Abstract
Background:
The prevalence of gestational diabetes mellitus (GDM), as a complex problem in pregnancy, is increasing all over the world, but most noticeable in developing countries.
Aims:
To estimate GDM prevalence and associated pregnancy features in the southern part of Bosnia and Herzegovina.
Methods:
A cross-sectional observational study was conducted from October 2010 through March 2011. A total of 285 pregnant women with singleton pregnancies participated and were asigned to the study in the order they came for their usual ante-natal clinic examination. They underwent an oral glucose tolerance test (OGTT) with 75 g of glucose. Information on OGTT results, maternal characteristics and pregnancy outcomes were collected from database and medical records.
Results:
Prevalence of GDM was 10.9% according to 1999 World Health Organisation (WHO) diagnostic criteria. Prenatal cigarette smoking, previous GDM, cesarean delivery rate and neonatal hypoglycemia were significantly more frequent in the GDM group compared to the group of pregnancies with normal glucose tolerance (p = 0.015, p < 0.001, p = 0.015, p = 0.002).
Conclusion:
This study presents a relatively high prevalence of GDM in Bosnia and Herzegovina. There is a need for large well-designed study on GDM prevalence and its other features.
Keywords: gestational diabetes mellitus, prevalence, perinatal outcome, risk factors
1. INTRODUCTION
Gestational diabetes mellitus (GDM), as a common pregnancy complication, is defined as glucose intolerance with onset or first recognition during pregnancy (1). GDM is associated with adverse perinatal outcome, and increased long-term risks of type 2 diabetes mellitus, metabolic syndrome and cardiovascular disorders for both mother and offspring (2-6). Hence, the impact of GDM on maternal and infant health is of great clinical and public health importance. The prevalence of the disorder is increasing all over the world, but most noticeable in developing countries (7). Like many developing countries, Bosnia and Herzegovina is experiencing increased prevalence of obesity and other risks of GDM development (8-10). The aim of this study was to determine GDM prevalence, maternal characteristics and pregnancy outcomes for the first time among women in Bosnia and Herzegovina by using the WHO diagnostic criteria.
2. MATERIALS AND METHODS
A cross-sectional observational study was conducted in the Herzegovina-Neretva County, southern part of Bosnia and Hezegovina from October 2010 through March 2011. Actually, the research was conducted at two public, ante-natal clinics in the western part of Mostar city and Čapljina city and in the University Hospital Mostar as a reference institution for deliveries in Southern-Western region of Bosnia and Herzegovina. The participants were pregnant women with singleton pregnancies aged 18 years or older who participated in this research during their usual ante-natal clinic visits. In the study period, as recommended for high-risk population, all pregnant women from the western part of Mostar and Čapljina city who consecutively attended local public antenatal clinic at 22-32 weeks of gestation underwent an oral glucose tolerance test (OGTT) with 75 g glucose due to one or more GDM risk factors or characteristic pregnancy complications: pre-pregnancy overweight and obesity (maternal body mass index ≥ 25kg/m2), family history of diabetes type 2, previous pregnancy complicated with GDM, birth weight ≥ 4000g, stillbirth with no clear obstetric cause or major cardiovascular / CNS malformation, history of polycystic ovary syndrome, current hypertensive disorder after 20 weeks of gestation, recurrence of glucosuria, and polyhydramnios. Those with no GDM risk factors were advised to participate in the study at 22-32 weeks of gestation, and they were informed about OGTT procedure and the possible effects in the same manner as we informed the high-risk pregnant women. Gestational age was determined by last menstrual period (LMP) and according to early ultrasound examination. Exclusion criteria were diabetes type 1 or 2 prior to pregnancy, multifetal pregnancy and age younger than 18. A total of 285 pregnant women finally participated in this study. All of them completed OGTT and gave birth at the University Hospital Mostar where the data were finally collected for the prevalence, maternal characteristics and pregnancy outcomes (premature delivery, cesarean delivery, large for gestational age/LGA, birth trauma, neonatal hypoglycemia, neonatal hyperbilirubinemia, early neonatal death). LGA was defined as a birth weight above the 90th percentile for gestational age. Neonatal hypoglycemia was defined as glucose level < 2.2 mmol/L or < 40mg/dl. Neonatal hyperbilirubinemia was defined as jaundice requiring phototherapy, except the one caused by ABO or Rh isoimmunisation. Each participant underwent a standard 2-h OGTT, with the use of a 75g dose of glucose, and with plasma glucose levels (PGL) measured fasting and 2-hour after ingestion. PGL was measured by means of enzymatic methods according to International Federation of Clinical Chemistry (IFCC). All participants were required to fast staring the night before.
Statistical analysis was performed using descriptive statistics, c2 test, Fisher’s exact test, and Student’s t-test for independent samples or Mann-Whitney U test depending on the sample distribution. Mean and standard deviations, as well as, median and interquartile range are reported for continuous variables. Frequency and percentage were reported for categorical variables. The distribution of the sample was tested by Kolmogorov-Smirnov test. Software system SPSS for Windows (11.0, SPSS Inc, Chicago, Illinois, USA) was used for statistical analysis of the obtained data. Statistical significant difference was at p < 0.05.
3. RESULTS
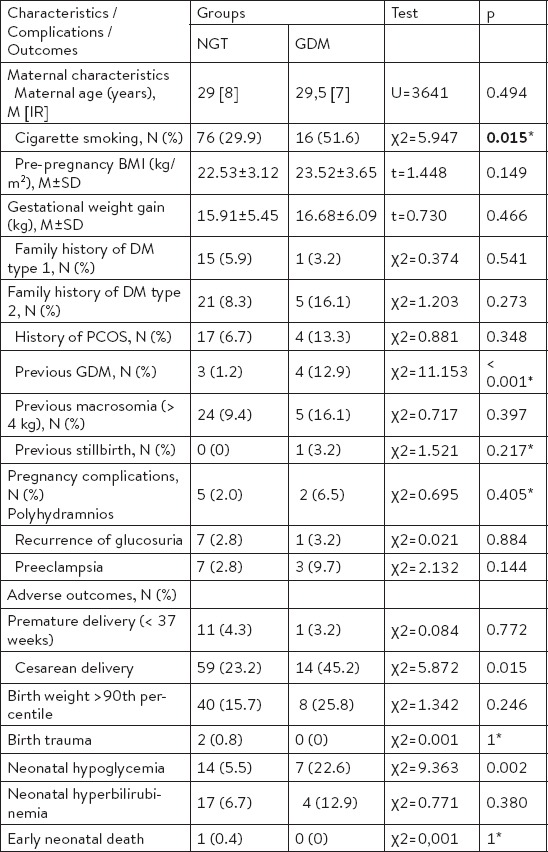
A total of 285 participants had a diagnostic OGTT and complete data regarding maternal characteristics and perinatal outcomes during the study period were obtained. Maternal and their newborns’ characteristics, and pregnancy outcomes are summarized in Table 1. The mean age of the participants was 29.8 ± 5.6 years (mean ± SD), and more than half of them were primiparas (51.2%). The GDM was diagnosed in 31 (10.9%) participants according to the WHO diagnostic criteria (Table 2). Cigarette smoking and previous GDM as GDM risk factors were significantly more frequent in the GDM group (p = 0.015, p < 0.001) compared to the group of pregnancies with normal glucose tolerance. Further, Table 2 shows differences in pregnancy complications and adverse perinatal outcomes of the participants according to GDM status. There were significant differences in cesarean deliveries and neonatal hypoglycemia (p = 0.015, p = 0.002).
Table 1.
Maternal and newborns’ characteristics, and pregnancy outcomes in the group of participants (n=285)
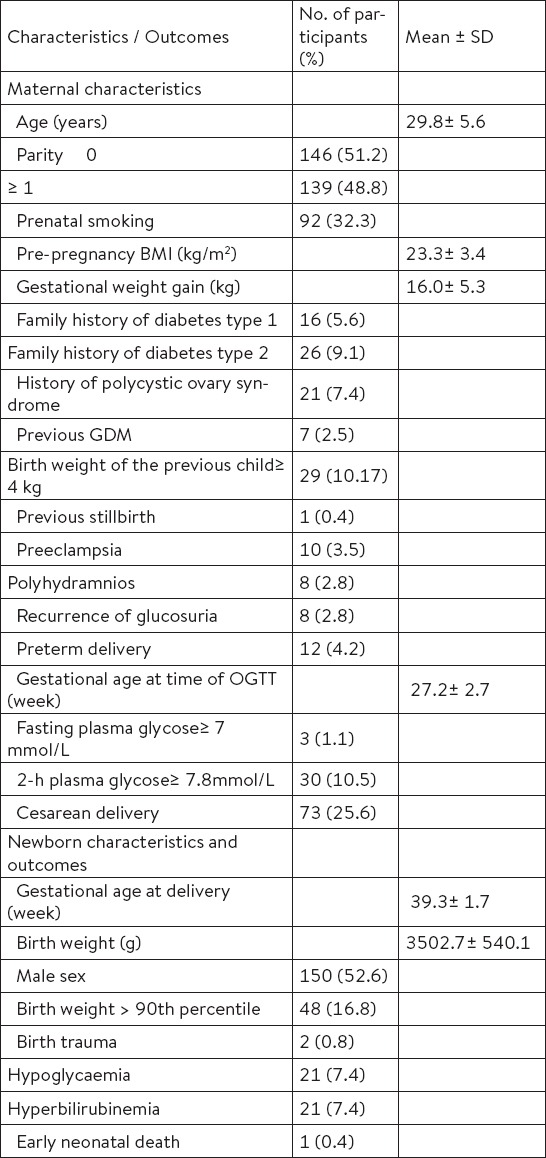
Table 2.
Comparison of the maternal characteristics, pregnancy complications and adverse outcomes between the groups of pregnancies with normal glucose tolerance (NGT) (n = 254) and GDM (n = 31)
4. DISCUSSION
Gestational diabetes mellitus has long been recognised as a complex problem in pregnancy due to significant levels of fetal and maternal morbidity (2, 4, 11). The prevalence of the disorder is increasing all over the world, but most noticeable in developing countries (7). The main objective of this study was to asses prevalence of GDM and associated risk factors as well as pregnancy outcomes in the Southern part of Bosnia and Herzegovina. This is the first study to estimate the prevalence of GDM in this region of developing countries. We found that the prevalence of GDM was 10.9% according to 1999 WHO criteria. GDM prevalence in European countries is most often reported as 2-6% of all pregnancies (12). Reported GDM prevalence is higher in the Southern Mediterranean seaboard but also in some other parts of Europe such as Ireland (10%) and Finland (10-11%) (12,13). Accordingly, our unselected population of pregnant women should be classified to the high GDM prevalence group (10.9%). This result is comparable with the studies published from Asian developing countries, including Bangladesh (9.6%), Malaysia (11.4%) and India (13.9%) (7, 14, 15). South Asian ethnic origin was observed as an independent risk of GDM and type 2 diabetes development (16, 17). It is well known that the prevalence of GDM varies widely worlwide according to the various diagnostic criteria that are of significantly different sensitivities and specificities (18). The variation may be also due to age and ethnic structure of the study population, dietary habits diversity, and socioeconomic factors. The estimated high prevalence of GDM in developing countries, including Bosnia and Herzegovina could be explanined by socioeconomic factors. Like many developing countries, Bosnia and Herzegovina is experiencing increased prevalence of obesity and other risks of GDM development due to socioeconomic status and lifestyle factors (8-10). It is likely that stressful events, dietary habits and sedentary life styles are responsible for the increase in obesity and GDM (19). In a previous study, Cullinan at al. found increased prevalence of GDM for women in the lowest socioeconomic group when compared to the highest, suggesting a strong association between socioeconomic status and GDM prevalence (20). Comparing maternal characteristics according to the GDM status we observed statistically significant association between GDM and prenatal cigarette smoking. Previous studies of associations between cigarette smoking and GDM are uncommon and conflicting (10). However, prevalence of cigarette smoking as a behavioural risk factor in our pregnant women population is worrying in comparison with some other reports (21). We also observed statistically significant association between onset of GDM and previous GDM. This data is consistent with the observations published in some other studies (22, 23). Previous studies reported older age as an independent risk factor for GDM, as well as increasing pre-pregnancy BMI and excessive gestational weight gain (10, 22-24). In the present study, the mean age of GDM participants as well as the mean pre-pregnancy BMI and the mean gestational weight gain was higher, but not statistically significant. In addition, family history of diabetes type 2, history of polycystic ovary syndrome, birth weight of the previous child ≥ 4 kg and previous stillbirth as GDM risk factors were more frequent in the GDM group compared to the group of pregnancies with normal glucose tolerance, but not significantly. Most of the previous studies that reported significant associations between GDM and listed maternal charasteristics (family history od diabetes type 2, increasing pre-pregnancy BMI, excessive gestational weigt gain, history of polycystic ovary syndrome, birth weight of the previous child ≥ 4 kg and previous stillbirth) had significantly higher number of participants and thus increased statistical power (10, 22-25). Analyzing specific GDM pregnancy complications and outcomes, we found significant difference between GDM group and normal tolerance glucose group in cesarean delivery rate and neonatal hypoglycemia. These data are consistent with previous published observations (26, 27).
Our study had some limitations. A cross-sectional design of our research could have resulted in selection bias because only women with singleton pregnancies who had an antenatal check-up during an observed time interval and in selected clinics participated in the study. Thus, our results may not be generalizable to all Bosnia and Herzegovina women. Further, our study did not have sufficient statistical power for maternal characteristics as GDM risk factors and pregnancy outcomes examination due to relatively small sample.
5. CONCLUSION
This study presents a relatively high prevalence of GDM in Bosnia and Herzegovina. There is a general observation that the prevalence of GDM is increasing in developing countries. Accordingly, it is important to increase awareness of GDM among medical professionals and pregnant women in Bosnia and Herzegovina. Ultimately, there is a need for large well-designed study on GDM prevalence and its other features in this region of the developing countries.
Footnotes
• Conlict of interest: none declared.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarinder U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity: what is the link? Curr Opin Obstet Gynecol. 2012;24:376–81. doi: 10.1097/GCO.0b013e328359f0f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292–301. doi: 10.1111/dme.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malcolm J. Through the looking glass: gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes Metab Res Rev. 2012;28:307–11. doi: 10.1002/dmrr.2275. [DOI] [PubMed] [Google Scholar]
- 6.Harreiter J, Dovjak G, Kautzky-Willer A. Gestational diabetes mellitus and cardiovascular risk after pregnancy. Womens Health. 2014;10:91–108. doi: 10.2217/whe.13.69. [DOI] [PubMed] [Google Scholar]
- 7.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, Datta M. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu)-a community based study. 2008;56:329–33. [PubMed] [Google Scholar]
- 8.Pilav A, Nissinen A, Haukkala A, Niksic D, Laatikainen T. Cardiovascular risk factors in the Federation of Bosnia and Herzegovina. Eur J Public Health. 2007;17:75–9. doi: 10.1093/eurpub/ckl066. [DOI] [PubMed] [Google Scholar]
- 9.Singh J, Huang CC, Driggers RW, Timofeev J, Amini D, Landy HJ, et al. The impact of pre-pregnancy body mass index on the risk of gestational diabetes. J Matern Fetal Neonatal Med. 2012;25:5–10. doi: 10.3109/14767058.2012.626920. [DOI] [PubMed] [Google Scholar]
- 10.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–83. [PubMed] [Google Scholar]
- 11.Hod M, Merlob P, Friedman S, Schoenfeld A, Ovadia J. Gestational diabetes mellitus. A survey of perinatal complications in the 1980s. Diabetes. 1991;40:S74–8. doi: 10.2337/diab.40.2.s74. [DOI] [PubMed] [Google Scholar]
- 12.Buckley BS, Harreiter J, Damm P, Chico A, Simmons D, Vellinga A, Dunne F DALI Core Investigator Group. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29:844–54. doi: 10.1111/j.1464-5491.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamberg S, Raitanen J, Rissanen P, Luoto R. Prevalence and regional differences of gestational diabetes mellitus and oral glucose tolerance tests in Finland. Eur J Public Health. 2012;22:278–80. doi: 10.1093/eurpub/ckq193. [DOI] [PubMed] [Google Scholar]
- 14.Jesmin S, Akter S, Akashi H, Al-Mamun A, Rahman MA, Islam MM, Sohael F, Okazaki O, Moroi M, Kawano S, Mizutani T. Screening for gestational diabetes mellitus and its prevalence in Bangladesh. Diabetes Res Clin Pract. 2014;103:57–62. doi: 10.1016/j.diabres.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Tan PC, Ling LP, Omar SZ. Screening for gestational diabetes at antenatal booking in a Malaysian university hospital: the role of risk factors and threshold value for the 50-g glucose challenge test. Aust N Z J Obstet Gynaecol. 2007;47:191–7. doi: 10.1111/j.1479-828X.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenum AK, Mørkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol. 2012;166:317–24. doi: 10.1530/EJE-11-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenum AK, Sommer C, Sletner L, Mørkrid K, Bærug A, Mosdøl A. Adiposity and hyperglycaemia in pregnancy and related health outcomes in European ethnic minorities of Asian and African origin: a review. Food Nutr Res. 2013:57. doi: 10.3402/fnr.v57i0.18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomić V, Petrović O, CrnčevićOrlić Ž, Mandić V. Gestational diabetes and pregnancy outcome-do we have right diagnostic criteria? J Matern Fetal Neonatal Med. 2013;26:854–9. doi: 10.3109/14767058.2013.776530. [DOI] [PubMed] [Google Scholar]
- 19.Hosler AS, Nayak SG, Radigan AM. Stressful events, smoking exposure and other maternal risk factors associated with gestational diabetes mellitus. Paediatr Perinat Epidemiol. 2011;25:566–74. doi: 10.1111/j.1365-3016.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 20.Cullinan J, Gillespie P, Owens L, Avalos G, Dunne FP. Is there a socioeconomic gradient in the prevalence of gestational diabetes mellitus? Ir Med J. 2012;105:21–3. [PubMed] [Google Scholar]
- 21.Moore Simas TA, Szegda KL, Liao X, Pekow P, Markenson G, Chasan-Taber L. Cigarette smoking and gestational diabetes mellitus in Hispanic woman. Diabetes Res Clin Pract. 2014;105:126–34. doi: 10.1016/j.diabres.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol. 2011;51:26–30. doi: 10.1111/j.1479-828X.2011.01292.x. Comment in: Hare A. Re: ‘Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines’. Teh WT, Teede HJ, Paul E. et al. ANZJOG 2011; 51: 26-30. Aust N Z J Obstet Gynaecol. 2011; 51: 383; author reply 383-4. [DOI] [PubMed] [Google Scholar]
- 23.Khan R, Ali K, Khan Z. Socio-demographic Risk Factors of Gestational Diabetes Mellitus. Pak J Med Sci. 2013;29:843–6. doi: 10.12669/pjms.293.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Cianni G, Volpe L, Lencioni C, Miccoli R, Cuccuru I, Ghio A, et al. Prevalence and risk factors for gestational diabetes assessed by universal screening. Diabetes Res Clin Pract. 2003;62:131–7. doi: 10.1016/j.diabres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 25.De Wilde MA, Veltman-Verhulst SM, Goverde AJ, Lambalk CB, Laven JS, Franx A, et al. Preconception predictors of gestational diabetes: a multicentre prospective cohort study on the predominant complication of pregnancy in polycystic ovary syndrome. Hum Reprod. 2014;29:1327–36. doi: 10.1093/humrep/deu077. [DOI] [PubMed] [Google Scholar]
- 26.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes-a systematic review of the WHO and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hay WW., Jr Care of the infant of the diabetic mother. Curr Diab Rep. 2012;12:4–15. doi: 10.1007/s11892-011-0243-6. [DOI] [PubMed] [Google Scholar]


