Abstract
Gold based nanoparticles with strong near infra-red (NIR) absorption are ideally suited for photothermal therapy (PTT) of brain tumors. The goal of PTT is to induce rapid heating in tumor tissues while minimizing thermal diffusion to normal brain. PTT efficacy is sensitively dependent on both nanoparticle concentration and distribution in tumor tissues. Nanoparticle delivery via passive approaches such as the enhanced permeability and retention (EPR) effect is unlikely to achieve sufficient nanoparticle concentrations throughout tumor volumes required for effective PTT. A simple approach for improving tumor biodsitribution of nanoparticles is the use of cellular delivery vehicles. Specifically, this review focuses on the use of monocytes/macrophages (Mo/Ma) as gold nanoparticle delivery vectors for PTT of brain tumors. Although the efficacy of this delivery approach has been demonstrated in both in vitro and animal PTT studies, its clinical potential for the treatment of brain tumors remains uncertain.
Keywords: Photothermal therapy, Gold nanoparticles, Macrophages, Brain tumor
Introduction
Numerous studies have demonstrated the utility of nanoparticles in both diagnostic and therapeutic medicine. This is due to a number of factors including the ability to control their shape, size and synthesis, as well as the ability to manipulate their optical properties (Lin et al. 2005). Gold-based nanoparticles are particularly attractive due to their low toxicity and ease of surface conjugation of biomolecules to facilitate molecular specificity (Hirsch et al. 2006). A variety of gold nanoparticles have been investigated for their utility in photothermal therapy (PTT) including gold nanorods (AuNR), gold-silica nanoshells (AuNS), gold nanocages, gold colloidal nanospheres, hollow gold nanoshells and gold-gold sulfide nanoparticles. The choice of nanoparticle for PTT will depend on a number of factors including maximum absorption wavelength, particle size and absorption and scattering cross sections. With the exception of gold colloidal nanospheres, optimum absorption of NIR light can be achieved by varying the dimension and/or composition of gold nanoparticles. This is an important consideration since NIR light has significant penetration in biological tissues (Oldenburg et al. 1998). The maximum absorption resonance of gold colloidal nanospheres occurs in the visible (approximately 530 nm) which makes them ill-suited for in vivo applications due to the limited tissue penetration of light at this wavelength. Since the photothermal transduction efficiencies (fraction of incident light converted to thermal energy) of the different types of gold nanoparticles do not vary by more than a factor of three, no gold nanoparticle has a significant advantage over the others (Cole et al. 2009). Compared to conventional dyes, the absorption cross section of gold nanoparticles can be many orders of magnitude higher (Loo et al. 2004) and, unlike dyes, they are photostable and do not undergo photobleaching thereby facilitating higher light energies and longer irradiation times. Taken together, these properties can be used to create localized heating or drug release, thus providing the basis for therapeutic applications.
Photothermal properties of gold nanoparticles
Photon interactions with nanoparticles are dominated by scattering and absorption. For the purposes of therapeutic applications, nanoparticles with high absorption and low scattering cross sections are desirable (Aslan et al. 2005). Light interacts with metal nanoparticles via the surface plasmon resonance (SPR) effect, i.e., the collective oscillation of free electrons at metallic surfaces which causes the nanoparticles to absorb and scatter electromagnetic radiation of wavelengths much larger than the particles. Excitation of surface electrons via photon absorption yields a heated electron gas that cools by exchanging its excitation energy with the nanoparticle lattice via electron-phonon interactions. The lattice itself cools within 100 ps by dissipating its energy to the surrounding medium via photon-photon interactions (Huang et al. 2006; Huang et al. 2008). Conversion of light energy to heat is optimized by using a laser at a wavelength that overlaps with the nanoparticle’s SPR wavelength which for gold, silver and copper occurs in the visible region (Link and El-Sayed 2003). This approach has been used in cancer therapeutics and is termed plasmonic photothermal therapy, or simply, photothermal therapy.
In the case of 40 nm gold colloidal nanospheres, the SPR wavelength occurs at approximately 530 nm corresponding to green light. Although significant absorption and, hence heating, is observed at 530 nm (Link and El-Sayed 1999), this wavelength has relatively shallow penetration in biological tissues which limits its therapeutic utility. In contrast, AuNS have a strong SPR in the NIR: the exact absorption peak depends on the ratio of gold shell to silica core thickness. As the shell thickness decreases, the peak absorption shifts to longer wavelengths due to increased coupling between the inner and outer shell surface plasmons (Prodan et al. 2003; Jain and El-Sayed 2007). Two absorption bands are observed for AuNR; a strong band in the near infrared (NIR) region due to the oscillation of electrons along the longitudinal axis, and a weaker band in the visible region (approximately 520 nm) due to transverse electronic oscillations (Huang et al. 2006; Huang et al. 2008). In this case, the peak absorption wavelength depends on the nanorod’s aspect ratio (length-to-width).
Photothermal therapy
As with any hyperthermic-based cancer therapy, the goal of nanoparticle-based hyperthermia is the destruction of tumor tissue while avoiding excessive heating of normal tissues – a common problem with well established hyperthermia techniques employing either radiofrequency or magnetic thermal ablation (Liu et al. 2008). Since biological tissues lack NIR-absorbing chromophores, the use of laser wavelengths in the “tissue optical window” (700 – 1000 nm) minimizes tissue heating. With the exception of gold colloidal nanospheres, gold nanoparticles have strong and tunable absorption in the NIR region so that the combination of two benign moieties, gold nanoparticles and NIR light, facilitates selective heating of tumors loaded with nanoparticles. For example, most AuNS PTT studies have employed diode lasers with wavelengths of 805–810 nm (Terentyuk et al. 2009). To achieve themal ablation, tissues are typically heated to temperatures exceeding 50°C, where tumors are selectively destroyed due to their poor vasculature and subsequent low heat tolerance.
Optimal PTT requires preferential localization and/or retention of nanoparticles in tumor tissues. Nanoparticle specificity is a natural consequence of the tumor vasculature which consists of rapidly formed defective vessels with large intercellular gaps (≤ 2 µm) allowing passage of large compounds from the circulation into tumors. This passive process is termed “enhanced permeability and retention (EPR)” (Maeda 2001). Significant accumulation of nanoparticles in tumors via the EPR effect is complicated by their removal from the circulation by Ma, in particular the Kupffer cells of the liver (Cuenca et al. 2006). A simple strategy to avoid detection by the immune system is to coat the surfaces of the nanoparticles with a polymer such as polyethylene glycol (PEG) (Hirsch et al. 2006). Although nanoshell accumulation via the EPR effect has been observed in a wide variety of tumors, the distribution is often very heterogeneous due to the imperfect tumor vasculature. As a result, it is not uncommon to find large regions within tumors with insufficient nanoparticle concentrations for effective PTT. This has provided the impetus for the development of “active” delivery approaches including the use of cell-based vectors such as Mo/Ma.
Limitations of the EPR effect
Although a great deal of progress in the development of multifunctional nanoscale vehicles for improved therapy has been achieved, the limited penetration of nanoagents, from the circulation into tumor tissue, is one of the main limitations to therapeutic efficacy. The distinctive tumor microenvironment, characterized by leaky vasculature and poor lymphatic drainage, enables the EPR effect, but unfortunately it is also one of its main barriers. The tumor microenvironment is heterogeneous consisting, in addition to cancer cells, of stromal cells, Mo and Mo-derived tumor associated macrophages (TAM), stem cells and a compact and dense extracellular matrix. These factors, combined with high interstitial fluid pressure and irregular blood supply found in tumor tissue, serve as significant barriers to inhibit penetration of nanoparticles (Tredan et al. 2007; Barua and Mitragotri 2014; Huynh and Zheng 2015; Maeda 2015). Increasing evidence suggests that nano-scale delivery systems are too large (ca 100 nm) to extravasate deep into tumor regions, and are limited to a penetration depth of only 1 to 2 cell layers from the nearest tumor capillaries (Fig. 1a). As gliomas grow their centers become necrotic due to the rapid proliferation of malignant cells within the core of the tumor at increasingly larger distances from the capillary blood supply. Although this isolation of cells renders the hypoxic areas of gliomas inaccessible to nanoparticle-based therapies via the EPR effect, this portion of the tumor is resected during surgery. It is in the resection margins, partially protected by the blood-brain barrier (BBB), where nanoparticle accumulation is desirable. Since the vasculature in this region is typically normal, the EPR effect is even less efficient than in solid tumors thus limiting the concentration of nanoparticles necessary for effective hyperthermia. While methods for improving tumor penetration of nanoparticles via the EPR effect have been vigorously pursued, recent discussions have focused on whether the EPR effect is actually clinically relevant since it appears to be observed only in animal models (Prabhakar et al. 2013). This skepticism originates from the lack of clinical evidence demonstrating improved therapeutic efficacy in contrast to the successes achieved in animal models.
Fig. 1.
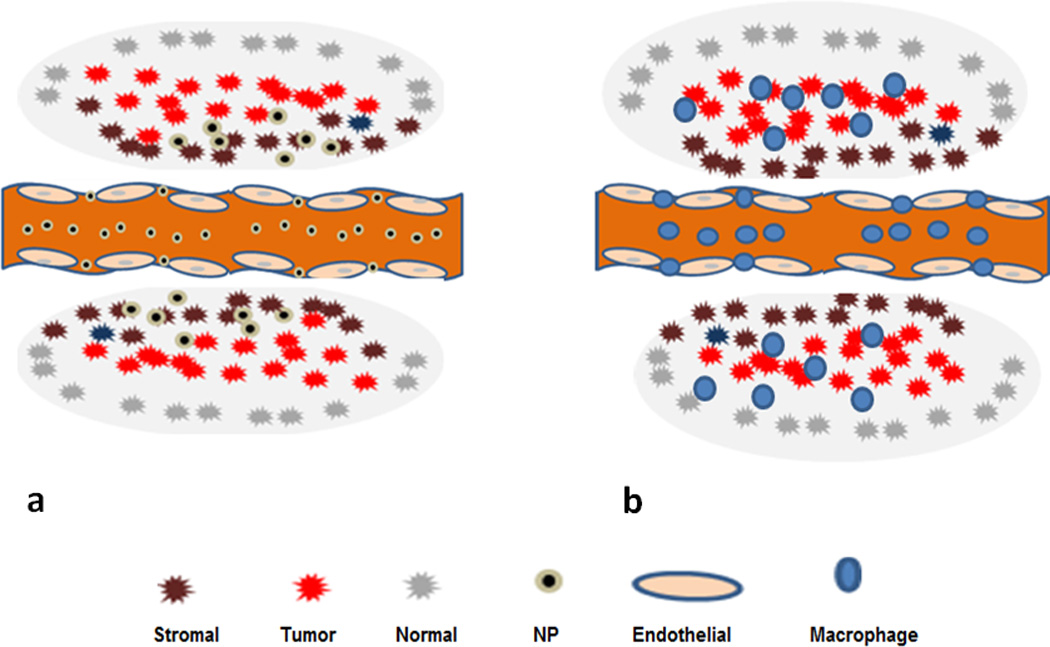
EPR effect vs cell-based delivery of nanoparticles. (a) Nanoparticles penetrate tumors via extravasation through gaps between the endothelial cells driven only by concentration gradients. The penetration of nanoparticles is limited due to tumor vasculature, heterogeneity, the presence of stromal cells and extra cellular matrix (gray region). (b) In contrast, loaded Ma are actively drawn to tumors and infiltrate the tumor tissue
Cell based delivery of nanoparticles
One method to overcome the limitations of the EPR effect is to use cells as delivery vehicles for nanoparticles. The ability of certain types of stem and immune cells to target tumors allows their utilization as vectors to deliver several types of anti-cancer payloads (Fischbach et al. 2013; Basel et al. 2014). Cells have the ability to target and infiltrate the tumor interstitium by an active process (Fig. 1b) and, as such, they can obtain access to tumors despite stromal barriers and the elevated interstitial pressure characteristic of most tumors. The cells are attracted to tumors by gradients of chemokines, cytokines, and growth factors. Of the cell types suited as delivery vectors i.e. neural stem cells, mesenchymal stem cells and Mo/Ma, the latter are particularly well suited as delivery vehicles for gold-based nanoparticles (Christie et al. 2015).
The ability of Mo/Ma to ingest large amounts of AuNS or AuNR for NIR PTT has been investigated by our group as well as by others (Choi et al. 2007; Kah et al. 2009; Baek et al. 2011, Chhetri et al. 2014; Trinidad et al. 2014). Our studies have employed Mo and Ma from mouse and rat respectively in combination with rat and human glioma cells. Previous studies have shown that size, shape and surface chemistry influence phagocytosis. For example, smaller particles are more readily phagocytized than larger ones as are particles with shapes similar to pathogens such as bacteria. The effects of surface chemistry on phagocytosis have been studied extensively. Generally, the addition of a polyethylene glycol coating (PEGylated) impairs phagocytosis by delaying opsonization, or adsorption of proteins which enhance phagocytosis (Kah et al. 2009). Electron microscopy of AuNS-loaded macrophages (MaNs) revealed AuNS, in small aggregates, inside vacuoles dispersed throughout the cellular cytoplasm (Choi et al. 2007; Baek et al. 2011).
In vitro macrophage-mediated AuNS PTT glioma model
Spheroid cultures, consisting of glioma cells have been used in a number of studies of the direct effects of treatment modalities on tumors since they represent an appealing alternative to monolayers and animal models. Multicell spheroids are three-dimensional aggregates of tumor cells that mimic micro-tumors prior to their vascularization. Tumor cells within spheroids show a higher degree of morphological and functional differentiation than cells grown in monolayer culture. They also display growth kinetics, metabolic rates, and resistance to therapy similar to tumor cells in vivo (Madsen et al. 2006). The ability of fluorescent labeled Mo/Ma to infiltrate into multicell tumor spheroids was investigated by Baek et al. (2011) using two-photon microscopy. Following 48 h of co-incubation, Mo/Ma were seen to be distributed throughout the spheroid. This was most probably caused by chemoatractive agents produced by tumor cells acting on the Mo/Ma. This hypothesis was tested in a collagen migration assay, consisting of two collagen layers, one containing a single tumor spheroid and the other containing distributed Mo/Ma. Spheroids were placed eccentrically in order to differentiate between random and induced cell migration. Mo/Ma migration occurred only in the region where the spheroid was closest to the Mo/Ma collagen layer, with the tumor cells migrating out of and the Mo/Ma migrating towards the spheroid, respectively (Hirschberg et al. 2010).
The dependence of the ratio of loaded Ma to tumor cells on PTT efficacy is a critical parameter. This has been investigated in hybrid spheroids, produced from a suspension mixture of tumor cells and MaNS or gold nanorod-loaded macrophages (MaNR). This technique produces spheroids of known tumor cell to Ma ratios with Ma distributed uniformly throughout the spheroid. Hybrid multicell human glioma spheroids, formed with the addition of empty or MaNS/MaNR, were studied in a series of experiments (Baek et al. 2011; Madsen et al. 2015]. The basic experimental setup is shown in Fig. 2.
Fig. 2.
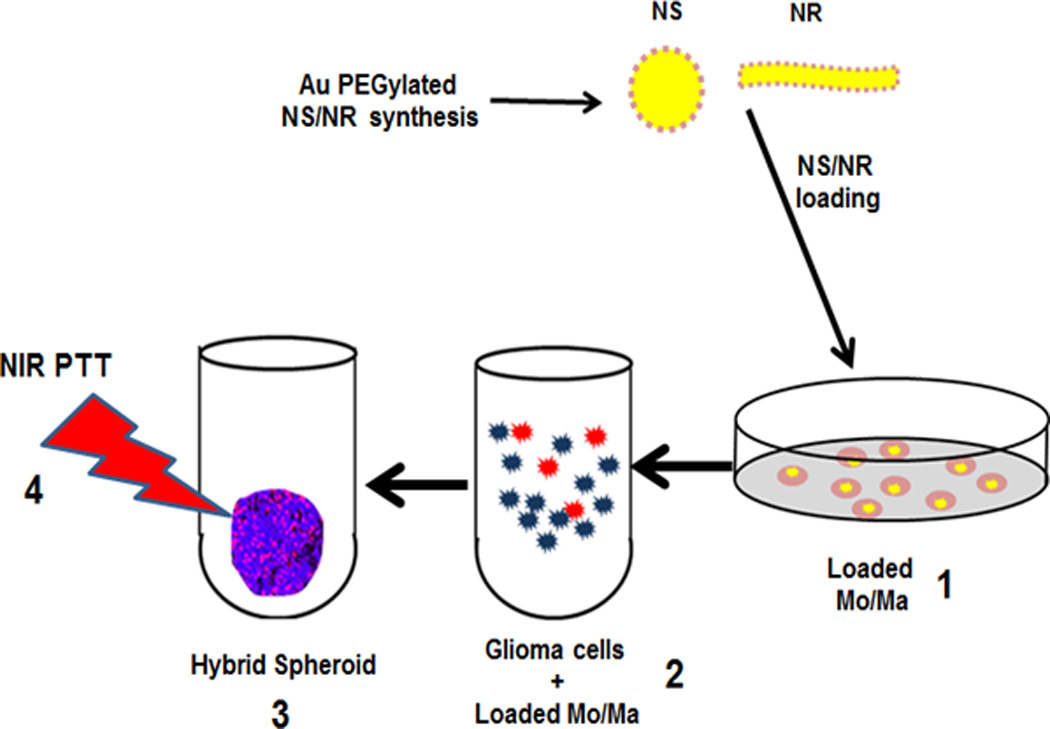
In vitro PTT of hybrid spheroids. 1) Mo/Ma co-incubated with either AuNS or AuNR. 2) Loaded Mo/Ma (red) combined with glioma cells (blue) at different ratios. 3) Hybrid spheroids of the two cell types are formed. The loaded Mo/Ma are distributed throughout the spheroid 4) NIR light treatment (PTT)
Baek et al. (2011) investigated the effects of exposure to laser NIR on multicell human glioma spheroids infiltrated with empty (containing no AuNS) or MaNS. The results demonstrated that: 1) murine Mo/Ma could efficiently take up bare or PEGylated AuNS, 2) MaNS infiltrated into glioma spheroids to the same or, in some cases, to a greater degree than empty Mo/Ma, 3) NIR laser irradiation of spheroids incorporating MaNS resulted in complete growth inhibition in an irradiance dependent manner and 4) spheroids infiltrated with empty macrophages had growth curves identical to untreated control cultures.
Employing a similar human glioma/Mo hybrid spheroid model Chhetri et al. (2014) compared the efficacy of AuNR-mediated PTT to that obtained using AuNS. Overall, the results showed that, despite the ability of Mo to ingest a larger number of AuNR compared to AuNS, on a per nanoparticle bases, AuNS demonstrated a greater photothermal efficiency. For example, hybrid spheroids consisting of a 5:1 ratio of glioma cells to MaNS exhibited significant growth inhibition when subjected to irradiances of 7 W cm−2. In contrast, no growth inhibition was observed for the MaNR hybrid spheroids, even at the highest irradiance investigated (28 W cm−2). Growth inhibition was observed at 28 W cm−2 when the nanorod concentration was increased, i.e., by forming hybrid spheroids with a 2:1 ratio of glioma cells to Ma. The increased efficacy of AuNS is likely due to their larger photothermal transduction cross-sections compared to AuNR.
Combined Ma-mediated PTT with other modalities
Combined treatment modalities to increase the efficacy of MaNs-mediated PTT have been examined in systems similar to the ones described above. The efficacy of photodynamic therapy (PDT), a commonly used treatment modality for certain types of cancer, has been shown to be enhanced by moderate hyperthermia (MHT). Employing a hybrid monolayer system similar to the spheroid cultures previously described, Trinadad et al. (2014) examined the ability of MaNS-mediated PTT to potentiate the cytotoxic effects of PDT for squamous cell head and neck carcinoma. Two different laser light wavelengths were employed simultaneously, one to activate the photosensitizer (λ = 670 nm) and the other for MaNS-mediated PTT (λ = 810 nm). The synergistic effect of the combined treatments on the viability of tumor cell monolayer cultures was clearly significant compared to each modality acting alone. For comparable PTT toxic effects, much lower laser power levels were required for combined treatment compared to PTT alone. The mechanism of synergism between PDT and PTT is, in part due to the inhibition of cellular repair and lowered activation energy of protein denaturation, caused by PDT, thus making the proteins more susceptible to thermal damage.
Madsen et al. (2016) recently described experiments that demonstrated a synergistic response of combined MHT induced by NIR activation of MaNS and commonly used chemotherapeutic agents. The model system consisted of monolayers of murine MaNS in co-culture with tumor cells. Suboptimal levels of both drugs and MHT were used. The results showed that MHT combined with cisplatin resulted in synergism while additive effects were observed for concurrent treatments of MHT and both doxorubicin and bleomycin. The three drugs are incorporated into DNA causing damage which often results in cell death unless the damage is repaired. Since hyperthermia inhibits DNA repair, it’s postulated that the addition of heat enhances the cytotoxicity of the chemotherapeutic agents via inhibition of DNA repair.
In vivo macrophage mediated AuNS PTT
In three of the five in vivo PTT brain tumor studies reported in the literature (Schwartz et al. (2009); Chen et al. 2010); Day et al. 2011) passive tumor accumulation of PEGylated gold nanoparticles (AuNS or gold nanocages) via the EPR effect was followed by NIR laser irradiation. In all three animal models, gold nanoparticle-mediated PTT was found to be highly effective based on tumor growth kinetics. In the remaining two studies, targeted approaches for improving tumor selectivity of AuNS were employed. Day et al. (2012) investigated the use of AuNS conjugated to vascular endothelial growth factor (VEGF) for vascular-targeted PTT of gliomas. It was shown that VEGF targeting doubled the proportion of AuNS bound to tumor vessels resulting in significant vascular disruption following NIR exposure. The normal brain was unharmed in all treatment and control animals. The study showed that PTT with vascular targeted AuNS is an effective inducer of vascular distruption in gliomas. To date, only one in vivo MaNS PTT study has been reported in the literature. Madsen et al. (2015) investigated PTT efficacy using a Sprague-Dawley rat glioma model. Animals received direct intracranial injection of C6 glioma cells. These newly implanted glioma cells, were used as a proxy to simulate infiltrating cells remaining in the resection margin following surgical removal of bulk tumor. Two days later, an equal number of MaNS were implanted in the glioma injection site followed by NIR laser irradiation. MaNS mediated PTT resulted in either complete tumor inhibition or delayed growth thus demonstrating the potential of this targeted PTT approach. As was observed in in vitro experiments, high energy levels (0.6 kJ) were required to achieve inhibition of tumor development. In related experiments, Yang et al. (2015) injected Ma into peri-tumoral sites in experimental squamous cell tumors in mice. Penetration of the Ma throughout the tumor was observed approximately 48 h after injection. NIR laser irradiation (PTT) of the tumor site led to significant and extensive cellular destruction seen evenly distributed within the tumor, immediately after treatment.
Tumor infiltration of indigenous Mo/Ma
The use of Mo/Ma as cell-based delivery vehicles for nanoparticles stemmed from the observation that they naturally infiltrate most solid tumors including brain tumors (Badie et al. 2000; Roggendorf et al. 2004; Carolina et al. 2013). TAM are frequently found in and around glioblastomas in both experimental animals and patient biopsies: it has been shown that TAM can constitute up to a third of the tumor mass in glioblastomas (Badie et al. 2000; Roggendorf et al. 2004). These observations indicate local synthesis of Mo/Ma chemo-attractive factors in gliomas and that inflammatory cells can pass through the compromised blood-brain tumor barrier (BBTB) and, most importantly, into the brain adjacent-to-tumor (BAT) region where post operatively, the concentration of infiltrating glioma cells is highest and recurrences most often occur (Petrecca et al. 2013). Mo trafficking into the CNS is dependent on cell–cell interactions that involve endothelial cells and astrocytes, as well as the local release of factors that promote BBB permeability (Hirschberg et al 2008; Mathews et al. 2011). Additionally, TAM infiltration has been positively correlated to the histological grade of the malignancies and the number of glioma initiating cells (GIC) (Liang et al. 2010).
Tumor infiltration of exogenous Mo/Ma
Intravenously injected Ma loaded with iron oxide nanoparticles have been shown to target experimental brain tumors (Valable et al. 2008). This indicates that the local synthesis of chemo attractive factors in gliomas also attract loaded inflammatory cells intravenously introduced into the circulation. These findings demonstrate that exogenous iron-oxide labeled Ma are capable of infiltrating small (less than 10 mm3) as well as large (25–70 mm3) glioma brain tumors in rats. Subsequent T2*-weighted MR imaging revealed the invasion of the entire tumor mass by the labeled Ma. Importantly, these authors demonstrated a higher density of loaded Mo/Ma at the tumor borders. In patients, surgical tumor resection is usually the first modality employed in the treatment of glioma. Rather than treating un-resected tumors, the therapeutic goal following surgical resection is the elimination of infiltrating tumor cells remaining in the margins of the resection cavity and in surrounding brain, where most tumor recurrences occur. These infiltrating nests of glioma cells are protected by the BBB which can vary from intact to partially compromised. The observation that loaded Ma migrate to and concentrate around tumor borders and BAT areas in the brain (i.e. the post-operative resection margin) is therefore of significant clinical relevance for therapy and, as such, one can envision clinical protocols such as the one illustrated in Figure 3.
Fig. 3.
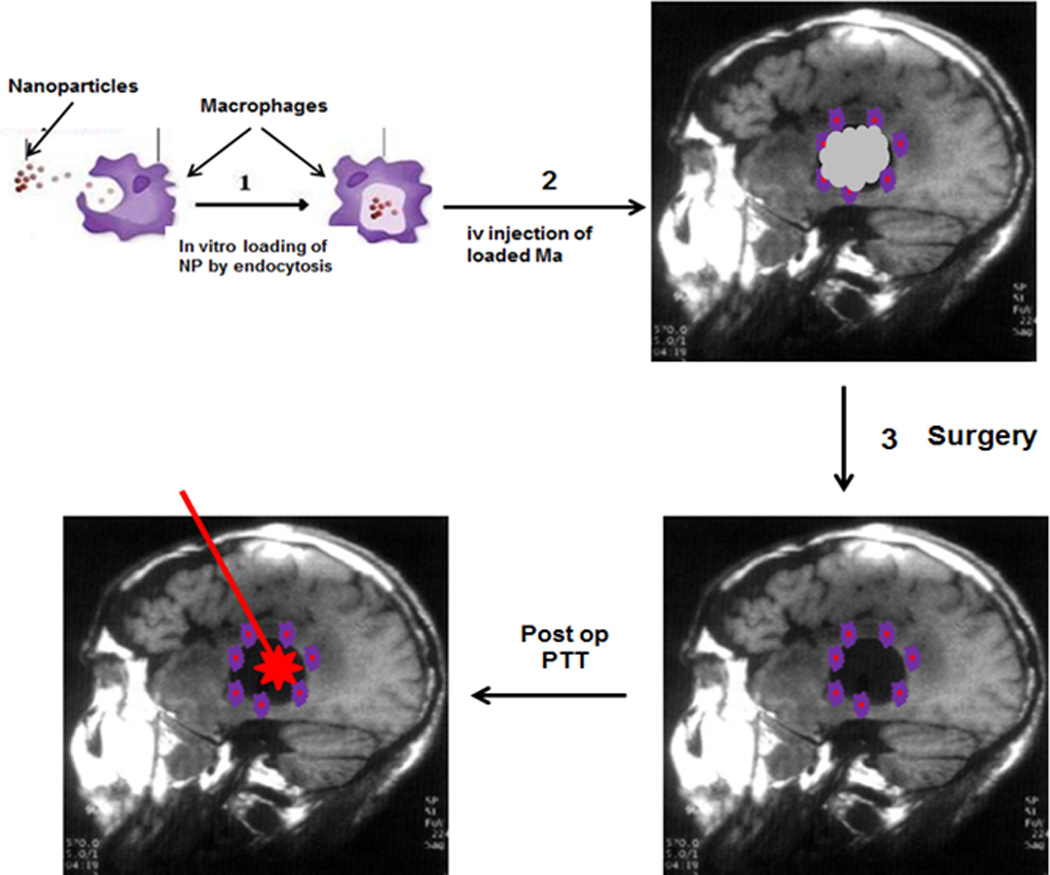
(1) Mo are harvested from the patient’s peripheral blood by leukapheresis and incubated ex vivo with AuNS forming MaNS. (2) The loaded Ma are re-injected i.v. into the patient and will home to the tumor border. (3) Following cytoreductive surgery, MaNs remain in the resection cavity wall and BAT region. (4) Intracavity NIR laser irradiation (PTT) is performed via an indwelling balloon catheter
Several methods which would increase the ability of exogenous loaded Ma to migrate through the BBB and into the BAT, making them available for postoperative therapeutic modalities, have been examined. Chemical opening of the BBB has been shown to increase the number of labeled Mo into normal brain in mice (Wu et al. 2006). Targeting this type of chemical BBB modification to specific sites in the brain is very difficult thereby reducing its applicability. In contrast, PDT has been shown to selectively open the BBB in a targeted limited volume (Hirschberg et al. 2008). The effects of PDT on the migration of i.v. injected exogenous Ma loaded with iron oxide nanoparticles in non-tumor bearing rats has been investigated by Madsen et al. (2013). The extent of loaded Ma infiltration was evaluated using MRI and histology. Superparamagnetic iron oxide (SPIO)-loaded Ma were administered i.v. immediately prior to PDT. Histopathological analyses failed to find evidence of iron oxide in normal rat brain. Accumulations of SPIO-loaded Ma were observed in both MR images and histological sections of non-tumor bearing rat brain following PDT-induced disruption of the BBB. The Ma infiltration in the brain was localized to the light irradiated areas, demonstrating that the delivery of the SPIO nanoparticles was site specific.
Conclusions
Mo/Ma are particularly well suited to the delivery of nanoparticles: they readily ingest a variety of gold nanoparticles and they have been shown to infiltrate multicell tumor spheroids. NIR laser irradiation of spheroids infiltrated with MaNS or MaNR (PTT) has been shown to inhibit growth in an irradiance dependent manner. In vitro studies have also confirmed the ability of MaNS PTT to enhance the cytotoxic effects of other treatment modalities such as PDT and chemotherapy. The few animal studies that have been performed provide proof-of-principle of Mo/Ma mediated PTT. Not only do these studies demonstrate MaNs migration into tumor tissues, but also effective tumor growth inhibition following NIR laser irradiation, i.e., PTT. Although these studies suggest that PTT with gold nanoparticle-loaded Mo/Ma is a promising adjuvant for the treatment of malignant gliomas, a number of challenges remain. The protocol envisioned in Fig. 3 has never been implemented either in experimental animals or patients and, perhaps more importantly, the fundamental question as to whether a sufficient number of intravenously administered loaded Ma are capable of reaching the BAT for effective PTT, remains unanswered.
It is important to note that gold nanoparticle mediated PTT has reached the stage of clinical trials. In a recently closed trial (October 2015), AuNS PTT safety and efficacy was evaluated for the treatment of refractory and/or recurrent head and neck cancer (ClinicalTrials.gov). An ongoing trial is investigating AuNS PTT efficacy in patients with primary and/or metastatic lung tumors while another trial is recruiting patients to evaluate the efficacy of this modality for the treatment of prostate cancer. As of the writing of this review, results have not been released from any of these trials.
Acknowledgments
The authors are grateful for support from the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the LAMMP Program NIBIB P41EB015890 at UCI. Steen Madsen was supported, in part, by the Tony and Renee Marlon Charitable Foundation.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Aslan K, Lacowicz J, Geddes CR. Plasmon light scattering in biology and medicine: new sensing approaches, visions and perspectives. Curr Opin Chem Biol. 2005;9(5):1367–5931. doi: 10.1016/j.cbpa.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie B, Schartner JM. Flow cytometric characterization of tumor associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–961. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104(2):439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basel MT, Shrestha TB, Bossmann SH, Troyer DL. Cells as delivery vehicles for cancer therapeutics. Therapeutic Delivery. 2014;5(5):555–567. doi: 10.4155/tde.14.24. [DOI] [PubMed] [Google Scholar]
- Carolina A, da Fonseca C, Badie B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol. 2013;2013:265608. doi: 10.1155/2013/264124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Gold nanocages as photothermal transducers for cancer treatment. Small. 2010;6(7):811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhetri S, Hirschberg H, Madsen SJ. Photothermal therapy of human glioma spheroids with gold-silica nanoshells and gold nanorods: a comparative study. Proceedings SPIE, Photonic Therapeutics and Diagnostics. 2014;8928:U1–U8. [Google Scholar]
- Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE. A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7(12):3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- Christie C, Madsen SJ, Peng Q, Hirschberg H. Macrophages as nanoparticle delivery vectors for photothermal therapy of brain tumors. Ther Deliv. 2015;6(3):371–384. doi: 10.4155/tde.14.121. [DOI] [PubMed] [Google Scholar]
- Cole JR, Mirin NA, Knight MW, Goodrich GP, Halas NJ. Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J Phys Chem C. 2009;113(28):12090–12094. [Google Scholar]
- Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107(3):459–466. doi: 10.1002/cncr.22035. [DOI] [PubMed] [Google Scholar]
- Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, Blaney SM, West JL. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ES, Zhang L, Thompson PA, Zawaski JA, Kaffes CC, Gaber MW, Blaney SM, West JL. Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine (London) 2012;7(8):1133–1148. doi: 10.2217/nnm.11.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach MA, Bluestone JA, Lim WA. sci tCell-based therapeutics: the next pillar of medicine. Sci Transl Med. 2013;5(179):1–6. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LR, Gobin AM, Lowery AR, Tam F, Halas NJ. Metal nanoshells. Ann Biomed Eng. 2006;334(1):15–22. doi: 10.1007/s10439-005-9001-8. [DOI] [PubMed] [Google Scholar]
- Hirschberg H, Uzal FA, Chighvinadze D, Zhang MJ, Peng Q, Madsen SJ. Disruption of the blood-brain barrier following ALA-mediated photodynamic therapy. Lasers Surg Med. 2008;40:535–542. doi: 10.1002/lsm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H, Baek SK, Kwon YJ, Sun CH, Madsen SJ. Bypassing the blood brain barrier: delivery of therapeutic agents by macrophages. Proc SPIE, Photonic Therapeutics and Diagnostics. 2010;7548:3Z1–3Z5. [Google Scholar]
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- Huynh E, Zheng G. Cancer nanomedicine: addressing the dark side of the enhanced permeability and retention effect. Nanomedicine (Lond.) 2015;10(13):1993–1995. doi: 10.2217/nnm.15.86. [DOI] [PubMed] [Google Scholar]
- Jain PK, El Sayed MA. Universal scaling of plasmon coupling in metal nanostructures: extension from particle pairs to nanoshells. Nano Lett. 2007;7:2854–2858. doi: 10.1021/nl071496m. [DOI] [PubMed] [Google Scholar]
- Kah JC, Wong KY, Neoh KG, Song JH, Fu JW, Mhaisalkar S, Olivo M, Sheppard CJ. Critical parameters in the pegylation of gold nanoshells for biomedical applications: an in vitro macrophage study. J Drug Target. 2009;17:181–193. doi: 10.1080/10611860802582442. [DOI] [PubMed] [Google Scholar]
- Lin AW, Lewinski NA, West JL, Halas NJ, Drezek RA. Optically tunable nanonoparticle contrast agents for early cancer deterction: Midel-based analysis of gold nanoshells. J Biomed Opt. 2005;10(6):064035. doi: 10.1117/1.2141825. [DOI] [PubMed] [Google Scholar]
- Link S, El-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331–366. doi: 10.1146/annurev.physchem.54.011002.103759. [DOI] [PubMed] [Google Scholar]
- Link S, El-Sayed MA. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B. 1999;103:4212–4217. [Google Scholar]
- Liu C, Mi CC, Li BQ. Energy absorption of gold nanoshells in hyperthermia therapy. IEEE Trans Nanobioscience. 2008;7(3):206–214. doi: 10.1109/TNB.2008.2002284. [DOI] [PubMed] [Google Scholar]
- Loo C, Lin A, Hirsch L, Lee MH, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–44. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- Madsen SJ, Sun CH, Tromberg BJ, Cristine V, DeMagalhaes N, Hirschberg H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med. 2006;38:555–564. doi: 10.1002/lsm.20350. [DOI] [PubMed] [Google Scholar]
- Madsen SJ, Gach HM, Hong SJ, Uzal FA, Peng Q, Hirschberg H. Increased nanoparticle-loaded exogenous macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg Med. 2013;45(8):524–532. doi: 10.1002/lsm.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen SJ, Christie C, Hong SJ, Trinidad A, Chhetri S, Peng Q, Uzal FA, Hirschberg H. Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers Med Sci. 2015;30(4):1357–1365. doi: 10.1007/s10103-015-1742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen SJ, Shih EC, Peng Q, Christie C, Krasieva T, Hirschberg H. Photothermal enhancement of chemotherapy mediated by gold-silica nanoshell-loaded macrophages: in vitro squamous cell carcinoma study. J Biomed Opt. 2016;21(1):018004. doi: 10.1117/1.JBO.21.1.018004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regulation. 2001;41(1):189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:1–152. doi: 10.1016/j.addr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Mathews MS, Chighvinadze D, Gach HM, Uzal FA, Madsen SJ, Hirschberg H. Cerebral edema following photodynamic therapy using endogenous and exogenous photosensitizers in normal brain. Lasers Surg Med. 2011;43:892–900. doi: 10.1002/lsm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Nanoengineering of optical resonances. Chem Phys Lett. 1998;288:243–247. [Google Scholar]
- Petrecca K, Guiot MC, Panet-Raymond V. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with Glioblastoma. J Neurooncol. 2013;111:19–23. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodan E, Radioff C, Halas NJ, Nordlander PA. A hybridization model for the plasmon response of complex nanostructures. Science. 2003;301:419–422. doi: 10.1126/science.1089171. [DOI] [PubMed] [Google Scholar]
- Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92:288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD. Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res. 2009;69(4):1659–1667. doi: 10.1158/0008-5472.CAN-08-2535. [DOI] [PubMed] [Google Scholar]
- Terentyuk GS, Maslyakova GN, Suleymanova LV, Khlebtsov NG, Khlebtsov BN, Akchurin GG. Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. J Biomed Opt. 2009;14(2):021016. doi: 10.1117/1.3122371. [DOI] [PubMed] [Google Scholar]
- Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- Trinidad A, Hong SJ, Peng Q, Madsen SJ, Hirschberg H. Combined concurrent photodynamic and gold nanoshell loaded macrophage-mediated photothermal therapies: an in vitro study on squamous cell head and neck carcinoma. Lasers Surg Med. 2014;46(4):310–318. doi: 10.1002/lsm.22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, Remy C. In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage. 2008;40(2):973–983. doi: 10.1016/j.neuroimage.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Wu J, Yang S, Luo H, Zeng L, Ye L, Lu Y. Quantitative evaluation of monocyte transmigration into the brain following chemical opening of the blood-brain barrier in mice. Brain Res. 2006;1098:79–85. doi: 10.1016/j.brainres.2006.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TD, Choi W, Yoon TH, Lee KJ, Lee JS, Jang HJ, Lee MG, Yim HS, Choi KM, Kim B, Lee JJ, Kim H, Lee DY, Jung KY, Baek SK. In vivo photothermal treatment by the peritumoral injection of macrophages loaded with gold nanoshells. Biomed Opt Express. 2015;7(1):185–193. doi: 10.1364/BOE.7.000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H. Glioma-initiating cells: A predominant role in microglia/macrophages tropism to glioma. J Neuroim. 2011;232(1–2):75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]


