Abstract
Patients with pathogenic truncating mutations in the prion gene (PRNP) usually present with prolonged disease courses with severe neurofibrillary tangle and cerebral amyloidosis pathology, but more atypical phenotypes also occur, including those with dysautonomia and peripheral neuropathy. We describe the neurological, cognitive, neuroimaging, and electrophysiological features of a 31-year-old man presenting with an orbitofrontal syndrome, gastrointestinal symptoms, and peripheral neuropathy associated with PRNP Q160X nonsense mutation, with symptom onset at age 27. The mutation was also detected in his asymptomatic father and a symptomatic paternal cousin; several members of prior generations died from early onset dementia. This is the first report of a family affected with the nonsense PRNP mutation Q160X displaying clear autosomal dominant disease in multiple family members and reduced penetrance. This case strengthens the evidence suggesting an association between PRNP truncating mutations and prion systemic amyloidosis. PRNP gene testing should be considered in any patient with atypical dementia, especially with early onset and neuropathy, even in the absence of a family history.
Keywords: Amyloidosis, DNA sequencing, dysautonomia, exome, mutation, peripheral neuropathy, prion dementia
INTRODUCTION
Most pathogenic mutations in the prion protein gene (PRNP) are point mutations causing a classical prion disease presentation of rapidly progressive dementia, cerebellar ataxia, and myoclonus or slower progressive parkinsonian and/or ataxic syndromes such as Gerstmann Sträussler Scheinker syndrome (GSS). Truncating mutations in PRNP are very rare, but have been reported due to nonsense variants or a two-base pair deletion resulting in a reading frame shift causing a premature stop codon [1–7]. These PRNP mutations leading to truncated prion protein expression, have been shown to cause a slowly progressive dementia, often mistaken for Alzheimer's disease (AD), with neurofibrillary tangles and deposition of prion (PrPSc)-amyloid in the form of cortical amyloid plaques with or without cerebral amyloid angiopathy [6, 8, 9]. Furthermore, some truncating PRNP mutations feature diarrhea, dysautonomia, and peripheral neuropathy. A large British kindred presenting with diarrhea, autonomic failure, and length-dependent axonal sensory peripheral neuropathy associated with truncating mutation Y163X, had deposition of PrP-amyloid in multiple peripheral organs including bowel and peripheral nerves [6]. This has led to the proposal of a new condition related to truncating PRNP mutations: PrP systemic amyloidosis [10]. We report a case of very early onset, slowly progressive prion disease associated with nonsense PRNP mutation Q160X identified by whole exome sequencing, with a novel presentation of orbitofrontal syndrome accompanied by gastrointestinal symptoms and subsequent sensori-motor polyneuropathy.
METHODS
Clinical assessment
The subject's surrogate provided written, informed consent for participation in our research program project grant and rapidly progressive dementia protocol. The subject assented to research participation. The University of California, San Francisco (UCSF) Committee on Human Research approved all research protocols. The subject underwent neurological examination, and completed neuropsychological assessments of language, memory, visuospatial, and executive function. Family medical history was obtained through discussions with family members and review of medical records. Magnetic resonance imaging (MRI) scans were obtained on a 3T GE system equipped with a birdcage headcoil. Structural MRI sequences as well as fluid-attenuated inversion recovery (FLAIR) sequences, diffusion weighted images (DWI), and apparent diffusion coefficient (ADC) maps were obtained. Electroencephalogram (EEG) recordings were obtained using a standard international 10–20 electrode placement with 18 channels. The recordings were obtained using a reference electrode and reformatted digitally into sequential bipolar and referential montages for review. Electrophysiology studies included motor and sensory nerve conductions studies with surface electrodes. Bilateral tibial, bilateral peroneal to the extensor digitorum brevis, left peroneal to the tibialis anterior, median, and ulnar motor nerve conduction studies were performed. Sensory nerve conduction studies included bilateral sural and superficial peroneal as well as left median, radial, and ulnar sensory nerves.
Exome analysis
Genomic DNA from the subject and both parents was prepared according to Illumina's TruSeq Sample Preparation (Illumina, CA, USA), and capture was performed with Illumina's TruSeq Exome Enrichment according to the manufacturer's instructions. Sequencing was performed in Illumina's HiSeq2000 using 100-bp paired end reads. Sequence alignment and variant calling were performed against the reference human genome (GRCh37) using Burrows Wheeler Alignment. Reads were processed and variants were called using the Genome Analysis Toolkit. Variants were annotated using Ingenuity Variant Analysis (Qiagen, CA, USA). The PRNP mutation and codon 129 genotype were confirmed by Sanger sequencing using standard methodology.
RESULTS
Case description
A 31-year-old right-handed man was referred with possible behavioral variant frontotemporal dementia (bvFTD) due to a 4-year history of a frontal dementia syndrome and gastric upset. He was premorbidly jovial, equable, very independent, and self-sufficient. Although he had no known learning disability, he was historically a below-average student, earning Cs and Ds in high school. He married at age 22, worked in a welding shop while studying applied technology in college, but required some tutoring. His marriage was characterized by mismanaged finances by him and his wife and his wife's substance abuse. He divorced at age 27 and moved in with his parents due to financial hardship. His mother noted behavioral changes, including apathy and poor judgment, a decline in his personal hygiene, and his needing frequent reminders to bathe. He also became irritable and impulsive, displaying angry outbursts disproportionate to the offending trigger. At around the same time, he developed constipation requiring frequent laxative use. Several months later he developed episodes of vomiting and diarrhea with abdominal pain. The episodes occurred suddenly, dissipated gradually after 5-7 days, and would recur every 6–10 weeks. Anti-emetics provided only partial relief of vomiting. At age 28, he quit his third year of college due to financial hardship and difficulty with coursework. He retained his current job and began a second one. At age 29, he was fired from both jobs; he had difficulty comprehending directions from supervisors and forgot to complete tasks despite frequent reminders. He subsequently became a busboy. Four months later, he displayed violent irritability and impulsivity, and was fired after threatening to harm coworkers and “blow up” the workplace. At home, during these occasional outbursts, he would hit his parents and 12-year-old brother. At age 30, he displayed new behavioral changes, addressing a former co-worker by a racial slur and stating out loud private thoughts. His speech became “broken and choppy,” he had long pauses during conversation, and his words were occasionally out of order. His speech was also more effortful with and occasional gibberish words (“just in the flak of the fug”) and word substitutions (“skill” instead of “school”). At around age 31, the patient developed clumsiness and balance problems. It was subsequently noted that his nausea and vomiting responded best to topical promethazine. His agitation responded to lorazepam 1 mg orally as needed. Quetiapine worsened his agitation, whereas donepezil, memantine, risperidone, and dextroamphetamine/amphetamine had no effects.
His past medical history included depression, hypertension, morbid obesity, and erectile dysfunction. He lost more than 200 pounds, at least partially intentionally, during the previous 5 years owing to changes in diet and exercise. At age 19, he had a concussion with brief loss of consciousness (less than 5 min) following a motor vehicle accident. He smoked half to one pack per day for 8 years until quitting at age 27. He drank heavily in college, with unknown quantity/frequency consumption but with no alcohol dependence. He had a remote history of prescription narcotics and marijuana use during marriage, but frequency and duration of use were unknown. His history also included visual hallucinations, beginning at age 5-7, when he periodically saw a man (a stranger) walk through his house. This figure appeared repeatedly for 11–13 years. At age 18, his family moved to a new house, and he stopped seeing the man. Beginning around this time, he saw a woman (a stranger) walk through his house. This figure appeared 2–3 times over 9 years. He stopped reporting hallucinations by age 27.
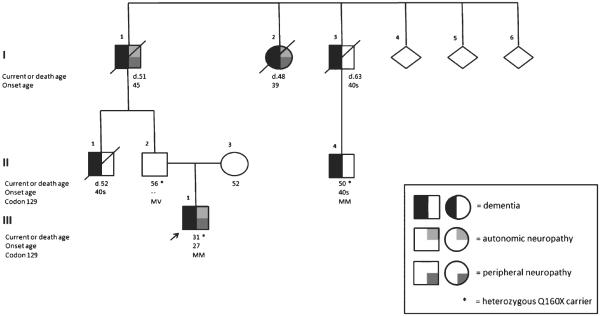
His mother was still alive at 52 and maternal family history was non-contributory. His paternal family history, however, was significant for an autonomic and cognitive disorder with neuropathy and gastric upset (Fig. 1). His 56-year-old father (II-2) was asymptomatic. His paternal uncle (II-1), who died at age 52, developed a digestive disorder in his 40 s, characterized by frequent postprandial loose stools, followed by memory and behavioral changes. In his 40 s, he uncharacteristically changed his profession numerous times before stopping work altogether. He asked questions repeatedly, and eventually required help with activities of daily living. The paternal grandfather (I-1), who died at age 51, had neuropathy, dysautonomia, and gastric upset beginning at age 45. At onset, he was hospitalized for frequent syncope due to severe orthostatic hypotension, abdominal pain, and lower extremity paresthesias (from knees to feet). He had erectile dysfunction, an unusual sweat pattern (predominantly truncal, left greater than right, with minimal groin and axilla involvement), unexplained weight loss over an unknown period of time, renal calculi and plantar ulcers. At age 48, he developed diarrhea, urinary incontinence, and memory impairment, which progressed to “nocturnal confusion.” He had behavioral changes, becoming agitated and paranoid. An infusion urogram showed bilateral calcifications within the collecting systems. A large flaccid bladder showed a markedly trabeculated contour consistent with chronic cystitis. A follow up study showed a left distal ureteric calculus with obstructive hydronephrosis. A computed tomography of the abdomen showed retroperitoneal lymphadenopathy. An exploratory abdominal laparotomy revealed adhesions and enlarged nodes. Excisional biopsies of omental, retroperitoneal, and gastro-celiac lymph nodes were consistent with chronic lymphadenitis with sinus histiocytosis. At age 50, his employer asked him to retire early because he was unable to perform his job duties. Two months later he was hospitalized for severe dysautonomia associated with hypotensive episodes leading to renal failure from recurrent hypoperfusion. He was noted to have poor lacrimation leading to exposure keratitis, bilateral Adie's pupil, presenile dementia, and plantar ulcers. His hypotension partially responded to fludrocortisone 0.2 mg per day and ergotamine 1 mg twice a day. Methylphenidate caused agitation and diarrhea. His cerebrospinal fluid (CSF) was remarkable only for elevated protein at 118 mg/dL. A computed tomography of the brain was noted to have “unexplained” atrophy. He died five months after this hospitalization and his dysautonomia was attributed to postinfluenzal autonomic neuropathy. No autopsy was performed. The grandfather's brother (I–3), who died at age 63, developed unspecified digestive and cognitive problems in his 40 s. The grandfather's brother has a son (II-4), age 50, with a similar condition. The grandfather's sister (I-2), who died at age 48, developed gastric upset at age 39. At age 42, she was hospitalized for recurring diarrhea without abdominal pain and unexplained weight loss over 3 years. At age 45, she had a second hospitalization for recurring nausea, vomiting, and diarrhea. At age 46, she was diagnosed with depression. At age 47, she developed orthostatic hypotension, which occasionally alternated with hypertension. At age 48, she had episodes of confusion diagnosed as a mild “organic brain syndrome,” but seven months later was diagnosed with Shy-Drager syndrome. Her cognitive syndrome worsened, and at age 51, she was diagnosed with presenile dementia and Shy-Drager syndrome. Shortly thereafter she was hospitalized due to flank pain, exposure keratitis, and renal calculi requiring nephrostomy, and died during this hospitalization. Autopsy showed enlarged ventricles, neuritic plaques, and neurofibrillary tangles in her hippocampi and midbrain oculomotor nuclei, as well as plaques in the cerebellum, all attributed to AD. The paternal family was of Austrian, Scottish, and Irish descent.
Fig. 1.
Pedigree of family affected with PRNP Q160X. An autosomal dominant inheritance pattern was noted. The proband's asymptomatic father was a confirmed heterozygous Q160X carrier. The proband's paternal grandfather had severe dysautonomia, peripheral neuropathy, and cognitive decline. The arrow denotes the proband. The asterisks correspond to genetically confirmed PRNP Q160X cases. MM, codon 129 methionine homozygosity; MV, codon 129 heterozygosity; d., death age.
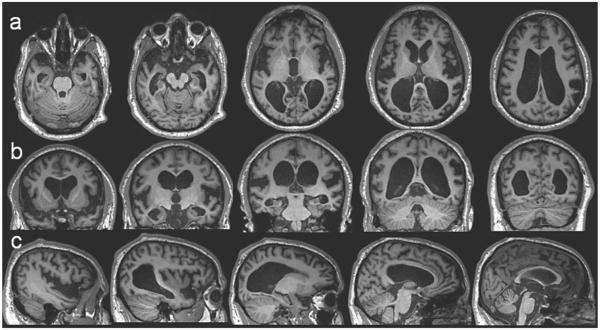
On physical examination at his first UCSF visit, 4 years after onset, he was moderately hypertensive, alert, and socially appropriate. His Mini-Mental State Exam score was 3/30, earning points only for orientation, one component of the 3-step command, and following the written command (Table 1). He was alert and cooperative, but his comprehension fluctuated throughout the exam, and he had difficulty following simple instructions. Speech was halting, effortful, but not dysarthric. He made grammatical errors, used neologisms, and his speech output was frequently unintelligible. Additional cognitive testing showed severe impairment in working and visual memory, speech/language (apraxia with relatively spared semantics), and frontal-executive function. Calculation and visuospatial skills were impaired, though he had poor comprehension of test directions. Verbal memory, though moderately impaired, was a relative strength. His pupils were slightly midriatic and he had an irregular left pupil and with sluggish constriction. He had decreased sense of temperature and joint position, with intact touch and vibration in the distal lower extremities. Deep tendon reflexes were preserved and symmetric. He had mildly increased tone, a postural hand tremor, a wide-based gait, and mild retropulsion. Brain MRI at the first visit revealed severe white matter atrophy (likely contributing to) enlarged ventricles in the bilateral temporal horns (Fig. 2), widened Sylvian fissures, and severe atrophy of orbitofrontal cortex, left greater than right parietal lobes, bilateral angular gyri, and bilateral anterior cingulate. The occipital lobe and cerebellum had relatively intact volumes. T2 and FLAIR sequence showed no cortical or subcortical signal abnormalities, and DWI and ADC sequences showed no evidence of restricted diffusion (not shown).
Table 1.
Longitudinal neuropsychological testing in proband with PRNP Q160X
| Baseline | 7 month follow up | 13 month follow up | |
|---|---|---|---|
| Mini-Mental State Examination | 3 (+state, 3-step command, +1 written command) | 5 (+1 state, +1 item recognition, +1 naming +1 repetition) | 2 (+1 state, +1 3-step command) |
| Digits forward/backward | 3/0 | Unable to test | 3/unable to test |
| Repetition | 1/5 | 1/5 | 0/5 |
| Sentence comprehension | 2/5 | 1/5 | Unable to test |
| Boston naming test | 2/15 | 1/15 | 0/15 |
| Benson figure copy | 0/17 | Unable to test | 1/17 |
| Benson figure recall | 0/17 | Unable to test | 0/17 |
Fig. 2.
Brain magnetic resonance imaging of the proband at age 31. Axial (a), coronal (b), and sagittal (c) T1-weighted images show severe symmetric temporal, perisylvian, parietal, and thalamic atrophy with associated ex-vacuo hydrocephalus.
By seven months later, at a second visit, his apathy had worsened: his activity was restricted to sitting around at his mother's workplace and watching TV at home. He was less irritable and impulsive. His physical aggression had stopped, but he still had occasional verbal outbursts, typically when he was asked to do tasks he did not want to do, for example, putting away laundry. He had reduced verbal output, and his verbal and reading comprehension were diminished. He enjoyed having the newspaper, but the extent to which he read it or understood content was unclear. He had new pain, numbness, and tingling in his hands and feet. His gait worsened mildly, and he required banister or wall support when descending stairs. He dressed, bathed, and ate independently, but needed assistance with toileting. He had bladder and bowel incontinence. Neurologic exam at the second visit showed no significant changes from the previous evaluation. He had no orthostatic hypotension. Sensory testing was invalid due to poor comprehension of interviewer commands. He was unable to follow instructions during many cognitive measures, but no significant changes were observed in testable domains. He continued to have severely impaired working memory, speech/language (speech apraxia with relatively spared semantics), and frontal-executive function (Table 1). Repeat brain MRI revealed no significant changes.
CSF collected at the second visit showed normal cell count, protein, glucose, and immunoglobulin G index, and no oligoclonal bands. Total tau (National Prion Disease Pathology Surveillance Center, NPDPSC) was normal at 183×pg/mL. CSF 14-3-3 (NPDPSC) was negative, and neuron-specific enolase (Mayo Laboratories, Rochester, MN) was normal at 9.8 ng/mL (reference <15 ng/mL). EEG revealed diffuse background slowing and attenuated cerebral activity, without epileptiform potentials or periodic discharges.
By six months later, at his third UCSF visit, he had developed new compulsive eating behaviors; he ate every food morsel he was served, regardless of the serving size and quantity of food he just previously consumed. He sought food in between meal times, and hoarded food in his room. He ate quickly and occasionally choked while eating solid foods. He drank liquids without choking. Despite these changes, his weight remained stable. He continued to have verbal outbursts, occurring a few times per week and usually during instances when his overeating was discouraged, but he responded to behavior redirection, and calmed down after a few minutes. He continued to accompany his mother to work, but now became upset if he could not follow her or not stand by her side around the office or at home. He developed insomnia, falling asleep nightly, but waking 4–6 hours later. Once awake, he compulsively turned on lights, opened cupboards looking for food, and released his pet dogs from their overnight kennel. He napped during the day, but had no excessive daytime somnolence. He compulsively chewed on pens, his fingernails, and nasal mucous. Verbal output declined further. He spoke in one- or two-word utterances only. Gabapentin 300 mg three times a day was attempted to treat neuropathic pain, but it was discontinued due to leg swelling. Nevertheless, he complained less frequently of pain, numbness, and tingling in his hands and feet. His gait remained unsteady with several near falls. He used no assistive device for ambulation. He needed more help with independent activities of daily living, including bathing and shaving. He was still able to comb his hair and make a cup of coffee. He no longer required assistance with toileting. Neurologic exam at the third visit showed no significant changes from the previous evaluation. Sensory testing was again invalid. He had no orthostatic hypotension. Cognitive testing revealed no significant changes in most domains, although language measures elicited some semantic loss (Table 1). Structural brain MRI and CSF showed no significant changes. Nerve conduction studies performed at the third visit provided evidence of a length-dependent primarily axonal sensorimotor polyneuropathy (Table 2).
Table 2.
Summary of abnormal electrophysiological findings in proband with PRNP Q160X*
| Latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | F-wave latency (ms) | |
|---|---|---|---|---|
| Motor nerve conduction studies | ||||
| Left peroneal to EDB | 9.8 (<6.0) | 0.4 (>2.0) | 28 (>38) | Absent (<56) |
| Right peroneal to EDB | 4.7 (<6.0) | 2.7 (>2.0) | 42 (>38) | Absent (<56) |
| Left tibial | 6.0 (<6.5) | 8.7 (>3.0) | 36 (>41) | 51.5 (<56) |
| Left ulnar | 2.8 (<3.7) | 9.3 (>5.0) | 43 (>50) | 30.9 (<31) |
| Peak latency (ms) | Amplitude (μV) | Conduction velocity (m/s) | ||
| Sensory nerve conduction studies | ||||
| Left sural | 4.6 <(4.8) | 3 (>6) | 40 (>42) | |
| Right sural | none (<4.8) | none (>6) | − (>42) | |
| Left superficial peroneal | 3.7 (<4.6) | 4 (>4) | 38 (>42) | |
| Right superficial peroneal | none (<4.6) | none (>4) | − (>42) | |
normal values appear in parentheses.
Genetics
Initial Sanger sequencing revealed no frontotemporal dementia-causing or AD-causing mutation. Exome sequencing revealed a nonsense PRNP mutation (Q160X), resulting in the introduction of a stop codon leading to premature truncation of translation, with codon 129 methionine homozygosity. His 56-year-old father carries the same mutation with codon 129 heterozygosity (MV), and is asymptomatic, whereas his mother did not have a PRNP mutation. His 50-year-old paternal cousin once removed (II-4) was later tested and found to have the same mutation with codon 129 methionine homozygosity (MM), and has similar symptoms to our proband.
DISCUSSION
This is the fourth patient with a PRNP Q160X mutation described in the literature, and the first with an orbitofrontal syndrome followed by gastric upset with vomiting and diarrhea, and sensorimotor polyneuropathy, as well as the first with an autosomal dominant family history featuring reduced penetrance. All affected relatives had gastric upset with diarrhea and dysautonomia, followed by a memory-predominant cognitive syndrome. The grandfather had these symptoms as well as prominent autonomic neuropathy, as also suggested by symptoms of our proband. The grandfather's sister had gastric upset, dysautonomia, and a cognitive syndrome, but no report of neuropathy. She received a neuropathologic diagnosis of AD; her amyloid plaques, in retrospect, were likely PrPSc.
Our proband presented with a slowly progressive dementia affecting behavior, memory, executive function, speech/language, and motor function, while relatively sparing visuospatial function. The most salient initial features of his behavioral syndrome were behavioral disinhibition, emotional lability, impaired judgment, distractibility and decreased social tact, consistent with cortico-subcortical orbitofrontal dysfunction. The orbitofrontal circuit plays a major role in the determination of strategies for environmentally elicited behavioral responses. Lesions in this system disconnect frontal monitoring systems from limbic inputs [11]. Although he was referred for suspected bvFTD, he did not meet revised criteria (i.e., in the first three years of illness, 3 of 6 of the following: disinhibition, apathy, loss of sympathy/empathy, perseverative/compulsive behaviors, hyperorality, and dysexecutive neuropsychological profile) [12]. He was apathetic, irritable, impulsive, and had declining personal hygiene, but he maintained many social graces 4 years after onset. He had executive impairment and abulia, but no true loss of empathy, compulsive behaviors, or hyperorality during early disease. The compulsive food seeking and eating behavior developed 4 years after symptom onset. His use of racial epithets and stating out loud private thoughts 3 years after symptom onset constituted socially inappropriate behavior, but its occasional occurrence contrasted with the typically pervasive disinhibition of bvFTD, which frequently dominates the behavior changes. Many of his social pragmatics were preserved. He met NIA-AA criteria for possible AD (i.e., dementia with insidious onset, progressive course, amnestic or non-amnestic presentation not due to other etiologies; possible AD allows atypical course or mixed etiology) [13]. His gastric upset with constipation/diarrhea and vomiting, erectile dysfunction, and sensorimotor neuropathy, along with his family history of dysautonomia and neuropathy, were consistent with transthyretin-mediated familial amyloid polyneuropathy (FAP-TTR) [14]. Going against this diagnosis, however, was his orbitofrontal syndrome with progressive cognitive decline without cardiac (restrictive cardiomyopathy, arrhythmia, or conduction disorder) or ocular (vitreous opacities) manifestations, the latter of which are typical of FAP-TTR [15]. His cognitive syndrome and neuropathy, as well as the family history of dementia and neuropathy with plantar ulcer, also were consistent with DNMT1-mediated hereditary sensory and autonomic neuropathy (HSAN1-DNMT1) [16], but inconsistent features included his and his family's prominent gastric upset, as well as the absence of sensorineural deafness, a hallmark sign of HSAN-DNMT1. [17]. He did not meet WHO criteria for possible or probable sporadic Creutzfeldt-Jakob disease (sCJD) (i.e., dementia with 2 of the following: myoclonus, pyramidal/extrapyramidal, visual/cerebellar signs, akinetic mutism, typical EEG, or CSF 14-3-3 if duration <2 years) or European criteria for possible or probable sCJD (identical to WHO, but includes certain MRI abnormalities) [18, 19]. He met UCSF criteria for possible sCJD because of progressive dementia, mild extrapyramidal sign of postural tremor, and higher focal cortical sign of aphasia [20]. Although neuropathological confirmation of the diagnosis is not yet available, his unique constellation of cognitive deficits, systemic symptoms, positive family history, and positive PrP truncating mutation status are strongly suggestive of PrP systemic amyloidosis.
Clinical presentations of the previously reported patients with the same mutation differed from our case. The first reported case was a 32-year-old man (codon 129 MM) with an unspecified dementia (diarrhea not×noted) and a family history of dementia in his father and brother [2]. The second was a 39-year-old man (codon 129 MV) with a clinical and neuropathologic diagnosis of AD (diarrhea not noted) with a family history of AD and diarrhea in his mother [4]. The third was a 38-year-old woman (codon 129×VV) with a clinical diagnosis of AD with transient diarrhea and a family history of AD in her mother [21].
Many PRNP mutations display reduced penetrance [18, 22], but no other reported family with the Q160X mutation displayed clear autosomal dominant disease with reduced penetrance, as suggested by the asymptomatic 56-year-old father (II-2). With the exception of the patient, all other affected relatives had disease onset around the fourth decade. However, we cannot completely rule out the possibility that the Q160X mutation displays complete penetrance with a highly variable age of onset, and that the asymptomatic father will develop symptoms at a later age. Additional clinical follow up of the family will be informative. The polymorphic codon 129 has been proposed to influence the phenotype (e.g., age of onset, duration) of both sporadic and genetic prion disease [23–25]. Its effect on age of onset in genetic kindreds, however, may be contingent upon the specific type of mutation, with evidence of influence in the setting of octapeptide repeat insertions [26], but no influence in D178 N or E200K mutations [27]. In this family, all affected family members with molecular PRNP testing had codon 129×MM heterozygosity, but it is unclear if this heterozygosity could explain the lack of symptoms in the father. Notably the second and third reported cases harboring the Q160X mutation had codon 129 genotypes MV and VV, respectively. This suggests the influence of additional genetic, epigenetic, environmental or stochastic factors on the expressivity of truncating PRNP gene mutations. This includes a possible differential phenotypic effect resulting from the association of the mutation with 129×M or 129 V haplotypes.
Truncating mutations generate a stop codon that signals the end of protein synthesis, with subsequent interruption of mRNA translation and expression of an incomplete or truncated protein. Truncating mutations of PRNP gene were first described at codon 145, which was associated with an AD-like dementia, with PrP-amyloid in the cortex and cerebral vasculature, as well as a severe tauopathy [3, 9]. Nonsense mutations at PRNP codons 163 [6, 7, 28], 178 [5], 186 [22], 226 [8], and 227 [8] have been reported previously, several of which have been associated with PrP-amyloid neuropathology and clinical syndromes consistent with HSAN, AD, or GSS. Though one patient with a Q227X mutation had a clinical diagnosis of frontotemporal dementia, the report did not provide sufficient information as to whether she met research criteria for bvFTD, and her progressive hypokinetic, rigid syndrome with dementia differed from our case [8]. We suspect that the neuritic plaques and neurofibrillary tangles of our proband's grandfather's sister's autopsy that at the time were thought to be suggestive of AD, were likely PrP-amyloid deposits, although prion immunohistochemistry was not performed. We suspect our proband and the grandfather's sister had PrP-amyloid deposition in her gastrointestinal tract, affecting enteral motility, which would be consistent with observations that some truncating mutations are associated with PrP-amyloid deposition in peripheral organs [10]. Prion protein that is truncated at or after codon 145 lacks the C-terminal glycosylphosphatidylinositol anchor but retains the amyloid binding domain, both features which may have a role in disease pathogenesis. In contrast, truncating mutations near the N-terminus that cause total loss of function have been observed in individuals without a personal or family history of neurodegenerative disease or neuropathy [22].
The present case strengthens the evidence suggesting an association between PRNP truncating mutations and PrP systemic amyloidosis [10]. It also illustrates the importance of exome sequencing in any patient with atypical dementia, especially early onset dementia with cyclic diarrhea or gastric upset and peripheral neuropathy, particularly with a positive family history.
ACKNOWLEDGMENTS
Supported by NIH/NIA R01 AG031189, P50AG 023501, P01 AG021601, AG10129, 2T32 AG023 481, NIH/NCRR UCSF-CTSI (UL1 RR024131), Hellman Family Foundation, and the Michael J. Homer Family Fund.
Footnotes
Authors' disclosures available online (http://j-alz.com/manuscript-disclosures/16-0300r1).
REFERENCES
- [1].Finckh U, Muller-Thomsen T, Mann U, Eggers C, Marksteiner J, Meins W, Binetti G, Alberici A, Hock C, Nitsch RM, Gal A. High prevalence of pathogenic mutations in patients with early-onset dementia detected by sequence analyses of four different genes. Am J Hum Genet. 2000;66:110–117. doi: 10.1086/302702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Finckh U, Muller-Thomsen T, Mann U, Eggers C, Mark-steiner J, Meins W, Binetti G, Alberici A, Sonderegger P, Hock C, Nitsch RM, Gal A. High frequency of mutations in four different disease genes in early-onset dementia. Ann N Y Acad Sci. 2000;920:100–106. doi: 10.1111/j.1749-6632.2000.tb06910.x. [DOI] [PubMed] [Google Scholar]
- [3].Ghetti B, Piccardo P, Spillantini MG, Ichimiya Y, Porro M, Perini F, Kitamoto T, Tateishi J, Seiler C, Frangione B, Bugiani O, Giaccone G, Prelli F, Goedert M, Dlouhy SR, Tagliavini F. Vascular variant of prion protein cerebral amyloidosis with tau-positive neurofibrillary tangles: The phenotype of the stop codon 145 mutation in PRNP. Proc Natl Acad Sci U S A. 1996;93:744–748. doi: 10.1073/pnas.93.2.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jayadev S, Nochlin D, Poorkaj P, Steinbart EJ, Mastrianni JA, Montine TJ, Ghetti B, Schellenberg GD, Bird TD, Leverenz JB. Familial prion disease with Alzheimer disease-like tau pathology and clinical phenotype. Ann Neurol. 2011;69:712–720. doi: 10.1002/ana.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Matsuzono K, Ikeda Y, Liu W, Kurata T, Deguchi S, Deguchi K, Abe K. A novel familial prion disease causing pan-autonomic-sensory neuropathy and cognitive impairment. Eur J Neurol. 2013;20:e67–e69. doi: 10.1111/ene.12089. [DOI] [PubMed] [Google Scholar]
- [6].Mead S, Gandhi S, Beck J, Caine D, Gajulapalli D, Carswell C, Hyare H, Joiner S, Ayling H, Lashley T, Linehan JM, Al-Doujaily H, Sharps B, Revesz T, Sandberg MK, Reilly MM, Koltzenburg M, Forbes A, Rudge P, Brandner S, Warren JD, Wadsworth JD, Wood NW, Holton JL, Collinge J. A novel prion disease associated with diarrhea and autonomic neuropathy. N Engl J Med. 2013;369:1904–1914. doi: 10.1056/NEJMoa1214747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Themistocleous AC, Kennett R, Husain M, Palace J, Mead S, Bennett DL. Late onset hereditary sensory and autonomic neuropathy with cognitive impairment associated with Y163X prion mutation. J Neurol. 2014;261:2230–2233. doi: 10.1007/s00415-014-7521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jansen C, Parchi P, Capellari S, Vermeij AJ, Corrado P, Baas F, Strammiello R, van Gool WA, van Swieten JC, Rozemuller AJ. Prion protein amyloidosis with divergent phenotype associated with two novel nonsense mutations in PRNP. Acta Neuropathol. 2010;119:189–197. doi: 10.1007/s00401-009-0609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kitamoto T, Iizuka R, Tateishi J. An amber mutation of prion protein in Gerstmann-Straussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun. 1993;192:525–531. doi: 10.1006/bbrc.1993.1447. [DOI] [PubMed] [Google Scholar]
- [10].Mead S, Reilly MM. A new prion disease: Relationship with central and peripheral amyloidoses. Nat Rev Neurol. 2015;11:90–97. doi: 10.1038/nrneurol.2014.263. [DOI] [PubMed] [Google Scholar]
- [11].Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- [12].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Schel-tens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hund E. Familial amyloidotic polyneuropathy: Current and emerging treatment options for transthyretin-mediated amyloidosis. Appl Clin Genet. 2012;5:37–41. doi: 10.2147/TACG.S19903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ueda M, Ando Y. Recent advances in transthyretin amyloidosis therapy. Transl Neurodegener. 2014;3:19. doi: 10.1186/2047-9158-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baets J, Duan X, Wu Y, Smith G, Seeley WW, Mademan I, McGrath NM, Beadell NC, Khoury J, Botuyan MV, Mer G, Worrell GA, Hojo K, DeLeon J, Laura M, Liu YT, Senderek J, Weis J, Van den Bergh P, Merrill SL, Reilly MM, Houlden H, Grossman M, Scherer SS, De Jonghe P, Dyck PJ, Klein CJ. Defects of mutant DNMT1 are linked to a spectrum of neurological disorders. Brain. 2015;138:845–861. doi: 10.1093/brain/awv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Klein CJ, Botuyan MV, Wu Y, Ward CJ, Nicholson GA, Hammans S, Hojo K, Yamanishi H, Karpf AR, Wallace DC, Simon M, Lander C, Boardman LA, Cunningham JM, Smith GE, Litchy WJ, Boes B, Atkinson EJ, Middha S, B Dyck PJ, Parisi JE, Mer G, Smith DI, Dyck PJ. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat Genet. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takada LT, Geschwind MD. Prion diseases. Semin Neurol. 2013;33:348–356. doi: 10.1055/s-0033-1359314. [DOI] [PubMed] [Google Scholar]
- [19].Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, Schuur M, Haik S, Collins SJ, Jansen GH, Stokin GB, Pimentel J, Hewer E, Collie D, Smith P, Roberts H, Brandel JP, van Duijn C, Pocchiari M, Begue C, Cras P, Will RG, Sanchez-Juan P. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Geschwind MD, Josephs KA, Parisi JE, Keegan BM. A 54-year-old man with slowness of movement and confusion. Neurology. 2007;69:1881–1887. doi: 10.1212/01.wnl.0000290370.14036.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guerreiro R, Bras J, Wojtas A, Rademakers R, Hardy J, Graff-Radford N. A nonsense mutation in PRNP associated with clinical Alzheimer's disease. Neurobiol Aging. 2014;35:2656, e2613–2656. doi: 10.1016/j.neurobiolaging.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, McLean CY, Tung JY, Yu LP, Gambetti P, Blevins J, Zhang S, Cohen Y, Chen W, Yamada M, Hamaguchi T, Sanjo N, Mizusawa H, Nakamura Y, Kitamoto T, Collins SJ, Boyd A, Will RG, Knight R, Ponto C, Zerr I, Kraus TF, Eigenbrod S, Giese A, Calero M, de Pedro-Cuesta J, Haik S, Laplanche JL, Bouaziz-Amar E, Brandel JP, Capellari S, Parchi P, Poleggi A, Ladogana A, O'Donnell-Luria AH, Karczewski KJ, Marshall JL, Boehnke M, Laakso M, Mohlke KL, Kahler A, Chambert K, McCarroll S, Sullivan PF, Hultman CM, Purcell SM, Sklar P, van der Lee SJ, Rozemuller A, Jansen C, Hofman A, Kraaij R, van Rooij JG, Ikram MA. Uitterlinden AG, van Duijn CM, Exome Aggregation C, Daly MJ, MacArthur DG (2016) Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med. 8:322ra329. doi: 10.1126/scitranslmed.aad5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gabizon R, Rosenman H, Meiner Z, Kahana I, Kahana E, Shugart Y, Ott J, Prusiner SB. Mutation in codon 200 and polymorphism in codon 129 of the prion protein gene in Libyan Jews with Creutzfeldt-Jakob disease. Philos Trans R Soc Lond B Biol Sci. 1994;343:385–390. doi: 10.1098/rstb.1994.0033. [DOI] [PubMed] [Google Scholar]
- [24].Ladogana A, Puopolo M, Croes EA, Budka H, Jarius C, Collins S, Klug GM, Sutcliffe T, Giulivi A, Alperovitch A, Delasnerie-Laupretre N, Brandel JP, Poser S, Kretzschmar H, Rietveld I, Mitrova E, Cuesta Jde P, Martinez-Martin P, Glatzel M, Aguzzi A, Knight R, Ward H, Pocchiari M, van Duijn CM, Will RG, Zerr I. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64:1586–1591. doi: 10.1212/01.WNL.0000160117.56690.B2. [DOI] [PubMed] [Google Scholar]
- [25].Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature. 1991;352:340–342. doi: 10.1038/352340a0. [DOI] [PubMed] [Google Scholar]
- [26].Mead S, Poulter M, Beck J, Webb TE, Campbell TA, Linehan JM, Desbruslais M, Joiner S, Wadsworth JD, King A, Lantos P, Collinge J. Inherited prion disease with six octapeptide repeat insertional mutation–molecular analysis of phenotypic heterogeneity. Brain. 2006;129:2297–2317. doi: 10.1093/brain/awl226. [DOI] [PubMed] [Google Scholar]
- [27].Minikel EV, Zerr I, Collins SJ, Ponto C, Boyd A, Klug G, Karch A, Kenny J, Collinge J, Takada LT, Forner S, Fong JC, Mead S, Geschwind MD. Ascertainment bias causes false signal of anticipation in genetic prion disease. Am J Hum Genet. 2014;95:371–382. doi: 10.1016/j.ajhg.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, Ghiso J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–130. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]