Abstract
Background
Simultaneous activation of the stellate ganglion (SGNA), the ligament of Marshall (LOM) and the ganglionated plexi (GP) often precedes the onset of paroxysmal atrial tachyarrhythmias (PAT).
Objective
To test the hypothesis that ablation of the LOM and the superior left GP (SLGP) reduces atrial vulnerability and results in remodeling of the stellate ganglion.
Methods
Nerve activity was correlated to PAT and ventricular rate (VR) at baseline, after ablation of the LOM and SLGP, and after AF. Neuronal cell death was assessed with Tyrosine hydroxylase (TH) and terminal deoxynucleotidyl transferase dUTP nick end label (TUNEL) staining.
Results
There were 4±2 PAT episodes per day in controls. None were observed in the ablation group; even though SGNA and VR increased from 2.2 μV (95% confidence interval (CI); 1.2 – 3.3 μV) and 80 bpm (CI 68 – 92 bpm) at baseline to 3.0 μV (CI 2.6 – 3.4 μV, p=0.046) and 90 bpm (CI 75 – 108 bpm, p=0.026) after ablation, and to 3.1 μV (CI 1.7 – 4.5 μV, p=0.116) and 95 bpm (CI 79 – 110 bpm, p=0.075) after AF. There was an increase in TH-negative cells in the ablation group and a 19.7% (CI, 8.6 – 30.8%) TUNEL-positive staining in both the left and right SG. None were observed in the control group.
Conclusion
LOM and SLGP ablation caused LSG remodeling and cell death. There was reduced correlation of the VR response and PAT to SGNA. These findings support the importance of SLGP and LOM in atrial arrhythmogenesis.
Keywords: Ablation, Superior Left Ganglion Plexi, Ligament of Marshall, Atrial Fibrillation
Introduction
Cardiac autonomic innervation consists of neurons from both the intrinsic and extrinsic autonomic nervous systems.1 The intrinsic autonomic nervous system is composed of multiple ganglionated plexus (GP) that are within the epicardial fat pads2. The neurons of the GPs also form extensive connections with the extrinsic cardiac nervous system which includes the stellate ganglion and the vagus nerve. The ligament of Marshall (LOM) has also been shown to be highly innervated with both sympathetic and parasympathetic neurons.3 Studies in anesthetized dogs have shown that the GP and the LOM function as the “integration centers” that modulate the autonomic interactions between the extrinsic and intrinsic cardiac autonomic systems.4, 5 To study the importance of the Intrinsic cardiac nerve activity (ICNA) and the extrinsic cardiac nerve activity (ECNA) in atrial arrhythmogenesis, Choi et al 6 simultaneously recorded nerve activity from the fat pads at the left superior pulmonary vein (LSPV)-left atrial (LA) junction, the LOM and the left stellate ganglion (LSG) in a canine model of pacing-induced atrial fibrillation (AF). The authors found that ICNA either alone or in collaboration with ECNA is an invariable trigger of paroxysmal atrial tachycardia (PAT) or paroxysmal AF (PAF). The importance of ICNA in atrial arrhythmogenesis is also supported by randomized clinical trials that showed the benefit of GP ablation in controlling AF.7 However, in spite of adding GP ablation to a pulmonary vein (PV) isolation procedure, the 2-year recurrence rate of PAF remained significant (26%). Because of the interaction between ICNA and ECNA, we hypothesize that GP and LOM ablation may result in the remodeling of the extrinsic cardiac nervous system. That remodeling process might play a role in the recurrence of AF after a catheter ablation procedure. To test that hypothesis, we monitored the left stellate ganglion nerve activity (SGNA) after GP and LOM ablation to determine if ablation of these structures resulted in changes to the SGNA in a canine model of PAT and PAF.6, 8 We also performed analysis on the LSG for structural remodeling and cell death. The results were used to test the hypothesis that disassociation of the ICNA and the ECNA via GP and LOM ablation results in an increase in SGNA with a reduced correlation to ventricular rate (VR), and structural remodeling of the LSG in ambulatory dogs.9, 10
Methods
The research protocol was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research institute, Indianapolis, Indiana, and conforms to the guidelines of the American Heart Association. Six mongrel dogs (three male, weight 20–30kg) underwent two sterile surgeries, the first to implant a radio transmitter to record nerve activity, and the second for LOM and superior left GP (SLGP) ablation and pacemaker implantation for high rate atrial pacing. For each surgery, anesthesia was induced with ketamine 5–10mg/kg and midazolam 0.1– 0.2mg/kg IV, and maintained with 2.0% isoflurane after intubation and mechanical ventilation.
Continuous Ambulatory Autonomic Nerve Recordings
During the 1st sterile surgery, a Data Sciences International (DSI; St. Paul, MN) D70-EEE or D70-CCTP was implanted to record the nerve activity as previously described.6 Briefly, a left thoracotomy was performed through the 2nd intercostal space, and a pair of bipolar electrodes was sutured onto the caudal half of the left stellate ganglion (LSG) to record SGNA. A second pair of bipolar electrodes was sutured onto the superior cardiac branch of the left vagal nerve 4 to 5 cm above the aortic arch to record vagal nerve activity (VNA). In dogs with a D70-EEE implantation (N=3), an additional bipolar pair of electrodes was inserted into the subcutaneous tissues of the thorax for electrocardiogram (ECG) recording. For dogs with the D70-CCTP implantation, the left vagal nerve recording was band-pass filtered from 5–100 Hz for a pseudo-ECG recording. For all devices, the transmitter and ground wires were inserted into a subcutaneous pocket. After 2 weeks of postoperative recovery, the radio-transmitter was turned on to record baseline nerve activity.
Superior Left Ganglionated Plexi and ligament of Marshall Ablation
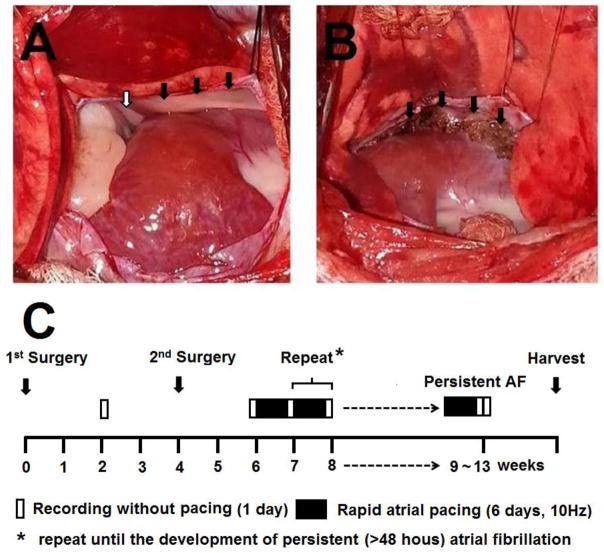
The 2nd sterile surgery was performed approximately 4 weeks after the radio transmitter was implanted. A left thoracotomy was performed through the 4th intercostal space. The heart was exposed and electrical cautery was used to ablate the LOM and the SLGP within the fat pad of the LSPV-LA junction (Figure 1A, B). A modified Medtronic Secura pacemaker (Medtronic Inc, Minneapolis, MN) was then implanted, and a pacing lead was sutured onto the LA appendage for intermittent high-rate atrial pacing.6, 11 After 2 weeks of recovery, post-ablation baseline activity was recorded (Figure 1C). High- rate (10 Hz) atrial pacing was then initiated and continued for 6 days, followed by 1 day of monitoring during which the atrial pacemaker was turned off. During this time, the rhythm was monitored to determine the presence of PAT or PAF. If AF was not present, the atrial pacing protocol was repeated until persistent (>48 hours) AF was documented. The dogs were then euthanized and the heart and stellate ganglion were harvested for histological analysis.
Figure 1. Ablation of the SLGP and LOM and the study protocol.
A, Ligament of Marshall (LOM, black arrowhead) and superior left ganglionated plexi (SLGP, white arrowhead). B, The scar after SLGP ablation (black arrowhead). C, Diagram of study protocol.
Data Analyses
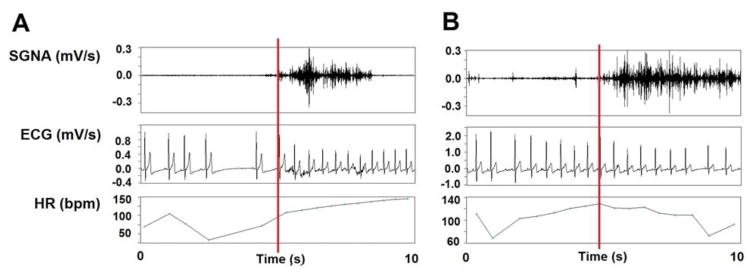
The signals were manually analyzed has previously described.6 The onset of each SGNA episode was used as the starting point for the analysis. To quantify the nerve activity, the average amplitude of nerve activity (aSGNA) was calculated over a 10-second window. Within each 10-second window, the signal was rectified and integrated. The result was then divided by the total number of samples. As depicted in Figure 2, the aSGNA and average ventricular rate (VR) was then determined 5 seconds prior to and 5 seconds after this time point to test the hypothesis that aSGNA is associated with VR elevation. A tachyarrhythmia was defined as an abrupt (>50 bpm/s) increase in the atrial rate to >200 bpm that persisted for at least 5 seconds.12 If the tachyarrhythmia was regular, it was defined as a PAT episode. If the tachycardia was irregular, it was defined as a PAF episode. In addition to manual analyses, custom-written software was used to calculate the average amplitude of the nerve activities (aSGNA and aVNA) as previously described.13 Bandpass filtering (5 to 100 Hz) was applied to the VNA recording to obtain an ECG for heart rate and arrhythmia analyses.8
Figure 2. Stellate ganglion nerve activity (SGNA) and ventricular rate (VR).
A was recorded at baseline. The onset of SGNA (red bar) was identified first, then the average VR 5 seconds prior to and 5 seconds after the onset of SGNA was determined. B, The same analyses was performed to compare the occurrence of SGNA to VR at baseline post-ablation. An example showing SGNA that induced little VR change is shown.
In addition to analyzing the dogs studied with the present research protocol, the data of 6 dogs with atrial pacing without GP or LOM ablation from a previous study6 were retrieved and analyzed de novo with the same methods to serve as control. All dogs within the control group underwent a single sterile surgery for DSI radio transmitter and atrial pacemaker implantation, without GP or LOM ablation. The atrial pacing protocol was the same as described above.
Histology
Tissue samples were obtained from the recording sites and fixed in 4% formalin for 45–60 minutes, followed by storage in 70% alcohol.14 The tissues were processed routinely, paraffin embedded and cut into 5-μm thick sections. Immunohistochemical staining was performed with antibodies against tyrosine hydroxylase (TH) using mouse monoclonal anti-TH (Accurate Chemical, Westbury, NY). All slides were examined manually under a BX60 microscope (Olympus, Tokyo, Japan) to determine if there were regions with decreased ganglionic cell density, pyknotic cell body or decreased TH staining. The first analysis was to compare the percentage of TH-negative cells11, 15 in each SG (ganglion cells that did not stain for TH). Five photos from both normal and damaged regions of each SG were captured using a 20X objective lens, in regions where ganglion cell density was the highest. If the region was too small for 5 photos, then the maximal number of photos was taken to cover the entire region. The number of TH-negative cells were counted manually using Photoshop CS6 (San Jose, CA), and a percentage was calculated to represent the ratio between TH-negative cells and total ganglion neuronal cells in each image.15 The mean percentage of TH-negative cell in five selected fields was used as the value for that SG.16 TUNEL staining was used to detect DNA fragmentation (cell death). The slides where then double stained for TH and TUNEL and examined them under a model TCS SP8 (Leica Microsystems, Wetzlar, Germany) Confocal Microscope.
Statistical analysis
Data were expressed as mean and 95% confidence interval (CI). Statistical comparison of variables during baseline, the averaged amplitude of the nerve activity or the incidence of arrhythmia before and after rapid atrial pacing was analyzed using paired t-test. Paired t-test for pairwise comparison was performed to compare the VR change during different stages of experiments. Correlation coefficient between percent TUNEL positive non-ganglion cells and ganglion cells were calculated accounting for the correlation of data from the same dog. The bootstrap method was used calculate the confidence interval (CI) of the correlation coefficient. The statistics were computed using the PASW Statistics (version 18; SPSS Inc, Chicago, IL) and SAS 9.2 (SAS Inc, Cary, NC). A two-sided P≤0.05 was considered to be statistically significant.
Results
Effects of SLGP and LOM Ablation on SGNA and ventricular rate
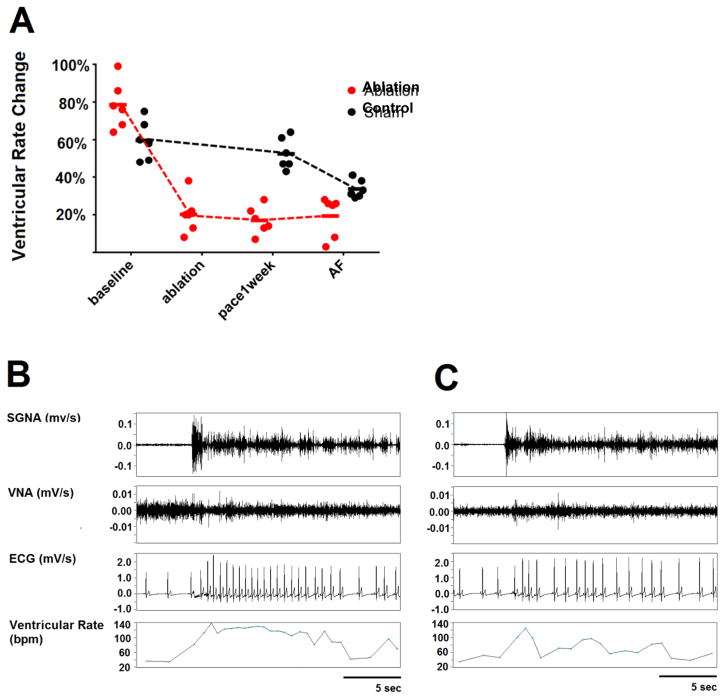
Table 1 shows the summary data of the quantification of nerve activity via time series integration, and the ventricular rate (VR) at baseline, after ablation, and after 1 week of rapid atrial pacing. In the ablation group, the SGNA increased after ablation as compared with baseline. In addition, as compared with the control group, dogs with ablation had significantly increased SGNA and VR. In spite of increased SGNA and VR, there was no correlation between the SGNA and VR after ablation. At baseline, there was a strong correlation as the occurrence of SGNA significantly increased VR by 78.5% (CI, 65.2 – 91.8%). However, after ablation, the SGNA-induced VR change decreased to 20.2% (CI, 9.5 – 30.9%, p=0.028 compared with baseline Figure 3A). SGNA increased VR by only 17.0% (CI, 9.3 – 24.7%, p=0.028) after one-week of rapid pacing and by only 19.3% (CI, 7.9 – 30.8%, p=0.028) during AF. Figure 3B is an example at baseline, in which an episode of sympathetic discharge led to a VR increase from 67 to 117 bpm (74.6% increment). This contrasts with Figure 3C, that shows that an episode of sympathetic discharge in a dog with SLGP and LOM ablation led to a VR increase from 62 to 73 bpm (17.7% increment).
Table 1.
Effects of Ganglionated Plexi and LOM Ablation on SGNA and VR
| Groups | Parameters | Baseline | Ablation Baseline | Pacing 1 week | AF |
|---|---|---|---|---|---|
| SLGP ablation | aSGNA (μV) | 2.2(1.2,3.3) | 3.0(2.6,3.4)* | 3.1(1.7,4.5) | 2.8(1.5,4.0) |
| VR (bpm) | 80(68,92) | 92(75,108)* | 95(79,110) | 156(142,170)*† | |
| Control | aSGNA (μV) | 2.1(1.9,2.3) | 2.3(1.7,2.9) | 2.2(2.0,2.5) | |
| VR (bpm) | 81(70,91) | 86(70,101) | 154(143,164)*† |
Data were expressed as mean and 95% confidence interval (CI). Data were analyzed by Wilcoxon Signed Ranks test. aSGNA, average amplitude of stellate nerve activity; VR, ventricular rate; bpm, beats per minute.
p<0.05 vs. Baseline,
p<0.05 vs. Ablation Baseline.
Figure 3. Effect of SLGP and LOM ablation on SGNA-induced VR changes.
A, Statistical dot plot showing that the SGNA-induced VR acceleration was markedly attenuated by SLGP ablation compared with the control group. B, An example at baseline shows that a burst of SGNA led to VR increase from 67 to 117 bpm (74.6% increment). C, An example after SLGP ablation shows that a burst of SGNA led to VR increase from 62 to 73 bpm (17.7% increment).
Effects of SLGP and LOM Ablation on PAT
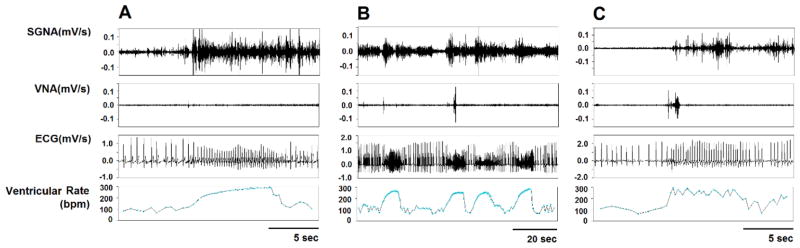
The PAT and PAF episodes were determined for each dog during the day of monitoring when the pacemaker was turned off. Analysis was performed in both the ablation and control groups at baseline, and 1 week after pacing. Analysis was also performed in the SLGP/LOM ablation group after ablation, but before the onset of rapid atrial pacing. For dogs that underwent rapid atrial pacing without SLGP/LOM ablation,6 the number of PAT and PAF episodes significantly increased with pacing. Figure 4A and 4B show typical PAT episodes. There were 4±2 episodes of PAT per day (include 2 PAF episodes, Figure 4C) at normal baseline. No episodes of PAT or PAF were observed in the SLGP/LOM ablation group after ablation either before or after the commencement of 4±2 weeks of rapid LA pacing.
Figure 4. The episodes of paroxysmal atrial tachyarrhythmia (PAT) and paroxysmal atrial fibrillation (PAF).
A, An example of PAT; B, 4 continuous PAT episodes within 90 seconds; C, An example of PAF.
Histology Studies
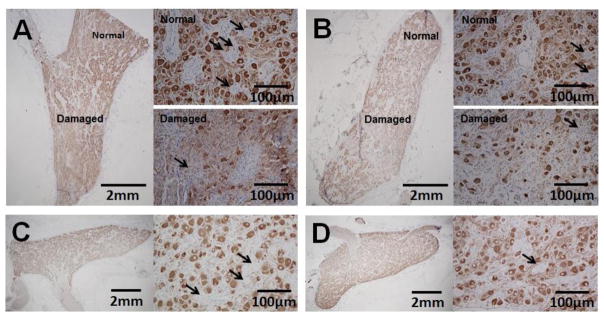
The stellate ganglia (SG) from 5 dogs were harvested for histological and immunohistochemical analyses. The remaining SG was not successfully harvested due to technical difficulties associated with extensive scar formation. Within each SG that was histologically analyzed, ganglion cells were observed that did not have an immunoreactivity to tyrosine hydroxylase (TH, as indicated in Figure 5). In the ablation group, within the left stellate ganglion (LSG), 10.7% (CI, 6.8 – 14.5%) of the ganglion cells stained negative for TH. The percentage of TH-negative ganglion cells was 7.7% (CI, 5.3 – 10.1% p=0.043) in the right stellate ganglion (RSG). The percentage of TH negative cells in the LSG control group was 5.53% (CI, 3.12 – 7.93%, p=0.043 compared with LSG in the ablation group), and the percentage of TH negative cells in the RSG control group was 5.12% (CI, 2.56 – 7.68%, p=0.043 compared with RSG in the ablation group).
Figure 5. Tyrosine hydroxylase (TH) staining of the SG.
Low magnification (1.25X objective lens) shows the presence of both a damaged region and a normal region in the same LSG (Panel A) and RSG (Panel B) of the ablation group. Images of these regions are also shown under a higher magnification (20X objective lens). Arrows point to ganglion cells that did not stain for tyrosine hydroxylase (TH negative). There was evidence of nuclear shrinking and pyknosis in the ganglion cells; Panels C and D show the control group LSG and RSG respectively.
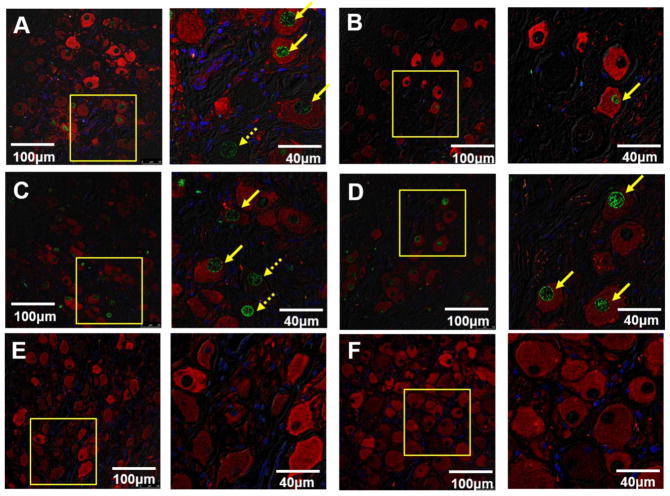
The slides from all available SG were then double stained for TH and TUNEL. As shown in the confocal immunofluorescent images in Figure 6, green shows positive TUNEL stain, red indicates the positive TH stain and blue is the DAPI stain of the nuclei. All 5 LSG (Figure 6A, 6C) and 2 RSG (Figure 6D) showed TUNEL-positive ganglion cells, and occasional TUNEL-positive ganglion cells were found in another 3 RSG (Figure 6B). The percentage of TUNEL-positive ganglion cells in LSG and RSG was 19.7% (CI 8.6 – 30.8%) and 12.8% (CI −2.1 – 27.7%), respectively. No TUNEL-positive ganglion cells were observed in the control group.
Figure 6. Confocal images of TH and TUNEL double staining of SG.
Green shows positive TUNEL stain, red indicates the positive TH stain and blue is the DAPI stain of the nuclei. There were 2 patterns observed in the SG of the GP ablation group. In the first pattern, LSG (A) have TUNEL-positive ganglion cells, and occasional TUNEL-positive ganglion cells were found in RSG (B). In the second pattern, both LSG (C) and RSG (D) have the same percentage of TUNEL-positive ganglion cells. There were no TUNEL-positive cells in the LSG (E) or RSG (F) of the control group.
Discussion
In the present study, we demonstrated that ablating the LOM and the SLGP significantly increased the SGNA, but reduced the correlation between the VR response and the occurrence of SGNA. In addition, SLGP/LOM ablation reduced atrial vulnerability as there were no episodes of PAT or PAF in this group despite prolonged intermittent rapid atrial pacing. Histological analysis demonstrated large areas of cellular damage within the stellate ganglion, and an increase in TH-negative and TUNEL-positive cells in the ablation group. These data indicate that the antiarrhythmic effects of SLGP and LOM ablation could be due to reduced communication between the extrinsic and intrinsic nervous systems of the heart. Even though there was increased SGNA, there was no evidence of increased atrial vulnerability.
Effects of ICNA on PAT
The canine intrinsic and extrinsic cardiac nervous systems are known to be anatomically similar to those in humans.17 Intrinsic cardiac neurons are found in the atria and are innervated with both sympathetic and parasympathetic neurons that are connected to both the spinal cord and medullary neurons.18 Choi et al6 demonstrated that there is a correlation between the ICNA and the ECNA in ambulatory dogs. All episodes of PAT and PAF were preceded by LOM nerve activity and/or super left ganglionated plexi (SLGP) nerve activity. A majority of these episodes (78%) also included ECNA co-activation. The results demonstrated that ICNA is an invariable trigger of atrial tachyarrhythmias in the rapid pacing dog model. In the present study, no episodes of either PAT or PAF were observed with LOM and SLGP ablation either before or after rapid LA pacing. These results support previous studies showing that ICNA plays an important role in atrial vulnerability.
Effects of SLGP Ablation on Persistent AF Inducibility
Rapid atrial pacing has been shown to induce neural remodeling that involved nerve sprouting of the intrinsic autonomic ganglia.19 The intrinsic autonomic ganglia have been shown to be capable of developing spontaneous neural activity independent of the extrinsic control.20 These neural activities could influence the development of AF by acting as triggers. In the present study even though no episodes of PAF or PAT were observed, all six dogs with SLGP/LOM ablation did eventually develop persistent AF, a finding that is compatible with the results of our previous studies.8, 11 This supports that ICNA could be a trigger mechanism for PAF or PAT and the rapid pacing in this group of dogs still develops an atrial substrate that is supportive of persistent AF. Ablation of the SLGP and LOM could be performed in addition to other standard ablation procedures such as PV isolation or left atrial maze to eliminate triggers and help to reduce AF vulnerability7, 21
Recently, acute studies have been performed to determine whether GP ablation can improve the success rate of AF ablation.4, 22, 23 These results showed that the cardiac autonomic nervous system forms an interconnected neural network composed of GPs and LOM; this network modulated and controls the release of neurotransmitters within the myocardium.4 These investigations also found that systematic GP ablation could increase the acute success rate of ablation for AF in dog models. Therefore, whether GP ablation could affect PAT in the long-term has not been fully clarified. Recently, Mao et al also demonstrated a decrease in atrial vulnerability acutely post GP ablation.24 However, after an 8 week follow up, atrial vulnerability increased which the authors concluded was a result of increased atrial nerve density. It is not known if there were any spontaneous PAT or PAF episodes in these dogs as monitoring between studies was not performed. A clinical study25 showed that recipients with orthotopic cardiac transplantation had a very low incidence (0.3%) of AF compared with the 21% incidence of AF in patients who have undergone coronary bypass surgery. The authors suggested that the complete isolation from both the extrinsic and intrinsic nervous system could contribute to the low incidence of AF in these patients. In the present study, we used cautery to ablate the LOM and SLGP but not the SG. The results support previous studies as there were no episodes of spontaneous PAT or PAF after ablation either before or after rapid pacing. These findings would support the addition of the ablation of the SLGP and LOM to the standard catheter ablation procedure. However, more studies need to be performed to evaluate the long term efficacy of SLGP and LOM ablation on AF vulnerability.
Effects of LOM Ablation on SG
The LOM derives from the embryonic left superior vena cava and is richly innervated by the autonomic nervous system.3 One of the major sympathetic nerves from the middle cervical and stellate ganglia courses along the LOM to innervate the heart. In the present study, we ablated the LOM, as well as the sympathetic nerves that originated from SG cells. The damaged axons led to physiological SG cell death that resulted in TUNEL-positive ganglion cells. All of LSG and 2 RSG had TUNEL-positive ganglion cells, indicating that neurons of the LOM come from both sides of the SG. Since the percentage of TUNEL-positive ganglion cells in LSG was 19.7%, the increased SGNA after ablation could be a result of a compensatory effect from the remaining healthy ganglion cells. The aSGNA increased from 2.2 μV at baseline to 3.0 μV after ablation, and up to 3.1 μV after 1 week of rapid pacing. In spite of increased aSGNA, VR responses to SGNA were blunted, as the SG was dissociated from the atria by LOM ablation.
Limitations
We did not perform right SGNA recording to test if that will also increase. The relationship between both sides of the SG was not determined. A second limitation is that the effective refractory periods or AF inducibility before and after GP ablation were not measured.
Conclusion
Ablation of the SLGP and LOM eliminated the occurrence of paroxysmal arrhythmias and reduced the correlation between the observed nerve activity and the VR even though there was increased SGNA. SLGP/LOM ablation also resulted in structural remodeling of the stellate ganglion demonstrating the connection between the ICNA and ECNA. Intrinsic nerve activity could influence the occurrence of atrial triggers that would increase atrial vulnerability to either PAT or PAF.
Acknowledgments
We thank Nicole Courtney, Jian Tan, Jessica Warfel and Christopher Corr for their assistance.
Funding Sources
This study was supported by NIH/NHLBI grants R01 HL71140, P01 HL78931, R41HL124741, the Charles Fisch Cardiovascular Research Award endowed by Dr Suzanne B. Knoebel of the Krannert Institute of Cardiology and by the strategic research initiative of the Indiana University Health/Indiana University School of Medicine
Footnotes
Disclosures
Drs Peng-Sheng Chen and Shien-Fong Lin have equity interest in Arrhythmotech, LLC. Indiana University has filed patent application related to the findings of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armour JA. Cardiac neuronal hierarchy in health and disease. AmJPhysiol RegulIntegrComp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 2.Hou Y, Zhou Q, Po SS. Neuromodulation for cardiac arrhythmia. Heart Rhythm Feb. 2016;13:584–592. doi: 10.1016/j.hrthm.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Makino M, Inoue S, Matsuyama TA, Ogawa G, Sakai T, Kobayashi Y, Katagiri T, Ota H. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol Jun. 2006;17:594–599. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 4.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. JAmCollCardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 5.Lin J, Scherlag BJ, Niu G, Lu Z, Patterson E, Liu S, Lazzara R, Jackman WM, Po SS. Autonomic elements within the ligament of Marshall and inferior left ganglionated plexus mediate functions of the atrial neural network. J Cardiovasc Electrophysiol Mar. 2009;20:318–324. doi: 10.1111/j.1540-8167.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation Jun 22. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic Denervation Added to Pulmonary Vein Isolation for Paroxysmal Atrial Fibrillation: A Randomized Clinical Trial. Journal of the American College of Cardiology Aug 8. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 8.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall J. On the development of the great anterior veins in man and mammalia: including an account of certain remnants of foetal structure found in the adult, a comparative view of these great veins in the different mammalia, and an analysis of their occasional peculiarities in the human subject. Phil Trans R Soc Lond. 1850;140:133–169. [Google Scholar]
- 10.Doshi RN, Wu TJ, Yashima M, Kim YH, Ong JJC, Cao JM, Hwang C, Yashar P, Fishbein MC, Karagueuzian HS, Chen PS. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–883. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 11.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swissa M, Zhou S, Paz O, Fishbein MC, Chen LS, Chen PS. A Canine Model of Paroxysmal Atrial Fibrillation and Paroxysmal Atrial Tachycardia. AmJPhysiol Heart CircPhysiol. 2005;289:H1851–H1857. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Zhao Y, Doytchinova A, et al. Using skin sympathetic nerve activity to estimate stellate ganglion nerve activity in dogs. Heart Rhythm Jun. 2015;12:1324–1332. doi: 10.1016/j.hrthm.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 15.Shen MJ, Hao-Che Chang X, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm Jan 26. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinda K, Tsai WC, Chan YH, et al. Intermittent left cervical vagal nerve stimulation damages the stellate ganglia and reduces the ventricular rate during sustained atrial fibrillation in ambulatory dogs. Heart Rhythm. 2015 Dec 1; doi: 10.1016/j.hrthm.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. AnatRec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- 18.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. ExpPhysiol. 2008;93:165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- 19.Chang CM, Wu TJ, Zhou SM, Doshi RN, Lee MH, Ohara T, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 20.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronically decentralized canine hearts. American Journal of Physiology. 1991;260:H713–H721. doi: 10.1152/ajpheart.1991.260.3.H713. [DOI] [PubMed] [Google Scholar]
- 21.Zheng S, Li Y, Han J, Zhang H, Zeng W, Xu C, Jia Y, Wang J, Guo K, Jiao Y, Meng X. Long-term results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. PloS one. 2013;8:e79755. doi: 10.1371/journal.pone.0079755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Scherlag BJ, Lu Z, Zhang Y, Liu S, Patterson E, Jackman WM, Lazzara R, Po SS. Inducibility of atrial and ventricular arrhythmias along the ligament of marshall: role of autonomic factors. J Cardiovasc Electrophysiol Sep. 2008;19:955–962. doi: 10.1111/j.1540-8167.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol Aug. 2008;1:184–192. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao J, Yin X, Zhang Y, Yan Q, Dong J, Ma C, Liu X. Ablation of epicardial ganglionated plexi increases atrial vulnerability to arrhythmias in dogs. Circ Arrhythm Electrophysiol Aug. 2014;7:711–717. doi: 10.1161/CIRCEP.113.000799. [DOI] [PubMed] [Google Scholar]
- 25.Khan M, Kalahasti V, Rajagopal V, et al. Incidence of atrial fibrillation in heart transplant patients: long-term follow-up. J CardiovascElectrophysiol. 2006;17:827–831. doi: 10.1111/j.1540-8167.2006.00497.x. [DOI] [PubMed] [Google Scholar]