Abstract
Background
Dry needling is an effective treatment for reducing pain associated with active myofascial trigger points (aMTrPs) in the short term. The duration of the benefits of this treatment have not been fully assessed.
Objective
This study attempts to determine whether the benefits of dry needling (DN) of a-MTrPs are sustained 6 weeks post-treatment.
Design
Follow-up of a prospective study
Setting
University
Participants
Forty-five subjects (13 males) with cervical pain > 3 months and a-MTrPs in the upper trapezius who completed 3 DN treatments, evaluated 6-weeks post-treatment.
Interventions
None
Main Outcome Measures
Primary Outcomes: Changes from baseline to follow-up in scores for the verbal analogue scale (VAS), Brief Pain Inventory (BPI), and MTrP status. MTrPs were rated: active (spontaneously painful), latent (painful only on compression) and non-palpable nodule. Responders: patients whose MTrP status changed from active to latent or non-palpable nodule (resolved).
Secondary Outcomes
Pain pressure threshold (PPT), Profile of Mood States, Oswestry Disability Index (ODI), SF-36, Cervical Range of Motion.
Results
Pain measures remained significantly improved 6 weeks post-treatment (p<.003), as did the SF-36 physical functioning score (.01) and ODI (p=.002). Side bending and PPT for subjects with unilateral MTrPs had sustained improvement (p=.002). The number of subjects with sustained MTrP response at 6 weeks was significant (p<.001). Comparing responders to non-responders, change in VAS and BPI were statistically significant (p = .006, p=.03), but PPT was not. Patients with higher baseline VAS have higher risk of not responding to DN, those with a greater drop in VAS from baseline have a higher probability of sustained response. One unit decrease in VAS at baseline results in a 6.3-fold increase in the odds of being a responder versus a non-responder (p = .008).
Conclusions
There is sustained reduction of pain scores after completion of DN, which is more likely with a greater drop in VAS. Patients with higher baseline VAS are less likely to respond to DN. Early intervention toward significant pain reduction is likely to be associated with sustained clinical response.
INTRODUCTION
Myofascial pain syndrome (MPS) is often a chronic condition whose prevalence varies from 15% to a life-long prevalence of 85% (1–3). There is lack of consensus about what constitutes criteria for diagnosis (4–7) and which measures are appropriate for clinical research outcomes (8). Recently, researchers have reported the results of surveys among health care providers to assess whether there is even a working consensus about diagnostic criteria, which tissue should be the treatment target, which measures are best for determining efficacy and should they be objective and/or self-reported outcomes (3,9). The results of these surveys identify a lack of agreement among clinicians and investigators on many of these points. Accordingly, there is a need for standardizing patient evaluations, interventions and treatment outcomes if we are to generate reliable evidence and influence practice. With these standards, treatment guidelines can be generated.
An approach, and it is one that our research team has selected, is to devise a standard evaluation for patients with MPS that is used for determining the diagnosis, the level of severity of the condition and outcomes sought to assign effectiveness (2,10). Toward this end, pain as well as the status of the myofascial trigger point (MTrP) were considered important. We posited that the MTrP was central to the MPS. Standardizing the examination of the MTrP, assessing its status in terms of being active or latent, and evaluating its responsiveness (or lack thereof) to treatment provides an opportunity for obtaining reliable data about the effectiveness of treatment. The Travell and Simons description states that an MTrP is a discrete, palpable nodule located within a taut band of skeletal muscle (6). When the MTrP is spontaneously painful, it is known as an active MTrP (a-MTrP). Strong digital pressure on an a-MTrP exacerbates the patient’s spontaneous pain complaint and mimics the patient’s familiar pain experience. When the MTrP is not spontaneously painful, but typical pain can be elicited when palpated or disturbed, it is regarded as a latent MTrP (l-MTrP). The l-MTrP is a nodule with the same physical characteristics as an a-MTrP, but requires palpation to elicit pain. Electromyographic, biochemical, and imaging studies have demonstrated notable differences between these two MTrP classifications, and also distinguish them from normal, non-painful tissue (11,12).
It has not yet been established that the MTrP is necessary for the pain syndrome. It is also not yet known whether the MPS “causes” the development of the MTrP. These questions remain of significant interest to the research community and to practitioners. If it could be shown that the MTrP is a necessary condition for MPS, it would serve as an objective, identifiable target for pain relief. Thus, a clear, definitive relationship between the MTrP and MPS would help advance the field.
Our research group has reported the results of a prospective intervention trial of dry needling for the treatment of subjects with chronic shoulder girdle/neck pain secondary to a-MTrPs in the upper trapezius muscle (13). In this study, we applied standardized evaluations that assessed objective findings and self-reported outcomes, including pain, mood, health status and disability measures and cervical range of motion. We also used ultrasound, Doppler and elastography to determine tissue properties of the upper trapezius muscle and the characteristics of MTrPs at baseline and after treatment. Our results demonstrated that 3 treatments (once per week for 3 weeks) of a-MTrPs using a standardized dry needling technique significantly reduced myofascial pain and changed the status of the MTrP from active to either latent or resolved (13). Other investigators have used a similar approach with positive therapeutic results. Several recent reviews and meta-analyses are available (14–17). This paper reports the findings of the same cohort 6 weeks after completion of the 3 dry needling treatments. Our aim for this manuscript is to assess the long-term treatment outcome of dry needling in a cohort of patients, receiving one course of treatment without any intervening subsequent treatment for MPS.
METHODS
The study was approved by the Chesapeake Institutional Review Board. All participants were consented by one of three of the authors. Participants were recruited from a university campus and surrounding area, received no remuneration for participation and ranged in age from 18–65 years. This was a convenience sample. All patients who participated in the intervention trial were asked to return for a follow-up evaluation 6 weeks after completion of their initial 3 dry needling treatments and were seen at the same clinic. Entry criteria included at least one palpable a-MTrP in the upper trapezius. Exclusion criteria included chronic fatigue syndrome, fibromyalgia, chronic Lyme disease, cervical radiculopathy, head/neck/shoulder girdle surgeries, new medication or change within 6 weeks, and current use of acupuncture. A general history and physical examination were completed on all participants and included inquiry about medication, dietary supplements, regular exercise (defined by at least 3 episodes of exercise for at least 30 minutes in duration. (2) A repeat of all baseline and treatment outcome measures was performed by 2 clinicians. There were 2 measures of pain used as primary outcome measures. These were a verbal analogue scale (VAS) (18), scored from 0 to 10 (0-no pain, 10-unbearable pain); and the Brief Pain Inventory (BPI) (19). Another primary outcome was change in trigger point status, as determined by palpation of the upper trapezius muscle by two clinicians (JS, LG), who have achieved good inter-rater reliability (13). MTrP status was scored as a change from a-MTrP to either l-MTrP or no palpable nodule. A secondary outcome measure used for pain was the pain pressure threshold (PPT) (20). PPT was obtained at 4 sites, following a standard procedure for assessing relative comparisons among the anatomical sites using a pressure algometer (Commander Algometer, Tech Medical, Salt Lake City, UT; http://www.jtechmedical.com/Commander/commander-algometer). Additional secondary outcomes included cervical range of motion (ROM) (flexion/extension, side bending, and rotation). Cervical ROM was determined using the Deluxe Cervical Range of Motion Instrument (CROM), model 12-1156 (Fabrication Enterprises, White Plains, NY) for determination of asymmetry. Patient reported outcomes included: Oswestry Disability Index (21), the MOS 36-Item Short-Form Health Survey (SF-36) (22), as well as a short version of the Profile of Mood States (POMS) (23). A full description of the instruments used and methods followed is available (2, 13).
Data Analysis
Analysis of covariance models were conducted to determine the impact of responder/non-responder status on pain metrics, adjusted for important variables. The adjustment variables are the baseline pain measure, side, age, gender, and exercise status. As these variables are deemed a priori important, they are included in all models whether or not significant. Least squares means were computed for VAS, BPI, and PPT for the change from baseline to 8 weeks and the change from 3 weeks to 8 weeks. A full longitudinal assessment was not performed since data were only available at two points in time.
Appropriate regression diagnostics were performed, including Q-Q plots to assess the normality assumption of the regression model, and residual plots to assess homoscedasticity. All models were deemed appropriate. Results for p-values are reported without adjustment for multiple testing.
We conducted an adjusted logistic regression analysis to determine how the baseline and change from baseline pain scores affect the odds ratio of being a responder versus a non-responder. It should be noted that the study was not designed to answer this question.
RESULTS
There were 45 patients, 13 males, with a mean age of 37 years who completed follow up at 8 weeks. All had received 3 dry needling treatments, which were completed 6 weeks prior, and had no intervening treatment for MPS.
Subject characteristics at baseline and 8 weeks are given in Tables 1(A) – (D) and provide the p-value of the t-test of comparison of means. Asymmetry of side bending for subjects with unilateral trigger points and PPT at the treated site are significantly improved from baseline (p=.002. Pain measures are all significantly improved except for VAS at the untreated site in patients with unilateral trigger points (p<.003). The SF-36 physical functioning score (p=.012) and the Oswestry Disability Index (p=.002) remained significantly improved from baseline.
Table 1.
| (A). Baseline and 8 Weeks Characteristics: Physical Findings (Mean ± SD) | ||||
|---|---|---|---|---|
| Characteristic | n | Baseline | Follow-up | p-value |
| Cervical ROM Extension | 33 | 73.4 ± 10.3 | 73.5 ± 10.9 | .91 |
| Cervical ROM Flexion | 33 | 52.8 ± 9.5 | 54.7 ± 8.2 | .28 |
| Rotation Asymmetry Unilateral | 16 | 6.6 ± 5.7 | 3.6 ± 4.1 | .12 |
| Rotation Asymmetry Bilateral | 17 | 4.6 ± 3.6 | 1.9 ± 3.2 | .06 |
| Side Bending Unilateral | 16 | 6.4 ± 3.7 | 1.9 ± 2.6 | .002 |
| Side Bending Bilateral | 17 | 4.1 ± 4.7 | 2.6 ± 4.2 | .31 |
| PPT Treated Site Unilateral | 16 | 7.4 ± 4.0 | 8.8 ± 4.1 | .009 |
| PPT Treated Site Bilateral | 17 | 6.7 ± 3.0 | 7.6 ± 2.8 | .39 |
| PPT Untreated Site Unilateral | 16 | 9.1 ± 4.0 | 8.8 ± 4.8 | .77 |
| PPT Untreated Site Bilateral | 17 | 8.3 ± 3.5 | 7.1 ± 2.2 | .27 |
| (B). Baseline and 8 Weeks Characteristics: Pain (Mean ± SD) | ||||
|---|---|---|---|---|
| Characteristic | n | Baseline | Follow-up | p-value |
| BPI | 27 | 3.2 ± 1.1 | 1.9 ± 1.4 | <.001 |
| VAS Treated Site Unilateral | 24 | 3.3 ± 2.0 | 1.3 ± 1.8 | .002 |
| VAS Treated Site Bilateral | 21 | 3.0 ± 1.4 | 1.2 ± 1.7 | .001 |
| VAS Untreated Site Unilateral | 23 | 1.1 ± 2.0 | 0.78 ± 1.44 | .41 |
| VAS Untreated Site Bilateral | 21 | 2.7 ± 1.2 | 1.1 ± 1.3 | <.001 |
| SF36 Pain | 29 | 61.5 ± 15.5 | 72.6 ± 15.2 | .003 |
| (C). Baseline and 8 Weeks Characteristics: Self-Reported Outcomes (Mean ± SD) | ||||
|---|---|---|---|---|
| Characteristic | n | Baseline | Follow-up | p-value |
| POMS Confusion | 29 | 0.21 ± 0.34 | 0.17 ± 0.21 | .51 |
| POMS Depression | 29 | 0.08 ± 0.20 | 0.08 ± 0.22 | >.99 |
| POMS Fatigue | 29 | 0.74 ± 0.78 | 0.49 ± 0.56 | .13 |
| POMS Tension | 29 | 0.43 ± 0.40 | 0.34 ± 0.62 | .50 |
| POMS Mood | 29 | −0.06 ± 1.50 | −0.58 ± 1.88 | .20 |
| POMS Vigor | 29 | 1.63 ± 0.90 | 1.75 ± 0.92 | .34 |
| POMS Anger | 29 | 0.10 ± 0.24 | 0.06 ± 0.13 | .39 |
| SF36 General Health | 29 | 78.4 ± 18.4 | 80.5 ± 14.3 | .24 |
| SF36 Mental Health | 29 | 79.3 ± 9.4 | 79.3 ± 14.5 | >.99 |
| SF36 Physical Functioning | 29 | 92.8 ± 9.8 | 95.9 ± 6.1 | .01 |
| SF36 Emotional | 29 | 85.6 ± 22.9 | 93.4 ± 9.3 | .08 |
| SF36 Physical Role | 29 | 87.9 ± 15.2 | 91.2 ± 12.2 | .28 |
| SF36 Social Functioning | 29 | 91.4 ± 12.5 | 93.1 ± 11.4 | .35 |
| SF36 Vitality | 29 | 60.3 ± 16.0 | 63.1 ± 16.9 | .30 |
| (D). Baseline and 8 Weeks Characteristics: Disability (Mean ± SD) | ||||
|---|---|---|---|---|
| Characteristic | n | Baseline | Follow-up | p-value |
| Oswestry Score | 28 | 8.7 ± 5.4 | 6.2 ± 4.7 | .002 |
The change in status of the MTrP from baseline was also measured. When the status of the MTrP changed from active to latent or non-palpable the subject was identified as a “responder.” The number of subjects who were responders at 3 weeks was highly significant and this was sustained at 8 weeks (p<.001).
Figures 1, 2, and 3 show the mean and standard deviation of VAS, BPI, and PPT, respectively, from baseline to 3 weeks, and 8 weeks. These results indicate that the VAS decreases from baseline at 3 weeks, and this decrease is sustained at 8 weeks for responders. Among responders, the VAS at 8 weeks was significantly lower than baseline. For non-responders, the VAS at 8 weeks is not significantly different from baseline. A similar pattern was observed for BPI. No significant trends were observed for PPT, although the PPT for responders at 8 weeks was significantly higher (less pain for the same amount of pressure) than that of non-responders. We proceeded to model the baseline to 8 week data and 3 week to 8 week data to report adjusted least squares means.
Figure 1.

Trends for VAS at baseline, 3 weeks and 8 weeks among responders and non-responders.
Figure 2.
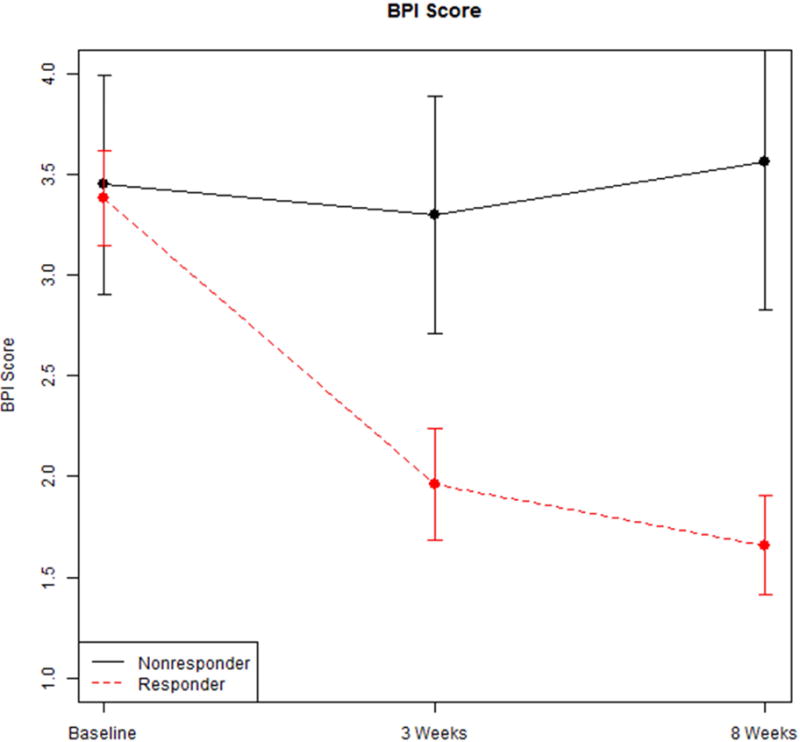
Trends for BPI at baseline, 3 weeks and 8 weeks among responders and non-responders.
Figure 3.
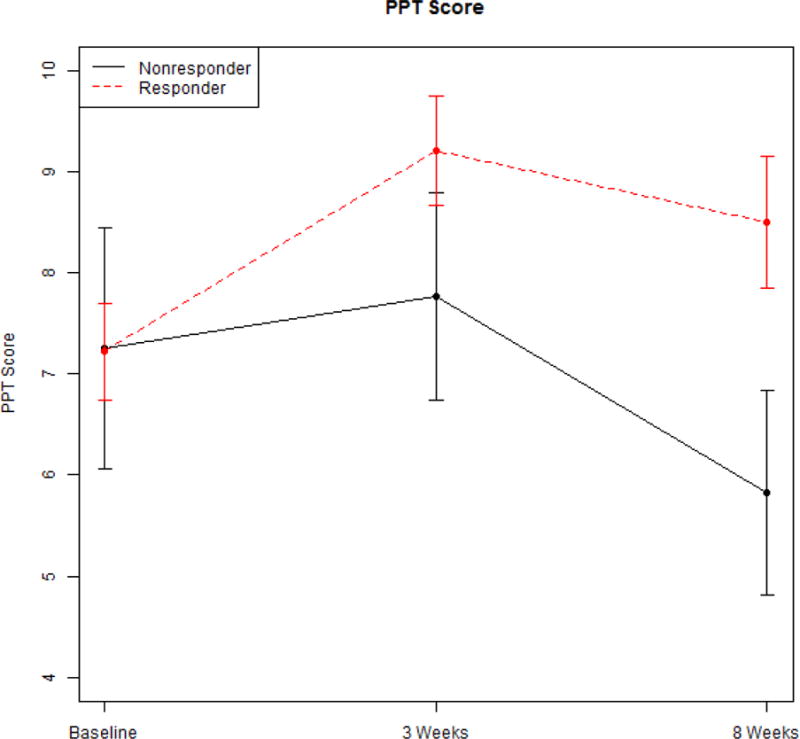
Trends for PPT at baseline, 3 weeks and 8 weeks among responders and non-responders.
Change from baseline to 8 weeks in VAS was statistically significant (p = .006) between responders (−2.29, S.E. = 0.27) and non-responders (−0.46, S.E. 0.60) (Table 4). Of the adjustment variables, only baseline VAS (p < .001) and age (p = .02) are statistically significant (see Table 5). Change from baseline to 8 weeks in BPI was also statistically significant (p = .026) between responders (−1.43, S.E. = 0.22; and non-responders (0.08, S.E. = 0.60 (see Table 6); and with no significant adjustment variables (see Table 7). Change from baseline to 8 weeks in PPT was not statistically significant (p = .17; data not shown) between responders (1.11, S.E., 0.57) and non-responders (−1.14, S.E. 1.57).
Table 4.
Least Squares Means of Change in VAS Score at 8 Weeks from Baseline Adjusted for Baseline, Site, Gender, Age, and Exercise Status
| Response | Mean Change in VAS | SE |
|---|---|---|
| Responders | −2.29 | 0.27 |
| Non-Responders | −0.46 | 0.60 |
Table 5.
Regression Estimates with Change in VAS Score at 8 Weeks from Baseline as Response Variable
| Parameter | Estimate | SE | t | p-value |
|---|---|---|---|---|
| Intercept | 3.41 | 0.91 | 3.74 | .001 |
| VAS Baseline | −0.73 | 0.14 | −5.27 | <.001 |
| Responders | −1.83 | 0.62 | −2.97 | .005 |
| Non-Responders | baseline | |||
| Bilateral | 0.17 | 0.45 | 0.39 | .70 |
| Unilateral | baseline | |||
| Age | −0.05 | 0.02 | −2.45 | .02 |
| Gender f | 0.49 | 0.53 | 0.94 | .36 |
| Gender m | baseline | |||
| Exercise Status no | −0.45 | 0.51 | −0.88 | .38 |
| Exercise Status yes | baseline |
Table 6.
Least Squares Means of Change in BPI Score at 8 Weeks from Baseline Adjusted for Baseline, Site, Gender, Age, and Exercise Status
| Response | Mean Change in BPI | SE |
|---|---|---|
| Responders | −1.43 | 0.22 |
| Non-responders | 0.08 | 0.60 |
Table 7.
Regression Estimates with Change in BPI Score at 8 Weeks from Baseline as Response Variable
| Parameter | Estimate | SE | t | p-value |
|---|---|---|---|---|
| Intercept | 1.21 | 1.26 | 0.96 | .35 |
| BPI Baseline | −0.28 | 0.21 | −1.36 | .19 |
| Responders | −1.51 | 0.63 | −2.41 | .03 |
| Non-responders | baseline | . | . | . |
| Bilateral | 0.11 | 0.44 | 0.24 | .81 |
| Unilateral | baseline | . | . | . |
| Age | −0.01 | 0.02 | −0.69 | .50 |
| Gender f | −0.05 | 0.47 | −0.1 | .92 |
| Gender m | baseline | . | . | . |
| Exercise Status no | 0.34 | 0.46 | 0.74 | .47 |
| Exercise Status yes | baseline | . | . |
Change from 3 weeks to 8 weeks in VAS was not statistically significant (p = .57) between responders (0.47, S.E. = 0.26) and non-responders (0.14, S.E. = 0.57). Similarly, change from 3 weeks to 8 weeks in BPI was not statistically significant (p = .91) between responders (−0.14, S.E. = 0.24) and non-responders (−0.06, S.E. = 0.62).
Our results, viewed from the perspective of baseline and change from baseline in pain scores, show that patients with higher baseline scores have a higher risk of not responding to dry needling. They also show that patients with a higher reduction in VAS pain scores from baseline score have a higher probability of responding to treatment. A one unit decrease in VAS score at baseline results in a 6.3-fold increase in the odds of being a responder versus a non-responder (p = .008). For VAS scores measured at 3 weeks, a one unit increase in change from baseline results in an 8-fold increase in the odds of being a responder versus a non-responder (p =.004). For VAS scores measured at 8 weeks, a one unit increase in change from baseline results in a doubling of the odds of being a responder versus a non-responder (p =.02).
DISCUSSION
MPS is a pain syndrome whose etiology is still being debated. Many, but not all, clinicians and investigators agree that an MPS diagnosis must include the presence of an MTrP (6,24,25). This study supports the view that MPS and a-MTrPs have a significant relationship, but their pathophysiology and mechanisms need to be better understood.
Pieces of the puzzle about MTrPs are unfolding. However, the relationships between the biochemical findings and tissue properties of MTrPs, and whether they play a key role in clinical response to treatment, remain debated. Additionally, the idea that a-MTrPs are the “pain generators” within MPS remains contested as well. Some groups suggest that the tissue in need of treatment is not necessarily the MTrP but rather the muscle (26,27). Another suggests it is fascia (28). Still others suggest that there are several contributors to pain development, such as arthritis or nerve root irritation (29,30). Regardless, the persistence of pain typically requires the development of central sensitization and/or changes in the dorsal horn (31). Sensitization of both peripheral and central afferents is responsible for the transition from normal to aberrant pain perception in the central nervous system that outlasts a noxious peripheral stimulus. Biochemical data suggest that a-MTrPs involve mechanisms of muscle nociception, sensitization and are therefore, potential sources of persistent pain (32,33). Our group has previously reported a link in pain reduction in patients with a-MTrPs and MPS to a change in status of the MTrP. The conversion of an a-MTrP from a spontaneously painful state to one requiring perturbation (latent) or a resolution of the finding is associated with a significant reduction in pain (13). A reduction in pain is accompanied by a decrease in central sensitization. The follow-up data reported in this manuscript demonstrate that the relationship between MTrP status change and pain reduction remains significant at 8 weeks. This observation supports the view that on average, persistent pain reduction is likely related to sustained MTrP status change or vice versa, but a causal relationship has not been established. That is, the change in status of the MTrP does not necessarily cause the decrease in pain, but rather both occur together.
Many have reported abnormalities of MTrPs associated with MPS that might help explain their pathophysiology and underlying association with the pain syndrome. These include increased spontaneous electrical activity (34), which is an indication of excessive ACh release at the motor endplate. This would lead to sarcomere contracture that could, in turn, produce local ischemia and hypoxia, resulting in the release of algogenic and vasoactive substances (e.g., inflammatory cytokines, neuropeptides and catecholamines) capable of activating and sensitizing peripheral nociceptors, in a-MTrPs and surrounding soft tissue (35–38).
The mechanism(s) by which dry needling of MTrPs might reduce myofascial pain is also somewhat speculative. These include its effects on the taut band, local ischemia and hypoxia and peripheral and central sensitization via neural mechanisms, increase in local milieu blood flow and oxygenation, change in the milieu of endogenous opioids, endorphins, cholinergic anti-inflammatory mediators and a modulatory effect on sensory neural impulses at the central nervous system level (39–46).
One study demonstrated that dry needling of active (but not latent) MTrPs can elicit motor unit potentials (MUPs) – in a time-locked manner – on the contralateral side of the body. Since only a-MTrPs featured contralateral MUPs, it suggests that one difference between a-MTrPs versus l-MTrPs is due to maladaptive neuroplastic changes in the central nervous system of both the sensory and motor arms. In addition, 38% of a-MTrPs did not feature contralateral MUPs, suggesting there may be degrees of central sensitization. This likely depends upon the chronicity of pain and maybe even the degree of neuroplasticity. This study demonstrated another recordable pathophysiological distinction that emphasizes the validity and importance of clinically differentiating active from latent MTrPs (47). Only a few papers have been published about the natural history of a-MTrPs and their relationship to MPS (30,48). None, to our knowledge, has addressed the natural history of treating a-MTrPs. In other words, if one has an effective treatment, how long does the treatment effect persist? Additionally, are there any potential patient or syndrome characteristics that might inform us about the post-treatment trajectory of patients? Such syndrome characteristics could include intensity and chronicity of pain, the presence of segmental and/or supraspinal sensitization, the amount of pain reduction following treatment, whether pain reduction is associated with a low PPT and improvements in self-reported outcome measures, the number and size of a-MTrPs, and whether the patient presented with bilateral a-MTrPs or only unilateral a-MTrPs at baseline. Which patients are at risk for recurrence and what are their patient profiles?
The findings from the prospective treatment trial reported here, suggest that patients receiving 3 weekly treatments of dry needling for an active MTrP have sustained benefit for at least 6 weeks following treatment. We believe this can be attributed to the effect of the dry needling they had received, since no intervening treatment was obtained to the best of our knowledge. Dry needling, the use of a high-gauge, solid, filiform no-bore needle without addition of solutions or local pharmacological agents, has had therapeutic application for nearly 50 years (24,48).
In this study, pain is the primary outcome, and the VAS and BPI were used to measure pain. The VAS is a uni-dimensional measure of pain intensity that we selected because of validity, ease and frequency of use in many different clinical settings (49,50). However, we also measured pain using two different multi-dimensional instruments, the BPI and the Bodily Pain Score of the SF-36. Results from these measures were similar to the VAS. Pain response was sustained during the follow-up period and remained statistically significantly different from baseline scores.
The algometer measurements remained significantly different form baseline only in the patients who had a unilateral a-MTrP. Those with bilateral involvement were only slightly better at 8 weeks (PPT score of 6.7 at baseline, 7.6 at 8 weeks). This was not statistically significantly different from baseline scores. Algometry and the recording of the PPT has been shown to be a very reliable measure and correlates well with MTrP sensitivity (51–53). It uses an instrument (algometer), hence is objective, but requires patient reporting of when the pain threshold is reached. The interpretation of what the PPT represents and whether it is providing the same information as a self-reported pain score is worthy of discussion. Fischer suggested that this type of measurement can be used reliably to quantify tenderness and to “diagnose pathological tenderness in muscles.” He further cites that there is a strong correlation between the PPT and tissue resistance, as evidenced by the taut muscular band, “muscle tone or consistency” and tissue compliance meters. This latter is a method of determining the “depth of penetration achieved by a unit of force applied” (51). These observations suggest that the PPT may provide different information than a self-report of pain.
Mood and health-related quality of life measures were not significantly different from baseline measures. However, the disability measure (Oswestry Disability Index) and the physical functioning score of the SF-36 demonstrated sustained improvement over baseline.
There were no statistically significant differences between baseline VAS or BPI scores between the responders and non-responders. The drop in VAS for the responders was clinically significant (>2.0 points). This suggests that a sustained response to treatment is more likely to be observed if there is a clinically meaningful drop in pain level at initial treatment. A decrease in VAS of 2 points is thought reach this threshold. The standard for clinically meaningful improvement for the PPT is an increase of 4kg/cm2 (54). The clinical response of the cohort improved by 2kg/cm2. In the earlier report about the efficacy of 3 dry needling techniques (13), there were no significant differences in PPT scores between patients with unilateral vs bilateral trigger points.
This study had several limitations. The follow-up phase initially began as a clinical assessment during which patients were asked to return for a physical examination and an assessment of their pain status. The research team decided to perform all the baseline and post-treatment assessments, which was approved. The first 8 subjects had only limited data for this follow-up and are not included in the 8 week evaluation. In addition, while we requested that study participants not initiate new treatments for myofascial pain; and they reported that they did not obtain such treatments, we did not confirm this through means other than self-reports. The cohort in this study was recruited primarily from a university campus and may not reflect the usual population of people with myofascial pain. In general, they were young, spent much time at computer terminals and may have a life-style different from an older population. We did perform regression estimates for changes in VAS and found that age was a significant variable, although participation in exercise was not, and we did not inquire about diet.
CONCLUSION
Three dry needling treatments for people with chronic myofascial pain and active myofascial trigger points resulted in a sustained reduction of pain, as measured by VAS and BPI, at 6 week follow-up after completion of treatment. Only patients with unilateral active MTrPs had sustained improvement in PPT scores and cervical side bending. Patients achieving a lower pain score after treatment a have sustained response to treatment.
Table 2.
Primary outcome for Treated Subjects with Bilateral Active Trigger Points
| Site | Baseline | Follow-up | Count |
|---|---|---|---|
| Treated | Active | Active | 6 |
| Active | Latent | 8 | |
| Active | Normal | 7 | |
| Untreated | Active | Active | 6 |
| Active | Latent | 8 | |
| Active | Normal | 3 |
Table 3.
Primary outcome for Treated Subjects with Unilateral Active Trigger Points
| Side | Baseline | Follow-up | Count |
|---|---|---|---|
| Treated | Active | Active | 7 |
| Active | Latent | 9 | |
| Active | Normal | 8 | |
| Untreated | Latent | Active | 0 |
| Latent | Latent | 11 | |
| Latent | Normal | 3 | |
| Normal | Active | 1 | |
| Normal | Latent | 1 | |
| Normal | Normal | 2 |
Acknowledgments
Funding for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (grant#1R01AR057348).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynn H Gerber, Center for the Study of Chronic Illness and Disability, George Mason University, Fairfax, VA 22030.
Siddhartha Sikdar, Bioengineering Department, George Mason University, 4400 Universoty Boulevard, Fairfax, VA 22030.
Jacqueline V. Aredo, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
Katee Armstrong, Center for the Study of Chronic Illness and Disability, George Mason University, Fairfax, VA 22030.
William F. Rosenberger, Department of Statistics, George Mason University, Fairfax, VA 22030.
Hui Shao, Department of Statistics, George Mason University, Fairfax, VA 22030.
Jay P. Shah, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151:157–160. [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber LH, Sikdar S, Armstrong K, et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM R. 2013;5:931–938. doi: 10.1016/j.pmrj.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tekin L, Akarsu S, Durmus O, Cakar E, Dincer U, Kıralp MZ. The effect of dry needling in the treatment of myofascial pain syndrome: A randomized double-blinded placebo-controlled trial. Clin Rheumatol. 2013;32:309–315. doi: 10.1007/s10067-012-2112-3. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn LJ. A discussion of fibrositis. Proc R Soc Med. 1913;6:27–35. doi: 10.1177/003591571300600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schade H. Untersuchungen in der Erkältungstrage: III. Uber den Rheumatismus, insbesondere den Muskelrheumatismus (myogelose) Müench Med Wochenschr. 1921;68:95–99. [Google Scholar]
- 6.Travell JG, Simons DG. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- 7.Borg-Stein J, Simons DG. Focused review: Myofascial pain. Arch Phys Med Rehabil. 2002;83(3 Suppl 1):S40–S49. doi: 10.1053/apmr.2002.32155. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21:427–445. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14:95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Rathbone AT, Kumbhare DA. Signs and Symptoms of Myofascial Pain: An International Survey of Pain Management Providers and Proposed Preliminary Set of Diagnostic Criteria. Pain Med. 2015 Dec 7; doi: 10.1111/pme.12780. [DOI] [PubMed] [Google Scholar]
- 11.Turo D, Otto P, Hossain M, et al. Novel Use of Ultrasound Elastography to Quantify Muscle Tissue Changes After Dry Needling of Myofascial Trigger Points in Patients With Chronic Myofascial Pain. J Ultrasound Med. 2015;34(12):2149–61. doi: 10.7863/ultra.14.08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubbard DR, Berkoff GM. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18:1803–1807. doi: 10.1097/00007632-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Gerber LH, Shah J, Rosenberger W, et al. Dry Needling Alters Trigger Points in the Upper Trapezius Muscle and Reduces Pain in Subjects With Chronic Myofascial Pain. PM R. 2015;7:711–718. doi: 10.1016/j.pmrj.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. Jrnl Amer Board Fam Med. 2010;25:5640–5646. doi: 10.3122/jabfm.2010.05.090296. [DOI] [PubMed] [Google Scholar]
- 15.Kietrys DM, Palombaro KM, Mannheimer JS. Dry needling for management of pain in the upper quarter and craniofacial region. Curr Pain Headache Rep. 2014;18:437. doi: 10.1007/s11916-014-0437-0. [DOI] [PubMed] [Google Scholar]
- 16.Dunning J, Butts R, Mourad F, Young I, Flannagan S, Perreault T. Dry needling: A literature review with implications for clinical guidelines. Phys Ther Rev. 2014;19:252–265. doi: 10.1179/108331913X13844245102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cagnie B, Castelein B, Pollie F, Steelan L, Verhoeyen H, Cools A. Evidence for the use of ischemic compression and dry needling in the management of myofascial trigger points in the upper trapezius in patients with neck pain: A systematic review. Amer J Phys Med Rehabil. 2015;94:573–83. doi: 10.1097/PHM.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 19.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 20.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 21.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 24.Simons DG, Travell JG, Simons LS. Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd. Vol. 1. Williams &Wilkins; Baltimore, MD, USA: 1999. [Google Scholar]
- 25.Cailliet R. Soft tissue pain and disability. F. A. Davis; Philadelphia, Pa, USA: 1977. [Google Scholar]
- 26.Mense S. Muscle Pain: Mechanisms and Clinical Significance. Deutches Arzt Int. 2008;105:214–219. doi: 10.3238/artzebl.2008.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Wang H, Gay R, et al. Quantitation of Myofascial Taut Bands. Arch PMR. 2016;97:67–72. doi: 10.1016/j.apmr.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stecco A, Gesi M, Stecco C, Stern R. Fascial Components of the myofascial pain syndrome. Curr Pain Headache Rep. 2013;17:352. doi: 10.1007/s11916-013-0352-9. [DOI] [PubMed] [Google Scholar]
- 29.Huntley AH, Srbely JZ, Zettel JL. Experimentally induced central sensitization in the cervical spine evokes postural stiffening strategies in healthy young adults. Gait Posture. 2015;41:652–657. doi: 10.1016/j.gaitpost.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Deitos A, Dussán-Sarria JA, Souza A, et al. Clinical Value of Serum Neuroplasticity Mediators in Identifying the Central Sensitivity Syndrome in Patients With Chronic Pain With and Without Structural Pathology. Clin J Pain. 2015;31(11):959–967. doi: 10.1097/AJP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R. Myofascial trigger points: relation to acupuncture and mechanisms of pain. Archives of Physical Medicine and Rehabilitation. 1981;62:114–117. [PubMed] [Google Scholar]
- 32.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 33.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Ge HY, Monterde S, Graven-Nielsen T, Arendt-Nielsen L. Latent myofascial trigger points are associated with an increased intramuscular electromyographic activity during synergistic muscle activation. J Pain. 2014;15:181–187. doi: 10.1016/j.jpain.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. Journal of Electromyography and Kinesiology. 2004;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Awad E. Interstitial myofibrositis: hypothesis of the mechanism. Arch Phys Med Rehabil. 1973;54:449–453. [PubMed] [Google Scholar]
- 37.Brendstrup P, Jespersen K, Asboe H. Morphological and chemical connective tissue changes in fibrositic muscles. Ann Rheum Dis. 1957;16:438–440. doi: 10.1136/ard.16.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosendal L, Larsson B, Kristiansen J, et al. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Lewit K. The needle effect in the relief of myofascial pain. Pain. 1979;6:83–90. doi: 10.1016/0304-3959(79)90142-8. [DOI] [PubMed] [Google Scholar]
- 40.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Archives of Physical Medicine and Rehabilitation. 2001;82:986–992. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- 41.Tough EA, White AR, Cummings TM, Richards SH, Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomised controlled trials. European Journal of Pain. 2009;13:3–10. doi: 10.1016/j.ejpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Gerwin RD, Dommerholt J, Shah JP. An expansion of Simons’ integrated hypothesis of trigger point formation. Current pain and headache reports. 2004;8:468–475. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 43.Srbely JZ, Dickey JP, Lowerison M, Edwards AM, Nolet PS, Wong LL. Stimulation of myofascial trigger points with ultrasound induces segmental anti-nociceptive effects: A randomized controlled study. Pain. 2008;139:260–266. doi: 10.1016/j.pain.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Srbely JZ, Dickey JP, Lee D, Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42:463–468. doi: 10.2340/16501977-0535. [DOI] [PubMed] [Google Scholar]
- 45.Chu J. Dry needling (intramuscular stimulation) in myofascial pain related to lumbosacral radiculopathy. European Journal of Physical Medicine and Rehabilitation. 1995;5:106–121. [Google Scholar]
- 46.Cagnie B, Dewitte V, Barbe T, Timmermans F, Delrue N, Meeus M. Physiologic effects of dry needling. Curr Pain Headache Rep. 2013;17:34. doi: 10.1007/s11916-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 47.Audette JF, Wang F, Smith H. Bilateral activation of motor unit potentials with unilateral needle stimulation of active myofascial trigger points. Am J Phys Med Rehabil. 2004;83:368–374. doi: 10.1097/01.phm.0000118037.61143.7c. [DOI] [PubMed] [Google Scholar]
- 48.Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial Trigger Points Then and Now: A Historical and Scientific Perspective. PM R. 2015;7(7):746–761. doi: 10.1016/j.pmrj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 50.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 51.Fischer AA. Pressure threshold meter: its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67:836–838. [PubMed] [Google Scholar]
- 52.Jaeger B, Reeves JL. Quantification of changes in myofascial trigger point sensitivity with the pressure algometer following passive stretch. Pain. 1986;27:203–210. doi: 10.1016/0304-3959(86)90211-3. [DOI] [PubMed] [Google Scholar]
- 53.Fischer AA. Pressure algometry over normal muscles. Pain. 1987;30:115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 54.Todd KH. Clinical versus statistical significance in the assessment of pain relief. Ann Emerg Med. 1996;27:439–441. doi: 10.1016/s0196-0644(96)70226-3. [DOI] [PubMed] [Google Scholar]


