Abstract
Purpose
The ability of low-frequency repetitive transcranial magnetic stimulation (rTMS) to enhance intracortical inhibition has motivated its use as a potential therapeutic intervention in focal hand dystonia (FHD). In this preliminary investigation, we assessed the physiologic and behavioral effects of multiple sessions of rTMS in FHD.
Methods
12 patients with FHD underwent five daily-sessions of 1Hz rTMS to contralateral dorsal premotor cortex (dPMC). Patients held a pencil and made movements that did not elicit dystonic symptoms during rTMS. We hypothesized that an active but non-dystonic motor state would increase beneficial effects of rTMS. Five additional patients received sham-rTMS protocol. The area under curve (AUC) of the motor evoked potentials and the cortical silent period (CSP) were measured to assess changes in corticospinal excitability and intracortical inhibition, respectively. Behavioral measures included pen force and velocity during handwriting and subjective report.
Results
Multiple-session rTMS strengthened intracortical inhibition causing a prolongation of CSP after 3 days of intervention and pen force was reduced at day 1 and 5, leaving other measures unchanged. 68% of patients self-reported as ‘responders’ at day 5, and 58% at follow-up. Age predicted responders.
Conclusions
A strong therapeutic potential of this rTMS paradigm in FHD was not supported but findings warrant further investigation.
Keywords: focal hand dystonia, rehabilitation, rTMS, writer's cramp, clinical
Introduction
The possibility to induce lasting changes in cortical excitability has motivated the investigation of repetitive transcranial magnetic stimulation (rTMS) as a therapeutic tool in a growing number of clinical applications (for review Edwards et al. 2008). The virtue of low-frequency rTMS to strengthen inhibition (Chen et al. 1997) has motivated its application in focal hand dystonia (FHD), a movement disorder associated with deficient inhibition throughout the central nervous system (for review Berardelli et al. 1998) including the motor cortex (Quartarone et al. 2003). FHD is an idiopathic neurological disorder of movement characterized by involuntary co-contractions of agonist and antagonist hand and forearm muscles that are sustained or repetitive (Sheehy and Marsden 1982). FHD can affect any voluntary muscle or group of muscles in the affected limb and is triggered primarily during specific tasks such as handwriting or playing a musical instrument (i.e., task-specific FHD).
Several studies have shown that low-frequency rTMS can alter deficient intracortical inhibition in the primary motor cortex (Siebner et al. 1999b; Siebner et al. 1999c; Murase et al. 2005). Murase and colleagues (2005) compared the excitability of intracortical inhibitory circuits in the primary motor cortex (M1) as measured by cortical silent period and hand-writing performance before and after low-frequency rTMS (0.2Hz) applied to the contralateral (left) M1, supplementary motor area (SMA), dorsal premotor cortex (dPMC), and sham stimulation. The highest success rate in terms of clinical improvement, decrease in pen force and increase in intracortical inhibition was observed after dPMC stimulation (Murase et al. 2005). Targeting the dPMC is reasonable as this region plays an important role in movement selection (Schluter et al. 2001) and is abnormally activated in imaging studies in patients with FHD (Ibanez et al. 1999).
A recent study applied 1Hz rTMS repeatedly over five days (900 pulses/day) in subjects with FHD rather than a single rTMS session (Borich et al. 2009). Compared to sham stimulation, this rTMS protocol reduced intracortical inhibition and improved handwriting velocity. These effects persisted for ten days following intervention, but clinical improvements were subtle (Borich et al. 2009).
There is converging evidence that the physiological and behavioral effects of rTMS critically depend on the “neural state” of the cortical area at the time of stimulation (Siebner and Rothwell 2003; Ziemann et al.). Based on the acute effects of occipital TMS on visual perception, it has been proposed that rTMS preferentially affects the attributes encoded by less active neural populations (Silvanto et al. 2007a; Silvanto et al. 2007b). We thus applied interventional rTMS while patients performed writing movements that did not trigger their dystonic symptoms to produce an active but non-dystonic motor state. We speculated that in this state, the “dystonic” neural populations (i.e., the populations producing FHD) would be less active than the normally functioning “non-dystonic” neural populations and thus, would be more susceptible to the inhibitory effects of rTMS. This hypothesis is supported by an rTMS study in which a single intervention of 30min inhibitory rTMS was applied to M1 while patients performed “nonsense” scribbling (without inducing symptoms) (Siebner et al. 1999b). In that study, 1Hz rTMS during “nonsense” writing reinforced intracortical inhibition and induced a short-lasting improvement in circle drawing. In this preliminary investigation, we adopted a multi-session approach and performed repeated applications of inhibitory 1Hz rTMS to the contralateral dPMC during non-dystonic writing motion. The goals of this study were: to assess clinical changes and investigate neurophysiologic responses to rTMS of the activated motor system in patients with FHD.
Methods
Subjects
17 subjects with task-specific FHD (age: 46.5±12.4 y) were studied. Due to the preliminary nature of the investigation, a sample of convenience with 5 subjects was used to test the protocol with a sham-rTMS intervention. Subjects were recruited from local clinics and web sites. The clinical history and physical exam of subjects is reported in Table 1 (Sheehy and Marsden 1982). Exclusion criteria were: (1) any neurologic condition other than FHD, (2) medication for dystonia, (3) botulinum toxin within the past three months, (4) seizure history, (5) pregnancy or (6) implanted medical devices (Rossi et al. 2009). All subjects gave written informed consent prior to participation according to the Declaration of Helsinki. The study was approved by the University of Minnesota General Clinical Research Center and Institutional Review Board.
Table 1. Clinical and demographic information for subjects with dystonia.
| Case | Group | Sex | Age | Hand | Duration of sx | Diagnosis | Clinical Pattern | Toxin Hx |
|---|---|---|---|---|---|---|---|---|
| 1 | Sham | F | 64 | R | 21yr | WC R | R 3rd digit flexion during writing and typing | Y, 1yr prior |
| 2 | Real | M | 63 | R | 7yr | MD R | R 3rd, 4th digit flexion during piano playing, writing, typing | Y, 2yr prior |
| 3 | Real | M | 41 | L | 3yr | MD R | R 4th, 5th digit flexion during writing, guitar playing | Y, 1.5yr prior |
| 4 | Real | M | 68 | L | 5yr | WC L | L 2nd-4 digit abnormal posturing during writing/typing | N |
| 5 | Sham | M | 46 | R | 6yr | WC R | R grip, wrist extensor spasms during writing/typing | Y, 3mo prior |
| 6 | Real | M | 50 | R | 2.5yr | WC R | R 2nd-3rd digit abnormal posturing during writing/calculator use | Y, 3mo prior |
| 7 | Real | M | 59 | R | 37yr | WC R | R 3rd digit abnormal posturing during writing/golfing | N |
| 8 | Real | M | 50 | R | 8yr | WC R | R 3rd digit abnormal posturing during writing/suturing/card dealing | Y, 5yr prior |
| 9 | Sham | M | 42 | R | 14yr | MD R | 2nd/3rd digit spasm during typing and classical guitar playing | N |
| 10 | Sham | M | 55 | L | 9yr | MD L | L 2nd digit flexion playing piano | N |
| 11 | Real | F | 49 | R | 5yr | MD R | R 5th digit extension during guitar picking/piano/writing/typing | N |
| 12 | Real | F | 67 | R | 2.5yr | WC R | R hand abnormal flexion/tremor during writing/mousing | Y, 1yr prior |
| 13 | Sham | F | 55 | R | 12yr | WC R | R hand abnormal flexion/tremor during writing/mousing/pinching | N |
| 14 | Real | F | 72 | R | 50yr | WC R | R 2nd-4th digit abnormal posturing during writing/mousing/typing | Y, 5yr prior |
| 15 | Real | F | 38 | R | 7mo | WC R | R hand abnormal posturing during writing | N |
| 16 | Real | F | 61 | R | 25yr | WC R | R hand abnormal posturing/cramping during writing/carpentry | Y,14yr prior |
| 17 | Real | M | 42 | R | 3yr | WC R | R hand abnormal posturing/fatigue during writing and typing | Y, 6mo prior |
M=male, F=female, R=right, L=left, WC=writer's cramp, MD=musician's dystonia
Study Design
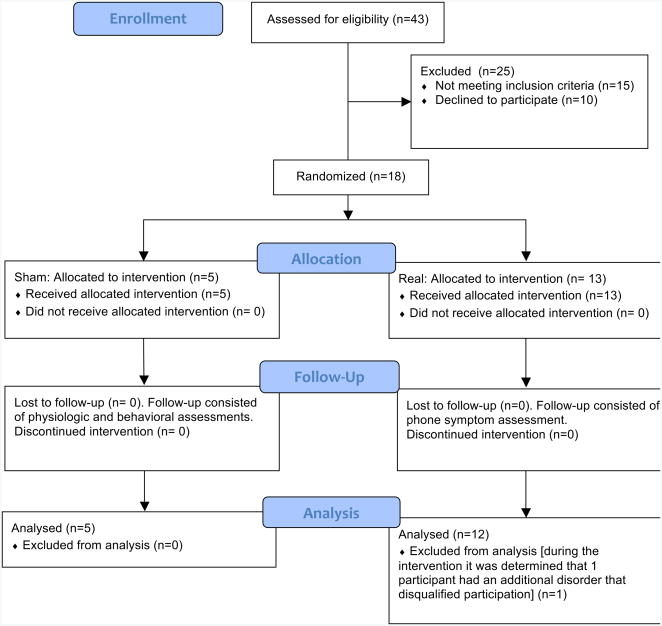
Subjects in the real intervention group (n=12) received only the real rTMS protocol with 10 day follow-up. Subjects in the sham group (n=5) were blinded to group assignment (Figure 1). These subjects were told there were two different levels of stimulation being tested. They initially received the sham stimulation paradigm and then crossed over to the real intervention protocol following the ten-day follow-up. However, due to possible contamination of results due to order effects, the results from the real intervention in the cross-over subgroup were not included in the real group analysis.
Figure 1.
Consort diagram demonstrating flow of subjects through study.
The intervention protocol consisted of one rTMS session (real or sham) given to the contralateral dPMC performed daily for five consecutive days. The primary behavioral measure was axial pen force and velocity during writing. Cortical excitability assessments were collected to assess changes in neurophysiologic response. Subjective symptom report was collected via phone interview after 10 days posttest.
Handwriting analysis
Handwriting analysis was performed following TMS testing using a computerized tablet (WACOM Co., Ltd, Japan), custom modified digitized pen and Oasis hardware and software package for data collection and analysis (UltraPen, Kiko Software, Doetinchem, The Netherlands). Subjects were instructed to write in their natural, self-paced style the sentence: ‘My country tis of thee,’ and perform a loop tracing condition. Each condition was repeated 4 times. Data were sampled at 215 Hz (resolution: 5,080 lpi, accuracy: ± 0.01” pressure range: 0-800 g). Mean axial pen force and velocity were the functional variables of interest. Handwriting was assessed after one and five rTMS sessions.
Assessment of cortical excitability
The primary time point of interest for all measures was results after five days of intervention. However, changes in excitability over time were also assessed to understand the evolution of the individual's electrophysiology. Thus, single pulse TMS assessments were taken after session 1, 3, and 5, whereas hand-writing was assessed after session 1 and 5 only. Subjects were seated comfortably in a reclining chair. Surface electrodes were affixed to the skin overlying the first dorsal interosseus (FDI) muscle of the involved hand using a belly/tendon montage. The electromyographic (EMG) activity of the resting FDI muscle was recorded at a sampling rate of 2560 Hz using a Cadwell Sierra EMG amplifier (Cadwell Laboratory, Washington) (sensitivity: 100 μv/div, filter: 20-2000 Hz). The EMG collected for each trial was 250 ms in duration, with 25 ms recorded prior to stimulus to assess pre-stimulus activity. Complete absence of pre-MEP activity was ensured by visual inspection during resting TMS data collection and verified during off-line analysis. Trials with >10 μV were excluded from analysis. TMS was performed with a 70-mm figure-of-eight coil connected to a Magstim 200 Rapid magnetic stimulator (Magstim Co., Whitland, Dyfed, UK). The handle of the coil was directed posterolaterally 45° to the mid-sagittal line of the head producing a posterior-anterior current flow in the brain (Orth and Rothwell 2004). We first determined the resting motor threshold (RMT), defined as the minimum intensity required to elicit MEP amplitude >50 μV peak-to-peak in at least 3/5 trials in the resting target muscle (Rossini et al. 1999) which was used to determine the intensity of stimulation for the single-pulse testing. The RMT was recalculated at each testing session with minimal variability between subjects [mean RMT: 48.1% max stimulator output, mean within subject SD 1.59% (range: 0-4.35%), CV=0.04].
The relation between increasing stimulus intensity and increase in MEP amplitude was assessed with TMS of M1 contralateral to the tested hand. MEP data were collected at intensities of 110, 120, 130, and 140% of RMT with a repetition rate of <0.1 Hz. Five trials were collected at each intensity in a pseudorandom order (gain: 200-500 μv/div, filter: 20-2000 Hz). The linear relationship between mean MEP amplitude and stimulus intensity was analyzed by calculating the slope and area under the curve (AUC) for each subject for each day. The area under the curve (AUC) was calculated through the integral of the linear line of best fit for the mean data points of each subject across the entire intensity range.
For CSP recording, IFCN committee guidelines were followed (Groppa et al. 2012a). Subjects performed an isometric abduction contraction of the index finger against a strain gauge coupled to a load cell. Force was transduced into an electrical signal displayed on an oscilloscope placed in front of the subject. Subjects were asked to produce a constant force at 20% of maximum voluntary isometric contraction marked on the screen, until instructed to relax. A single TMS pulse was applied 2-3s after contraction initiation and subjects were instructed to relax 2-3s after stimulation. Eight trials were recorded with a minimum 20s rest interval between each trial. The duration of the CSP was measured on a trial-by-trial basis and was delineated by the first superimposed TMS-evoked EMG spike (onset) and the return of activity to 50% of prestimulus EMG signal (offset) (Siebner et al. 2000; Kimberley et al. 2009). The mean CSP duration was calculated for each block of measurements. The duration of CSP is thought to be related to intracortical GABAergic synapse-mediated inhibition in the stimulated cortical region (Inghilleri et al. 1993; Chen and Hallett 1999). Measures of CSP have been shown to be reliable in repeated measures studies to determine an effect of intervention within a group of subjects (Orth and Rothwell 2004; Borich et al. 2009).
Subjective Symptom and Safety Assessment
Subjects rated their perceived symptom change using a Likert scale (-3=significant worsening, 0=no change, 1=mild, 2=moderate/clinically meaningful, 3=significant improvement). An adverse events questionnaire was administered prior to and after each session, as well as at follow-up.
rTMS intervention
In each rTMS session, 1800 biphasic stimuli were given to the dPMC in the hemisphere contralateral to the dystonic hand. Low-frequency rTMS (1Hz) was administered using a figure-of-eight coil (Magstim Rapid2, Magstim Co., Whitland, Dyfed, UK). The stimulation site for dPMC was defined as 2 cm anterior and 1 cm medial to the previously defined hotspot for FDI activation. This site was chosen based on localization from a previous PET studies (Fink et al. 1997; Schluter et al. 1998) and previous FHD intervention studies (Murase et al. 2005; Borich et al. 2009). In real-rTMS sessions, stimulus intensity was set at 90% of RMT. In the sham-rTMS sessions, all stimulation variables were identical but the coil was held orthogonally to the head to prevent biological effects of stimulation delivery to the head (Lisanby et al. 2001).
During the intervention, subjects were seated in a reclining chair with feet elevated. The stimulation was delivered while patients engaged in an active motor task that did not trigger their dystonic symptoms. This task varied based on each individual's severity of symptoms. Subjects were instructed to hold a ‘built-up’ pencil in their natural grip and place the tip to paper if possible without triggering any dystonia symptoms. If this was possible, they were then instructed to perform “nonsense” scribbling movements. Subjects were queried regarding possible dystonic symptoms and monitored for dystonic posturing by investigators. Three subjects were unable to put pencil to paper without eliciting symptoms, thus they held the pencil and drew in the air above the paper.
Statistical Analysis
To compare the effects of real- to sham-rTMS interventions multivariate linear mixed effects regression models were implemented using the SAS PROC MIXED procedure (SAS Institute, Cary, NC). This model has the benefit of accounting for missing data, thus addressing the unequal sample sizes between the two groups. Dependent variables of the mixed effects model including slope, AUC, change from baseline in CSP, handwriting pressure and velocity. Independent variables included baseline, group, day and the interaction of group and day. Repeated statement was used to account for the correlation from repeated measures within each subject and treatment. The overall treatment effect by day was evaluated by a Type 3 test. The difference in least square means between TMS and control and its p-value were calculated for each day. Subjective symptom change was reported qualitatively and was used to determine “responder” or “non-responder”. A criterion for responder was set as a rating of 2 or greater. Binary logistic regression was then performed with the dependent variable ‘responder’ to examine potential measures that could predict a responder vs. non-responder.
Results
All patients tolerated the experimental procedures well without major adverse events. Two patients reported mild headache after a real rTMS intervention and one patient noticed fatigue.
Single-pulse TMS Assessment
Assessment of excitability changes from baseline revealed an increase in excitability of intracortical circuits generating the CSP (Fig 2C) while having no consistent effects on the slope or AUC (Fig. 2D), reflective of general corticospinal excitability.
Figure 2.
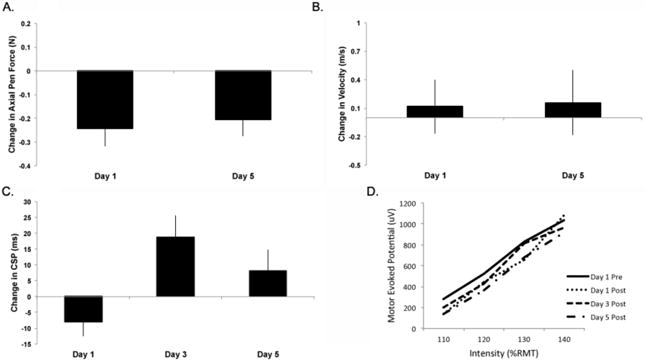
Outcome measures from group receiving real stimulation [mean (SE), n=12]. A. Mean change axial pen force from baseline (SE) was reduced, Day 1: -0.25 (0.08) N, p=0.043 that was maintained until Day 5: -0.21 (0.07) N, p=0.018. B. Change in velocity from baseline with no differences found. C. Change in cortical silent period (CSP). There was a significant effect of day (p=0.029) with demonstrated lengthening of the silent period between day 1 and 3 of 19.0 (9.2) ms (p=0.0054). D. Slope of Stimulus/Response for single pulse measures at 110 – 140% resting motor threshold. Demonstrated is an apparent reduction in slope but no significant differences across day.
Compared to baseline, mean CSP duration increased by 19ms (18% change) after three days of real rTMS. While there was still a numerical increase in CSP duration, this increase was not statistically significant on day 5 (7.5% change). In the comparison between groups, there was a significant effect of group (F=6.01, p=0.02). Type 3 tests within the Real group revealed a significant effect of Day (t=3.29, df=41, p=0.029), with a pairwise comparison difference between Day 1 and 3 (t=-2.94, df=41, p=0.0054). There was no change in the sham group. CSP baseline values, change scores and the mean percent change from baseline is reported in Table 2.
Table 2. Cortical silent period mean values.
| Baseline (ms) | CSP (ms) | Day 1 ΔCSP (ms) | % change | CSP (ms) | Day 3 ΔCSP (ms) | % change | CSP (ms) | Day 5 ΔCSP (ms) | % change | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sham | 117.34 (17.7) | 115.29 (18.4) | -2.06 (10.1) | -1.75 | 120.61 (12.0) | 3.27 (6.8) | 2.79 | 118.95 (20.2) | 1.61 (15.7) | 1.4 |
| Real | 106.30 (7.5) | 98.45 (9.7) | -7.85 (4.5) | -7.34 | 117.28 (6.4) | 18.77 (7.0) | 17.66 | 106.24 (7.1) | 8 (6.9) | 7.5 |
Multivariate linear mixed effects regression models did not demonstrate a significant effect of group. Within the individual groups there was no effect of visit on the slope (F=0.304, df=3, p=0.82) or AUC (F=0.38, df=3, p=0.77). This suggests that the intervention did not induce consistent shifts in the gain function of corticospinal excitability, that is, the relative increase in MEP amplitude with increasing stimulus intensity (Fig 2D).
Handwriting analysis
There was a significant effect of baseline axial pen force (t=-2.45, df = 12, p=0.031) and a group x day interaction on day 5 (t=-2.09, df= 12, p=0.047). When each group was evaluated separately, there was no change across sessions in the sham-rTMS group or in the real-rTMS group for writing velocity (Figure 2B). However, a significant reduction in axial pen force was found across sessions following real rTMS. Type 3 test revealed a significant effect of day (F=3.29, p=0.029) and differences in least squares means demonstrated a reduction after the first real-rTMS intervention (-0.24N, t= 2.32, p=0.043) that was maintained at the conclusion of the interventional period (-0.21N, t=-2.54, p=0.018)(Figure 2A).
Effects of sham rTMS
Data are reported for the 5 subjects who received sham stimulation for qualitative comparison across day (Fig 3). No subjects reported being aware the intervention was sham. In a qualitative assessment of AUC, there is a distinct increase observable at posttest day 1 and 3.
Figure 3.
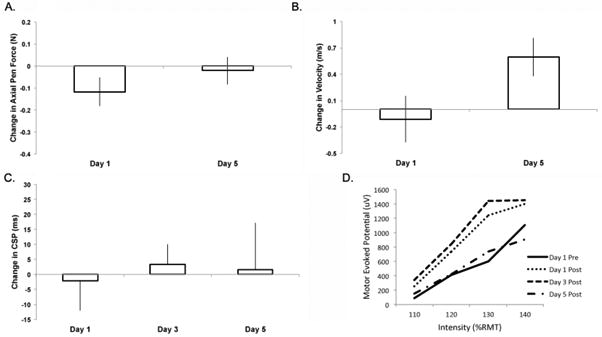
Outcome measures from group receiving sham stimulation [mean (SE), n=5]. A. Change in axial pen force from baseline. B. Change in velocity from baseline. C. Change in cortical silent period (CSP). D. Slope of Stimulus/Response for single pulse measures at 110 – 140% resting motor threshold. There were no significant differences in the sham group.
Subjective report
Subjects were considered a ‘responder’ if they reported a change ≥2. Some subjects in the sham group reported changes between 0-1, but no subjects were considered ‘responders’ during the sham intervention phase. In the real rTMS group, a total of 58% of subjects were responders for at least 1 session. At Day 1 and 3 there were 4 responders, Day 5 there were 8 and at Day 15, 7 responders. Aside from the planned assessments, 3 subjects contacted investigators to inquire if they may participate again, as they reported meaningful symptom improvements that lasted for several months.
Regression analysis
Binary logistic regression of ‘responder’ (n=8) with the following covariates was assessed in two ways. First, an analysis of response with only age and duration of symptoms was run to determine if demographic information could predict response. Second, day 1 change was added to the prediction model. In a stepwise method: age, symptom duration, day 1 change in CSP, baseline CSP and day 1 change in axial pen force, and baseline AUC, were analyzed. For the purposes of the regression analysis, a subject was classified a ‘responder’ if they met the set criterion at day 3 or 5. For the first analysis, a significant model was found with age and symptom duration as covariates (Chi-square 4.12, p=0.048, Cox & Snell pseudo R2=0.300). This model predicted the correct response 82.4% of time. This suggests that increasing age and symptom duration negatively predicts responder. Interestingly, the model was also significant with only age as a predictor (Chi-square 4.12, p=0.042). In the second regression assessment the additional measured variables were included stepwise into the model. When additional covariates were added, the model failed to reach significance. Additionally, when any dependent measure or combination of measures was used without age, no significant prediction occurred.
Discussion
The use of rTMS as a potential therapeutic agent in dystonia is attractive because rTMS is non-invasive and can reinforce intracortical inhibition known to be dysfunctional in FHD (Siebner et al. 2003; Huang et al. 2004). The majority of studies to date have investigated a single application of stimulation to assess potential effects. Adopting a multi-session approach, this preliminary investigation failed to support our hypothesis that a five-day course of 1Hz rTMS of the dPMC contralateral to the dystonic hand during non-symptom inducing writing would produce physiological or clinically meaningful changes, however, noteworthy effects were observed. The repeated sessions of rTMS (1) strengthened intracortical inhibition, albeit transiently, as indicated by a longer duration of the CSP peaking after 3 days of stimulation and (2) induced a significant change in handwriting. Patients exerted less pen force during a standardized writing task without changing writing velocity, which was not seen in the sham group. This change corresponded to over half the individuals reporting clinical symptom improvement. Finally, logistic regression identified the age of the patient as the strongest predictor of clinical response to the rTMS intervention.
In the present study, the prolongation in CSP peaked after 3 days of stimulation and became less consistent after completion of the intervention. This speaks against the notion that the reinforcing effects of repeated low-frequency rTMS on intracortical inhibition simply accumulate over time with the number of applications, at least when delivered daily. Although our protocol strengthened intracortical inhibition at the cortical level, this reinforcement did not correspond to the behavioral change. Specifically, at post intervention assessment on day 1, there was a decrease in handwriting pressure and a decrease in CSP indicating decreased intracortical inhibition; and at day 5, there was significant handwriting pressure reduction, but not a significant CSP change. As mentioned previously, handwriting was not assessed at Day 3, so conclusions about this increase can't be directly drawn. It is interesting to note the apparent increase in sham AUC that is not observed in the real group. Though not assessed statistically, this could suggest that the rTMS prevented the increase observed in the sham. This is an area for future investigation. Overall, there appears to be dissociation between these measures which suggests that the link between the level of intracortical inhibition as measured by a single TMS paradigm and the severity of clinical symptoms might not be strong. Thus, the change in CSP duration may not be useful in predicting the clinical efficacy of an interventional rTMS protocol. Change in handwriting pressure has been reported by others following rTMS (Siebner et al. 1999a) and other therapeutic interventions (Zeuner et al. 2008). Whether the increased pressure in subjects with dystonia is measuring the dystonic impairment of handwriting or a compensatory strategy to stabilize the tip of the pen on the tablet during handwriting remains unclear (Zeuner et al. 2007). However, our results do suggests that the axial writing pressure represents a measure that is sensitive to changes in handwriting induced by interventional protocols in FHD.
The novel feature of the present study was to apply rTMS to left dPMC for 5 consecutive days during a non-dystonic motor task. Patients were asked to produce nonsense writing movements while holding a pen in their dystonic hand, but to avoid any symptoms. The rationale behind applying rTMS during an active, non-dystonic state was to more specifically target the motor circuits that produce the dystonic movements (Siebner et al. 1999a). It is possible that our hypothesis was incorrect, and this in fact reduced responsiveness. Indeed recent work has demonstrated that motor activity prior or during theta-burst stimulation to M1 can modify the stimulation-induced effects on corticosponal excitability (Gentner et al. 2008; Huang et al. 2008). It is also possible that since the active task required a certain degree of sensory awareness training to ensure lack of dystonic cramping, this could have influenced the effects of the rTMS alone. Further, although we visually monitored for lack of dystonic cramping, it is possible that low level of dystonic cramping did occur during the task. Another study used dual-site TMS to test state-dependent modulation of premotor-to-motor inhibition in FHD. Patients with FHD, but not healthy controls, expressed premotor-to-motor inhibition at rest while this form of inhibition was absent during movement in both groups (Beck et al. 2009). Therefore, it might be reasonable to first reinstate normal premotor-to-motor inhibition at rest in FHD before trying to influence motor circuits in an active motor state. One might also argue for the opposite strategy by applying rTMS in the presence of dystonia. During the presence of dystonia, deficient inhibition is most obvious and thus, the inhibitory effects might more specifically reinforce inhibition in those circuits subserving dystonia.
In this preliminary study, there were subjects with different types of hand dystonia and large range of symptom duration. An ideal study would have sufficient n to be able to stratify subjects within various groups. Another strategy shown to be effective for determining effects in studies with small n is the single subject design analysis (Kimberley and Di Fabio 2010). These studies would help evaluate subpopulations of FHD that are most likely to benefit from rTMS intervention.
It is also important to note, that although a robust effect was not observed in our measures, eight subjects reported “mild-moderate improvement” during the intervention week and seven subjects reported persisting improvement for at least ten days. No improvements in symptom rating were observed for subjects in the sham group at follow-up.
Interestingly, age was the strongest factor assessed that was associated with predicting responders to the intervention. Age was negatively associated with responder to the rTMS intervention paradigm, such that the older the subject: the less likely to be a responder. The strength of the model increased when adding symptom duration and change in CSP from baseline. The reason for age of subject to be a significant predictor of response is beyond the scope of this investigation but may be due to issues of decreasing neuroplasticity with age (Hutchinson et al. 2002).
In conclusion, though the primary goal of the investigation to produce a robust effect in FHD was not achieved, results do demonstrate short-term changes in behavioral, physiologic and clinical measures that support further inquiry into the therapeutic potential of rTMS for individuals with FHD. Future work should explore various parameters of stimulation delivery when investigating the relationship between rTMS, cortical neurophysiology and behavior in FHD and employ other measures of intracortical excitability (e.g. paired pulse assessment of intracortical excitability cortico-cortico excitability with dual-site TMS (Groppa et al. 2012b). Lastly, the salient patient characteristics associated with positive response to rTMS merits further inquiry.
Acknowledgments
Funding of work: This work is supported, in part, by M01-RR00400 National Center for Research Resources, National Institutes of Health and by the Dystonia Medical Research Foundation.
Footnotes
Financial Disclosure/conflict of interest: No authors have any conflict of interest.
References
- Beck S, Houdayer E, Richardson SP, Hallett M. The role of inhibition from the left dorsal premotor cortex in right-sided focal hand dystonia. Brain Stimul. 2009;2:208–214. doi: 10.1016/j.brs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Borich MR, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restor Neurol Neuros. 2009;27:55–65. doi: 10.3233/RNN-2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Can J Neurol Sci. 1999;26:163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clin Neurophysiol. 2003;114:938–944. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Talelli P, Rothwell JC, Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7:827–840. doi: 10.1016/S1474-4422(08)70190-X. [DOI] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Fujiyama H, Garry MI, Levin O, Swinnen SP, Summers JJ. Age-related differences in inhibitory processes during interlimb coordination. Brain Res. 2009;1262:38–47. doi: 10.1016/j.brainres.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Sole J, Siebner HR. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Munchau A, Deuschl G, Ruschworth MFS, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage. 2012b;62:500–509. doi: 10.1016/j.neuroimage.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC, Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Mov Disord. 2004;19:54–59. doi: 10.1002/mds.10627. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Koboyashi M, Horkan M, Pascual-Leone A, Alexander MP, Schlaug G. Age-related differences in movement representation. Neuroimage. 2002;17:1720–1728. doi: 10.1006/nimg.2002.1309. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer's cramp. Neurology. 1999;53:96–105. doi: 10.1212/wnl.53.1.96. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Kimberley TJ, Borich MR, Prochaska KD, Mundfrom SL, Perkins AE, Poepping JM. Establishing the definition and inter-rater reliability of cortical silent period calculation in subjects with focal hand dystonia and healthy controls. Neurosci Lett. 2009;464:84–87. doi: 10.1016/j.neulet.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley TJ, Di Fabio RP. Visualizing the Effects of rTMS in a Patient Sample: Small N vs. Group Level Analysis. PLoS ONE. 2010;5:e15155. doi: 10.1371/journal.pone.0015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King NK, Kuppuswamy A, Strutton PH, Davey NJ. Estimation of cortical silent period following transcranial magnetic stimulation using a computerised cumulative sum method. J Neurosci Meth. 2006;150:96–104. doi: 10.1016/j.jneumeth.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiat. 2001;49:460–463. doi: 10.1016/s0006-3223(00)01110-0. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki T, Sakata-Igasaki M, Mima T, Ikeda A, Shibasaki H. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005;128:104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W, Pauri F. Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. EEG Cl N Su. 1999;52:171–185. [PubMed] [Google Scholar]
- Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Sheehy MP, Marsden CD. Writers' cramp-a focal dystonia. Brain. 1982;105:461–480. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- Siebner H, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999a;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Auer C, Ceballos-Baumann A, Conrad B. Has repetitive transcranial magnetic stimulation of the primary motor hand area a therapeutic application in writer's cramp? EEG Cl N Su. 1999b;51:265–275. [PubMed] [Google Scholar]
- Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, Frackowiak RS, Bhatia KP. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain. 2003;126:2710–2725. doi: 10.1093/brain/awg282. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell JC. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Reinforcement of intracortical inhibitory circuitry by low-frequency repetitive transcranial magnetic stimulation in patients with writer's cramp. Neurology. 1999c;52:529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Mentschel C, Auer C, Lehner C, Conrad B. Repetitive transcranial magnetic stimulation causes a short-term increase in the duration of the cortical silent period in patients with Parkinson's disease. Neurosci Lett. 2000;284:147–150. doi: 10.1016/s0304-3940(00)00990-3. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. Eur J Neurosci. 2007a;26:523–528. doi: 10.1111/j.1460-9568.2007.05682.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur J Neurosci. 2007b;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Teo JT, Schneider SA, Cheeran BJ, Fernandez-del-Olmo M, Giunti P, Rothwell JC, Bhatia KP. Prolonged cortical silent period but normal sensorimotor plasticity in spinocerebellar ataxia 6. Mov Disord. 2008;23:378–385. doi: 10.1002/mds.21847. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner KE, Peller M, Knutzen A, Hallett M, Deuschl G, Siebner HR. Motor re-training does not need to be task specific to improve writer's cramp. Mov Disord. 2008;23:2319–2327. doi: 10.1002/mds.22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner KE, Peller M, Knutzen A, Holler I, Munchau A, Hallett M, Deuschl G, Siebner HR. How to assess motor impairment in writer's cramp. Mov Disord. 2007;22:1102–1109. doi: 10.1002/mds.21294. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]