polynucleotide phosphorylase. Prog Nucl Acids Res. 1963;1:93–133. [Google Scholar]
- Hajnsdorf E, Régnier P. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc Natl Acad Sci USA. 2000;97:1501–1505. doi: 10.1073/pnas.040549897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnsdorf E, Braun F, Haugel-Nielsen J, Régnier P. Polyadenylylation destabilizes the rpsO mRNA of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:3973–3977. doi: 10.1073/pnas.92.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Yagi M, Morita T, Aiba H. Hfq binding at RhlB-recognition region of RNase E is crucial for the rapid degradation of target mRNAs mediated by sRNAs in Escherichia coli. Mol Microbiol. 2011;79:419–432. doi: 10.1111/j.1365-2958.2010.07454.x. [DOI] [PubMed] [Google Scholar]
- Jarrige A-C, Brechemier-Baey D, Mathy N, Duche O, Portier C. Mutational analysis of polynucleotide phosphorylase from Escherichia coli. J Molecular Biology. 2002;321:397–409. doi: 10.1016/s0022-2836(02)00645-9. [DOI] [PubMed] [Google Scholar]
- Jensen KG. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J Bacteriol. 1993;175:3401–3407. doi: 10.1128/jb.175.11.3401-3407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A, Vanzo NF, Sousa S, Dreyfus M, Carpousis AJ. Function in Escherichia coli of the non-catalytic part of RNase E: role in the degradation of ribosome-free mRNA. Mol Microbiol. 2002;45:1231–1243. doi: 10.1046/j.1365-2958.2002.03104.x. [DOI] [PubMed] [Google Scholar]
- Leroy A, Vanzo NF, Sousa S, Dreyfus M, Carpousis AJ. Function in Escherichia coli of the non-catalytic part of RNase E: role in the degradation of ribosome-free mRNA. Molecular Microbiol. 2002;45:1231–1243. doi: 10.1046/j.1365-2958.2002.03104.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Pandit S, Deutscher MP. Polyadenylation of stable RNA precursors in vivo. Proc Natl Acad Sci USA. 1998;95:12158–12162. doi: 10.1073/pnas.95.21.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. Analysis of the function of Escherichia coli poly(A) polymerase I in RNA metabolism. Mol Microbiol. 1999;34:1094–1108. doi: 10.1046/j.1365-2958.1999.01673.x. [DOI] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. Polynucleotide phosphorylase functions both as a 3′ - 5′ exonuclease and a poly(A) polymerase in Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:11966–11971. doi: 10.1073/pnas.220295997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol Microbiol. 2003;50:645–658. doi: 10.1046/j.1365-2958.2003.03724.x. [DOI] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. The majority of Escherichia coli mRNAs undergo post-transcriptional modification in exponentially growing cells. Nucleic Acids Res. 2006;34:5695–5704. doi: 10.1093/nar/gkl684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. Ribonuclease P processes polycistronic tRNA transcripts in Escherichia coli independent of ribonuclease E. Nucleic Acids Res. 2007;35:7614–7625. doi: 10.1093/nar/gkm917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Kushner SR. Bacterial/archaeal/organellar polyadenylation. WIREs RNA. 2010;2:256–276. [Google Scholar]
- Mohanty BK, Kushner SR. Deregulation of poly(A) polymerase I in Escherichia coli inhibits protein synthesis and leads to cell death. Nucleic Acids Res. 2013;41:1757–1766. doi: 10.1093/nar/gks1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Maples VF, Kushner SR. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol. 2004;54:905–920. doi: 10.1111/j.1365-2958.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- Mohanty BK, Maples VF, Kushner SR. Polyadenylation helps regulate functional tRNA levels in Escherichia coli. Nucleic Acids Res. 2012;40:4589–4603. doi: 10.1093/nar/gks006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty BK, Giladi H, Maples VF, Kushner SR. Analysis of RNA decay, processing, and polyadenylation in Escherichia coli and other prokaryotes. Methods Enzymol. 2008;447:3–29. doi: 10.1016/S0076-6879(08)02201-5. [DOI] [PubMed] [Google Scholar]
- O’Hara EB, Chekanova JA, Ingle CA, Kushner ZR, Peters E, Kushner SR. Polyadenylylation helps regulate mRNA decay in Escherichia coli. Proc Natl Acad Sci U S A. 1995;92:1807–1811. doi: 10.1073/pnas.92.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka H, Ishikawa H, Morita T, Aiba H. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc Natl Acad Sci U S A. 2011;108:13059–13064. doi: 10.1073/pnas.1107050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow MC, Liu Q, Kushner SR. Analysis of mRNA decay and rRNA processing in Escherichia coli in the absence of RNase E-based degradosome assembly. Mol Microbiol. 2000;38:854–866. doi: 10.1046/j.1365-2958.2000.02186.x. [DOI] [PubMed] [Google Scholar]
- Ow MC, Perwez T, Kushner SR. RNase G of Escherichia coli exhibits only limited functional overlap with its essential homologue, RNase E. Mol Microbiol. 2003;49:607–622. doi: 10.1046/j.1365-2958.2003.03587.x. [DOI] [PubMed] [Google Scholar]
- Ow MC, Liu Q, Mohanty BK, Andrew ME, Maples VF, Kushner SR. RNase E levels in Escherichia coli are controlled by a complex regulatory system that involves transcription of the rne gene from three promoters. Mol Microbiol. 2002;43:159–171. doi: 10.1046/j.1365-2958.2002.02726.x. [DOI] [PubMed] [Google Scholar]
- Perwez T, Kushner SR. RNase Z in Escherichia coli plays a significant role in mRNA decay. Mol Microbiol. 2006;60:723–737. doi: 10.1111/j.1365-2958.2006.05124.x. [DOI] [PubMed] [Google Scholar]
- Py B, Causton H, Mudd EA, Higgins CF. A protein complex mediating mRNA degradation in Escherichia coli. Molecular Microbiol. 1994;14:717–729. doi: 10.1111/j.1365-2958.1994.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Py B, Higgins CF, Krisch HM, Carpousis AJ. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature. 1996;381:169–172. doi: 10.1038/381169a0. [DOI] [PubMed] [Google Scholar]
- Py B, Higgins CF, Krisch HM, Carpousis AJ. A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature. 1996;381:169–172. doi: 10.1038/381169a0. [DOI] [PubMed] [Google Scholar]
- Rott R, Zipor G, Portnoy V, Liveanu V, Schuster G. RNA polyadenylation and degradation in cyanobacteria are similar to the chloroplast but different from Escherichia coli. J Biol Chem. 2003;278:15771–15777. doi: 10.1074/jbc.M211571200. [DOI] [PubMed] [Google Scholar]
- Sauer E, Weichenrieder O. Structural basis for RNA 3′-end recognition by Hfq. Proc Natl Acad Sci U S A. 2011;108:13065–13070. doi: 10.1073/pnas.1103420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg B, Huang J, Cohen SN. The Streptomyces coelicolor polynucleotide phosphorylase homologue, and not the putative poly(A) polymerase, can polyadenylate RNA. J Bacteriol. 2003;185:7273–7278. doi: 10.1128/JB.185.24.7273-7278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H, Littauer UZ. Purification and characterization of polynucleotide phosphorylase from Escherichia coli. J Biol Chem. 1977;252:6885–6888. [PubMed] [Google Scholar]
- Stead MB, Marshburn S, Mohanty BK, Mitra JPCL, Ray D, Hughes T, Kushner SR. Analysis of E. coli RNase E and RNase III activity in vivo using tiling microarrays. Nucleic Acids Res. 2010;39:3188–3203. doi: 10.1093/nar/gkq1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzo NF, Li YS, Py B, Blum E, Higgins CF, Raynal LC, Krisch HM, Carpousis AJ. Ribonuclease E organizes the protein interactions in the Escherichia coli RNA degradosome. Genes Dev. 1998;12:2770–2781. doi: 10.1101/gad.12.17.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- Wisniewski JR, Rakus D. Quantitative analysis of the Escherichia coli proteome. Data in brief. 2014;1:7–11. doi: 10.1016/j.dib.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Cohen SN. RNA degradation in Escherichia coli regulated by 3′ adenylation and 5′ phosphorylation. Nature. 1995;374:180–183. doi: 10.1038/374180a0. [DOI] [PubMed] [Google Scholar]
- Yehudai-Resheff S, Schuster G. Characterization of the E. coli poly(A) polymerase: nucleotide specificity, RNA-binding affinities and RNA structure dependence. Nucleic Acids Res. 2000;28:1139–1144. doi: 10.1093/nar/28.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.