Abstract
Purpose
Visual inspection (VI) of bubbles in the chest drainage unit does not differentiate a true leak of alveolopleural fistula (APF) from a false leak. We hypothesized that detection of elevated levels of carbon dioxide, increase in oxygen content, or both, in pleural gas upon the administration of supplemental oxygen would accurately identify APF.
Description
Prospective study comparing pleural gas analysis (GA) with VI to detect APF after surgical lobectomy (n = 50).
Evaluation
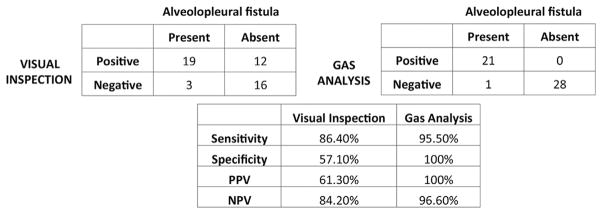
APF was found in 22 (44%) patients at the time of analysis. VI revealed air bubbles in 31 (62%) patients, indicating the presence of APF, of whom 12 (38.7%) were false leaks. VI failed to identify APF in 3 (6%) patients that resulted in post–tube removal pneumothorax. By contrast, GA accurately demonstrated APF in 21 patients, with only one false negative and no false positives. GA demonstrated better sensitivity (95.5% vs 86.4%), specificity (100% vs 57.1%), positive predictive value (100% vs 61.3%), and negative predictive value (96.6% vs 84.2%) compared to VI.
Conclusions
Pleural gas analysis is an effective technique to detect APF and can facilitate timely and safe chest tube removal.
Despite major advances in thoracic surgery, little improvement has been made in chest drainage since the inception of the water seal bottle system [1]. In this system, bubbles in the water column as detected by visual inspection (VI) are believed to represent the egress of air from an alveolopleural or bronchopleural fistula (true leak) [2]. However, VI is prone to error because the presence of air bubbles is dependent on patient effort, tube position, and the presence of fluid or clots in the tube, among other factors [3]. Bubbles may be observed even without an APF (false leak) in the setting of a large pleural space after lung resection [2], the introduction of air into the pleural cavity from the tube exit site, or reverse air flow in the chest tube [4]. Reverse air flow allows air in the tube to be sucked back into the pleural cavity during inspiration, and subsequent cough or forceful expiration produces bubbles that can be misinterpreted as an APF.
If no acid-base disorder is present, the partial pressure of alveolar carbon dioxide (CO2) is ~40 mm Hg, equivalent to 5.6% CO2 at sea level with atmospheric pressure of 760 mm Hg. In the presence of an alveolopleural communication, alveolar gas containing CO2 escapes into the pleural cavity. Hence, elevated CO2 in gas collected from the chest drainage system can indicate an APF. However, CO2 can persist in the pleural cavity after resolution of an APF. In such circumstances, an increase in pleural O2 with nasally administered supplemental O2 (SupO2) could differentiate an APF from high pleural CO2 resulting from delayed diffusion out of the pleural cavity. Therefore, we hypothesized that pleural gas analysis (GA) would accurately identify APF and distinguish between true and false leaks. Accordingly, we determined the pleural CO2 and O2 levels that would indicate an APF and compared the efficacy of pleural gas analysis with the current standard of visual inspection.
Technology and Technique
Patient Population
We first determined the pleural CO2 and O2 levels in patients without APF (APF-negative) who had chest tubes for pleural effusion and those with APF observed in the operating room after lung decortication (APF-positive, n = 10 each). Then, we prospectively compared VI and GA in patients (n = 50) undergoing surgical lobectomy. The study was approved by the Institutional Review Board of Northwestern University.
Chest Tube Management and Visual Inspection for Air Leak
All patients had a single 24F or 28F chest tube that was kept on suction at −20cm after operation and switched to water seal drainage on day 1. For VI, the patient was asked to cough five times and then take five deep breaths. This sequence was repeated twice. The first sequence eliminated any trapped air in the pleural space and tubing. The result of VI was considered positive if air bubbles were detected during the second sequence.
Pleural Gas Analysis
The GA was performed by connecting a Datex analyzer (GE Healthcare, Inc, OK) to the sampling port of the chest draining system (Atrium, Inc, Hudson, NH). Measurements were performed while the tubes were on water seal drainage. Patients were breathing room air with SaO2 above 92%. The analyzer was first connected to the chest drainage system and CO2 and O2 were recorded. SupO2 was then administered nasally. Patients were allowed to take deep breaths for 1 minute, after which pleural CO2 and O2 levels were recorded.
End Point and Statistical Analysis
If pneumothorax developed after chest tube removal, APF was considered to be present. Patients were followed up for the development of pneumothorax until 3 weeks after operation. Statistical analysis was performed with Microsoft Excel 2011 (Microsoft Corp, Redmond, WA) and GraphPad Prism, version 6 (GraphPad Software, Inc, San Diego, CA). Two-tailed Student and Fisher exact t tests were used as appropriate. Statistical significance was defined at p < 0.05.
Clinical Experience
Pleural CO2 and O2 Levels in Patients With and Without APF
The mean CO2 in the APF-negative patients was 0.9 ± 0.28%, and O2 was 14.9 ± 1.8%. SupO2 up to 10 L/min in APF-negative patients did not change the pleural O2 composition (Fig 1) with the variation less than 2%. By contrast, APF-positive patients revealed a CO2 of 4.9 ± 1.3%, O2 17.0 ± 1.2%, and increase in pleural O2 of 2% or more with SupO2 (p < 0.01). Incremental levels of SupO2 demonstrated that 5 L/min was adequate to achieve an increase in pleural O2 of 2% or more for all APF-positive patients. We concluded that pleural CO2 less than 1%, and increase in O2 less than 2% with SupO2 5 L/min, or both, would indicate an absence of APF. Additionally, CO2 above 1% with an increase in O2 of 2% or more with SupO2 5 L/min would be consistent with APF, whereas CO2 above 1% but an increase in O2 below 2% would suggest recently resolved APF.
Fig 1.
Pleural CO2 and increase in O2 with supplemental O2 in patients with (APF-positive, cross mark) and without (APF-negative, circle) alveolopleural fistula.
Discordance Between VI and GA in Patients Undergoing Lobectomy
Next, we prospectively compared VI and GA in patients undergoing lobectomy (n = 50). The mean age of the study cohort was 53.1 ± 11.0 years, and the male to female ratio was 29:21. All patients had postresection predicted forced expiratory volume in first second and diffusion capacity of lung for carbon monoxide above 40%. Thirty patients (60%) underwent a right-sided procedure, and 20 (40%) underwent a left-sided procedure. Twenty-nine (58%) had upper, 19 (38%) had lower, and 2 (4%) had middle lobectomies. When the staff surgeon deemed that the fluid output had reached the removal threshold, a comparison between VI and GA was performed. The mean duration for the fluid output to fall below the individual surgeon’s threshold from the day of operation was 1.8 ± 0.7 days. At this time, VI revealed bubbles suggesting APF in 31 (62%) patients. However, GA indicated APF in only 19 of these 31 patients (Table 1). Hence, 12 of 31 (38.7%) patients with bubbles on VI were deemed to have a false leak. The size of leak between those with false and true leak was not different (2.4 ± 1.1 vs 2.5 ± 0.8 chambers, p = 0.8). To confirm false leak, we clamped the tube in the first 4 patients, and their chest roentgenograms at 4 hours were normal. The tubes were removed without the development of pneumothorax. In the subsequent 8 patients, the tubes were successfully removed without a clamp trial, resulting in the patients’ discharge. None of these 12 patients experienced pneumothorax during 3 weeks of postoperative follow-up.
Table 1.
Correlation Between Visual Inspection and Gas Analysis for Alveolopleural Fistula
| Gas Analysis | Visual Inspection
|
|
|---|---|---|
| Bubbles Present | Bubbles Absent | |
| Positive for APF | 19 (38%) | 2 (4%) |
| Negative for APF | 12 (24%) | 17 (34%) |
APF = alveolopleural fistula.
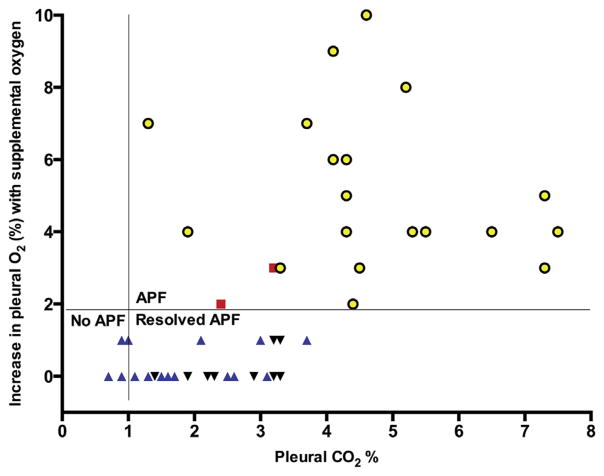
The VI revealed no leak in 19 (38%) patients, whereas the GA indicated APF in 2 of these 19 patients. Tube removal in these 2 patients resulted in the development of a large pneumothorax on a roentgenogram obtained within 2 hours. Hence, we reason that VI might not be sensitive to detect small APF that do not cause bubbles but can still lead to pneumothorax. The pleural CO2 and increase in O2 levels with SupO2 of 5 L/min and the correlation of GA and VI in the lobectomy patients is shown in Figure 2 and Table 1.
Fig 2.
Pleural CO2 and increase in O2 with supplemental O2 at 5 L/min in patients undergoing surgical lobectomy. Symbols represent correlation between gas analysis (GA) and visual inspection (VI). (yellow circles = GA-positive VI-positive; red squares = GA-positive VI-negative; inverted black triangles = GA-negative VI-positive; blue triangles = GA-negative VI-negative.)
Concordance Between VI and GA in Patients Undergoing Lobectomy
There was concordance between VI and GA in 36 (72%) patients (Table 1). In 17 (34%) patients, no APF was detected by both VI and GA. One of these patients experienced a moderate-size pneumothorax, detected on chest roentgenogram after tube removal, for which a pleural pigtail catheter was placed. There were no technical errors during tube removal, indicating that both test results were falsely negative. Repeated gas analysis after catheter placement was consistent with the presence of APF, suggesting that the prior tube had become nonfunctional.
In 19 (38%) patients there was confirmation of APF by both methods. These patients were serially monitored to determine discrepancy between the two techniques. Tubes were removed if the result of VI was negative for APF. A greater proportion of patients had positive test results for APF by VI every day (Table 2). As demonstrated earlier (Table 1), a positive VI test result but negative GA result indicates a false air leak. Therefore, VI overestimated the prevalence of APF on each postoperative day.
Table 2.
Serial Analysis of Visual Inspection and Gas Analysis in Patients Deemed to Have APF on Day 0 by Both Techniques
| Day | 0a | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Air leak on visual inspection | 19 (100%) | 14 (74%) | 12 (63%) | 7 (37%) | 4 (16%) | 3 (11%) |
| Gas analysis positive for APF | 19 (100%) | 10 (53%) | 5 (26%) | 4 (21%) | 2 (10%) | 2 (10%) |
Day 0 represents the start of serial analysis when the fluid output decreased to below removal threshold for the surgeon. The mean duration for the fluid output to fall below the threshold from the day of operation was 1.82 ± 0.71 days.
APF = alveolopleural fistula.
Comparison of Visual Inspection and Gas Analysis After Lobectomy
We reasoned that the 19 patients with positive test results on VI and GA, the 2 patients who did not have a leak on VI despite a positive GA test result but experienced pneumothorax upon tube removal, and the patient who experienced pneumothorax despite both test results being negative had APF. Therefore, the prevalence of APF was 22 of the 50 (44%) patients. Accordingly, GA demonstrated better sensitivity (95.5% vs 86.4%), specificity (100% vs 57.1%), positive predictive value (100% vs 61.3%), and negative predictive value (96.6% vs 84.2%) in the detection of APF, as shown in Figure 3.
Fig 3.
Comparison of visual inspection and gas analysis for detection of alveolopleural fistula after lobectomy. (NPV = negative predictive value; PPV = positive predictive value.)
Comment
After lung operations, APF remains an important predictor of postoperative morbidity and mortality [5]. Based on the current method of VI, the prevalence of air leaks immediately after lung resection, generally accepted to represent APF, can exceed 75% [2]. Furthermore, it has been reported that 50% of emphysema patients and up to 25% of patients with normal lungs can experience prolonged air leaks, assumed to indicate persistent APF, after lung resection [6]. The question whether or not to leave chest tubes on water seal or suction also remains highly debatable, with studies showing conflicting results [7]. Our data suggest that a significant proportion of patients observed to have air leaks on VI might not actually have APF. Therefore, these studies could have been confounded by the inclusion of patients with false leaks. Although surgeons can perform clamp trials when they suspect false air leaks, the results are inconsistent. We conclude that VI overestimates the incidence of APF and might lead to unnecessary delays in chest tube removal, thereby increasing patient morbidity and hospital stay.
Digital chest drainage systems have been developed for more accurate assessment of APF. Despite providing a more objective analysis, they suffer from some of the same limitations present in the analog system. For example, a large pleural space or the introduction of air into the pleural cavity around the tube exit site will still register a leak in these systems, suggesting APF. Pleural GA provides a more precise assessment of APF by analyzing gas content rather than air flow. Intriguingly, there is strong evidence that high CO2 impairs lung healing by suppressing alveolar type II cell proliferation [8, 9]. Hence, we further hypothesize that high CO2 in the pleural cavity might impair the lung healing process. The monitoring of pleural gases, therefore, also provides an opportunity to investigate and modulate the environment to promote lung healing. However, this needs to be validated in subsequent studies.
It is important to note that tubes should be patent during GA. Kinking of the tube or clot formation might reduce accuracy, as was likely the case in the patient who experienced pneumothorax despite a negative test result. There were no patients with false negative GA result when there was “tidaling” in the chest drainage unit or in the setting of a positive air leak on VI, both indicative of a patent chest tube. Therefore, the accuracy of GA might approach 100% when the tube is functional. In the rare event that the tube is nonfunctional and there is no air leak on VI, a false negative GA would not have attributable risk because the tube should be removed in that situation based on the current standard. Further, any resulting pneumothorax in such a situation would likely not be immediately life-threatening because large APF in the presence of a nonfunctional tube would have caused a large pneumothorax even before GA. We also propose that real-time measurement of CO2 and O2 might be a more valuable tool if integrated into chest drainage systems because monitoring the gas trends would likely eliminate false negatives. This approach can increase the cost of chest drainage systems. However, given that the CO2 and O2 sensors are relatively inexpensive and are based on infrared spectroscopy, the additional cost should be small and comparable with that of the existing digital systems. Furthermore, we anticipate that it would prove to be cost effective when balanced against early tube removal, prevention of postremoval pneumothorax, and early hospital discharge.
Footnotes
Disclaimer
The Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, and The Annals of Thoracic Surgery neither endorse nor discourage use of the new technology described in this article.
Disclosures and Freedom of Investigation
No property or the tested technology was purchased, borrowed, or donated to the study. The authors had full freedom of investigation including control of the design of the study, methods used, outcome parameters, analysis of data, and production of the written report.
References
- 1.Meyer JA. Gotthard Bulau and closed water-seal drainage for empyema, 1875–1891. Ann Thorac Surg. 1989;48:597–9. doi: 10.1016/s0003-4975(10)66876-2. [DOI] [PubMed] [Google Scholar]
- 2.Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis. 2014;6:271–84. doi: 10.3978/j.issn.2072-1439.2013.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varela G, Jimenez MF, Novoa N. Portable chest drainage systems and outpatient chest tube management. Thorac Surg Clin. 2010;20:421–6. doi: 10.1016/j.thorsurg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Stouby A, Neckelmann K, Licht PB. Reverse airflow in certain chest drains may be misinterpreted as prolonged air leakage. World J Surg. 2011;35:596–9. doi: 10.1007/s00268-010-0943-0. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg. 2010;89:1327–35. doi: 10.1016/j.athoracsur.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 6.DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006;82:197–206. doi: 10.1016/j.athoracsur.2006.02.050. discussion 206–7. [DOI] [PubMed] [Google Scholar]
- 7.Qiu T, Shen Y, Wang MZ, et al. External suction versus water seal after selective pulmonary resection for lung neoplasm: a systematic review. PLoS One. 2013;8:e68087. doi: 10.1371/journal.pone.0068087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT. Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci. 2014;71:831–45. doi: 10.1007/s00018-013-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vohwinkel CU, Lecuona E, Sun H, et al. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem. 2011;286:37067–76. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]