Abstract
Background
Increased IL-17A production has been associated with more severe asthma, however the mechanisms whereby IL-17A may contribute to IL-13-driven pathology in asthma remain unclear.
Objective
We sought to gain mechanistic insight into how IL-17A can influence IL-13-driven responses.
Methods
The effect of IL-17A on IL-13-induced airway hyperresponsiveness (AHR), gene expression, mucus hypersecretion, and airway inflammation was assessed using in vivo models of IL-13-induced lung pathology and in vitro culture of murine fibroblast cell lines and primary fibroblasts, and human epithelial cell lines or primary human epithelial cells exposed to IL-13, IL-17A, or IL-13 and IL-17A.
Results
Compared to mice given intratracheal IL-13 alone, those exposed to IL-13 and IL-17A displayed augmented AHR, mucus production, airway inflammation and IL-13-induced gene expression. In vitro, IL-17A enhanced IL-13-induced gene expression in asthma-relevant murine and human cells. In contrast to the exacerbating influence of IL-17A on IL-13-induced responses, co-exposure to IL-13 inhibited IL-17A-driven antimicrobial gene expression in vivo and in vitro. Mechanistically, in both primary human and murine cells, IL-17A-driven elevation of IL-13-induced gene expression was associated with enhanced IL-13-driven STAT6 activation.
Conclusions
Our data suggest that IL-17A contributes to asthma pathophysiology by increasing the capacity of IL-13 to activate intracellular signaling pathways such as STAT6. These data represent the first mechanistic explanation of how IL-17A may directly contribute to the pathogenesis of IL-13-driven pathology.
Keywords: Asthma, IL-13, IL-17A, STAT6, Cytokines, Signal transduction
Graphical Abstract
Introduction
IL-13 has been ascribed a central pathogenic role in experimental models of asthma, as exogenous IL-13 induces mucus hypersecretion, airway hyperresponsiveness (AHR), and airway remodeling.1-4 Moreover, eliminating IL-13 signaling abrogates allergen-driven responses and selective restoration of IL-13 signaling in airway epithelial cells or smooth muscle cells is sufficient to recapitulate most features of allergic asthma.5, 6 This suggests that IL-13-induced responses in pulmonary structural cells are critical to asthma pathogenesis. Recently, Th17 cytokines have been shown to influence the development and severity of asthma, and murine models eliciting mixed Th2/Th17 responses are associated with more severe airway inflammation and dysfunction than purely Th2 driven models.7, 8 Similar to IL-13, IL-17A signaling in airway smooth muscle cells also influences AHR,9 suggesting that the convergence of IL-13 and IL-17A signaling in pulmonary structural cells may contribute to the pathogenesis of severe forms of allergic asthma.
In humans, IL-17A levels in lung biopsies, sputum, and serum correlate with asthma severity,10-14 and a unique subset of IL-4+/IL-17A+ CD4+ T cells (Th2/Th17 cells) was recently identified in the blood, bronchoalveolar lavage (BAL) fluid, and lungs of asthmatics.15-17 Asthmatics with a predominance of Th2/Th17 cells in the BAL fluid (“Th2/Th17 predominant”) exhibited the greatest airway obstruction, hyperreactivity, and steroid use relative to “Th2 predominant” or “Th2/Th17 low” subgroups.16 While these studies suggest a link between IL-17A and severe asthma in humans, the mechanisms whereby IL-17A influences disease severity, especially in individuals with mixed Th2/Th17 phenotypes, remain unclear.
Recent studies directed at determining how IL-17A regulates the development of IL-13-induced lung pathology have examined the impact of different doses or cellular sources of IL-17A.7, 9, 18, 19 However, an important unanswered question is how interactions between IL-13 and IL-17A at the cellular and molecular level influence asthma pathogenesis. Hence, the aim of the present study was to determine molecular mechanisms by which IL-17A enhances IL-13 pathology in severe asthma using in vivo models of IL-13-induced lung pathology and in vitro cultures of relevant cell types.
Methods
For a complete description of the materials and methods used in the murine and in vitro experiments, please see the Methods section in this article's Online Repository at www.jacionline.org
Human subjects and nasal epithelial cell sampling
Nasal epithelial cells (NEC) were obtained from healthy donors at Cincinnati Children's Hospital Medical Center. Cells were collected following informed consent, and all studies were approved by institutional IRB (IRB#2008-0711). Nasal mucosa was sampled as described elsewhere.20 To expand primary NECs, cells were resuspended in BEGM (Lonza, Walkersville, MD) and cultured in an upright T25 flask, changing medium every 48 hours. Once confluent, cells were expanded in a T25 flask lying flat until confluent. 50,000 cells were then transferred into 24-well plates and allowed to reach confluence. Once confluent, cells were treated with cytokines as described.
Statistical analysis
Data are expressed as mean + SEM. To determine differences between multiple groups, one-way ANOVA followed by the Tukey-Kramer test was used. For comparison between two groups, a Student's t-test was performed. Figures were produced and statistics were analyzed using GraphPad Prism 5.
Results
IL-17A increases IL-13-induced pathology in vivo
To examine the effect of IL-17A on IL-13-induced lung pathology, A/J mice were treated intratracheally (i.t.) with PBS, IL-13 (2μg or 5μg), IL-17A (2μg or 5μg), or both cytokines on days 0, 3, and 6. Airway hyperresponsiveness (AHR), airway inflammation, mucus production, and gene expression were assessed on day 7. While treatment with IL-17A alone failed to alter AHR, treatment with IL-13 induced AHR (Fig. 1A & 1B). Treatment with 5μg but not 2μg of IL-17A enhanced AHR induced by 5μg of IL-13 (Fig. 1B). Treatment with 5μg of IL-13 and IL-17A did not increase Th2 cytokine expression in the BAL fluid or total IgE levels in the serum (Supp. Fig. 1A & 1B). Further, although neither cytokine alone was administered at sufficiently high concentrations to increase total BAL cell numbers, simultaneous treatment with IL-13 and IL-17A increased BAL cell numbers (Fig. 1C & 1D), primarily due to an increased frequency of neutrophils (Fig. 1E & 1F). To examine goblet cell hyperplasia, we assessed levels of CLCA3, a protein highly expressed in murine goblet cells, in lung homogenates from cytokine-treated animals (Fig. 1G). IL-17A treatment did not increase CLCA3 protein in the lung, but treatment with IL-13 induced CLCA3 production in a dose-dependent manner, and IL-13-induced CLCA3 levels were further enhanced by both doses of IL-17A. Thus, IL-17A exacerbates IL-13-driven AHR, airway inflammation, and mucus production.
Fig 1.

Dose-dependent effects of IL-13 and IL-17A in vivo. AHR in A/J mice treated with IL-13 or IL-17A i.t. (A,B), total cell counts from BAL fluid of cytokine treated animals (C,D), frequency of macrophages, lymphocytes, neutrophils, and eosinophils in the BAL fluid (E,F), CLCA3 and αTubulin expression and densitometry in total lung homogenates (G), total lung expression of IL-13 (H) and IL-17A (I) induced transcripts following cytokine treatment. #, ##, and ### indicate P<0.05, <0.01, and < 0.001 vs PBS. +, ++, and +++ in indicate P<0.05, <0.01, and < 0.001 vs IL-13. Mean + SEM of n=4-9 mice per group (pooled over 3 independent experiments) are shown.
Reciprocal co-regulation of IL-13- and IL-17A-induced genes in vivo
Transcript levels of IL-13-induced genes were assessed in animals treated with 5μg of IL-13 and IL-17A since consistent enhancement of IL-13-induced AHR, inflammation, and mucus were observed at these doses. Treatment with IL-13 induced the expression of Tff2, Arg1, C3, Alox15, Ca2, and the gene encoding the IL-13 decoy receptor, Il13ra2 (Fig. 1H). While IL-17A treatment alone had little impact on the expression of these genes, co-treatment with IL-17A enhanced IL-13-induced expression of these genes by nearly 3-fold. IL-17A-mediated enhancement of IL-13-induced lung pathology was not a result of increased expression of the IL-13 receptor, as type II IL-4/IL-13 receptor (Il13ra1, Il4ra) transcript levels were unchanged (Supp. Fig. 2A & 2B).
In contrast to the enhancing effect of IL-17A on IL-13-induced gene expression, we found that expression of S100a8, S100a9, Lcn2, Csf2, Cebpb, and Cebpd was increased in animals exposed to IL-17A alone, but diminished in animals co-exposed to IL-17A and IL-13 (Fig. 1I). The inhibitory effect of IL-13 on IL-17A-induced gene expression was also independent of changes in IL-17A receptor levels, as expression of IL-17A receptor subunits (Il17ra, Il17rc) was unaltered (Supp. Fig. 2C & 2D). Collectively, these data suggest that there is reciprocal co-regulation of IL-13- and IL-17A-induced genes in vivo.
IL-13Rα2 is not required for IL-17A-mediated enhancement of IL-13 pathology
Despite its reported role as an IL-13 inhibitor,21-23 allergen-driven AHR is attenuated in IL-13Rα2−/− mice,24 suggesting that IL-13Rα2 may be required for maximal induction of AHR. To determine if enhanced expression of Il13ra2 contributes to IL-17A-mediated enhancement of IL-13-driven pathology, IL-13-induced AHR, inflammation, and gene expression were measured in wild-type (WT) BALB/c or IL-13Rα2−/− mice following i.t. treatment with 5μg of cytokines. While AHR was attenuated in IL-13Rα2−/− animals relative to WT, treatment with IL-17A still enhanced IL-13-driven AHR in IL-13Rα2−/− mice (Fig. 2A). Despite differences in the magnitude of basal, and IL-13-induced AHR, IL-17A enhanced IL-13-induced AHR by ~1.4-fold in both WT and IL-13Rα2−/− animals (Supp. Fig. 3A) suggesting that the effects of IL-17A were comparable in both strains. Similar to our observations in A/J mice, IL-17A enhanced IL-13-driven changes in BAL cellularity (Fig. 2B), increased the frequency of neutrophils in the BAL (Fig. 2C & 2D), and increased IL-13-driven transcript levels in the lung (Fig. 2E). Further, although IgE was significantly elevated in the serum of IL-13Rα2−/− mice relative to WT, antibody levels were not altered by cytokine treatment in either strain (Supp. Fig. 3B). IL-13 also diminished the expression of IL-17A-induced genes in both WT and IL13Rα2−/− mice (Fig. 2F). Collectively, these data suggest that the ability of IL-17A to enhance IL-13-induced lung pathology is not dependent on IL-13Rα2. Further, as IL-17A enhanced IL-13-induced pathology in both A/J (Fig. 1) and BALB/c animals (Fig. 2), this suggests these in vivo observations are not limited to a single mouse strain.
Fig 2.
IL-13Rα2 is not required for IL-17A-mediated enhancement of IL-13-induced pathology. AHR in WT or IL-13Rα2−/− mice treated with IL-13 or IL-17A i.t. (A), total cell counts from BAL fluid of cytokine treated animals (B), frequency of macrophages, lymphocytes, neutrophils, and eosinophils in the BAL fluid (C,D), total lung expression of IL-13 (E) and IL-17A (F) induced transcripts following cytokine treatment. # and ## indicate P<0.05 and <0.01 vs PBS. + and +++ indicate P<0.05 and <0.001 vs IL-13 (A,B) or IL-17A (F). Mean + SEM of n=5-11 mice per group (pooled over 3 independent experiments) are shown.
Reciprocal co-regulation of IL-13- and IL-17A-induced genes in vitro
To confirm that changes in gene expression reflected changes in transcriptional activity and not altered inflammatory cell recruitment, we expanded our studies to an in vitro analysis of fibroblasts, an IL-13- and IL-17A-responsive structural cell type. Initial dose-finding experiments using NIH/3T3 fibroblasts revealed strong induction of IL-13-responsive genes C3 and Il13ra2 at 100 ng/ml (Supp. Fig. 4). Subsequently, IL-13-induced gene expression was examined over a 24-hour period. As in the whole lung, treatment with IL-13 by itself again augmented both C3 and Il13ra2 expression, while IL-17A failed to increase Il13ra2 expression and resulted in only modest expression of C3 (Fig. 3A & 3B). Co-treatment with IL-13 and IL-17A significantly elevated Il13ra2 and C3 expression relative to media or IL-13 stimulated cells, as early as 2 hours and maximally between ~16-20 hours post treatment. Similar to in vivo, IL-13 inhibited IL-17A-induced expression of Cebpd and Lcn2 (Fig. 3C & 3D). Again, no effect was seen on the expression of either IL-13 or IL-17A receptor components (Supp. Fig. 5). Taken together, these data recapitulate our in vivo observations and establish that IL-17A enhances IL-13-induced gene expression in a single cell population independent of the lung microenvironment.
Fig 3.

Reciprocal co-regulation of IL-13- and IL-17A-induced genes occurs in vitro. Real-time PCR analysis of C3 (A), Il13ra2 (B), Cebpd (C), and Lcn2 (D) expression in NIH/3T3s stimulated with IL-13, IL-17A, or both cytokines over 24 hours. ## and ### indicate P<0.01 and <0.001 vs Medium. +, ++, and +++ in indicate P<0.05, <0.01, and < 0.001 vs IL-13 (A, B) or IL-17A (C). Mean + SEM of 4-8 replicates per condition shown. Results show 1 of 3 independent experiments.
IL-17A-mediated enhancement of IL-13-induced gene expression requires functional IL-13 and IL-17A signaling complexes in same cell
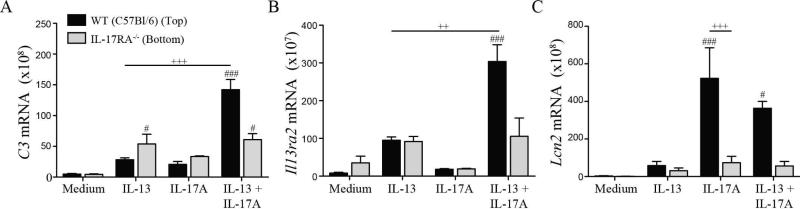
Given the time frame in which IL-17A enhanced IL-13-induced gene expression, the role of soluble IL-17A-induced factors was examined. To address this, WT C57BL/6 or IL-17RA−/− pulmonary fibroblasts were cultured in Transwell plates. In co-cultures consisting of WT fibroblasts on the top and bottom wells of the Transwell, IL-17A enhanced IL-13-induced gene expression, whereas in co-cultures consisting of IL-17RA−/− fibroblasts it did not (Supp. Fig. 6). In co-cultures of WT and IL-17RA−/− cells, IL-13 induced similar levels of C3 and Il13ra2 expression (Fig. 4A & 4B). However, in WT and IL-17RA−/− co-cultures treated with both cytokines, IL-17A enhanced IL-13-dependent C3 and Il13ra2 expression in the WT cells but not the IL-17RA−/− cells. IL-17A-induced expression of Lcn2 was abrogated in IL-17RA−/− fibroblasts, confirming their insensitivity to IL-17A (Fig. 4C). Overall, these data demonstrate that IL-17A-induced mediators cannot enhance IL-13-induced gene expression in the absence of functional IL-17A receptors, suggesting that IL-17A acts directly on IL-13-responsive cells to increase IL-13-induced transcriptional activity.
Fig 4.
IL-17A directly signals on IL-13-responsive cells to enhance gene expression. Real-time PCR analysis of C3 (A), Il13ra2 (B), and Lcn2 (C) expression in co-cultured WT or IL-17RA−/− lung fibroblasts stimulated with IL-13, IL-17A, or both cytokines for 24 hours. # and ### indicate P<0.05 and <0.001 vs Medium. ++ and +++ indicate P<0.01 and < 0.001 vs IL-13 (A, B) or IL-17A (C). Mean + SEM of 3 replicates per condition shown. Results show 1 of 3 independent experiments.
IL-17A does not enhance the stability of IL-13-induced transcripts
IL-17A synergizes with innate cytokines (TNFα, IL-1β) by increasing target mRNA stability.25-27 To determine whether IL-17A also regulates the stability of IL-13-induced transcripts, NIH/3T3 cells were incubated with IL-13 alone or in combination with IL-17A for 16 hours, then de novo transcription was blocked with actinomycin D (ActD) and mRNA degradation was evaluated over a 24-hour period. As expected, IL-17A improved the stability of TNFα-induced Csf3 and Lcn2 (Supp. Fig. 7). However, the half-life of IL-13-induced C3 (t1/2=6.3) and Il13ra2 (t1/2=5.4) mRNA was slightly reduced rather than enhanced by IL-17A (C3, t1/2=3.9; Il13ra2, t1/2=4.6) (Fig. 5A & 5B), suggesting that mRNA stabilization does not contribute to IL-17A-mediated enhancement of IL-13-induced C3 or Il13ra2 expression. Consistent with this observation, comparably greater levels of C3 and Il13ra2 transcripts were detected in cells that were stimulated with IL-13 and IL-17A both at the time of ActD addition and 24 hours post treatment (Supp. Fig. 8). Thus, in contrast to the central mechanism driving synergy between IL-17A and TNFα, these data suggest that IL-17A augments IL-13-induced transcript levels independently of mRNA stabilization.
Fig 5.

IL-17A does not stabilize IL-13-induced transcripts. Percentage mRNA remaining of IL-13 induced C3 (A) and Il13ra2 (B) in NIH/3T3s stimulated with IL-13 or IL-13 + IL-17A for 16 hours and incubated with ActD. Real-time PCR analysis of transcript levels was evaluated over 24 hours and t½ was calculated. Mean + SEM of 4-8 replicates per condition shown. Results show 1 of 3 independent experiments.
IL-17A enhances IL-13-driven STAT6 phosphorylation
As IL-13-driven lung pathology requires STAT6 expression,5 we evaluated the hypothesis that IL-17A augments IL-13-driven pathology by promoting activation of STAT6. To test this, NIH/3T3 cells were incubated with medium containing IL-13, IL-17A, or both cytokines, and STAT6 phosphorylation (pSTAT6) was evaluated by Western blot. Stimulation with IL-13 induced STAT6 phosphorylation (Fig. 6A). While IL-17A did not increase pSTAT6, co-treatment with IL-17A enhanced IL-13-driven STAT6 activation (Fig. 6A). Pre-treatment of NIH/3T3s with cycloheximide did not limit the ability of IL-17A to enhance IL-13-dependent STAT6 phosphorylation (Fig. 6B). In fact, pSTAT6 levels were comparably increased (~1.7 fold) in DMSO and cycloheximide exposed cultures (Fig. 6C), suggesting that IL-17A-mediated enhancement of IL-13-dependent pSTAT6 does not require de novo protein synthesis. We also assessed STAT6 phosphorylation in cytokine-treated NIH/3T3 cells by phosphoflow cytometry. Compared to IL-13 exposed cells, cells stimulated with IL-13 and IL-17A demonstrated significantly greater levels of pSTAT6 after 2 and 5 minutes of stimulation (Fig. 6D, Supp. Fig. 9). While these changes appear modest, analyses of area under the curve (AUC) show an ~9% increase in total pSTAT6 levels in cells exposed to IL-13 and IL-17A (AUC 9782) compared to those treated with IL-13 alone (AUC 8991).
Fig 6.

IL-17A directly enhances IL-13-driven pSTAT6. pSTAT6/STAT6 levels in NIH/3T3 cells pretreated with DMSO (A) or cycloheximide (B) then stimulated with cytokines for 5 minutes, (C) densitometry of pSTAT6/STAT6 expression shown in (A,B), geometric mean fluorescence intensity of pSTAT6 by phosphoflow in NIH/3T3s stimulated with cytokines over 1 hour (D), pSTAT6 and STAT6 expression and associated densitometry in whole lung homogenates from cytokine-treated A/J mice (mean + SEM of n=7-10 mice per group, pooled from 3 independent experiments) (E), real-time PCR analysis of C3 and Il13ra2 (F) or Lcn2 (G) expression in WT and STAT6−/− lung fibroblasts stimulated with cytokines for 24 hours. #, ##, and ### indicate P<0.05, <0.01, and <0.001 vs PBS or Medium. +, ++, and +++ indicate P<0.05 , <0.01, and <0.001 vs IL-13. Cyclohexamide experiments display 1 of 3 independent experiments performed. Phosphoflow cytometry and real-time PCR results show mean + SEM of 4-6 replicates per condition shown. Results show 1 of 2 independent experiments.
Based on these in vitro observations, we wanted to determine whether IL-17A also enhanced IL-13-driven STAT6 phosphorylation in vivo. Thus, pSTAT6 and total STAT6 levels were measured in whole lung homogenates from A/J mice treated with PBS, 5μg IL-13, 5μg IL-17A, or 5μg of both cytokines by Western blot. 24 hours following the final cytokine exposure, pSTAT6 was detectable in IL-13-treated animals, but not PBS or IL-17A exposed animals, and IL-13-induced pSTAT6 levels were further heightened by IL-17A (Fig. 6E). Thus, we observe an association between increased pulmonary pSTAT6 levels, AHR, airway inflammation, and mucus production in animals exposed to IL-13 and IL-17A.
While STAT6 is the dominant signaling pathway activated in response to IL-13, additional pathways have been implicated in IL-13 signaling.28-31 To determine whether these signaling pathways may also contribute to IL-17A-mediated enhancement of IL-13 responses, we cultured lung fibroblasts from BALB/c WT or STAT6−/− mice in medium containing IL-13, IL-17A, or both cytokines and assessed the expression of IL-13- (Fig. 6E) and IL-17A- (Fig. 6F) induced genes. In WT cells, treatment with IL-13 induced the expression of both Il13ra2 and C3, and expression was augmented by IL-17A. In contrast, IL-13-induced Il13ra2 and C3 expression was reduced in STAT6−/− fibroblasts and IL-17A did not notably enhance expression further. IL-17A-induced expression of Lcn2 was inhibited by IL-13 in cells from WT but not STAT6−/− animals (Fig. 6F), implying that inhibition of IL-17A signaling requires IL-13-induced STAT6 activation. Taken together, these findings suggest that IL-17A directly elevates IL-13-induced gene expression via STAT6 activation, leading to enhanced IL-13-dependent lung pathology.
Reciprocal co-regulation by IL-13 and IL-17A in human cells
To determine if IL-17A also augmented IL-13 activity in human cells, initial dose-finding experiments were carried out in normal human bronchial epithelial (NHBE) cells (ATCC CRL-4051). While SERPINB4 expression was elevated at all IL-13 concentrations tested (5-100 ng/ml), IL13RA2 was only induced by 100 ng/ml (Supp. Fig. 10A & 10B). A dose of 100 ng/ml was selected for further study to ensure that potential regulatory influences of IL-13Rα2 would be present (Supp. Fig. 4). To test the ability of IL-17A to enhance IL-13-induced gene expression in primary human cells, nasal epithelial cells (NEC) were collected from 7 healthy volunteers and IL-13-induced gene expression was assessed after treatment with IL-13, IL-17A or both IL-13 and IL-17A. Treatment with IL-13 induced the expression of SERPINB4 and IL13RA2 (Fig. 7A), and although IL-17A alone did not remarkably influence expression of these genes, treatment with both cytokines further elevated IL-13-induced transcript levels in the majority of individuals. Moreover, IL-13 also inhibited IL-17A-induced LCN2 expression (Fig. 7B), demonstrating that our observations in mice are consistent in human primary cells.
Fig 7.

Reciprocal co-regulation by IL-13 and IL-17A is conserved in human cells. Real-time PCR analysis of SERPINB4 or IL13RA2 (A) and LCN2 (B) expression in primary human NECs (n=7) stimulated with IL-13, IL-17A, or both cytokines for 24 hours. pSTAT6 and STAT6 levels and associated densitometric analysis in Caco-2s (C), A549s (D) stimulated with cytokines for 5 minutes or NECs (n=2) stimulated with cytokines for 15 minutes (E). Caco-2 and A549 immunoblots are representative of 2 and 3 independent experiments, respectively.
To determine if IL-17A also enhanced IL-13-driven STAT6 activation in human cells, human epithelial cell lines were incubated with medium containing IL-13, IL-17A, or both cytokines, and pSTAT6 levels were assessed by assessed Western blot. Both Caco-2 cells (Fig. 7C) and A549 cells (Fig. 7D) demonstrated greater levels of IL-13-induced pSTAT6 when co-stimulated with IL-17A. Finally, to confirm that IL-17A increased IL-13-induced pSTAT6 levels in primary human cells, NECs from 2 donors were additionally exposed to cytokines and pSTAT6 levels were assessed by Western blot. As shown in Fig. 7E, cells from both donors demonstrated IL-13-induced pSTAT6, which was further enriched in the presence of IL-17A. Overall, these results demonstrate that IL-17A enhances IL-13 activity across a panel of asthma-relevant human epithelial cells, suggesting a plausible mechanistic explanation for increased asthma severity in individuals who simultaneously produce measurable levels of both IL-13 and IL-17A.
Discussion
Despite reports of elevated IL-17A production in severe asthmatics,10-17 little is known about how IL-17A influences IL-13-driven responses. We propose a novel pathway where IL-17A enhances IL-13-induced signaling and gene expression both in vitro and in vivo. IL-17A-mediated enhancement of IL-13 signaling and gene expression occurs rapidly, and requires IL-17RA expression in IL-13-responsive cells. It is not a result of altered IL-13 receptor expression, and it is independent of molecular processes driving synergy between IL-17A and other pro-inflammatory mediators (i.e., TNFα). Mechanistically, we observe that IL-17A enhances IL-13-dependent activation of a key mediator of allergic responses - STAT6.
Whether increased STAT6 phosphorylation is sufficient to explain IL-17A-mediated enhancement of IL-13 activity is unclear. However, our observation that IL-17A does not enhance IL-13-induced gene expression in STAT6−/− cells suggests that other IL-13-activated pathways (IRS-2, STAT3)29-31 are not required. Although it is currently unclear how IL-17A regulates STAT6 phosphorylation, as de-phosphorylation and nuclear export of pSTAT6 is limited upon binding to DNA,32 IL-17A signaling may increase the number of available pSTAT6 binding sites, retaining increased levels of pSTAT6 in the nucleus and thus facilitating pSTAT6 accumulation. Supporting this possibility, NF-κB and C/EBP binding sites are found in the promoters of IL-17A-enhanced IL-13-induced genes.33-36 IL-17A-mediated inhibition of SOCS protein induction, previously demonstrated to negatively regulate IL-13 signaling,37-39 may also play a role. However, the enhancing effects of IL-17A on IL-13-induced STAT6 activation are exerted rapidly (≤ 5 min). As such, it is likely that the effects of IL-17A on IL-13 are related to membrane proximal factors, perhaps those directly interacting with the IL-13R complex, or associated kinases. To this end, IL-17A signaling may directly alter the activity of intracellular phosphatases previously reported to regulate pSTAT6 accumulation, including PP2A,40 PTP1B,41 SHP-1,42 or SHP2.43 Alternatively, as IL-17A activates TRAF6, an E3 ubiquitin ligase, it is conceivable that ubiquitination of STAT6 or other IL-13 signaling components may be altered in IL-17A-exposed cells. Consistent with this possibility, STUB1,44 GRAIL,45 and CBL-b46 have been shown to influence IL-13-driven responses through ubiquitination-dependent pathways. These possibilities are the subject of ongoing investigation our laboratory.
While our data are consistent with previous reports demonstrating IL-17A-mediated enhancement of IL-13 pathology,7, 8, 17, 47-49 protective roles for IL-17A in asthma have been reported in mouse models of asthma18, 19 and RSV-induced AHR50. In asthma models, it appears that, when protective, IL-17A is produced primarily by γδ T cells. For example, resolution of airway inflammation after allergen challenge is dependent on IL-17A produced by γδ T cells19. Similarly, while widespread inhibition of IL-17A production was associated with reduced IL-13-driven pathology (suggesting a pro-asthmatic role for IL-17A), inhibition of IL-17A production by γδ T cells exacerbated IL-13-driven pathology.18 How IL-17A-producing γδ T cells may limit IL-13-induced lung pathology remains unclear, however, additional factors produced by these cells may influence how IL-17A-derived signals are integrated. For example, others have shown that while IL-17A blockade limited allergen-induced airway eosinophilia and cytokine production in WT mice, IL-17A blockade exacerbated these parameters in IL-22−/− mice,51 suggesting that the cytokine milieu influences protective versus pro-inflammatory effects of IL-17A. Alternatively, our observation that IL-17A-mediated enhancement of IL-13-induced gene expression requires IL-13 and IL-17A signaling in the same cell suggests that where IL-17A is produced is also an important determinant of its role in asthma pathology. While γδ T cells can be found equally distributed in the alveolar spaces, perivascularly, and within the visceral pleura, with slightly lower distribution near the smooth muscle and in the lamina propria, αβ T cells (the likely source of IL-13) are found primarily in the alveolar spaces52, suggesting that the unique ability of γδ-derived IL-17A to control IL-13-induced AHR may be related to its inability to influence IL-13-exposed cells. As we did not observe altered frequency of IL-17A+ γδ T cells (or any T cell subset producing IL-13 or IL-17A) in response to exogenous cytokine administration (Supp. Fig. 11), it is unlikely that the increased IL-13-driven pathology observed in our model was due to altered γδ T cell activity.
Another observation that has potentially broad implications is that IL-13 antagonizes IL-17A-induced expression of innate anti-microbial genes (Lcn2, S100A8, S100A9, Csf2) in a STAT6-dependent manner. As IL-17A-induced gene products are important for limiting the growth of bacterial and fungal microorganisms, our data may explain the link between atopic disease and disordered microbial colonization.53-56 Further, these results agree with a recent report demonstrating that “Th2-high” asthmatics exhibit less Th17-associated gene expression and IL-13 blockade exacerbates Th17-associated inflammation.57 Moreover, we contend that this counter regulation of IL-17A-induced gene expression by IL-13 may also explain why recent endotyping/phenotyping studies have failed to consistently identify a role for Th17 cells or IL-17A in severe asthma.58-61 Finally, as reliably detecting IL-17A protein is difficult in even individuals with identified “Th17-high” asthma,57 it is likely that identification of patients with Th17-involved disease will be difficult, as both the primary cytokine signal (IL-17A),57, 62-65 and the downstream gene expression may be suppressed by ongoing Th2 inflammation. This challenge in identifying individuals with IL-17A associated asthma may suggest that inclusion of many individuals with IL-17A-independent disease may have limited the efficacy of anti-IL-17RA therapy in moderate to severe asthmatics in a recent clinical trial.66
Collectively, our findings provide a mechanistic underpinning for the association between elevated IL-17A levels and severe asthma. Given the essential role for IL-13 and STAT6 in AHR, airway inflammation, and mucus overproduction, our finding that IL-17A enhances IL-13-driven STAT6 phosphorylation has important implications for the pathogenesis of IL-13-induced disease. Moreover, it is interesting to note that common triggers of asthma exacerbations, such as upper respiratory tract infections with viral or bacterial pathogens, are potent triggers for Th17-associated cytokine production. As such, it is plausible that similar interactions between IL-17A and IL-13 may also drive changes in disease status following infection.
Methods
Mice
Male and female A/J, BALB/c, C57BL/6 (Jackson Laboratories), IL-13Rα2−/− (BALB/c) (gift from Dr. Gurjit Khurana-Hershey), IL-17RA−/− (C57BL/6) (Amgen), and STAT6−/− (BALB/c) (gift from Dr. Fred Finkelman) mice were housed in a specific pathogen-free facility. Procedures were approved by Cincinnati Children's Hospital Medical Center IACUC.
Treatment protocol
A/J, BALB/c, and IL-13Rα2−/− mice were treated intratracheally (i.t.) with PBS, 2μg or 5μg of rIL-13, 2μg or 5μg of rIL-17A, or a combination of both cytokines (BioLegend, eBioscience) on days 0, 3 and 6, and sacrificed on day 7 to measure AHR.
Analysis of AHR
AHR was evaluated using the Airway Pressure Time Index (APTI) technique as described previously.1 In brief, mice were anaesthetized, intubated, and respirated at a rate of 120 breaths/minute with a constant tidal volume (0.2 ml) and paralyzed with decamethonium bromide (25 mg/kg). After a stable baseline was achieved, acetylcholine (50 mg/kg) was injected into the inferior vena cava and dynamic airway pressure (cm H2O × sec) was recorded for 5 minutes. After AHR measurements, lung segments were snap-frozen for subsequent analysis by real-time PCR and Western blot. To collect BAL fluid, lungs were lavaged three times with a 1.0-ml aliquot of cold Hanks’ balanced salt solution (Invitrogen). Recovered lavage fluid (70 to 80%) was centrifuged (300g for 8 min) and the cell pellet resuspended in 1.0 ml of 10% FBS in 1X PBS. Total cells were counted with a hemocytometer. Slides were prepared by cytocentrifugation (Cytospin 4, Thermo Scientific), and stained with Diff-Quik (Dade Behring). Differential counts were determined using morphologic criteria under a light microscope with evaluation of ≥ 500 cells/slide.
Determination of Th2 Cytokine and IgE Concentration
BAL fluid and serum samples were collected from animals following AHR measurements and centrifuged at 3000 × g for 5 minutes to remove cell debris. BAL fluid concentrations of IL-4, IL-5, IL-9, and IL-10 and total serum IgE concentrations were measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience).
Cell culture and cytokine treatment
All cells were maintained in a humidified incubator at 37°C and 5% CO2. NIH/3T3s, A549s, and Caco-2s were maintained in DMEM supplemented with 10% FBS, 1% L-glutamine, and 1% mixture of penicillin and streptomycin. Lung fibroblasts were maintained in RPMI supplemented with 10% FBS, 1% L-glutamine, a 1% mixture of penicillin and streptomycin, and 0.1% β-mercaptoethanol. During serum starvation, cells were grown in culture medium supplemented with 0.1% FBS. HBEC3-KT cells (ATCC CRL-4051) were maintained in K-SFM supplemented with Epidermal Growth Factor 1-53 and Bovine Pituitary Extract (Invitrogen). All cells were stimulated with IL-13 (100 ng/ml), TNFα (10 ng/ml), IL-17A (100 ng/ml), IL-13 + IL-17A, or TNFα + IL-17A (eBioscience), unless otherwise noted, and harvested at indicated times.
Lung fibroblast cell culture
Lungs from BALB/c, C57BL/6, IL-17RA−/−, and STAT6−/− mice were excised, minced, and placed in 6 ml of serum-free RPMI containing Liberase CI (0.5 mg/ml, Roche), DNase I (0.5 mg/ml, Sigma), 1% L-glutamine, a 1% mixture of penicillin and streptomycin, and 0.1% β-mercaptoethanol at 37°C for 45 minutes. The tissue was forced through a 70-micron cell strainer, and red blood cells were lysed with ACK lysis buffer (Invitrogen). Cells were washed with RPMI containing 10% FBS, cultured for 2 hours in a T25, and then transferred to a new T75 flask until confluent. Passage of cells 3–4 times with Trypsin-EDTA (0.25%, Invitrogen) removed macrophage or monocyte contaminants. For transwell assays, lung fibroblasts were co-cultured in polyester (10 μm) Transwell plates (Corning) in complete culture medium to confluence, then incubated in serum starve medium supplemented IL-13, IL-17A, or both cytokines. RNA was isolated at 24 hours.
mRNA stability assay
NIH/3T3 cells were grown in complete culture medium to confluence, then incubated in serum starve medium supplemented with IL-13, TNFα, IL-13 + IL-17A, or TNFα + IL-17A. Actinomycin D (5 μg/ml) (Sigma) was added to cell cultures 12 (TNFα ± IL-17A) or 16 (IL-13 ± IL-17A) hours following cytokine treatment and RNA was isolated prior to the addition of actinomycin D and 2 hours (TNFα ± IL-17A) or 24 hours (IL-13 ± IL-17A) following the addition of actinomycin D.
Cycloheximide assay
NIH/3T3 cells were grown in complete culture medium to confluence, then incubated in serum starve medium supplemented with cycloheximide (5 μg/ml) or DMSO (0.05% v/v) 2 hours prior to cytokine stimulation. Cells were stimulated in medium supplemented with IL-13, IL-17A, or both cytokines for 15 minutes. Protein lysates were immunoblotted for phospho-STAT6 (Tyr641) and total STAT6 (Cell Signaling).
Flow cytometry
NIH/3T3 cells were grown in complete culture medium to confluence, then incubated in serum starve medium supplemented with IL-13, IL-17A, or both cytokines for 5, 15, 30, and 60 minutes. Cells were incubated with Trypsin-EDTA (0.05%, Invitrogen) for 5 minutes at 37°C, then fixed for 10 minutes with 2% paraformaldehyde at room temperature, and permeabilized in 90% methanol for 30 minutes at 4°C. Cells were stored at −80°C in 90% methanol until staining. Cells were stained for 1 hour at 4°C with anti-phospho-STAT6-PE (pY641) (BD Biosciences). To evaluate cytokine production by αβ and γδ T cells, 1 × 106 lung cells were stimulated with PMA (100 ng/ml) and ionomycin (1 μg/ml) for 16 hours, then Brefeldin A and monensin (eBioscience) were added for 4 hours. Cells were fixed, permeabilized and stained with anti-CD4-PE-Cy7 (RM4–5), anti CD90.2-Brilliant Violet 605 (53-2.1; BD Pharmingen), anti-CD3-Brilliant Violet 650 (145-2C11; BD Pharmingen), Anti TCRβ-eFluor 450 (H57-597) anti-IL-17A-Ax647 (eBio17b7) and anti-IL-13-PE (eBio13A). All mAbs were from eBioscience unless otherwise indicated.
RNA purification and real-time PCR
Total cellular RNA was extracted using TRI Reagent (Molecular Research Center) according to the manufacturer's protocol and reverse transcribed using SuperScript II reverse transcriptase (Invitrogen). To assess IL-13 and IL-17A-induced message expression in lung sections and cell cultures, we used quantitative real-time PCR with SYBR Green mix (Biorad). Expression levels were normalized to S14 (mouse) or S13 (human).
Western blot of whole lung homogenate
Total cellular protein was extracted from PBS or cytokine treated animals by homogenizing lung sections in RIPA buffer supplemented with a protease and phosphatase inhibitor cocktail (Halt). Protein lysates were immunoblotted for CLCA3 (Abcam), phospho-STAT6 (Tyr641) (Cell Signaling), total STAT6 (Cell Signaling), or αTubulin (Cell Signaling) according to the manufacturer's protocol.
Supplementary Material
Key Messages.
IL-17A directly enhances IL-13-associated pathology by increasing IL-13-initiated STAT6 signaling.
IL-13 antagonizes IL-17A activity through a STAT6-dependent mechanism.
These findings provide the first mechanistic insight into how IL-17A can contribute to the development of more severe asthma, and may explain previous observations of disordered microbial colonization in individuals with atopic disorders.
Capsule Summary.
IL-17A enhances IL-13-induced STAT6 activation, leading to increased IL-13-driven transcripts and lung pathology. IL-17A-mediated enhancement of IL-13 activity is observed in both mouse and human cells, suggesting that IL-17A may directly contribute to asthma severity.
Acknowledgments
Funded by NHLBI R01 HL122300 (IPL).
Abbreviations Used
- ActD
Actinomycin D
- AHR
Airway hyperresponsiveness
- ANOVA
Analysis of variance
- AUC
Area under the curve
- BAL
Bronchoalveolar lavage
- BEGM
Basic epithelial cell growth medium
- CLCA3
Chloride channel, calcium activated 3
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme-linked immunosorbent assay
- IL
Interleukin
- i.t.
Intratracheal
- NEC
Nasal epithelial cells
- PBS
Phosphate buffered saline
- SEM
Standard error of the mean
- STAT6
Signal transducer and activator of transcription 6
- WT
Wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–75. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–9. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 6.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, et al. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med. 2011;208:853–67. doi: 10.1084/jem.20100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–35. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakashin H, Hirose K, Maezawa Y, Kagami S, Suto A, Watanabe N, et al. IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med. 2008;178:1023–32. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–54. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–7. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 12.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–97. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–8. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 15.Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–30. e1–4. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinyanjui MW, Shan J, Nakada EM, Qureshi ST, Fixman ED. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J Immunol. 2013;190:3859–68. doi: 10.4049/jimmunol.1200506. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med. 2010;182:464–76. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guajardo JR, Schleifer KW, Daines MO, Ruddy RM, Aronow BJ, Wills-Karp M, et al. Altered gene expression profiles in nasal respiratory epithelium reflect stable versus acute childhood asthma. J Allergy Clin Immunol. 2005;115:243–51. doi: 10.1016/j.jaci.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daines MO, Hershey GK. A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses. Evidence for intracellular stores of IL-13 receptor alpha -2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002;277:10387–93. doi: 10.1074/jbc.M108109200. [DOI] [PubMed] [Google Scholar]
- 23.Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–9. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Sivaprasad U, Gibson AM, Ericksen MB, Cunningham CM, Bass SA, et al. IL-13 receptor alpha2 contributes to development of experimental allergic asthma. J Allergy Clin Immunol. 2013;132:951–8. e1–6. doi: 10.1016/j.jaci.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–41. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 26.Hartupee J, Liu C, Novotny M, Sun D, Li X, Hamilton TA. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J Immunol. 2009;182:1660–6. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henness S, Johnson CK, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A augments TNF-alpha-induced IL-6 expression in airway smooth muscle by enhancing mRNA stability. J Allergy Clin Immunol. 2004;114:958–64. doi: 10.1016/j.jaci.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, et al. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umeshita-Suyama R, Sugimoto R, Akaiwa M, Arima K, Yu B, Wada M, et al. Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor alpha chain 1: redundancy of requirement of tyrosine residue for STAT3 activation. Int Immunol. 2000;12:1499–509. doi: 10.1093/intimm/12.11.1499. [DOI] [PubMed] [Google Scholar]
- 30.Wery-Zennaro S, Letourneur M, David M, Bertoglio J, Pierre J. Binding of IL-4 to the IL-13Ralpha(1)/IL-4Ralpha receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS Lett. 1999;464:91–6. doi: 10.1016/s0014-5793(99)01680-4. [DOI] [PubMed] [Google Scholar]
- 31.Sozzani P, Hasan L, Seguelas MH, Caput D, Ferrara P, Pipy B, et al. IL-13 induces tyrosine phosphorylation of phospholipase C gamma-1 following IRS-2 association in human monocytes: relationship with the inhibitory effect of IL-13 on ROI production. Biochem Biophys Res Commun. 1998;244:665–70. doi: 10.1006/bbrc.1998.8314. [DOI] [PubMed] [Google Scholar]
- 32.Chen HC, Reich NC. Live cell imaging reveals continuous STAT6 nuclear trafficking. J Immunol. 2010;185:64–70. doi: 10.4049/jimmunol.0903323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon MR, Parikh AA, Pritts TA, Fischer JE, Cottongim S, Szabo C, et al. Complement component C3 production in IL-1beta-stimulated human intestinal epithelial cells is blocked by NF-kappaB inhibitors and by transfection with ser 32/36 mutant IkappaBalpha. J Surg Res. 1999;82:48–55. doi: 10.1006/jsre.1998.5503. [DOI] [PubMed] [Google Scholar]
- 34.Juan TS, Wilson DR, Wilde MD, Darlington GJ. Participation of the transcription factor C/EBP delta in the acute-phase regulation of the human gene for complement component C3. Proc Natl Acad Sci U S A. 1993;90:2584–8. doi: 10.1073/pnas.90.7.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.David MD, Bertoglio J, Pierre J. Functional characterization of IL-13 receptor alpha2 gene promoter: a critical role of the transcription factor STAT6 for regulated expression. Oncogene. 2003;22:3386–94. doi: 10.1038/sj.onc.1206352. [DOI] [PubMed] [Google Scholar]
- 37.Dickensheets HL, Venkataraman C, Schindler U, Donnelly RP. Interferons inhibit activation of STAT6 by interleukin 4 in human monocytes by inducing SOCS-1 gene expression. Proc Natl Acad Sci U S A. 1999;96:10800–5. doi: 10.1073/pnas.96.19.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hebenstreit D, Luft P, Schmiedlechner A, Duschl A, Horejs-Hoeck J. SOCS-1 and SOCS-3 inhibit IL-4 and IL-13 induced activation of Eotaxin-3/CCL26 gene expression in HEK293 cells. Mol Immunol. 2005;42:295–303. doi: 10.1016/j.molimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Hebenstreit D, Luft P, Schmiedlechner A, Regl G, Frischauf AM, Aberger F, et al. IL-4 and IL-13 induce SOCS-1 gene expression in A549 cells by three functional STAT6-binding motifs located upstream of the transcription initiation site. J Immunol. 2003;171:5901–7. doi: 10.4049/jimmunol.171.11.5901. [DOI] [PubMed] [Google Scholar]
- 40.Woetmann A, Brockdorff J, Lovato P, Nielsen M, Leick V, Rieneck K, et al. Protein phosphatase 2A (PP2A) regulates interleukin-4-mediated STAT6 signaling. J Biol Chem. 2003;278:2787–91. doi: 10.1074/jbc.M210196200. [DOI] [PubMed] [Google Scholar]
- 41.Lu X, Malumbres R, Shields B, Jiang X, Sarosiek KA, Natkunam Y, et al. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112:4098–108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–6. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- 43.Tao B, Jin W, Xu J, Liang Z, Yao J, Zhang Y, et al. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol. 2014;193:2801–11. doi: 10.4049/jimmunol.1303463. [DOI] [PubMed] [Google Scholar]
- 44.Wei Q, Sha Y, Bhattacharya A, Abdel Fattah E, Bonilla D, Jyothula SS, et al. Regulation of IL-4 receptor signaling by STUB1 in lung inflammation. Am J Respir Crit Care Med. 2014;189:16–29. doi: 10.1164/rccm.201305-0874OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahoo A, Alekseev A, Obertas L, Nurieva R. Grail controls Th2 cell development by targeting STAT6 for degradation. Nat Commun. 2014;5:4732. doi: 10.1038/ncomms5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, et al. E3 ubiquitin ligase Cblb suppresses proallergic T cell development and allergic airway inflammation. Cell Rep. 2014;6:709–23. doi: 10.1016/j.celrep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang F, Wachi S, Thai P, Loukoianov A, Tan KH, Forteza RM, et al. Potentiation of IL-19 expression in airway epithelia by IL-17A and IL-4/IL-13: important implications in asthma. J Allergy Clin Immunol. 2008;121:1415–21. 21, e1–3. doi: 10.1016/j.jaci.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, et al. IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation. Thorax. 2013;68:717–23. doi: 10.1136/thoraxjnl-2012-202404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–63. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 52.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol. 2005;78:1086–96. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 53.Allen HB, Vaze ND, Choi C, Hailu T, Tulbert BH, Cusack CA, et al. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol. 2014;150:260–5. doi: 10.1001/jamadermatol.2013.8627. [DOI] [PubMed] [Google Scholar]
- 54.Clausen ML, HC SL, Krogfelt KA, Andersen PS, Agner T. In Vivo Expression of Antimicrobial Peptides in Atopic Dermatitis. Exp Dermatol. 2015 doi: 10.1111/exd.12831. [DOI] [PubMed] [Google Scholar]
- 55.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135:25–30. doi: 10.1016/j.jaci.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 58.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136:323–33. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persson H, Kwon AT, Ramilowski JA, Silberberg G, Soderhall C, Orsmark-Pietras C, et al. Transcriptome analysis of controlled and therapy-resistant childhood asthma reveals distinct gene expression profiles. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–95. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–8. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newcomb DC, Boswell MG, Huckabee MM, Goleniewska K, Dulek DE, Reiss S, et al. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol. 2012;188:1027–35. doi: 10.4049/jimmunol.1102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127:1006–13. e1–4. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182:5317–21. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson MS, Ramalingam TR, Rivollier A, Shenderov K, Mentink-Kane MM, Madala SK, et al. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Ralpha2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–64. doi: 10.1053/j.gastro.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 1.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.