Abstract
BACKGROUND
Loss of donor-mediated immune antitumor activity after allogeneic hematopoietic stem-cell transplantation (HSCT) permits relapse of hematologic cancers. We hypothesized that immune checkpoint blockade established by targeting cytotoxic T-lymphocyte–associated protein 4 with ipilimumab could restore antitumor reactivity through a graft-versus-tumor effect.
METHODS
We conducted a phase 1/1b multicenter, investigator-initiated study to determine the safety and efficacy of ipilimumab in patients with relapsed hematologic cancer after allogeneic HSCT. Patients received induction therapy with ipilimumab at a dose of 3 or 10 mg per kilogram of body weight every 3 weeks for a total of 4 doses, with additional doses every 12 weeks for up to 60 weeks in patients who had a clinical benefit.
RESULTS
A total of 28 patients were enrolled. Immune-related adverse events, including one death, were observed in 6 patients (21%), and graft-versus-host disease (GVHD) that precluded further administration of ipilimumab was observed in 4 patients (14%). No responses that met formal response criteria occurred in patients who received a dose of 3 mg per kilogram. Among 22 patients who received a dose of 10 mg per kilogram, 5 (23%) had a complete response, 2 (9%) had a partial response, and 6 (27%) had decreased tumor burden. Complete responses occurred in 4 patients with extramedullary acute myeloid leukemia and 1 patient with the myelodysplastic syndrome developing into acute myeloid leukemia. Four patients had a durable response for more than 1 year. Responses were associated with in situ infiltration of cytotoxic CD8+ T cells, decreased activation of regulatory T cells, and expansion of subpopulations of effector T cells in the blood.
CONCLUSIONS
Our early-phase data showed that administration of ipilimumab was feasible in patients with recurrent hematologic cancers after allogeneic HSCT, although immune-mediated toxic effects and GVHD occurred. Durable responses were observed in association with several histologic subtypes of these cancers, including extramedullary acute myeloid leukemia. (Funded by the National Institutes of Health and others; ClinicalTrials.gov number, NCT01822509.)
Allogeneic hematopoietic stem-cell transplantation (HSCT) is the only cure for many patients who have advanced hematologic cancers, principally through the induction of a graft-versus-tumor effect.1 Unfortunately, more than one third of patients who have undergone transplantation have a relapse of disease.2 The prognosis for these patients is poor; the majority die within 1 year after relapse despite salvage chemotherapy, donor-lymphocyte infusion, or retransplantation.3–5
Immune escape (i.e., tumor evasion of the donor immune system) contributes to relapse after allogeneic HSCT, and immune checkpoint inhibitory pathways probably play an important role.6 The engagement of cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) and programmed death 1 (PD-1) receptors by their respective ligands B7-1 and B7-2 and PD-L1 and PD-L2 inhibits effector T-cell function. Tumor cells often express these ligands, thereby selectively blocking antitumor immunity. In murine models, PD-1 blockade has led to an increase in graft-versus-host-disease (GVHD),7 whereas selective blockade of CTLA-4 to treat late relapse after transplantation has augmented graft-versus-tumor effects without accelerating GVHD.8
We hypothesized that CTLA-4 blockade with ipilimumab may induce a graft-versus-tumor effect in patients with relapse after allogeneic HSCT and lead to a clinical response without excessive immune-related toxic effects. CTLA-4 blockade could provide a novel tool for immune modulation to treat or prevent relapse after allogeneic HSCT.
Studies9,10 have shown that ipilimumab (Yervoy, Bristol-Myers Squibb), a human IgG1 kappa monoclonal antibody, specifically blocks CTLA-4. We previously found that a single low dose of ipilimumab (0.1 to 3.0 mg per kilogram of body weight) had acceptable side-effect and adverse-effect profiles and did not result in clinically significant GVHD. Responses were seen in three patients with lymphoma. In that study, T-cell activation was observed,11,12 but patients received only one planned infusion of ipilimumab and doses higher than 3.0 mg per kilogram were not tested. These observations prompted the initiation of the current study of ipilimumab to evaluate the safety and efficacy of sustained CTLA-4 blockade in patients with relapse after allogeneic HSCT.
METHODS
PATIENTS
Patients were eligible if they had received a diagnosis of progressive or persistent leukemia, lymphoma, multiple myeloma, or a neoplasm with myelodysplastic or myeloproliferative features 3 months or more after allogeneic HSCT, had had no immune suppression for at least 4 weeks, and had no history of grade III or IV acute GVHD. Other eligibility criteria were 20% or more donor-derived CD3+ T-cell chimerism, no history of autoimmune disease, and an absence of active infection.
STUDY DESIGN AND OVERSIGHT
This phase 1/1b, open-label, investigator-initiated, single-group, multicenter study was designed to determine the maximum tolerated dose, characterize toxic effects, and assess the efficacy and pharmacodynamic features of ipilimumab in patients with recurrent hematologic cancer after allogeneic HSCT. Patients were enrolled from April 2013 through March 2015. In the phase 1 portion of the study, the original design included five patients who would receive ipilimumab at a dose of 3.0 mg per kilogram, and this dose would be escalated in a second cohort of five patients who would receive 10.0 mg per kilogram if no more than one manifestation of dose-limiting toxicity was observed. Additional patients could take the place of patients who discontinued the study because of early disease progression if the patients who discontinued the study did not complete the period of observation for toxic effects.
Next, in a phase 1b dose-expansion phase that was designed to better define toxicity and assess preliminary efficacy, an additional 15 patients were enrolled and received the maximum tolerated dose. Ipilimumab was administered intravenously over the course of 90 minutes every 21 days for four courses unless unacceptable toxic effects or clear disease progression occurred. Patients who did not have disease progression or unacceptable toxic effects could continue to receive maintenance therapy every 12 weeks for up to 60 weeks.
The study was designed solely by the authors, and the regulatory sponsor was the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute. The study drug was provided by the manufacturer, Bristol-Myers Squibb, to CTEP, which distributed it to the participating sites. The manufacturer had no other role in the study. The institutional review board at each site approved the protocol (available with the full text of this article at NEJM.org), and the study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All the participants provided written informed consent. The data analysis and writing of the manuscript were performed solely by the authors with no third-party assistance. All the authors were in agreement regarding submission of the manuscript and vouch for the completeness and accuracy of the data and analysis and the fidelity of the study to the protocol.
CLINICAL ASSESSMENTS
Patients underwent evaluations every week for the first 3 weeks, then every 3 weeks through week 12, and then monthly. Assessments of acute and chronic GVHD were made with the use of standard criteria.13,14
Dose-limiting toxic effects were defined as grade III or IV acute GVHD, grade 4 hematologic toxic effects unrelated to the underlying disease, or grade 3 nonhematologic toxic effects that did not improve to grade 1 with a 3-week dose delay. Immune-related adverse events that resolved to grade 1 or lower with the use of glucocorticoids were not considered to be dose-limiting toxic effects. We designated the observation period for toxic effects to be 12 weeks to capture delayed immunologic toxic effects. Responses were assessed with the use of standard disease-specific criteria (listed in the protocol) after 6 and 12 weeks of induction therapy and every 12 weeks thereafter during maintenance therapy.
IMMUNOLOGIC ANALYSIS
Correlative studies were performed at baseline and at response assessments. These studies included the following: immunophenotypic analysis of peripheral-blood mononuclear cells by means of flow cytometry and mass cytometry; immunohistochemical staining and RNA sequencing of formalin-fixed, paraffin-embedded skinbiopsy specimens; and cytokine and chemokine assays of plasma samples. Additional methods are described in the Supplementary Appendix, available at NEJM.org.
STATISTICAL ANALYSIS
All patients who received the study drug were included in the safety and efficacy analyses. Exploratory pharmacodynamic correlative studies were analyzed descriptively and graphically, and group comparisons were made with the use of the exact Wilcoxon rank-sum test. All P values were two-sided, and P values of less than 0.05 were considered to indicate statistical significance. All analyses were performed with the use of SAS software, version 9.3 (SAS Institute) and the statistical program R, version 2.13.2 (the CRAN project [http://cran.r-project.org]). A detailed statistical analysis plan is provided in the Supplementary Appendix.
RESULTS
PATIENTS AND TREATMENT
A total of 28 patients were enrolled at six sites. Baseline characteristics of the patients are summarized in Table 1. Diagnoses included acute myeloid leukemia (in 12 patients, including 3 with leukemia cutis and 1 with a myeloid sarcoma), Hodgkin’s lymphoma (in 7), non-Hodgkin’s lymphoma (in 4), and the myelodysplastic syndrome (in 2). In addition, 1 patient each had multiple myeloma, myeloproliferative neoplasm, and acute lymphoblastic leukemia. Eight patients (29%) had previously had grade I or II acute GVHD, and 16 patients (57%) had previously had chronic GVHD.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Total (N = 28) |
|---|---|
| Male sex — no. (%) | 16 (57) |
| Age at enrollment — yr | |
| Median | 58 |
| Range | 22–75 |
| Diagnosis — no. (%) | |
| Acute myeloid leukemia | 12 (43) |
| Relapse with extramedullary disease | 4 (14) |
| Hodgkin’s lymphoma | 7 (25) |
| Non-Hodgkin’s lymphoma | 4 (14) |
| Myelodysplastic syndrome | 2 (7) |
| Multiple myeloma | 1 (4) |
| Myeloproliferative neoplasm | 1 (4) |
| Acute lymphoblastic leukemia | 1 (4) |
| Time since HSCT — days | |
| Median | 675 |
| Range | 198–1830 |
| Donor type — no. (%) | |
| Unrelated | 15 (54) |
| Related | 13 (46) |
| Graft source — no. (%) | |
| Peripheral blood | 22 (79) |
| Bone marrow | 5 (18) |
| Umbilical cord | 1 (4) |
| Conditioning intensity — no. (%) | |
| Myeloablative | 11 (39) |
| Reduced intensity | 17 (61) |
| Prior treatment of relapse after allogeneic HSCT — no. (%) |
20 (71) |
| Chemotherapy | 17 (61) |
| Radiation therapy | 6 (21) |
| Donor-lymphocyte infusion | 6 (21) |
| Prior GVHD — no. (%) | |
| Grade I or II acute | 8 (29) |
| Chronic | 16 (57) |
| Limited | 8 (29) |
| Extensive | 8 (29) |
GVHD denotes graft-versus-host disease, and HSCT hematopoietic stem-cell transplantation.
The median time from transplantation to initial treatment with ipilimumab during the study was 675 days (range, 198 to 1830), and the median time from relapse to initial administration of ipilimumab was 97 days (range, 0 to 1415). Patients had received a median of three prior treatment regimens, excluding transplantation (range, 1 to 14), and 20 patients (71%) had previously received treatment for relapse after transplantation, including chemotherapy in 17 patients (61%), radiation therapy in 6 patients (21%), and donor-lymphocyte infusions in 6 patients (21%). The median pretreatment peripheral-blood donor T-cell chimerism was 99% (range, 58 to 100).
SAFETY
Adverse events that occurred in all patients are listed in Table 2. Six patients received 3 mg of ipilimumab per kilogram (five patients were initially enrolled, but one patient could not be assessed for toxic effects because of early progression, and another patient took that patient’s place, according to the protocol). Chronic GVHD of the liver developed in one patient; this was the only dose-limiting toxic effect in that cohort. Immune-related adverse events were observed in two patients (grade 2 pneumonitis in one patient and grade 2 diarrhea in one patient). These events were rapidly reversed with glucocorticoids and did not preclude further administration of ipilimumab.
Table 2.
Adverse Events and Outcomes of Treatment.*
| Variable | No. of Patients |
|---|---|
| Adverse event | |
| Patients who could be evaluated for adverse events | 28 |
| GVHD — dose-limiting toxicity | |
| Chronic GVHD of liver | 3 |
| Acute GVHD of gut | 1 |
| Immune-related adverse events | |
| Death | 1 |
| Pneumonitis | |
| Grade 4 | 1 |
| Grade 2 | 2 |
| Colitis, grade 3 | 1 |
| Immune thrombocytopenic purpura, grade 2 | 1 |
| Diarrhea, grade 2 | 1 |
| Other adverse events of grade 3 or higher regardless of attribution, or grade 1 or 2 events at least possibly related to ipilimumab |
|
| Acute kidney injury, grade 3 | 1 |
| Corneal ulcer, grade 3 | 1 |
| Thrombocytopenia, grade 3 or 4 | 9 |
| Neutropenia, grade 3 or 4 | 3 |
| Anemia, grade 3 or 4 | 2 |
| Pleural effusion, grade 3 | 1 |
| Limb edema, grade 1 or 2 | 6 |
| Outcome of treatment | |
| Patients who received maximum administered dose (10 mg/kg) | 22 |
| Complete response | 5 |
| Partial response | 2 |
| Stable disease | 6 |
| Progression of disease | 9 |
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
On the basis of the safety profile at a dose of 3 mg of ipilimumab per kilogram and evidence that 10 mg per kilogram may have greater efficacy than 3 mg in melanoma,15 a dose-escalation cohort of 7 patients was enrolled to receive a dose of 10 mg per kilogram (2 patients could not be evaluated for toxic effects because of early progression events and discontinued the study; 2 other patients took their place, according to the protocol). A subsequent dose-expansion cohort of an additional 15 patients was then enrolled; these patients received ipilimumab at a dose of 10 mg per kilogram.
Among the 22 patients who received 10 mg of ipilimumab per kilogram, dose-limiting toxic effects included two cases of chronic GVHD of the liver and one case of grade II acute GVHD of the gut, all of which resolved with glucocorticoids but precluded further administration of ipilimumab. Immune-related adverse events, which occurred in 3 patients, included grade 2 immune thrombocytopenia (in 1 patient), grade 3 colitis (in 1), and pneumonitis (1 patient with grade 2 pneumonitis and 1 patient with grade 4 pneumonitis). These toxic effects were treated with glucocorticoids, and in 2 patients, glucocorticoids were tapered and ipilimumab was resumed within 3 weeks. The third patient died 42 days after receiving the initial dose of ipilimumab after grade 3 colitis and grade 4 pneumonitis developed. No infectious complications of grade 3 or higher were observed.
The clinical presentation of GVHD and immune-related adverse events can be similar, so biopsy specimens of the affected site were obtained whenever feasible to distinguish pathologically between these potential causes. Two patients with colitis underwent colon biopsies. One of these patients had pathological features consistent with typical ipilimumab-related colitis, including a mixture of chronic inflammation in the lamina propria, acute neutrophilic inflammation in the crypts, and minimal apoptosis. The other patient had typical GVHD features, including crypt epithelial-cell apoptosis in the absence of inflammation (Fig. S1 in the Supplementary Appendix).
In total, ipilimumab was discontinued because of dose-limiting toxic effects in 5 patients: 4 patients with GVHD and 1 patient with severe immune-related adverse events. Overall, the median number of doses received was 4 (range, 1 to 8). A total of 15 patients (54%) completed the full course of induction therapy, and 6 patients (21%) received maintenance therapy.
CLINICAL RESPONSE
In the cohort of patients who received 3 mg of ipilimumab per kilogram, no responses were noted. One patient with extramedullary acute myeloid leukemia of the breast had a presumed tumor flare after two doses of ipilimumab and then had a 27% decrease in the size of the mass at the end of induction; the reduced size was maintained for 12 additional weeks (Fig. S2 in the Supplementary Appendix).
The best responses among the 22 patients who received 10 mg of ipilimumab per kilogram are listed in Table 2. Seven of 22 patients (32%) met disease-specific criteria for a response. All patients who had a response had baseline donor T-cell chimerism in the blood of 99% or higher. Five patients had an objective complete response (23%), including all 3 patients with leukemia cutis (Fig. 1A through 1D, and Fig. S3 in the Supplementary Appendix), 1 with myeloid sarcoma involving lymph nodes, and 1 with smoldering myelodysplastic syndrome developing into acute myeloid leukemia with marrow involvement.
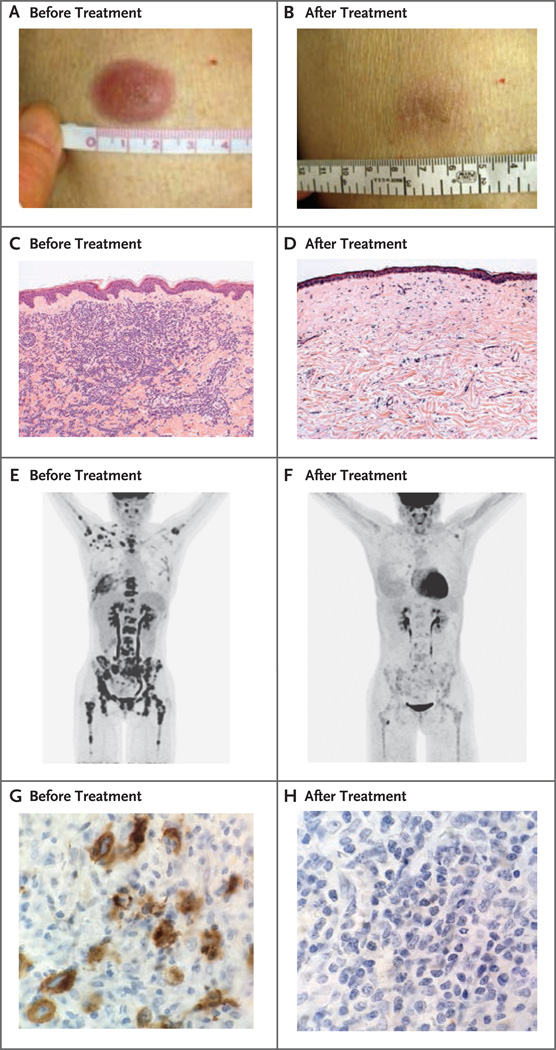
Figure 1. Clinical and Histopathological Responses to Ipilimumab in Patients with Leukemia Cutis and Hodgkin’s Lymphoma.
Panel A shows a lesion in the skin of a patient with leukemia cutis before treatment, and Panel B shows the clinical response after treatment. Panel C shows sheets of neoplastic cells before treatment in the same patient, and Panel D shows the histopathological response after treatment, with fibrosis and sparse chronic inflammation without evidence of cancer (hematoxylin and eosin, low magnification in both panels). Panel E shows a positron-emission tomographic and computed tomographic (PET-CT) image before treatment and Panel F shows the response on PET-CT after treatment in a patient with Hodgkin’s lymphoma. Panel G shows a bone marrow biopsy specimen in the same patient before treatment, with staining for CD30 showing extensive malignant lymphocyte infiltration (high magnification), and Panel H shows a bone marrow biopsy specimen after treatment, with no evidence of cancer (high magnification).
Two patients had a partial response, 1 with widespread Hodgkin’s lymphoma involving the bones and bone marrow (Fig. 1E through 1H) and 1 with lung plasmacytomas. Six additional patients who did not meet formal response criteria had a reduction in the tumor burden, including the patient with extramedullary acute myeloid leukemia of the breast, 3 patients with Hodgkin’s lymphoma who had had a relapse very soon after receiving multiple courses of post-transplantation therapy and remained in the study with stable disease for up to 1 year, a patient with cutaneous T-cell lymphoma, and a patient with acute myeloid leukemia who had a 28% reduction in marrow blasts. In total, 13 of 22 patients (59%) had a reduction in the tumor burden after receiving ipilimumab.
With a median follow-up time of 15 months (range, 8 to 27) among survivors, the 1-year overall survival rate was 49% (Fig S4 in the Supplementary Appendix). The median duration of response had not been reached. Two of the three patients with leukemia cutis remained in complete remission at 12 and 15 months, and the patient with the myelodysplastic syndrome developing into acute myeloid leukemia remained in complete remission at 16 months. The patient with lung plasmacytomas had a partial response without progression for more than 21 months. Clinical response was not associated with the patient’s age, the intensity of the conditioning regimen, the stem-cell source, the time since transplantation, or prior donor-lymphocyte infusion. All seven patients with a complete or partial response, as compared with 62% of patients who did not have a response, had some prior GVHD (P = 0.08).
IMMUNOLOGIC EFFECTS
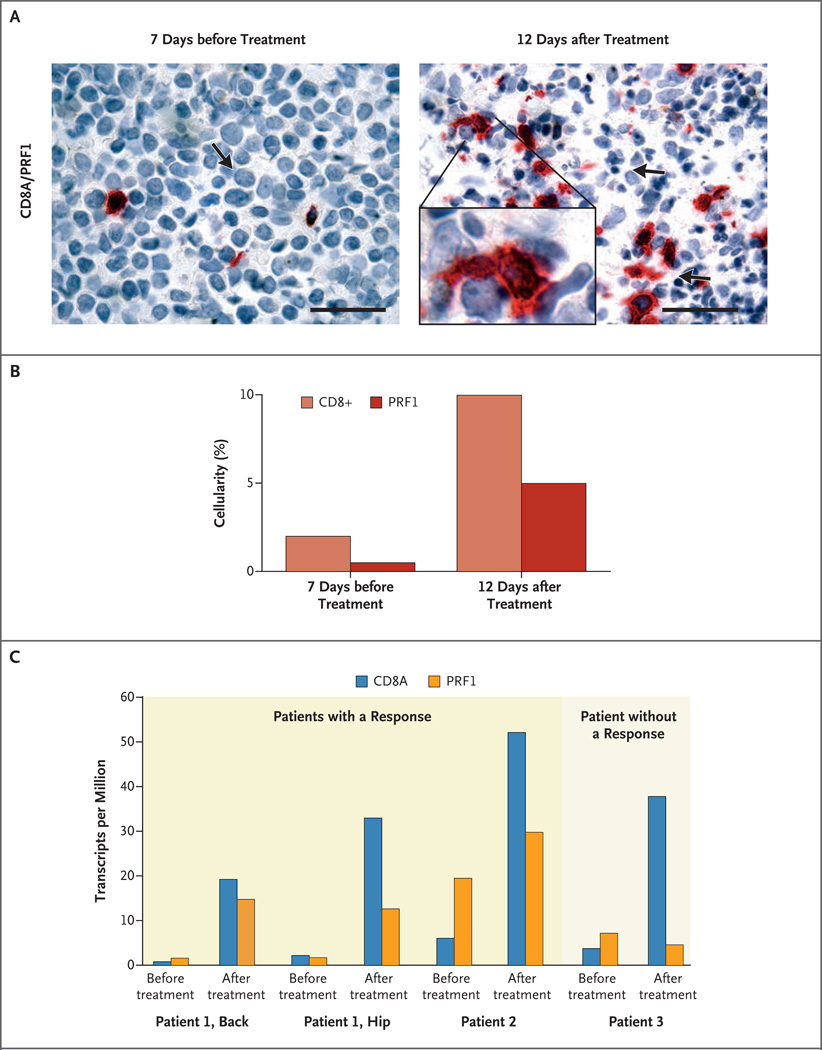
In exploratory studies, we assessed the association between clinical outcomes and the immunologic responses to ipilimumab both at the site of disease and in the blood. In three patients with leukemia cutis who had a complete response, pretreatment skin-biopsy specimens showed dense infiltration with leukemia cells, and post-treatment biopsy specimens confirmed elimination of leukemia cells and replacement with mild fibrosis and chronic inflammation (Fig. 1A through 1D, and Fig. S3 in the Supplementary Appendix). Serial skin-biopsy specimens in one of these patients showed increased infiltration of CD8+ T cells 12 days after the patient received a single dose of ipilimumab (Fig. 2A). Increased cytotoxicity, as evidenced by staining for perforin granules, paralleled the increased CD8+ T-cell infiltration (Fig. 2A and 2B).
Figure 2. Immunohistochemical and Transcriptional Analysis of In Situ Leukemic Responses to Ipilimumab.
Panel A shows immunohistochemical images (at high magnification) of costaining for CD8+ T cells (red) and perforin (brown) before and after the initial dose of ipilimumab in a patient with leukemia cutis (Patient 1) who had a complete remission. The arrow on the left side of the panel indicates a leukemic blast; the arrows on the right side of the panel indicate cells undergoing apoptosis. The scale bars represent 50 µm. The inset (right) shows CD8+ T cells (red) contacting multiple leukemic cells with perforin staining (brown). Panel B shows quantitation by means of visual estimation for the stains in Panel A. Panel C shows gene-expression analyses of CD8A and PRF1 from biopsy specimens obtained before and after treatment with ipilimumab in two patients with leukemia who had a response and one patient who did not have a response. Biopsy specimens from two different sites of leukemia cutis (the back and hip) were obtained from Patient 1 before and after treatment.
To further explore this finding, we performed RNA sequencing from biopsy specimens obtained at sites of leukemic involvement in two patients who had a response and one patient who did not have a response (Fig. 2C). Consistent with the immunohistochemical analysis, gene-expression data showed up-regulation of both CD8A (by a factor of 8.5 to 22.0) and PRF1 (perforin) (by a factor of 1.5 to 9.0) in association with the clinical response to ipilimumab. In one patient who had a response, increased expression of CD8A and PRF1 after the administration of ipilimumab was detected at two different sites of relapse. In contrast, the patient who did not have a response had up-regulation of CD8A (by a factor of 10.0) without increased PRF1 expression (PRF1 expression changed by a factor of −1.5).
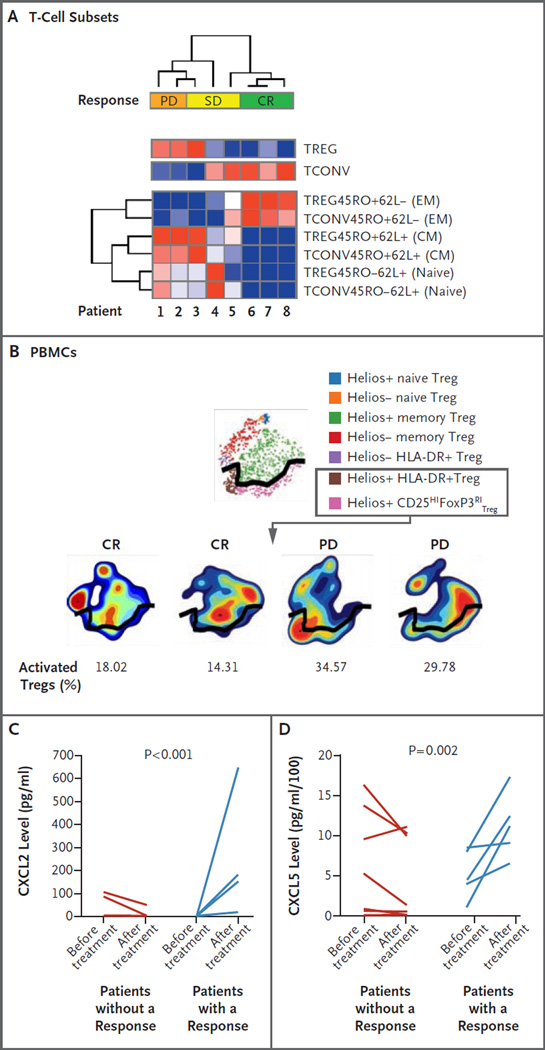
To probe the systemic effects of ipilimumab on T-cell immunity, we evaluated changes in CD4+ regulatory T-cell (Treg) and CD4+ conventional T-cell (Tcon) populations in peripheral blood 8 weeks after the patients began to receive ipilimumab. Flow cytometry (Fig. 3A) showed that patients who had a complete response or stable disease had fewer CD4+ Treg cells and more CD4+ Tcon cells than patients with progressive disease. Using mass cytometry (Fig. 3B), we found that activated CD4+ Treg cells were present at a 50% lower frequency in two patients who had a response than in two patients who had disease progression (additional details are provided in the Supplementary Appendix).
Figure 3. Systemic Immunologic Correlative Studies in Patients Who Received Ipilimumab, According to Response Status.
Panel A is a heat map showing the results of unsupervised clustering. These results are expressed as the percentage of T-cell subsets, as assessed by means of flow cytometry of peripheral-blood mononuclear cells (PBMCs) 8 weeks after administration of ipilimumab, in patients with progressive disease (PD), stable disease (SD), or a complete response (CR). The top two rows represent the percentage of total regulatory T-cells (Tregs) and conventional T-cells (Tconvs), and the bottom six rows represent T-cell subsets of these two populations, including Treg effector memory (EM) cells, Tconv EM cells, Treg central memory (CM) cells, Tconv CM cells, Treg naive cells, and Tconv naive cells. Minimum values (dark blue) and maximum values (dark red) in each row are shown. Plus signs indicate positive expression, and minus signs negative expression. Panel B shows a mass cytometric analysis of PBMCs at 8 weeks after administration of ipilimumab. Shown are activated Tregs (the population of cells below the black dividing line) in two patients who had a complete response as compared with two patients whose best response was PD. Cells expressing the Helios, HLA-DR, and CD25HIFoxP3RI markers are considered to be activated Tregs. Panel C shows levels of chemokine (C-X-C motif) ligand 2 (CXCL2), and Panel D shows levels of chemokine (C-X-C motif) ligand 5 (CXCL5) in the plasma of patients at baseline as compared with 8 weeks after treatment with ipilimumab, according to response status. Patients with a response included four patients with a CR and one with SD and some antitumor activity.
In plasma samples obtained from patients who had a response to ipilimumab, we observed significant increases in chemokines that are critical for leukocyte trafficking, including the interleukin-8 receptor ligands and chemokine (C-X-C motif) ligands 2 (CXCL2) (Fig. 3C) and 5 (CXCL5) (Fig. 3D), but not in other chemokines such as CXCL6, interleukin-1 receptor type I, angiopoietin-1 and angiopoietin-2, and vascular endothelial growth factor (data not shown). Finally, we observed that low pretreatment levels of soluble major histocompatibility complex class I polypeptide–related sequence A positively predicted a clinical response (P = 0.006) (Fig. S5 in the Supplementary Appendix). Taken together, these data began to elucidate immunologic responses both in situ and systemically during CTLA-4 blockade in patients with relapse after allogeneic HSCT.
DISCUSSION
Treatment options for patients with relapse of disease after allogeneic HSCT are limited. In our early-phase study, we found that CTLA-4 blockade with ipilimumab induced clinically significant remissions in patients with recurrent cancer after transplantation. Although no objective responses were seen at a dose of 3 mg per kilogram, 7 of 22 patients (32%) who received 10 mg per kilogram had a response; this suggests that the antibody dose may be important after transplantation. Five patients had complete remission and 4 patients who had a response continued to have a durable remission for more than 1 year. These observations suggest that CTLA-4 blockade may be effective after allogeneic HSCT by inducing a dormant graft-versus-tumor response.
Stimulation of a graft-versus-tumor effect arouses concern regarding the potential exacerbation of GVHD; however, GVHD that precluded additional administration of ipilimumab developed in 4 of 28 patients; each of these patients had a response to glucocorticoids. Immune-related adverse events that are typical of ipilimumab were also observed in 6 patients. After dose delay and the administration of glucocorticoids, 5 of these patients were able to receive additional ipilimumab without recurrence of the adverse effects. However, in 1 patient, immune-related adverse effects led to treatment-related death. Since our study included only patients who had undergone transplantation 3 months or more previously, no conclusions can be drawn regarding the safety of ipilimumab in the early post-transplantation period.
Patients with relapse after allogeneic HSCT are in a unique position to benefit from checkpoint blockade. One study has suggested that immune escape after transplantation can be mediated by reduced expression of costimulatory molecules and loss of the recipient’s mismatched HLA haplotype.16 Donor-derived T cells also express high levels of checkpoint receptors.17 Dysfunction of donor antitumor CD8+ T cells may play a crucial role in the biology of relapse after transplantation.18 Although donor-lymphocyte infusions may partially reverse this dysfunction, agents such as anti–CTLA-4 antibodies can be adjusted more easily than cellular products to selectively augment graft-versus-tumor effects without inducing severe GVHD. Extramedullary myeloid leukemias, which are typically refractory to standard therapies, may provide a particularly rich environment for antigen presentation that makes them sensitive to checkpoint blockade.19,20 Indeed, all three patients with leukemia cutis had a complete response, as did a patient with myeloid sarcoma. GVHD developed in the three patients with leukemia cutis; this suggests that the occurrence of GVHD may be associated with a clinical response.
Our immunohistochemical and gene-expression data indicated that in patients with leukemia cutis, ipilimumab drove infiltration of cytotoxic CD8+ T cells to the site of disease. These data were notable for CD8+ T-cell up-regulation both in patients who had a response and in those who did not, but a lack of in situ cytotoxic function in patients who did not have a response suggests that local factors may suppress antileukemic immunity by preventing the acquisition of a cytotoxic phenotype, rather than by blocking T-cell infiltration.
In contrast, analysis of peripheral blood showed decreased CD4+ Tregs and increased CD4+ effector T cells in patients who had a response. Moreover, CD4+ Tregs in patients who had a response were less activated than those in patients who did not have a response. In patients who had a response, peripheral T cells were also enriched for more differentiated CD62L− effector memory T-cell subsets. CD62L is a lymphnode “homing receptor” that is down-regulated during T-cell activation to allow for lymph-node egress and subsequent encounter with cognate antigen in the peripheral tissues. Increased CD62L− effector memory T cells may be an indirect effect of ipilimumab, reflecting activation, homing, and differentiation of CD4+ effector T cells as CTLA-4+ Tregs are depleted. The apparent differential effects of ipilimumab that we observed on CD4+ and CD8+ T cells in the peripheral blood and tissue suggest that immunologic correlative studies should not be restricted to one tissue compartment. Indeed, recent data have revealed localization-dependent heterogeneity in T-cell homeostasis.21
Our chemokine analysis suggests that greater activation of the interleukin-8 pathway is associated with improved antitumor immunity, which may facilitate a clinical response. Moreover, we found that pretreatment soluble MHC class I polypeptide–related sequence A was negatively correlated with the clinical outcome in patients after transplantation, as it is in patients with melanoma22; this suggests that it could be further evaluated as a predictive biomarker for response to checkpoint blockade, with potential usefulness in human cancers more broadly. These exploratory observations of immunologic correlates with clinical outcome will need to be validated in larger cohorts of patients who receive CTLA-4 blockade.
In summary, CTLA-4 blockade was a feasible approach for the treatment of patients with relapsed hematologic cancer after transplantation. Complete remissions with some durability were observed, even in patients with refractory myeloid cancers.
Supplementary Material
Acknowledgments
We thank the patients who participated in this study; the study research nurses, research coordinators, mid-level practitioners, and site staff for their support of the study; Carol Reynolds, Ph.D., for running the flow cytometric assays; Irene Ghobrial, M.D., Lee Greenberger, Ph.D., Keting Chu, Ph.D., and Jun Xu, Ph.D., for support of the study through the Blood Cancer Research Partnership; and Megan Hiserodt, B.A., for editorial assistance with an earlier version of the manuscript.
Supported by grants (5R01CA183559-03, 5R01CA183560-03, and 5UM1CA186709-02) from the National Institutes of Health, the Cancer Therapy Evaluation Program of the National Cancer Institute, the Leukemia and Lymphoma Society Therapy Accelerator Program, Pasquarello Tissue Bank, and Dana–Farber Cancer Institute Department of Medical Oncology and Center for Immuno-Oncology.
Appendix
The authors’ affiliations are as follows: the Department of Medical Oncology, Dana–Farber Cancer Institute and Harvard Medical School (M.S.D., H.T.K., P.B., R.L., A.S., A.P.L., M.H., M.S., F.S.H., C.J.W., V.T.H., C. Cutler, J.K., E.P.A., J.H.A., P.A., J.R., R.J.S.), the Bone Marrow Transplant Program, Beth Israel Deaconess Medical Center and Harvard Medical School (D.A.), the Bone Marrow Transplant Program, Massachusetts General Hospital Cancer Center and Harvard Medical School (Y.-B.C.), the Departments of Dermatology (N.R.L.) and Pathology (S.R.G., J.L.H., S.J.R.), Dana–Farber and Brigham and Women’s Cancer Center, and the Dana–Farber Cancer Institute, Center for Molecular Oncologic Pathology (M.B., C.W.Z.) — all in Boston; Broad Institute of Massachusetts Institute of Technology and Harvard (P.B., C.J.W.) and Neon Therapeutics (M.S.R.) — both in Cambridge; the Blood and Marrow Transplant Program, University of California, San Diego, Moores Cancer Center, La Jolla (C. Costello, E.D.B.); Colorado Blood Cancer Institute, Denver (P.M.); Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD (H.S.); and the Blood and Marrow Transplant Group of Georgia at Northside Hospital, Atlanta (A.B.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Antin JH. Graft-versus-leukemia: no longer an epiphenomenon. Blood. 1993;82:2273–2277. [PubMed] [Google Scholar]
- 2.Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 3.Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1160–1168. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Thanarajasingam G, Kim HT, Cutler C, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1713–1718. doi: 10.1016/j.bbmt.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soiffer RJ. Donor lymphocyte infusions for acute myeloid leukaemia. Best Pract Res Clin Haematol. 2008;21:455–466. doi: 10.1016/j.beha.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125:3393–3400. doi: 10.1182/blood-2015-02-567453. [DOI] [PubMed] [Google Scholar]
- 7.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. Journal Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 8.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162:6368–6377. [PubMed] [Google Scholar]
- 9.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 10.Kuhns MS, Epshteyn V, Sobel RA, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates the size, reactivity, and function of a primed pool of CD4+ T cells. Proc Natl Acad Sci U S A. 2000;97:12711–12716. doi: 10.1073/pnas.220423597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Bashey A, Zhong R, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells. Biol Blood Marrow Transplant. 2011;17:682–692. doi: 10.1016/j.bbmt.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 14.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 16.Vago L, Kimi Perna S, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 17.Toffalori C, Cavattoni I, Deola S, et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood. 2012;119:4813–4815. doi: 10.1182/blood-2012-02-411686. [DOI] [PubMed] [Google Scholar]
- 18.Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123:1412–1421. doi: 10.1182/blood-2013-08-523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koc Y, Miller KB, Schenkein DP, Daoust P, Sprague K, Berkman E. Extramedullary tumors of myeloid blasts in adults as a pattern of relapse following allogeneic bone marrow transplantation. Cancer. 1999;85:608–615. doi: 10.1002/(sici)1097-0142(19990201)85:3<608::aid-cncr11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Yoshihara S, Ando T, Ogawa H. Extramedullary relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: an easily overlooked but significant pattern of relapse. Biol Blood Marrow Transplant. 2012;18:1800–1807. doi: 10.1016/j.bbmt.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Thome JJ, Yudanin N, Ohmura Y, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koguchi Y, Hoen HM, Bambina SA, et al. Serum immunoregulatory proteins as predictors of overall survival of metastatic melanoma patients treated with ipilimumab. Cancer Res. 2015;75:5084–5092. doi: 10.1158/0008-5472.CAN-15-2303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.