Abstract
Objective
Examine for individual factors that may predict response to inhibitory repetitive transcranial magnetic stimulation (rTMS) in focal hand dystonia (FHD); present method for determining the optimal stimulation to increase inhibition in a given patient; and examine individual responses to prolonged intervention.
Design
A single-subject design to determine optimal parameters to increase inhibition for a given subject and to employ the selected parameters 1/wk for 6 weeks, with 1 wk follow up, to determine response.
Setting
Clinical research laboratory
Participants
A volunteer sample of 2 subjects with FHD. One participant had TMS responses indicating impaired inhibition, the other had responses within normal limits.
Interventions
1200 pulses of 1 Hz rTMS delivered using 4 different stimulation site/intensity combinations: primary motor cortex (M1) at 90% or 110% resting motor threshold (RMT); dorsal premotor cortex (PMd) at 90% or 110% of RMT. The parameters producing the greatest within-session increase in cortical silent period (CSP) duration were then used as intervention.
Main outcome measures
Response variables included handwriting pressure and velocity, subjective symptom rating, CSP, and short-latency intracortical inhibition and facilitation.
Results
The individual with baseline TMS responses indicating impaired inhibition responded favorably to the repeated intervention, with reduced handwriting force, increase in CSP and subjective report of “moderate” symptom improvement at 1-wk follow-up. The individual with normal baseline responses failed to respond to the intervention. In both subjects, 90% RMT to PMd produced greatest lengthening of CSP and was used as intervention.
Conclusions
An individualized understanding of neurophysiologic measures may be indicators of responsiveness to inhibitory rTMS in focal dystonia, with further work needed to determine 3 likely responders vs. non-responders.
Keywords: focal hand dystonia, rehabilitation, rTMS, writer's cramp, neuromodulation, dystonia
Focal hand dystonia (FHD) is an enigmatic disorder typically characterized by muscle spasms, involuntary movements and abnormal posturing. The pathophysiology is multifactorial with both genetic and environment contributions.1 Symptoms often arise after repeated activity of the hand during an occupation or hobby. There are a number of subtypes of FHD that differ in terms of symptom type and severity, provoking task, and underlying neural pathophysiology. A defining characteristic of FHD appears to be a loss of inhibition at multiple levels of the central nervous system.2 Although there are commonalities between subtypes of FHD, symptoms and neural pathophysiology are individual and task-specific. Critically, curative treatments for FHD do not currently exist and most group examinations of treatments have not demonstrated meaningful benefit. There is, however, evidence of benefit from various interventions when response is examined on an individual basis.3, 4 In this highly variable disorder, prudent examination of baseline characteristics and individual responses to an intervention may elucidate critical determinants of responders vs. non-responders.
Transcranial magnetic stimulation (TMS) is a non-invasive method to activate cortical tissue to quantify neural excitability and, when applied repetitively (rTMS), transiently up-regulate5 or down-regulate cortical activation.6 Considering the decreased cortical inhibition found in FHD, rTMS provides a non-invasive tool to address this issue. Previously, low-frequency rTMS applied to the dorsal premotor cortex (PMd) and also the primary motor cortex (M1) have been shown to transiently increase inhibitory cortical activity while reducing altered kinematics and subjective symptoms in FHD after a single application.7, 8 These effects were short-lived and did not produce lasting changes in brain behavior or symptom improvement, but provided important foundation for further investigation. A trial of repeated applications of low-frequency (1 Hz) rTMS applied to the PMd over five consecutive days led to increases in intracortical inhibition and improved handwriting kinematics that persisted for at least ten days following stimulation.9 Although these findings were encouraging, the symptom improvement was modest and some participants did not demonstrate any response to the intervention. Most recently, a similar investigation that delivered inhibitory rTMS while participants actively performed a hand motion task produced ‘moderate improvement’ in 68% of patients with FHD.10 This variable response to rTMS is evident in other disorders11 as well as in healthy subjects.12, 13 The sources of this variability have not been characterized comprehensively; however, in FHD it is likely that a variety of issues could affect response. These include type of FHD, age, duration of symptoms, genetic factors, and baseline characteristics. The high variability of response limits the therapeutic usefulness of these techniques at present which may be preventing potential benefit to some individuals who would improve from the intervention.
Another consideration when comparing interventional rTMS approaches is the parameters of stimulation. In addition to the frequency of TMS pulse delivery; location and intensity of stimulation can also be adjusted, which may alter the response to rTMS. In FHD, rTMS has been applied to different motor cortical regions with robust responses noted from PMd stimulation7, 9 in some cases and M1 in others.8, 14 Stimulation can also be applied above (suprathreshold) or below (subthreshold) the threshold for evoking a response in the target muscle. Previous work has typically employed subthreshold rTMS to minimize potential confounding effects of stimulus spread to adjacent cortical regions and potential afferent effects from repeated activation of the target muscle and associated sensory receptors.9, 15, 16 Other work has argued that suprathreshold rTMS may produce more robust cortical and behavior effects.17 Indeed, it is possible that the ideal stimulation parameters are not ‘diagnosis-specific’, but rather ‘individual-specific’. Further, baseline characteristics may influence the response to an intervention. Thus, a method to elucidate individual responses to an intervention in a patient population is needed.18,19
It is evident that one episode of rTMS will not produce significant lasting effects on cortical excitability, motor behavior or symptoms, and it appears likely that repeated administrations of rTMS is necessary to produce lasting and meaningful effects. But, given the significant testing burden associated with long-term application of rTMS to assess efficacy, particularly in a rare disorder, it is desirable to determine optimal stimulation parameters prior to large-scale studies and elucidate factors that may predict response, so appropriate design considerations will be made with future studies. Thus, the purposes of this study were to conduct a single-subject design in two subjects with FHD to: 1. Examine individual factors that may predict response to inhibitory rTMS in FHD; 2. present a method for determining the optimal stimulation parameters to increase inhibition within a given patient; and 3. determine individual physiologic and behavioral responses to the selected parameters of stimulation over a prolonged intervention.
Methods
Subjects
One 48-year-old male and one 39-year-old female with right hand, task-specific FHD were studied. Clinical details are given in Table 1. Subjects were recruited from a patient contact list. The clinical history and physical exam of both subjects was consistent with simple writer’s cramp.20 Exclusion criteria were: (1) any neurologic condition other than FHD, (2) medication for dystonia, (3) botulinum toxin injection within the past four months, (4) seizure history, (5) pregnancy, (6) metal in head, or (7) implanted medical devices.21 All subjects gave written informed consent, according to the Declaration of Helsinki, prior to participation. The study was approved by the University of X and the Clinical Translational Science Institute and Institutional Review Board.
Table 1.
Subject demographics
| Subject | Sex | Age | Duration of sx | Clinical pattern | Toxin Hx |
|---|---|---|---|---|---|
| 1 | F | 39 | 1yr7mo | R hand abnormal posturing during writing | N |
| 2 | M | 48 | 1yr6yr | R grip, wrist extensor spasms during writing/typing |
Y, 1yr 3mo prior |
M = male, F = female, R = right, L = left, N = no, Y= yes
Phase 1 Determine greatest inhibitory response: The preliminary experiment consisted of one rTMS session with 1200 pulses at 1 Hz given to the hemisphere contralateral to the affected (dominant) hand, which was the left hemisphere for both subjects, at one of the following parameters: 90% resting motor threshold (RMT) to PMd, 110% RMT to PMd, 90% RMT to M1, or 110% RMT to M1. This protocol was repeated once/ week for each of the stimulation parameter combinations. The parameter combinations were randomly sequenced for each subject. Excitability measures were taken immediately before (baseline) and after (posttest) the rTMS for each session.
Handwriting analysis
Handwriting analysis was performed using a computerized tableta, custom modified digitized penb, and MovAlyzeR® hardware and software packagec for data collection and analysis. Subjects were instructed to write in their natural, self-paced style ‘My country tis of thee,’ with visual feedback given. This was repeated three times. Data were sampled at 215 Hz (resolution: 5,080 lpi, accuracy: ± 0.01” pressure range: 0–800 g). Mean axial pen pressure and velocity were the variables of interest.
Assessment of cortical excitability
Subjects were seated comfortably in a reclining chair. Surface electrodes were affixed to the skin overlying the first dorsal interosseus (FDI) muscle of the involved hand using a belly/tendon montage. The electromyographic (EMG) activity of the right FDI muscle was recorded at a sampling rate of 2560 Hz using a Cadwell Sierra EMG amplifierd (Cadwell Laboratory, Washington) (sensitivity: 100µv/div, filter: 20–2000Hz). TMS was performed with a 70-mm figure-of-eight coil connected to a Magstim 200 Rapid magnetic stimulatore (Magstim Co., Whitland, Dyfed, UK). The handle of the coil was directed posterolaterally 45° to the mid-sagittal line of the head producing a posterior-anterior current flow in the brain.22 To find the optimal position for activating the FDI muscle, the coil was positioned over the approximate location of maximal sensitivity for FDI muscle activation. Single-pulse monophasic magnetic stimuli were delivered over the M1 contralateral to the dystonic hand at approximately 0.1 Hz. Starting at an intensity of 55% of maximum stimulator output, the intensity level was adjusted until a motor evoked potential (MEP) was elicited. Coil position was systematically moved 1cm anterior, posterior, medial and lateral to the presupposed hotspot until TMS evoked a maximal MEP. This location was marked on the scalp with a pen and the coil was kept in this position for single pulse TMS measurements of the M1. We first determined the RMT, defined as the minimum intensity required to elicit MEP amplitude >50 µV peak-to-peak in at least 3 of 5 trials in the resting target muscle.23 The RMT was then used to individually adjust the stimulus intensity for subsequent assessment of the short intracortical inhibition (SICI) and short intracortical facilitation (ICF) and CSP.
Cortical Silent Period
Single TMS pulses were applied at an intensity of 120% RMT during an isometric contraction of the contralateral FDI muscle. During isometric contraction, the MEP is followed by a period of electromyographic silence. This period of EMG quiescence is known as the CSP and is delineated by the first superimposed TMS-evoked EMG spike (CSP onset) and the return of activity to 50% of prestimulus EMG signal (CSP offset).24 The duration of CSP is used as a measure of intracortical excitability and is thought to be related to GABAergic synapse-mediated inhibition in the stimulated cortical region,25, 26 with a longer CSP duration indicating increased inhibition.
For CSP recording, subjects performed an isometric abduction contraction of the index finger against a strain gauge coupled to a load cell. Force was transduced into an electrical signal displayed on an oscilloscope placed in front of the subject. Subjects were asked to produce a constant force at approximately 20% of maximum voluntary isometric contraction indicated on a laptop screen, until instructed to relax. A single TMS pulse was applied 2–3 s after contraction initiation and subjects were instructed to relax 2–3 s after stimulation. Eight trials were recorded with a minimum 20s rest interval between each trial. The duration of the CSP was measured on a trial-by-trial basis and mean CSP duration was calculated for each block of measurements and defined as the duration between TMS stimulation artifact and a return to 50% of prestimulus EMG activation. Measures of CSP have been shown to be reliable in repeated measures studies to determine an effect of intervention within a group of subjects.9, 22 CSP was the primary outcome measure used to determine maximal effectiveness of the Phase 1 parameter assessment. This measurement was chosen due to previous work indicating CSP to be an abnormal characteristic in FHD, modifiable with rTMS.24
Paired Pulse Assessments
Paired-pulse MEPs were collected to assess SICI and ICF, which are thought to mediated by GABAergic and glutamatergic pathways respectively.27 A subthreshold (90% RMT) conditioning pulse was delivered at a short (3ms) or long (10ms) interstimulus interval prior to 1mV test pulse delivery.27, 28 Paired TMS stimuli were delivered with two Magstim 200 devicese connected through a Magstim BiStim2 Modulee through a standard 70-mm coil over the previously identified location of FDI activation while the hand was at rest. Eight trials were collected for each ISI alternating between ISIs every two trials to minimize priming effects. Peak-to-peak amplitudes were identified offline for each MEP collected. Data were then indexed to the mean single pulse (SP) MEP amplitude (n=8 trials) for the same assessment session (PP MEP/SP MEPX̄).29
Subjective Symptom and Safety Assessment
Subjects rated their perceived symptom change using a 7 point Likert scale (−3=significant worsening, 0=no change, 3=significant improvement).8, 9 Scores were collected each week. To monitor for unintended stimulation effects, an adverse events questionnaire was administered prior to and after each session, as well as at follow-up.
rTMS intervention
In each rTMS session, subjects received 1200 biphasic stimuli applied to the PMd in the hemisphere contralateral to the dystonic hand. Low-frequency rTMS was administered continuously at 1 Hz using a figure-of-eight coile in the same orientation as used for cortical excitability measurements (Magstim Rapid2, Magstim Co., Whitland, Dyfed, UK). The stimulation site for PMd was defined as 2 cm anterior and 1 cm medial to the previously defined hotspot for FDI activation. This site was chosen based on localization from a previous positron emission tomography study30, 31 and previous FHD intervention studies.7, 9, 10 During the intervention, subjects were seated in a reclining chair with feet elevated. The stimulation was delivered while patients were awake but at rest.
Design
In Phase I, an ABA design was used separately for each of the four interventions with measurements (A) occurring before and after each intervention (B). Changes in mean CSP were calculated for each intervention (posttest – baseline). The combination with the greatest positive change (i.e., lengthening in CSP indicating increased inhibition) was used for the rTMS intervention in Phase II.
Phase II, Determine response to repeated intervention: This phase began 1 month after the completion of the Phase I. The protocol consisted of a similar design as Phase I with intervention repeated 1×/week for 6 weeks with 1-week follow up, but the intervention used was only the optimal stimulation parameter combination determined in Phase I and testing only occurred at the beginning of each visit. That is, the design was: AB AB AB AB AB AB A, where A is comprehensive behavioral and excitability testing, B is the optimal stimulation parameter established in Phase I (but now, applied weekly for 6 weeks). The final testing session was a 1-week follow up after 6 weeks of intervention.
Statistical Analysis
To determine effectiveness of the intervention in Phase II, within each participant, single-subject analysis included visual inspection of graphed data to evaluate changes across time.32 A 95% confidence interval (CI) was determined for all continuous variables at baseline (week 0). Measures were graphed at each week and responses outside of the CI were considered significant.19
Baseline characteristics
For the purposes of qualitative assessment of individual characteristics, we used previous literature to determine responses that were within normal limits and which responses in each individual were outside that range. A 99% CI for CSP was calculated based upon an average of reported values in healthy participants24, 33–37 Any SICI value <1 suggests a lack of inhibitory response and was considered abnormal and any ICF response <1 would indicate a lack of facilitatory response (for review, see38). These values are listed in Table 2.
Table 1.
Baseline Characteristics
| Measure | Healthy (99% CI) | Subject 1 | Subject 2 |
|---|---|---|---|
| CSP (ms) | 132 (110 to 154) | 37±2.9† | 114±12.9 |
| SICI | <1 | 1.3±1.4† | 0.16±0.7 |
| ICF | >1 | 1.6±1.6 | 1.1±2.4 |
=different from normal range, CSP=cortical silent period, SICI=short intracortical inhibition, ICF=intracortical facilitation, CI=confidence interval
Results
Phase I, Determine greatest inhibitory response
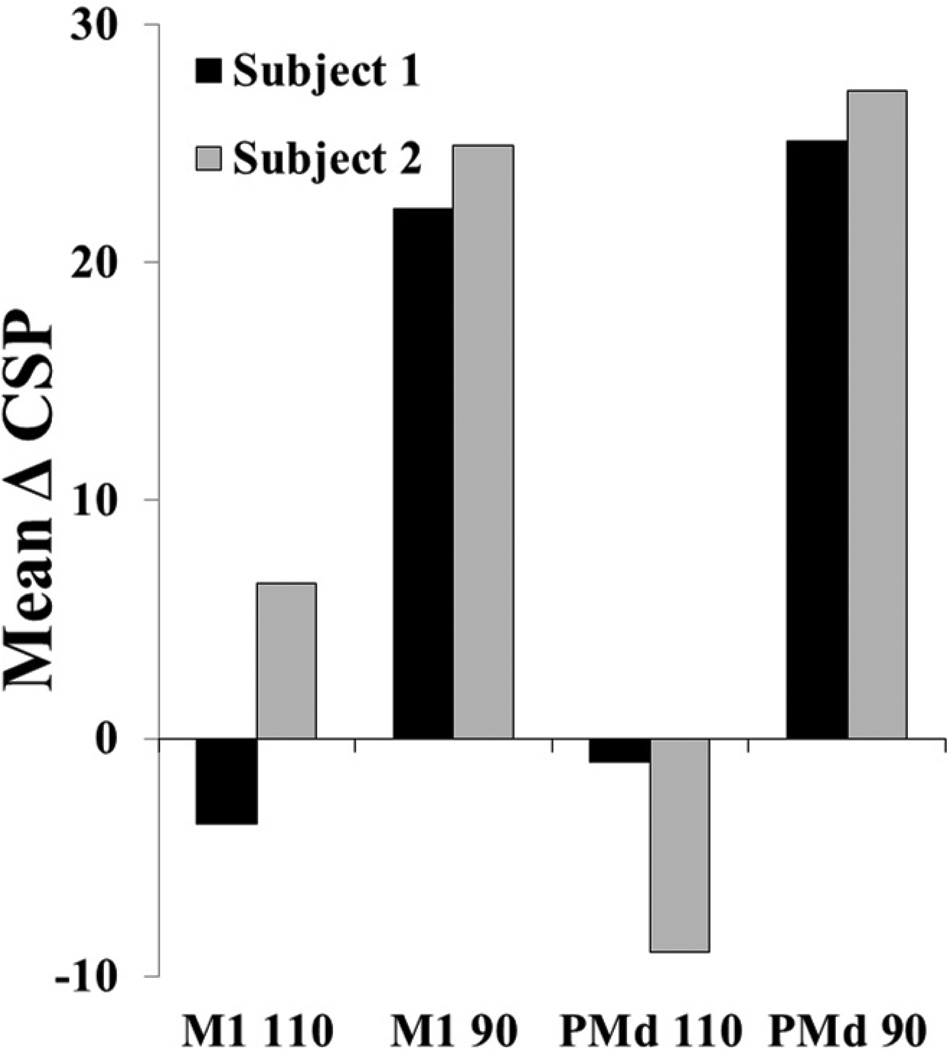
Both subjects demonstrated the greatest increase in CSP duration in response to rTMS applied over PMd 90% (Fig 1). These parameters were then used in Phase II.
Figure 1.
Phase I mean cortical silent period (CSP) change score within session. M1: primary motor cortex, dorsal premotor cortex (PMd), 90% or 110% of resting motor threshold. The largest mean change for both participants was 90% PMd.
Phase II, Determine response to repeated intervention
In this single subject design case series with 2 participants, one subject demonstrated significant beneficial changes and one subject had no meaningful improvements. In a qualitative assessment of baseline characteristics that may have influenced response, it is notable that the participant that responded to the intervention (Subject 1) had CSP and SICI responses greatly outside the range of normal. The participant that did not respond (Subject 2) had responses that were within normal limits.
Handwriting
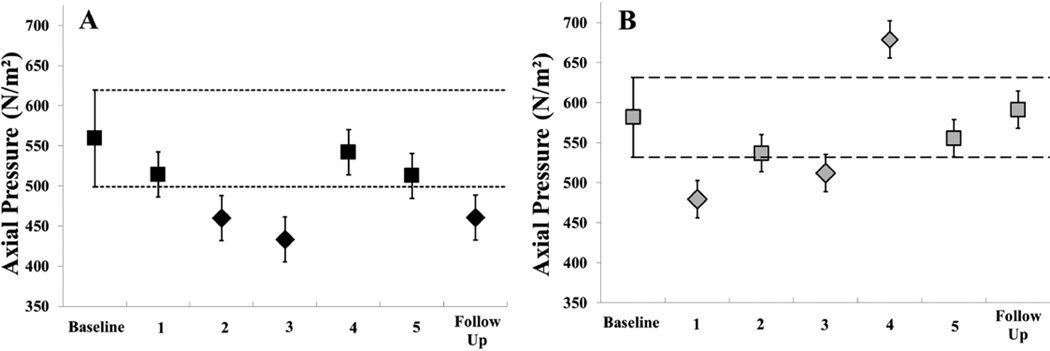
Handwriting assessments for Subject 1 demonstrated a decrease over time with significant reduction in handwriting pressure at week 2, 3, and at follow-up (Fig 2A). Despite changes in pressure, velocity (Table 3) remained stable with a significant increase on week 3, corresponding to the greatest reduction in pressure. Subject 2 demonstrated variable responses in handwriting assessments with two measurements of significant decrease (week 1 and 2) and one measurement of increase (week 4) (Fig 2 B). Velocity measure remained stable throughout (Table 3).
Figure 2.
Phase II handwriting results. Mean axial pen pressure at baseline, each week of the intervention, and at 1 week follow up. 95% confidence interval established at baseline and represented by the short dashed line for (A) Subject 1 (black) and long dashed line for (B) Subject 2 (gray). Square markers indicate non-significant and diamond markers indicate significant difference from baseline.
Table 2.
Handwriting velocity (Mean ± SD) (m/s)
| Subject | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Follow up |
|---|---|---|---|---|---|---|---|
| 1 | 1.28 ± 0.24 |
1.22 ± 0.10 |
1.08 ± 0.06 |
1.16 ± 0.10 |
0.82 ± 0.14 |
1.06 ± 0.10 |
1.27 ± 0.24 |
| 2 | 4.48 ± 1.35 |
5.49 ± 0.60 |
6.31 ± 0.89 |
5.72 ± 0.20 |
5.41 ± 0.26 |
6.50 ± 0.36 |
5.75 ± 0.62 |
Subjective Report
Subjective report of symptom change did not directly correspond to the handwriting changes but generally reflected meaningful improvement by Subject 1 and no change or worsening by Subject 2 (Table 4).
Table 3.
Subjective report of symptom change
| Subject | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Follow up |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | +1.5 | +1.5 | +1.5 | +2 |
| 2 | 0 | 1 | 0 | −0.5 | −1 | −0.5 |
0=no change, +1 = mild improvement, +2 = moderate improvement, +3 = much improvement, −1= mild worsening, −2= moderate worsening, −3 much worsening
Cortical Excitability
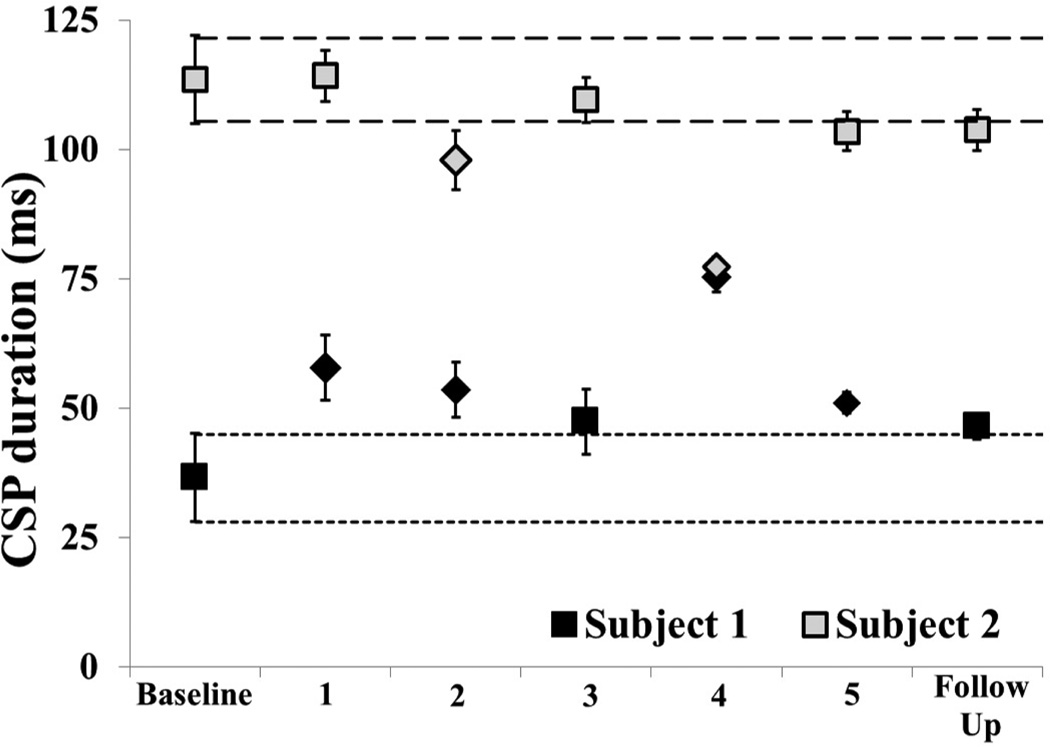
Cortical excitability measures reflected a similar pattern of change, with Subject 1 displaying significant reductions in excitability measures and Subject 2 showing no change or an increase in excitability. CSP measurements are displayed on the same graph to demonstrate the difference in baseline measurements. Subject 1 displayed an increase in CSP (indicating lengthening or increased inhibitory activity) at every time point (Fig 3). Subject 2 displayed no change or a decrease in CSP (indicating reduction in inhibition) at week 2 and 4 (Fig 3).
Figure 3.
Phase II Cortical silent period (CSP) length. 95% confidence interval established at baseline and represented by the short dashed line for Subject 1 (black) and long dashed line for Subject 2 (gray). Square markers indicate non-significant and diamond markers indicate significant difference from baseline.
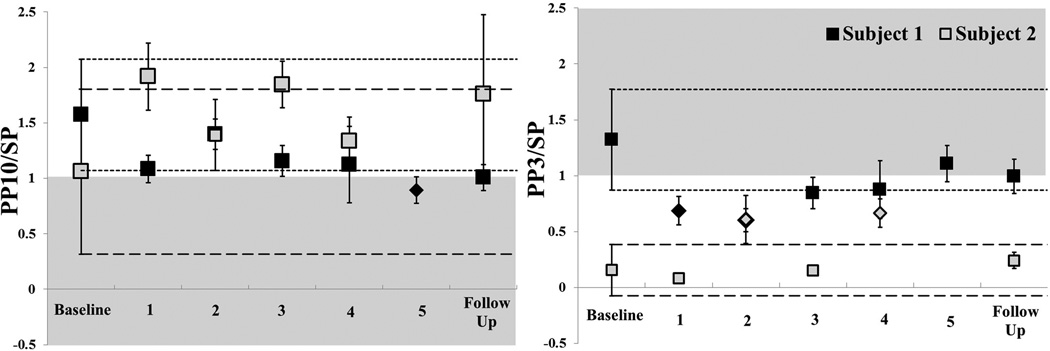
Paired pulse assessments indicate that Subject 1 did not display a SICI response at baseline, given the mean index was >1. At the beginning of the intervention, however, SICI was observed (ρ) and maintained until week 4. Subject 2 displayed a robust SICI response at baseline that was reduced across the intervention time period, but remained <1 (Fig 3). Both subjects demonstrated ICF at baseline. Subject 1 had a baseline measure of approximately 1 that increased inconsistently (Fig 4). Subject 2 demonstrated highly variable ICF at baseline measure that was non-significantly reduced and less variable across sessions (Fig 4).
Figure 4.
Phase II Paired Pulse Assessment. 95% confidence interval established at baseline and represented by the short dashed line for Subject 1 (black) and long dashed line for Subject 2 (gray). Square markers indicate non-significant and diamond markers indicate significant difference from baseline. The shaded area represents abnormal response for each test. A: Short intracortical facilitation (SICF) and B: Short intracortical inhibition (SICI). Subject 1’s responses (black) lengthened (normalized) but did not achieve significance. Subject 2’s responses (grey) shortened.
Discussion
The variable nature of response to non-invasive neuromodulation suggests that one interventional paradigm is not likely to be effective for all individuals.39 In clinical interventions involving patient populations, single-subject analyses allow individual exploration of data while maintaining a scientific assessment of change. Averaged responses and group statistics can fail to demonstrate very different responses to intervention.18 In this exploratory assessment of responder vs. non-responder in 2 participants, visual inspection points to pretest excitability as a potential factor in predicting response. Subject 2 had a mean CSP at baseline within the range of age-matched healthy individuals24 while Subject 1 showed an extremely short CSP suggesting dysfunctional GABAb-mediated cortical excitability40 and reduced SICI, suggesting abnormal GABAa-ergic activity.41 Mean SICF was not abnormal but was highly variable. The relationship between SICI and CSP for Subject 1 suggests excitability is generally being influenced by the intervention, with both measures demonstrating an increased inhibition. In Subject 2, there is not a clear relationship in the measures. It has been reported that people with focal dystonia have a higher variability in measures that healthy people24, and at pretest, Subject 1 did have more variability than Subject 2, which could be another potential indicator. Other work in focal hand dystonia has investigated responders and non-responders in rTMS and found age to be the most significant predictor of positive response.10 In this case, Subject 1 was 9 years younger which could also have influenced the outcome. These results suggest that future work may need to consider baseline neurophysiologic responses as factors to help determine potential biomarkers for responders to an intervention. Studies using electroencephalography and neuroimaging connectivity analysis can also be employed to determine patient-specific needs and assess individual responses to intervention. Genetic influences in response to modulation cannot be overlooked and may also be an important component to determine likely responders to a given intervention paradigm.42
This work also presented a viable model for determining individual response to intervention prior to beginning an extended intervention period. In both participants, 90% RMT to PMCd was the most effective modulator of CSP length. This result is similar to other work that also compared location of stimulation and found PMC to be the most effective location for beneficial effect in FHD.7 But, given that both participants did not benefit, further work is needed to determine responders to the intervention. Finally, these results indicate that rTMS delivered 1× per week for 6 weeks can be safely delivered and may result in improved subjective and objective measures in some patients. Given the nature of the single-subject case series, results cannot be generalized to the larger population with writer’s cramp, but do lend evidence that continued investigation is warranted. These results also highlight the importance of single-subject assessment for individual analysis and the importance of future work to determine markers for response to non-invasive neuromodulation.
Limitations
Single-subject designs allow for individual examination of response, but inherent in the design is the limitation that it does not allow for generalization to the population and must be considered in the context of the individual participants. In this experiment, only two brain locations were tested, but additional areas may be worthy of investigation. The location for the rTMS intervention was based on the change of CSP duration following single session rTMS. Although previous work has demonstrated impaired CSP in FHD, thus it is logical to attempt to modulate this measure, it may not be the key factor associated with improved function. The process of tailoring the location and intensity of stimulation may need to include re-testing to confirm an optimal response. Finally, the observation that severely impaired neurophysiologic responses were associated with a positive result whereas, the opposite result occurred in the patient with normal values, is conspicuous. Although no definitive conclusions can be derived from two subjects, the contrasting responses shown here are stark and implore careful consideration of baseline inhibition relative to normal in future trials.
Conclusion
An individualized understanding of neurophysiologic measures may be indicators of responsiveness to inhibitory rTMS in focal dystonia, with further work needed to determine likely responders vs. non-responders.
Acknowledgments
Funding of work: This work is supported, in part, by M01-RR00400 National Center for Research Resources, National Institutes of Health and by the Dystonia Medical Research Foundation.
ABBREVIATIONS
- CI
confidence interval
- CSP
cortical silent period
- PMd
dorsal premotor cortex
- EMG
electromyographic
- FDI
first dorsal interosseus
- FHD
focal hand dystonia
- ICF
intracortical facilitation
- MEP
motor evoked potential
- M1
primary motor cortex
- RMT
resting motor threshold
- rTMS
repetitive transcranial magnetic stimulation
- SICI
short-latency intracortical inhibition
- SP
single pulse
- TMS
transcranial magnetic stimulation
Footnotes
Financial Disclosure/conflict of interest: No authors have any conflict of interest.
SUPPLIERS
WACOM Co., Ltd, 2-510-1 Toyonodai, Kazo-shi, Saitama, 349-1148, Japan
Kiko Software, Hof van Nancy 1, 7007KH Doetinchem, Netherlands
Neuroscript LLC MovAlyzeR®, 435 E Carson Dr, Tempe, AZ, USA
Cadwell Laboratories, Inc., 909 N. Kellogg Street, Kennewick, WA 99336 USA
MagStim Co., Whitland, Dyfed, UK, 70-mm figure-of-eight coil, 200 Rapid magnetic stimulator, Magstim BiStim2 Module
References
- 1.Hallett M. Pathophysiology of writer's cramp. Human Movement Science. 2006;25(4–5):454–463. doi: 10.1016/j.humov.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 3.Zetterberg L, Halvorsen K, Färnstrand C, Aquilonius S-M, Lindmark B. Physiotherapy in cervical dystonia: six experimental single-case studies. Physiotherapy theory and practice. 2008;24(4):275–290. doi: 10.1080/09593980701884816. [DOI] [PubMed] [Google Scholar]
- 4.Byl NN, Nagajaran S, McKenzie AL, Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in patients with focal hand dystonia: a case series. Archives of Physical Medicine & Rehabilitation. 2003;84(10):1505–1514. doi: 10.1016/s0003-9993(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 7.Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005;128(Pt 1):104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- 8.Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999;52(3):529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 9.Borich MR, Arora S, Kimberley TJ. Lasting effects of repeated rTMS application in focal hand dystonia. Restorative neurology and neuroscience. 2009;27:55–65. doi: 10.3233/RNN-2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimberley TJ, Borich MR, Arora S, Siebner HR. Multiple sessions of low-frequency repetitive transcranial magnetic stimulation in focal hand dystonia: clinical and physiological effects. Restorative neurology and neuroscience. 2013;31(5):533–542. doi: 10.3233/RNN-120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theilig S, Podubecka J, Bosl K, Wiederer R, Nowak DA. Functional neuromuscular stimulation to improve severe hand dysfunction after stroke: does inhibitory rTMS enhance therapeutic efficiency? Experimental neurology. 2011;230(1):149–155. doi: 10.1016/j.expneurol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133(4):425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 13.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. The Journal of Physiology. 2010;588(13):2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant'Angelo A, Romano M, et al. Homeostatic-like plasticity of the primary motor hand area is impaired in focal hand dystonia. Brain. 2005;128(Pt 8):1943–1950. doi: 10.1093/brain/awh527. [DOI] [PubMed] [Google Scholar]
- 15.Carey JR, Evans CD, Anderson DC, Bhatt E, Nagpal A, Kimberley TJ, et al. Safety of 6-Hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22(2):185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- 16.Siebner H, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999;52(3):529–537. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 17.Lang N, Harms J, Weyh T, Lemon RN, Paulus W, Rothwell JC, et al. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clinical Neurophysiology. 2006;117(10):2292–2301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Kimberley TJ, Di Fabio RP. Visualizing the Effects of rTMS in a Patient Sample: Small N vs. Group Level Analysis. PLoS ONE. 2010;5(12):e15155. doi: 10.1371/journal.pone.0015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H, Kimberley TJ, Durfee WK, Dressler BL, Steil C, Carey JR. Combined statistical analysis method assessing fast versus slow movement training in a patient with cerebellar stroke: a single-case study. Physical therapy. 2013;93(5):649–660. doi: 10.2522/ptj.20120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehy MP, Marsden CD. Writers' cramp-a focal dystonia. Brain. 1982;105(Pt 3):461–480. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- 21.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clinical Neurophysiology. 2004;115(5):1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de Noordhout AM, Paulus W, et al. Applications of magnetic cortical stimulation. The International Federation of Clinical Neurophysiology. Electroencephalography & Clinical Neurophysiology - Supplement. 1999;52:171–185. [PubMed] [Google Scholar]
- 24.Kimberley TJ, Borich MR, Prochaska KD, Mundfrom SL, Perkins AE, Poepping JM. Establishing the definition and inter-rater reliability of cortical silent period calculation in subjects with focal hand dystonia and healthy controls. Neurosci Lett. 2009;464(2):84–87. doi: 10.1016/j.neulet.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen R, Hallett M. The time course of changes in motor cortex excitability associated with voluntary movement. Canadian Journal of Neurological Sciences. 1999;26(3):163–169. doi: 10.1017/s0317167100000196. [DOI] [PubMed] [Google Scholar]
- 26.Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol (Lond) 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- 27.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol (Lond) 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol (Lond) 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda F, Gangitano M, Thall M, Pascual-Leone A, Maeda F, Gangitano M, et al. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clinical Neurophysiology. 2002;113(3):376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 30.Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. Journal of neurophysiology. 1997;77(4):2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- 31.Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121(Pt 5):785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- 32.Kazdin AE. Single-case research designs: Methods for clinical and applied settings. New York: Oxford University Press; 1982. [Google Scholar]
- 33.Säisänen L, Julkunen P, Niskanen E, Hukkanen T, Mervaala E, Karhu J, et al. Short-and intermediate-interval cortical inhibition and facilitation assessed by navigated transcranial magnetic stimulation. Journal of neuroscience methods. 2011;195(2):241–248. doi: 10.1016/j.jneumeth.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Wassermann EM, Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clinical Neurophysiology. 2002;113(7):1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- 35.Ngomo S, Leonard G, Moffet H, Mercier C. Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. Journal of neuroscience methods. 2012;205(1):65–71. doi: 10.1016/j.jneumeth.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Koski L, Schrader LM, Wu AD, Stern JM. Normative data on changes in transcranial magnetic stimulation measures over a ten hour period. Clinical Neurophysiology. 2005;116(9):2099–2109. doi: 10.1016/j.clinph.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Fritz C, Braune HJ, Pylatiuk C, Pohl M. Silent period following transcranial magnetic stimulation: a study of intra- and inter-examiner reliability. Electroencephalography & Clinical Neurophysiology. 1997;105(3):235–240. doi: 10.1016/s0924-980x(97)96675-3. [DOI] [PubMed] [Google Scholar]
- 38.Hallett M. Neurophysiology of dystonia: the role of inhibition. Neurobiology of disease. 2011;42(2):177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22(11):2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stetkarova I, Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clinical Neurophysiology. 2013;124(2):339–345. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109(1):127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 42.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of physiology. 2008;586(23):5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]