Abstract
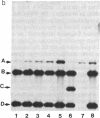
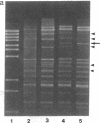
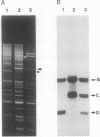
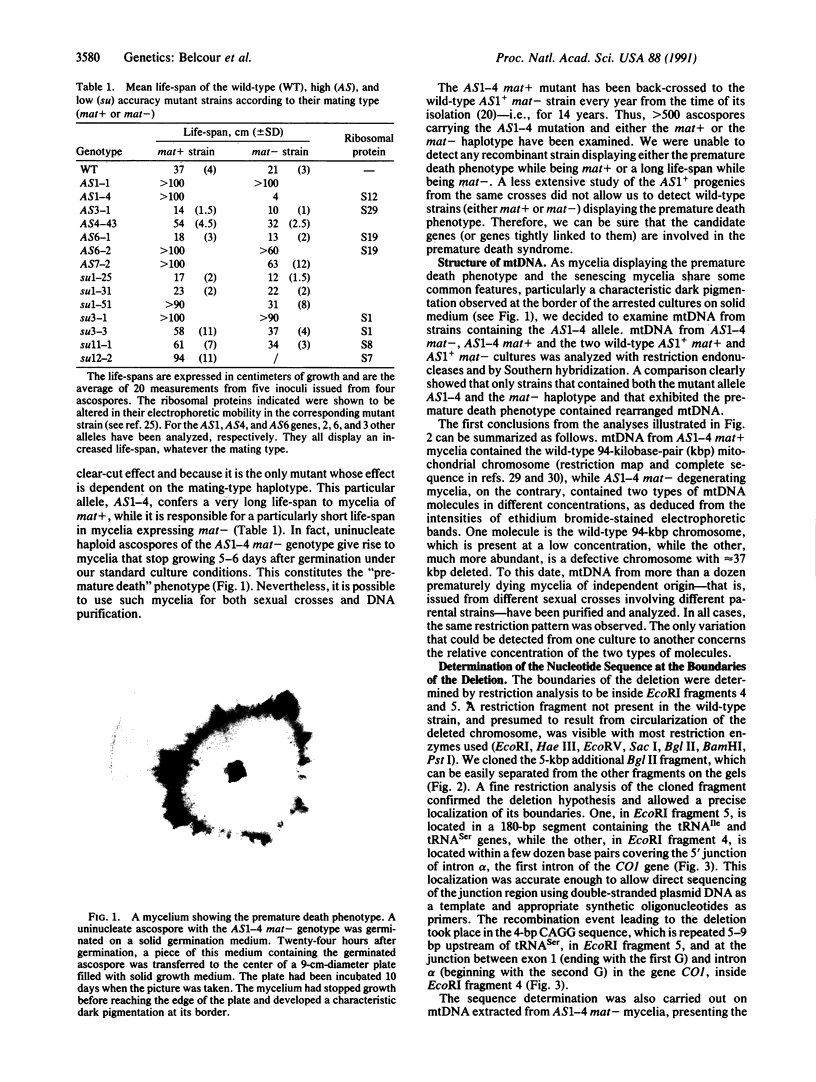
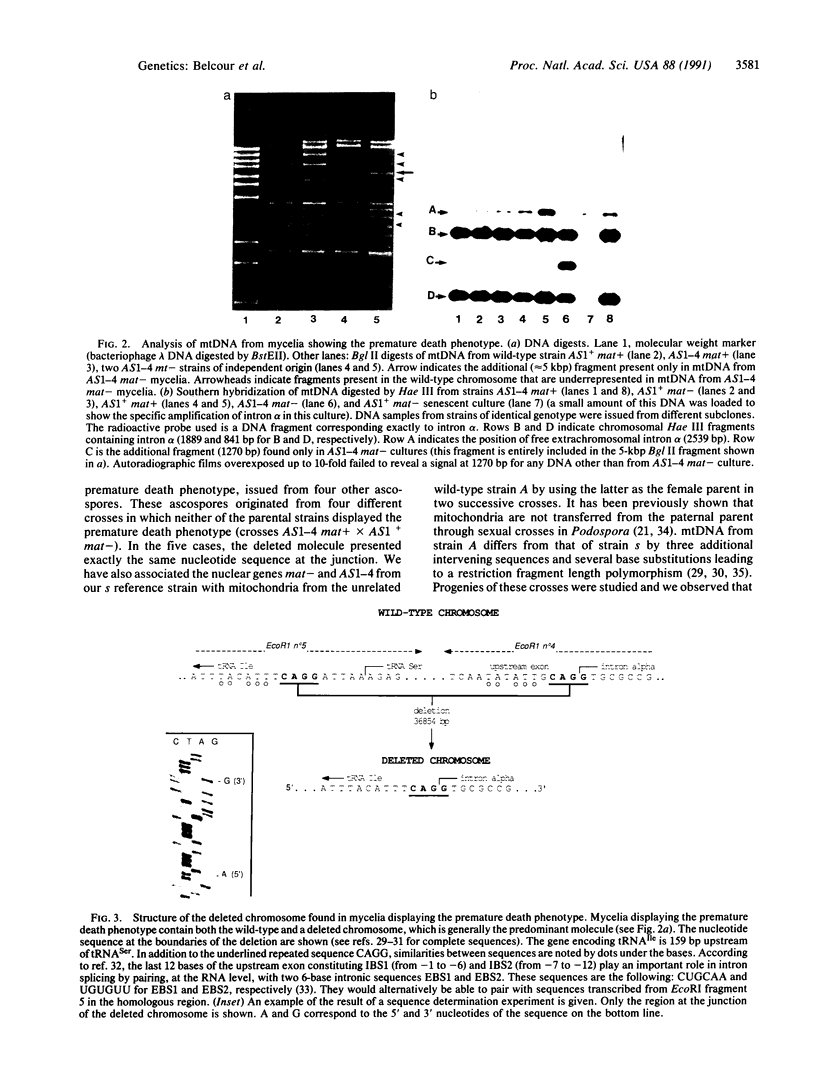
In the filamentous fungus Podospora anserina, the association of two nuclear genes inevitably leads to a "premature death" phenotype consisting of an early end of vegetative growth a few days after ascospore germination. Mycelia showing this phenotype contain a mitochondrial chromosome that always bears the same deletion. One of the break points is exactly at the 5' splice site of a particular mitochondrial intron, suggesting that the deletion event could result from molecular mechanisms also involved in intron mobility. One of the nuclear genes involved in triggering this site-specific event belongs to the mating-type minus haplotype; the other is a mutant allele of a gene encoding a cytosolic ribosomal protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Grant D. M., Stohl L. L., Bottorff D. A., Nargang F. E., Lambowitz A. M. Nucleotide sequence of the Varkud mitochondrial plasmid of Neurospora and synthesis of a hybrid transcript with a 5' leader derived from mitochondrial RNA. J Mol Biol. 1988 Nov 5;204(1):1–25. doi: 10.1016/0022-2836(88)90594-3. [DOI] [PubMed] [Google Scholar]
- Akins R. A., Kelley R. L., Lambowitz A. M. Mitochondrial plasmids of Neurospora: integration into mitochondrial DNA and evidence for reverse transcription in mitochondria. Cell. 1986 Nov 21;47(4):505–516. doi: 10.1016/0092-8674(86)90615-x. [DOI] [PubMed] [Google Scholar]
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Augustin S., Müller M. W., Schweyen R. J. Reverse self-splicing of group II intron RNAs in vitro. Nature. 1990 Jan 25;343(6256):383–386. doi: 10.1038/343383a0. [DOI] [PubMed] [Google Scholar]
- Belcour L., Begel O. Mitochondrial genes in Podospora anserina: recombination and linkage. Mol Gen Genet. 1977 May 20;153(1):11–21. doi: 10.1007/BF01035991. [DOI] [PubMed] [Google Scholar]
- Belcour L., Vierny C. Variable DNA splicing sites of a mitochondrial intron: relationship to the senescence process in Podospora. EMBO J. 1986 Mar;5(3):609–614. doi: 10.1002/j.1460-2075.1986.tb04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad-Webb H., Perlman P. S., Zhu H., Butow R. A. The nuclear SUV3-1 mutation affects a variety of post-transcriptional processes in yeast mitochondria. Nucleic Acids Res. 1990 Mar 25;18(6):1369–1376. doi: 10.1093/nar/18.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. II. Properties of mutant DNA and multimeric circular DNA from senescent cultures. Mol Gen Genet. 1979 Mar 27;171(3):239–250. doi: 10.1007/BF00267578. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M., Nelson J. DNA sequence and secondary structures of the large subunit rRNA coding regions and its two class I introns of mitochondrial DNA from Podospora anserina. J Mol Evol. 1989 Mar;28(3):242–255. doi: 10.1007/BF02102482. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., MacNeil I. A., Domenico J., Matsuura E. T. Excision-amplification of mitochondrial DNA during senescence in Podospora anserina. DNA sequence analysis of three unique "plasmids". J Mol Biol. 1985 Oct 20;185(4):659–680. doi: 10.1016/0022-2836(85)90052-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., McNally K. L., Domenico J. M., Matsuura E. T. The complete DNA sequence of the mitochondrial genome of Podospora anserina. Curr Genet. 1990 May;17(5):375–402. doi: 10.1007/BF00334517. [DOI] [PubMed] [Google Scholar]
- Dequard-Chablat M., Coppin-Raynal E., Picard-Bennoun M., Madjar J. J. At least seven ribosomal proteins are involved in the control of translational accuracy in a eukaryotic organism. J Mol Biol. 1986 Jul 20;190(2):167–175. doi: 10.1016/0022-2836(86)90290-1. [DOI] [PubMed] [Google Scholar]
- Herbert C. J., Labouesse M., Dujardin G., Slonimski P. P. The NAM2 proteins from S. cerevisiae and S. douglasii are mitochondrial leucyl-tRNA synthetases, and are involved in mRNA splicing. EMBO J. 1988 Feb;7(2):473–483. doi: 10.1002/j.1460-2075.1988.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jamet-Vierny C., Begel O., Belcour L. Senescence in Podospora anserina: amplification of a mitochondrial DNA sequence. Cell. 1980 Aug;21(1):189–194. doi: 10.1016/0092-8674(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Levings C. S., 3rd, Brown G. G. Molecular biology of plant mitochondria. Cell. 1989 Jan 27;56(2):171–179. doi: 10.1016/0092-8674(89)90890-8. [DOI] [PubMed] [Google Scholar]
- Levra-Juillet E., Boulet A., Séraphin B., Simon M., Faye G. Mitochondrial introns aI1 and/or aI2 are needed for the in vivo deletion of intervening sequences. Mol Gen Genet. 1989 May;217(1):168–171. doi: 10.1007/BF00330957. [DOI] [PubMed] [Google Scholar]
- Matsuura E. T., Domenico J. M., Cummings D. J. An additional class II intron with homology to reverse transcriptase in rapidly senescing Podospora anserina. Curr Genet. 1986;10(12):915–922. doi: 10.1007/BF00398289. [DOI] [PubMed] [Google Scholar]
- Michel F., Lang B. F. Mitochondrial class II introns encode proteins related to the reverse transcriptases of retroviruses. Nature. 1985 Aug 15;316(6029):641–643. doi: 10.1038/316641a0. [DOI] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörl M., Schmelzer C. Group II intron RNA-catalyzed recombination of RNA in vitro. Nucleic Acids Res. 1990 Nov 25;18(22):6545–6551. doi: 10.1093/nar/18.22.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Bennoun M. Genetic evidence of ribosomal antisuppressors in Podospora anserina. Mol Gen Genet. 1976 Sep 23;147(3):299–306. doi: 10.1007/BF00582881. [DOI] [PubMed] [Google Scholar]
- Picard-Bennoun M. Introns, protein syntheses and aging. FEBS Lett. 1985 May 6;184(1):1–5. doi: 10.1016/0014-5793(85)80640-2. [DOI] [PubMed] [Google Scholar]
- RIZET G. Sur l'impossibilité d'obtenir la multiplication végétative ininterrompue et illimitée de l'ascomycète Podospora anserina. C R Hebd Seances Acad Sci. 1953 Oct 12;237(15):838–840. [PubMed] [Google Scholar]
- RIZET G. Sur la longévité des souches de Podospora anserina. C R Hebd Seances Acad Sci. 1953 Nov 2;237(18):1106–1109. [PubMed] [Google Scholar]
- Rötig A., Cormier V., Blanche S., Bonnefont J. P., Ledeist F., Romero N., Schmitz J., Rustin P., Fischer A., Saudubray J. M. Pearson's marrow-pancreas syndrome. A multisystem mitochondrial disorder in infancy. J Clin Invest. 1990 Nov;86(5):1601–1608. doi: 10.1172/JCI114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsard-Chanet A., Begel O. Insertion of an LrDNA gene fragment and of filler DNA at a mitochondrial exon-intron junction in Podospora. Nucleic Acids Res. 1990 Feb 25;18(4):779–783. doi: 10.1093/nar/18.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U., Riederer B., Mörl M., Schmelzer C., Stahl U. Self-splicing of the mobile group II intron of the filamentous fungus Podospora anserina (COI I1) in vitro. EMBO J. 1990 Jul;9(7):2289–2298. doi: 10.1002/j.1460-2075.1990.tb07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel-Rogol B. L., King J., Bertrand H. Unstable mitochondrial DNA in natural-death nuclear mutants of Neurospora crassa. Mol Cell Biol. 1989 Oct;9(10):4259–4264. doi: 10.1128/mcb.9.10.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C., Walldorf U., Hug P., Gehring W. J. Fruit flies with additional expression of the elongation factor EF-1 alpha live longer. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7520–7521. doi: 10.1073/pnas.86.19.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierny C., Keller A. M., Begel O., Belcour L. A sequence of mitochondrial DNA is associated with the onset of senescence in a fungus. Nature. 1982 May 13;297(5862):157–159. doi: 10.1038/297157a0. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Webster G. C., Webster S. L. Decline in synthesis of elongation factor one (EF-1) precedes the decreased synthesis of total protein in aging Drosophila melanogaster. Mech Ageing Dev. 1983 Jun;22(2):121–128. doi: 10.1016/0047-6374(83)90105-7. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Horrum M. A., Cummings D. J. Are mitochondrial structural genes selectively amplified during senescence in Podospora anserina? Cell. 1982 Jun;29(2):505–515. doi: 10.1016/0092-8674(82)90167-2. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Servidei S., Gellera C., Bertini E., DiMauro S., DiDonato S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature. 1989 May 25;339(6222):309–311. doi: 10.1038/339309a0. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]