Abstract
Nischarin, a novel intracellular protein, was originally identified as a binding partner for the α5β1 integrin. Here we show that Nischarin also interacts with members of the PAK family of kinases. The amino terminus of Nischarin preferentially binds to the carboxy-terminal domain of PAK1 when the kinase is in its activated conformation. Nischarin binding to PAK1 is enhanced by active Rac, with the three proteins forming a complex, while expression of the α5β1 integrin also increases the Nischarin/PAK1 association. Interaction with Nischarin strongly inhibits the ability of PAK1 to phosphorylate substrates. This effect on PAK kinase activity closely parallels Nischarin's ability to inhibit cell migration. Conversely, reduction of endogenous levels of Nischarin by RNA interference promotes cell migration. In addition, PAK1 and Nischarin colocalize in membrane ruffles, structures known to be involved in cell motility. Thus, Nischarin may regulate cell migration by forming inhibitory complexes with PAK family kinases.
Keywords: cell migration, integrin, Nischarin, PAK
Introduction
Cell migration is a complex process that requires the spatial and temporal coordination of many proteins. During migration, cells extend membrane protrusions, establish adhesive contacts, exert force to move the cell body, and ultimately retract the rear portion of the cell. These events involve actin filament extension regulated by the Arp2/3 complex, as well as actinomyosin-mediated contractility (Pollard and Borisy, 2003). Members of the integrin family of heterodimeric cell surface receptors also play a key role in cell migration. Integrins and certain associated cytosolic proteins provide a structural linkage between the proteins of the extracellular matrix and the actin cytoskeleton (Liu et al, 2000; Sastry and Burridge, 2000). Further, integrins help to regulate an intricate network of signaling pathways needed for the control of migration (Hood and Cheresh, 2002; Juliano, 2002). Members of the Rho family of GTPases are critically important in regulating the actin cytoskeleton (Bishop and Hall, 2000; Ridley, 2001). However, a number of other signaling components have been implicated in cell migration, including focal adhesion kinase, Src, Crk, PI-3-kinase and MAP kinases, as has been reviewed elsewhere (Alahari et al, 2002).
The Rho family GTPases Rac and CDC42 regulate the formation of membrane protrusions involved in motility (Ridley, 2001). Among the key downstream effectors of CDC42 and Rac are members of the PAK family of serine/threonine kinases (Kumar and Vadlamudi, 2002; Bokoch, 2003). Based on their structures, PAKs have been divided into two groups: group 1 consists of PAK1–3, while group 2 consists of PAK4–6. Group 1 PAKs have a Rac/CDC42-binding domain and an overlapping autoinhibitory domain in the amino terminus, and a kinase domain in the carboxy terminus (Bagrodia and Cerione, 1999). Inactive group 1 PAKs exist as autoinhibited dimers. Upon GTPase binding, PAKs undergo a conformational change that separates the autoinhibitory domain from the kinase domain (Parrini et al, 2002). This induces kinase activity and autophosphorylation at several sites, including Thr 423 in the activation loop (Buchwald et al, 2001; Chong et al, 2001). Outside of the kinase- and GTPase-binding domains, group 2 PAKs are quite different from group 1 and their regulation may be distinct (Dan et al, 2002). PAKs are primarily localized in the cytoplasm in resting cells; however, activated PAKs translocate to focal adhesions and membrane ruffles (Sells et al, 2000). The exact mechanism of PAK regulation of the actin cytoskeleton and cell migration is not fully understood. PAKs phosphorylate and inhibit myosin light-chain kinase, leading to reduced actinomyosin contractility (Sanders et al, 1999). In addition, PAKs phosphorylate and activate LIM kinase leading to increased phosphorylation of cofilin, which plays a role in actin severing (Edwards et al, 1999) and regulation of actin filament turnover (Carlier et al, 1999). However, kinase-independent contributions of PAKs to cell motility have also been described (Sells et al, 1999).
In addition to Rac and CDC42, group 1 PAKs also associate with a variety of other proteins including Nck, filamin, paxillin, Merlin, p41-Arc, G-protein βγ subunits, Cool/Pix exchange factors, and certain cytoplasmic tyrosine kinases (Manser et al, 1998; Xia et al, 2001; Brown et al, 2002; Feng et al, 2002; Kumar and Vadlamudi, 2002; Vadlamudi et al, 2002; Bokoch, 2003; Kissil et al, 2003; Vadlamudi et al, 2004). Among the negative regulators of PAK are several kinases, including protein kinase A (Howe and Juliano, 2000), while the phosphatases PP2A and POPX1/2 dephosphorylate PAK and thus inhibit its activity (Koh et al, 2002; Kumar and Vadlamudi, 2002). In summary, PAKs interact with the cytoskeleton in several ways that influence cell motility; conversely, a number of proteins associate with PAKs and regulate their functions.
One important way in which integrins influence cellular behavior is by the interaction of their cytoplasmic tails with intracellular proteins (Liu et al, 2000). Many investigators have sought proteins that bind to integrin tail regions and might thus be downstream effectors. Pursuing this approach, we identified a novel protein termed Nischarin that interacts preferentially with the α5 cytoplasmic domain. Overexpression of Nischarin in fibroblasts led to changes in cytoskeletal organization and to a profound inhibition of cell migration (Alahari et al, 2000). Nischarin also strongly inhibited Rac-driven cell migration and invasion of carcinoma cells, with the observations suggesting that Nischarin might act primarily by affecting PAK (Alahari, 2003).
In the current report we demonstrate that Nischarin binds selectively to PAKs via its N-terminus, with preferential binding to the ‘open' conformation of the kinase. Nischarin also inhibits the ability of PAK1 to phosphorylate substrates. The ability of Nischarin to inhibit PAK kinase activity closely parallels its inhibition of cell migration. Further, Nischarin and PAK1 colocalize in areas of membrane ruffling associated with cell motility. Finally, the reduction of endogenous Nischarin levels results in enhanced cell migration via a process that seems to involve PAK activation. These studies suggest that Nischarin is a key regulator of PAKs in the context of cell migration. Further, since Nischarin also binds the α5β1 integrin, this may provide a means for localized control of PAK function.
Results
The N-terminal domain of Nischarin binds to the C-terminal domain of PAK1
Previous work on the effect of Nischarin on Rac-driven cell migration suggested a functional linkage between Nischarin and PAKs (Alahari, 2003). To determine if the link was physical, the ability of Nischarin and PAK1 to form intracellular complexes was examined. Nischarin is a large protein with an integrin α5-binding region as its only functionally defined domain (Alahari et al, 2000) (see Figure 1A). To begin to isolate the region responsible for binding PAK, Nischarin was initially subdivided into two large domains, the N-terminus (aa 1–802) and the C-terminus (aa 970–1354), with the sites of truncation chosen based on a predicted lack of secondary structure (http://www.embl-heidelberg.de/predictprotein/predictprotein.html). Most of the proline- and alanine-rich region, residues 803–969, was not included in either construct because truncated proteins containing this region were not stable (data not shown). Cos-7 cells were transfected with Myc epitope-tagged Nischarin constructs and V5 epitope-tagged PAK1; Nischarin was immunoprecipitated with anti-Myc antibody and the immunoprecipitates were probed for associated PAK1. The presence of PAK1 was readily detectable upon probing the immunoblots with the anti-V5 antibody in immunoprecipitates of full-length Nischarin or the N-terminus, but not the C-terminus (Figure 1B). Reciprocal immunoprecipitation of V5-PAK1 with the anti-V5 antibody co-immunoprecipitated Myc-Nischarin or the N-terminus (Figure 1C). The unrelated protein β-galactosidase bound neither PAK1 nor Nischarin in these assays.
Figure 1.
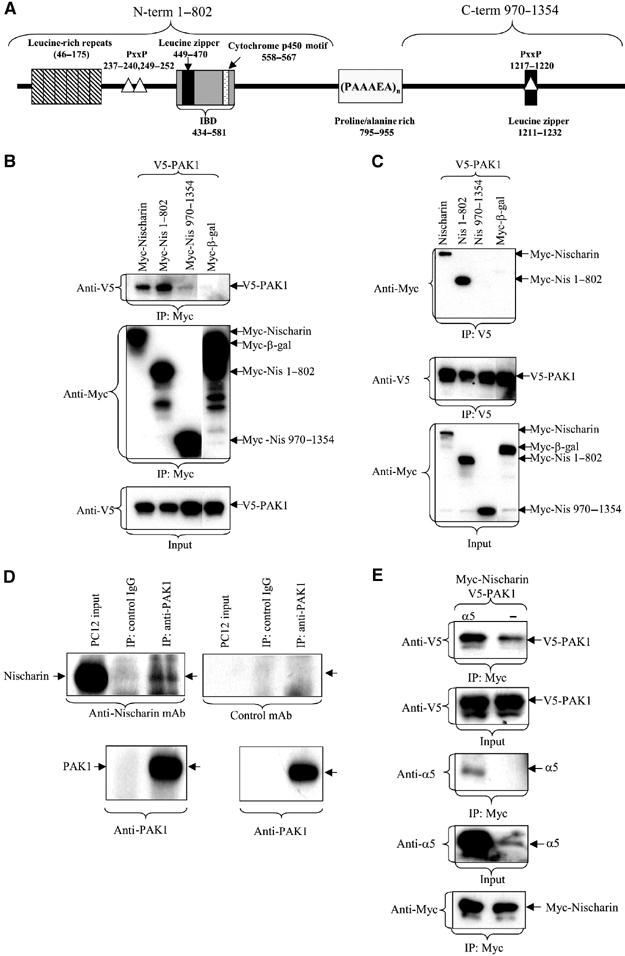
Nischarin/PAK1 interactions. (A) Nischarin domains. Regions homologous to known protein motifs as defined by BLAST analysis are shown. These include the leucine-rich repeats, leucine zipper motifs, potential SH3 binding sites (PXXP) and the cytochrome P450 cysteine heme-iron ligand signature. The integrin-binding domain (IBD) as defined in initial studies (Alahari et al, 2000) is also noted. (B, C) Nischarin's PAK-binding region. Cos-7 cells were cotransfected with Myc-Nischarin, Myc-Nis 1–802, Myc-Nis 970–1354, or Myc-β-galactosidase and V5-PAK1. At 48 h after transfection, the cells were lysed and the tagged proteins were immunoprecipitated with a 1:100 dilution of monoclonal anti-Myc or anti-V5 antibody. The blots were probed for the Myc and V5 epitopes. (D) Interaction of endogenous PAK and Nischarin. PC12 cells were lysed in modified RIPA buffer and lysates were immunoprecipitated with an agarose-conjugated rabbit polyclonal anti-PAK1 (N20) or a control agarose-conjugated IgG overnight at 4°C. Immunoblots were probed with a monoclonal anti-Nischarin antibody or an irrelevant mAb. (E) Effect of α5β1 on PAK/Nischarin interactions. V5-PAK1 and Myc-Nischarin were expressed in Cos-7 cells with or without coexpression of the integrin α5 subunit. Immunoprecipitation and Western blotting were as described above.
To isolate the region within the N-terminus that mediated complex formation, further deletion analysis was performed (Supplementary Figure 1). Thus the regions 1–415 and 416–624 exhibited strong binding, while 625–802 exhibited weaker binding. Division of the 1–415 region into two separate segments (1–217 and 218–415) disrupted PAK complex formation. Thus, several regions within the Nischarin amino terminus are able to contribute to the formation of PAK/Nischarin complexes.
Supporting the importance of this interaction, the Nischarin–PAK association takes place between endogenous proteins as well as during protein overexpression. Thus immunoprecipitation of endogenous PAK from PC12 cells results in co-immunoprecipitation of endogenous Nischarin (Figure 1D).
Since Nischarin interacts with the α5β1 integrin (Alahari et al, 2000), it is important to determine if the binding of Nischarin to PAK is affected by its interaction with integrin. As seen in Figure 1E, overexpression of the α5 subunit enhanced the binding of coexpressed Nischarin and PAK1. Further, all three components were found in the Nischarin immunoprecipitate, suggesting a simultaneous association between PAK, α5β1 and Nischarin.
The regulatory domain of PAK1 spans residues 1–248 of its N-terminus, while amino acids from 248 to 545 comprise the kinase domain. To identify which region of PAK1 interacts with Nischarin, the two domains were separately expressed in Cos-7 cells along with full-length Myc-Nischarin. Nischarin interacted with the kinase domain of PAK1 (248–545), but not with the regulatory domain (Figure 2A); this was consistently seen in reciprocal immunoprecipitations of these proteins (Figure 2B). Since Nischarin specifically interacts with the kinase domain of PAK1, we hypothesized that the interaction of Nischarin with full-length PAK1 would be dependent on the activation state of the kinase. To test this, Myc-Nischarin was coexpressed with the PAK1 activation loop mutant V5-PAK1-T423E; the threonine to glutamic acid substitution mimics the phosphorylation of T423 and partially activates the kinase (Sells et al, 1997; King et al, 2000; Chong et al, 2001). Myc-Nischarin was also coexpressed with V5-PAK1-K299R, a kinase-dead version of the protein (Sells et al, 1997). Nischarin immunoprecipitated via its Myc tag showed enhanced co-precipitation of PAK1-T423E, as compared to wild-type (WT) PAK1, while co-precipitation of PAK1-K299R was dramatically reduced (Figure 2C). These data indicate that the activation of PAK1 facilitates its interaction with Nischarin.
Figure 2.
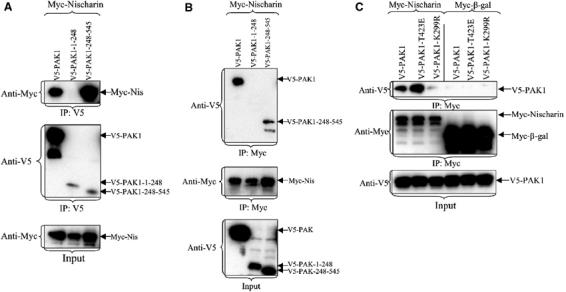
The Nischarin-binding domain of PAK1. (A, B) Nischarin interacts with the kinase domain of PAK1. Full-length V5-PAK1, V5-PAK-1-248 or V5-PAK1-248-545 was cotransfected with Myc-Nischarin. The extracts were immunoprecipitated with the anti-V5 (A) or anti-Myc (B) antibodies and the immunoprecipitates were immunoblotted for the indicated epitopes. (C) Nischarin interacts with active PAK1. Cos-7 cells were cotransfected with the Myc-Nischarin or Myc-β-galactosidase and V5-PAK1, constitutively active V5-PAK1-T423E, or kinase-dead V5-PAK1-K299R. After transfection, the Myc-tagged proteins were immunoprecipitated and the blots probed with anti-Myc and anti-V5 antibodies.
PAK and Nischarin colocalize in membrane ruffles
Since Nischarin both binds to PAK and affects cell migration, one might anticipate that PAK and Nischarin would be found together in subcellular compartments associated with cell movement. To test this, rat embryonic fibroblasts were cotransfected with GFP-Nischarin and with Myc-tagged PAK1. Transfected cells were plated on fibronectin substrata and the subcellular distributions of PAK1 and Nischarin were visualized by confocal fluorescence microscopy. Both PAK1 and Nischarin were widely distributed in the cytoplasm and seemed associated with vesicular structures in the perinuclear area. However, both proteins were also enriched in membrane ruffles, and image superposition clearly indicated regions of colocalization (Figure 3Aiii). When PAK1 mutants were examined, it was obvious that kinase-dead K299R PAK1 failed to show significant membrane colocalization with GFP-Nischarin (Figure 3B). By contrast, expression of the active T423E mutant resulted in enhanced colocalization with GFP-Nischarin (Figure 3C). Higher power images of cell protrusions suggested that PAK is present at the leading edge of the protrusion, while GFP-Nischarin overlaps with PAK just behind the edge (Figure 3Ciii′). These results show that PAK1 and Nischarin colocalize in ruffles, cellular structures known to be involved in cell migration, and that Nischarin preferentially localizes with active PAK1.
Figure 3.
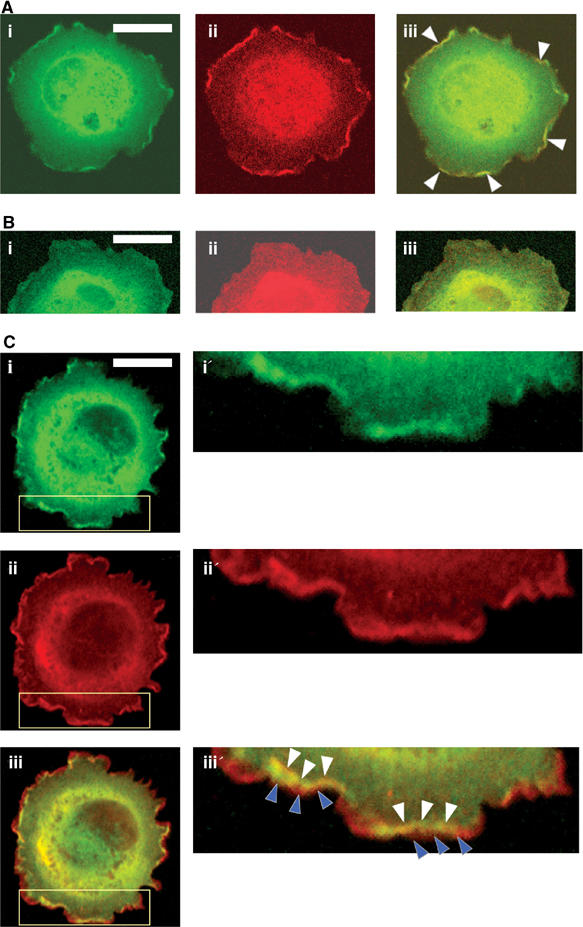
Colocalization of PAK and Nischarin in membrane ruffles. Rat embryonic fibroblasts were transiently transfected with GFP-Nischarin and (A) Myc-PAK1 or (B) Myc-K299R-PAK1 or (C) Myc-T423E-PAK1. After serum starvation, the cells were replated on fibronectin-coated coverslips for 45 min, stained with anti-Myc antibody and observed using an Olympus confocal fluorescence microscope with a × 60 lens. (A) GFP fluorescence is shown in green (i); anti-Myc-PAK1 staining is shown in red (ii); an overlay image is shown in (iii) where yellow indicates colocalization of Nischarin and PAK1 (B, C) Similar images of GFP-Nischarin and (B) Myc-K299R-PAK1 or (C) Myc-T423E-PAK1. Images i′–iii′ show enlargements of part (yellow box) of the i–iii images for Myc-T423E-PAK1 (white arrowheads point to colocalization of PAK and Nischarin in ruffles; blue arrowheads show PAK staining at the far edges of the cell). Scale bar: 20 μm.
Nischarin selectively inhibits PAK kinase activity
Given that Nischarin preferentially associates with activated PAK1, we hypothesized that Nischarin may influence PAK kinase activity. To test this, we transiently cotransfected Cos-7 cells with V5-tagged PAK1 and with Myc-Nischarin or Myc-β-gal. Immunoprecipitated PAK proteins were tested for their kinase activity using myelin basic protein as a substrate. As shown in Figure 4A, serum stimulation strongly increased PAK kinase activity, while coexpression of full-length Nischarin blocked the increase in PAK activity; by contrast, coexpression of β-galactosidase had no effect. Interestingly, full-length Nischarin was required for effective inhibition of PAK kinase activity, whereas the amino-terminal (1–802) or carboxy-terminal (970–1354) fragments, or other fragments of Nischarin (data not shown) had no effect on PAK activation.
Figure 4.
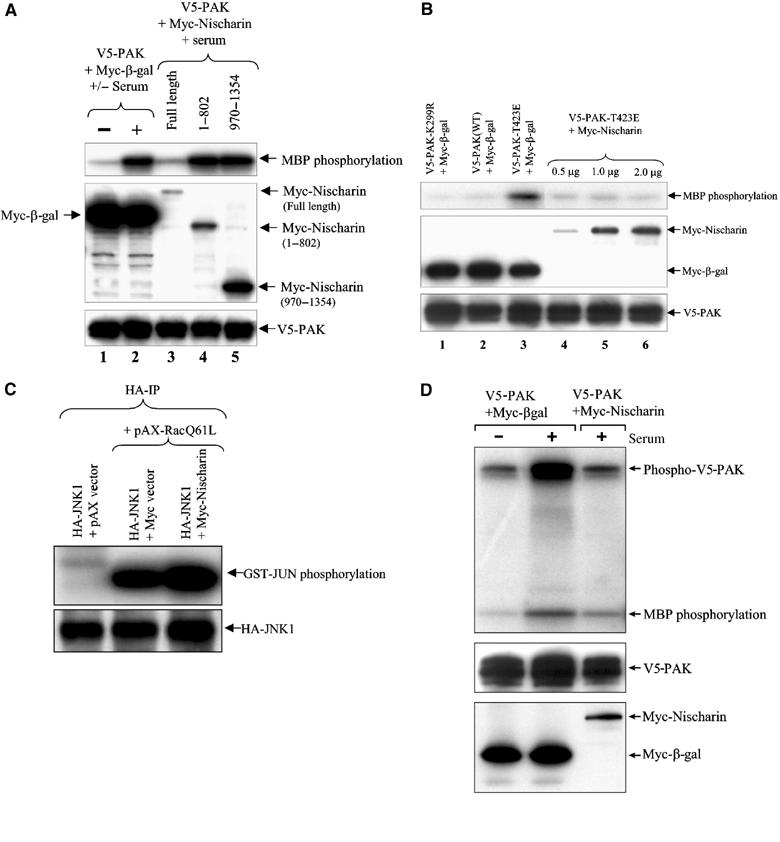
Kinase activity assays. (A) Nischarin inhibits serum-stimulated PAK activity. Immunoprecipitates were made from Cos-7 cells transfected with the indicated constructs. The cells shown in lanes 2–5 were stimulated with serum. Upper panel: the immunoprecipitates were used in in vitro kinase assays using myelin basic protein (MBP) as a substrate; middle panel: the lysates were blotted with anti-Myc antibody; lower panel: the lysates were blotted with anti-V5 antibody. (B) Nischarin inhibits T423E PAK activity. Immunoprecipitates were made from Cos-7 cells transfected with the indicated constructs. Upper panel: the immunoprecipitates were used in in vitro kinase assays as above; middle panel: the lysates were blotted with anti-Myc antibody; lower panel: the lysates were blotted with anti-V5 antibody. (C) Nischarin does not affect JNK activity. Cell lysates made from Cos-7 cells transfected with the indicated combinations of pAX vector, HA-JNK1, pAX-RacQ61L and Myc-Nischarin were immunoprecipitated with anti-HA antibody and the immunoprecipitates were used to detect JNK activation as described (Alahari, 2003). Upper panel: phosphorylation of GST-JUN; lower panel: immunoblotting with anti-HA antibody. (D) Nischarin inhibits autophosphorylation of PAK1. This assay was similar to that of (A) except that the kinase assay was for 5 min and the gel was run to allow visualization of the PAK band. Upper panel: phosphorylation of V5-PAK1 and MBP; middle panel: PAK levels in the IP; lower panel: Nischarin or β-gal levels in the lysate.
These experiments demonstrated that Nischarin blocks serum-mediated activation of PAK1. However, they did not distinguish between direct effects on PAK1 itself and blockade of the signaling pathway leading to PAK activation. To investigate whether Nischarin acts directly on PAK, a constitutive, partially activated form of PAK1 (PAK1-T423E) was used in kinase assays. As seen in Figure 4B, the kinase activity of immunoprecipitated PAK1-T423E was inhibited by Nischarin. Similar to the results with WT PAK1, the activity of T423E was inhibited only by full-length Nischarin, but not by truncated forms of Nischarin (data not shown). Thus Nischarin seems to inhibit directly the ability of PAK1 to phosphorylate substrates.
To determine whether the effect of Nischarin on PAK kinase activity is selective, we examined the action of Nischarin on c-Jun kinase (JNK) (Bishop and Hall, 2000). Rac strongly increased JNK activity, but Nischarin had no inhibitory effect on this process (Figure 4C). This indicates that Nischarin selectively inhibits PAK activity without affecting other Rac-driven kinases.
As an initial approach to understanding the mechanism by which Nischarin inhibits PAK1, we tested whether Nischarin blocks the autophosphorylation of PAK1 that is part of its activation process or, alternatively, whether Nischarin could serve as a competitive substrate for PAK1. As seen in Figure 4D, overexpression of Nischarin inhibited PAK autokinase activity in parallel with inhibition of substrate phosphorylation. Further, use of an antibody that recognizes the phosphorylated form of one of the autoactivation sites on PAK1 demonstrated that phosphorylation at this site was inhibited by Nischarin overexpression (Supplementary Figure 2). These observations suggest that Nischarin can inhibit the activation of PAK1. By contrast, we found little evidence that Nischarin could serve as a substrate for PAK1 and thus block phosphorylation of other substrates by competition. For example, partially purified, in vitro expressed Nischarin was not significantly phosphorylated by active GST-PAK1 under conditions where myelin basic protein was abundantly phosphorylated (Supplementary Figure 3). This suggests that Nischarin is a poor substrate for PAK1 and thus unlikely to compete with other substrates.
Nischarin levels modulate PAK-induced cell migration
As discussed above, PAK has been shown to play an important role in cell migration. Nischarin inhibits both cell migration (Alahari et al, 2000; Alahari, 2003) and PAK kinase activity; thus, it is important to understand the relationship between these two actions of Nischarin. To investigate this issue, we examined the effects of Nischarin and its fragments on migration stimulated by an active form of PAK1 (T423E). Thus, CHO B2 α27 cells (which express human alpha 5 integrin) were transfected with PAK1-T423E or with kinase-dead PAK (PAK1-K299R), and were cotransfected with Nischarin or the N-terminal (1–802) or C-terminal (970–1354) domains of Nischarin. Subsequent to transfection, cell migration assays were performed. As shown in Figure 5A, PAK1-T423E stimulated migration in the CHO cells, and this was strongly inhibited by overexpression of Nischarin. Interestingly, the kinase-dead version of PAK1 did not stimulate migration in this system. Consistent with our data on kinase inhibition, only full-length Nischarin was able to inhibit PAK1-T423E-driven migration (Figure 5A), while the N- or C-terminal domains had no effect. Thus there is a close parallel between Nischarin's ability to block PAK kinase activity and its ability to inhibit PAK-driven cell migration.
Figure 5.
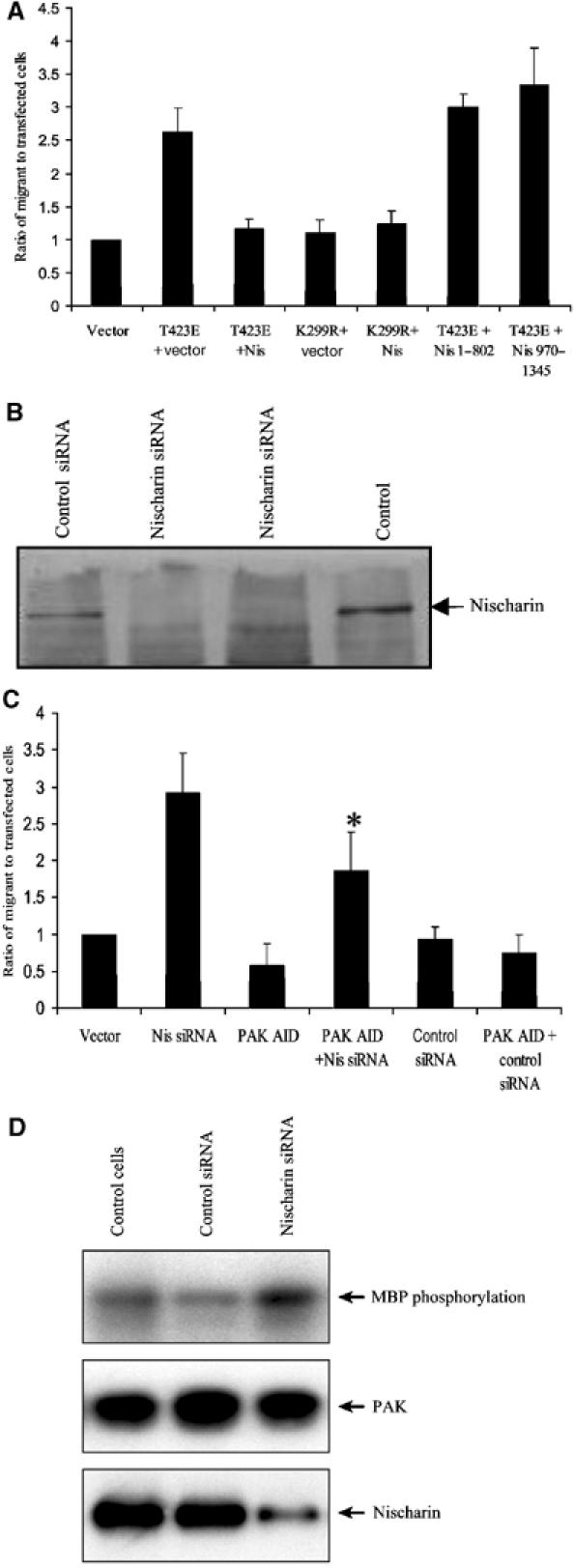
Effects of Nischarin on PAK-induced migration. (A) Overexpression of full-length Nischarin inhibits PAK-driven migration. CHO B2-α27 cells were transiently transfected with vector alone, with V5-PAK1-T423E plus Myc-vector, full-length Myc-Nischarin, Myc-Nischarin (1–802) or Myc-Nischarin (970–1354). Other cells were transfected with V5-PAK1-K299R plus Myc-Nischarin or vector control. A β-gal plasmid was also used to mark all transfectants. Cells were plated in transwells, and the β-gal-expressing cells migrating through the transwells were counted. (B) Effects of siRNA on Nischarin levels. PC12 cells were transfected with pcDNA-CD4 and 150 nM anti-rat Nischarin siRNA or control siRNA (anti-human MDR1). At 48 h after transfection, the CD4-positive cells were selected with anti-CD4-coated Dynabeads®. The cells were lysed and equal amounts of protein were used for SDS–PAGE. An anti-Nischarin antibody was used for Western blotting. Two separate lanes are shown for cells treated with siRNA for Nischarin. (C) Effects of siRNA on cell migration. The haptotactic migration of PC12 cells was examined using a Transwell assay. Membrane inserts were coated with 10 μg/ml collagen. Cells were transfected with 150 nM anti-Nischarin siRNA or with control siRNA, as well as with a vector expressing β-gal. Some sets of cells were cotransfected with a construct that expresses the PAK1 autoinhibitory domain (AID). Cells were plated in transwells and the β-gal-expressing cells migrating through the transwells were counted. Results are the means and standard errors of six determinations. (*) The difference between the Nis siRNA and Nis siRNA+AID samples was significant at the 0.01 level. (D) Effects of siRNA on PAK activity. PC12 cells were transfected with 150 nM Nischarin siRNA or control siRNA. At 48 h after transfection, the cells were lysed and endogenous PAK was immunoprecipitated. The immunoprecipitate was used in an in vitro kinase assay with MBP as a substrate. The upper panel shows MBP phosphorylation, the middle panel the amount of PAK in the immunoprecipitate and the lower panel the amount of Nischarin in the lysate.
Since overexpression of Nischarin inhibits PAK-driven cell migration, we wished to see if reducing endogenous levels of Nischarin would affect cell motility. Thus, PC12 cells (which have endogenous Nischarin) were transfected with an siRNA oligonucleotide targeted to Nischarin, or with a control oligonucleotide. The migratory ability of these cells was then tested. The anti-Nischarin siRNA, but not the control siRNA, caused a substantial reduction in the amount of endogenous Nischarin (Figure 5B). The anti-Nischarin siRNA, but not the control, also dramatically stimulated cell migration (Figure 5C). Interestingly, coexpression of a PAK fragment comprising the autoinhibitory domain significantly reversed the increase in migration caused by the anti-Nischarin siRNA. This domain can block PAK kinase activity, suggesting that the stimulation of migration caused by the siRNA was due to increased PAK activity. To pursue this further, we treated PC12 cells with anti-Nischarin siRNA or control siRNA, immunoprecipitated the endogenous PAK and tested its kinase activity. As seen in Figure 5D, transfection with anti-Nischarin siRNA caused an increase in the activity of endogenous PAK. It should be noted that only a fraction of the cells were transfected, so that only a portion of the total pool of PAK was affected by siRNA-mediated modulation of Nischarin. In summary, these results suggest that reducing levels of endogenous Nischarin can promote cell motility by permitting enhanced activation of PAK.
The PAK/Nischarin interaction is modulated by active Rac and requires the open form of PAK
Since the above observations suggest that the Nischarin–PAK interaction significantly affects cell migration behavior, we decided to further investigate other aspects of PAK/Nischarin binding. Binding of Rac1/CDC42 is an important step in the activation of PAK (Buchwald et al, 2001; Parrini et al, 2002). Since the data of Figure 2C suggest that Nischarin may bind preferentially to active PAK1, this implies that the binding of an active Rac1 to PAK1 should enhance the Nischarin/PAK1 interaction. Further, since introduction of a Y40C effector site mutation into Rac1 disrupts Rac1 binding to PAK1 (Westwick et al, 1997), this should reduce Rac's ability to enhance PAK1/Nischarin complex formation. As predicted, coexpression of active Rac1Q61L led to the enhancement of Nischarin co-immunoprecipitation with PAK1 (by about 3.5-fold) (Figure 6A and Supplementary Figure 4). By contrast, coexpression of mutant Rac1Q61L/40C resulted in less co-immunoprecipitation of PAK1 with Nischarin as compared to Rac1Q61L (Figure 6B). These data demonstrate that Rac activation of PAK1 enhances the formation of the Nischarin/PAK1 complex and this is dependent on the ability of Rac to bind to PAK1. Further, the presence of Nischarin does not inhibit the ability of PAK1 to bind Rac1, rather these proteins can form a tripartite complex.
Figure 6.
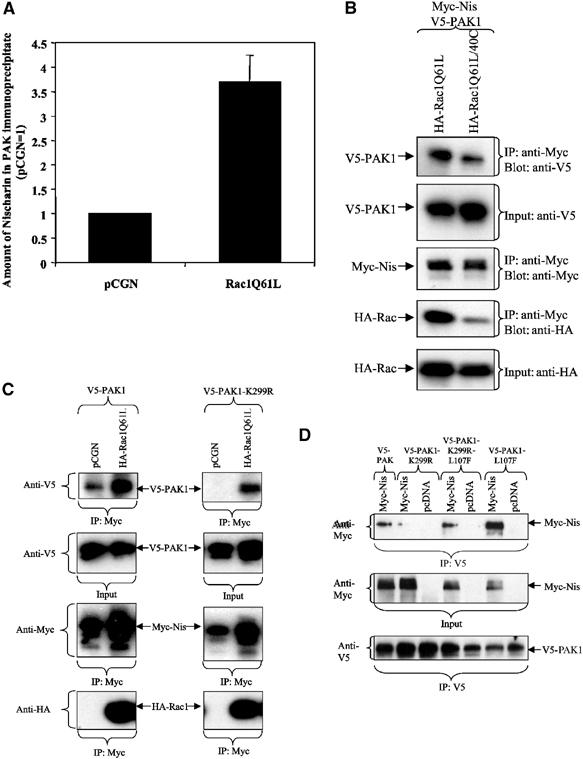
Effects of Rac and of PAK conformation on PAK/Nischarin binding. (A) Rac enhances Nischarin/PAK1 interaction. Myc-Nischarin and Myc-PAK1 were cotransfected into Cos-7 cells with HA-Rac1Q61L or pCGN vector control. After immunoprecipitation of PAK1 with polyclonal PAK1 antibody (N20), the levels of co-immunoprecipitating Myc-Nischarin were determined by Western blotting and were quantified on a Fluor-S MultiImager (Bio-Rad) and normalized to the vector control. The error bars show standard deviation (N=6). (B) Rac40C is less effective at promoting PAK–Nischarin interaction. Myc-Nischarin and V5-PAK1 were cotransfected with HA-Rac1Q61L or HA-Rac1Q61L/40C. Myc-Nischarin was immunoprecipitated from cytoplasmic lysates and immunoblots were performed to detect the indicated epitopes. (C) Rac increases the binding of both WT and kinase-dead PAK1 to Nischarin. Myc-Nischarin was coexpressed with HA-Rac1Q61L and with V5-PAK1 or V5-PAK1-K299R. The lysates were immunoprecipitated with anti-Myc and the immunoprecipitates blotted with antibodies to the indicated epitopes. (D) Nischarin binds to the open conformation of PAK1. Cos-7 cells were transfected with Myc-Nischarin or pcDNA and V5-PAK1, V5-PAK1-L107F, V5-PAK1-K299R or V5-PAK1-K299R-L107F. PAK1 was immunoprecipitated with the anti-V5 antibody. The input and immunoprecipitates were blotted for the respective epitope tags of the transfected proteins.
While the data of Figures 6A and B indicate that the activation PAK1 facilitates the interaction of Nischarin with PAK1's kinase domain, it does not determine whether kinase activity itself is necessary for this interaction. Activation of group 1 PAKs involves a relaxation of the association between the N-terminal autoinhibitory domain and the C-terminal kinase domain (Lei et al, 2000; Parrini et al, 2002); thus, the ‘open' conformation may be the preferred configuration for Nischarin binding. This concept was tested in two ways. First, active Rac was coexpressed with Nischarin and with either WT PAK1 or the K299R mutant of PAK1 that lacks kinase activity. As seen in Figure 6C, active Rac strongly promoted Nischarin binding to both forms of PAK1. Second, we examined the effect of the L107F mutation that inhibits the association of the regulatory and kinase domains of PAK1. As seen in Figure 6D, the presence of this mutation increased the binding of Nischarin to both WT PAK1 and K299R PAK1. Thus these data strongly suggest that Nischarin binds to the ‘open' conformation of PAK1, but that kinase activity per se is not required.
The interaction between Nischarin and PAK1 is direct
As shown above, PAK and Nischarin clearly exist in intracellular complexes. However, the interaction may be mediated by direct contact between these two proteins or through an unknown intermediary protein. To determine if Nischarin directly interacts with PAK1, Nischarin's N-terminus (1–802)-6xHis was expressed in an Escherichia coli-based transcription/translation system. This prokaryotic system should not contain any proteins that might mediate the PAK1/Nischarin interaction. Expression of a modified transcription factor TGV-6xHis (Ye and Juliano, 2002) was used as a negative control. Incubation of GST-PAK1-248-545-K299R with N-terminus-6xHis resulted in the formation of specific complexes, while nonspecific interactions were absent (Figure 7A). These data support the idea that the complex formed by Nischarin and PAK1 is mediated by a direct interaction between these two proteins. Similar results were obtained when the Nischarin N-terminus was expressed in a eukaryotic transcription–translation system and allowed to interact with GST-PAK1. Further, while WT GST-PAK1 bound Nischarin, full-length kinase-dead PAK1 did not, further supporting the hypothesis that PAK must be able to attain its ‘open' conformation to bind Nischarin (Figure 7B).
Figure 7.
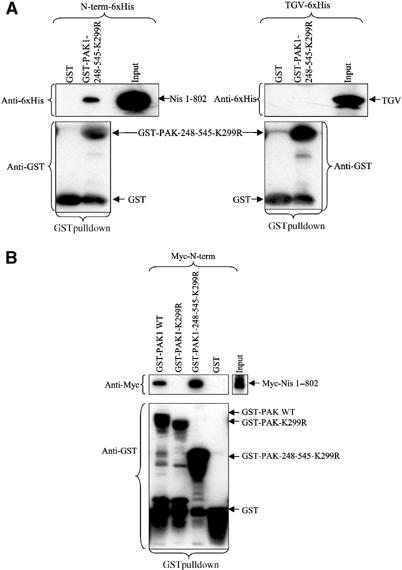
In vitro binding of Nischarin and PAK1. (A) In vitro binding of Nischarin N-terminus to GST-PAKs. The 6xHis-tagged N-terminus and the transcription factor TGV-6xHis were expressed in the E. coli Expressway™ In Vitro Protein Synthesis System. Lysates containing the N-terminus-6xHis or TGV-6xHis were incubated with GST-PAK1-248-545-K299R or GST and bound proteins were isolated on glutathione–Sepharose 4B beads. The immunoblots were probed with anti-6xHis and anti-GST monoclonal antibodies. (B) Binding of rabbit reticulocyte expressed Nischarin N-terminus to GST-PAKs. The N-terminus Myc fragment was expressed in the TnT® rabbit reticulocyte transcription/translation system. Binding to GST fusion protein was conducted as in (A). The immunoblots were probed with anti-Myc and anti-GST monoclonal antibodies.
Nischarin interacts with group 1 and 2 PAKs
To examine the potential of Nischarin to interact with other PAK isoforms, human PAK1, PAK4 and PAK5 were coexpressed in Cos-7 cells with Nischarin linked to EGFP at its C-terminus. Immunoprecipitation of PAK1, PAK4 and PAK5 by their Myc tags co-precipitated the coexpressed Nischarin-EGFP, without precipitating EGFP itself (Figure 8). Thus, Nischarin interacts with PAK isoforms from both groups 1 and 2.
Figure 8.
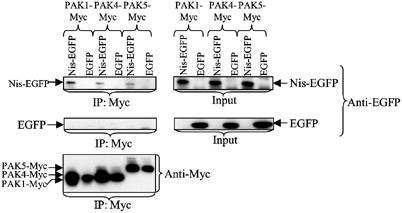
Interaction of Nischarin with PAK4 and PAK5. Nischarin-EGFP or EGFP and Myc-PAK1, Myc-PAK4 or Myc-PAK5 were coexpressed in Cos-7 cells. The PAK proteins were immunoprecipitated from lysates via the Myc epitope. The immunoprecipitates were immunoblotted for the presence of Myc and EGFP epitopes. The left column demonstrates binding of Nischarin-EGFP to PAK1, PAK4 and PAK5 (upper panel), whereas EGFP alone did not co-precipitate with the various PAKs (mid-panel). The right panels are loading controls.
Discussion
Cell migration is an intricate process involving several signal transduction pathways, including a key role for members of the PAK family of kinases (Bokoch, 2003). Thus it is important that PAK activity in various regions of the cell be orchestrated in a coordinated manner. As described above, the novel protein Nischarin potently inhibits both PAK activity and cell migration. Therefore, understanding the relationship between Nischarin and PAK family kinases may provide key insights into the regulation of motility.
The N-terminal domain (1–802) of Nischarin interacts with the C-terminal (248–545) kinase domain of PAK1. Several independent sites within the N-terminus are able to bind PAKs to some degree, raising the possibility that a single molecule of Nischarin may be able to bind more than one molecule of PAK. However, the precise stoichiometry of Nischarin–PAK binding has not yet been established. The existence of several distinct binding regions on Nischarin is not unusual for such a large, complex protein; for example, the regulator protein FIP200 binds to focal adhesion kinase via several distinct segments (Abbi et al, 2002). It should be noted that IRAS, the human ortholog of Nischarin, binds to the adaptor protein IRS-4 (Sano et al, 2002), but the consequences of this are unclear.
Nischarin not only binds to PAK1 but also strongly inhibits PAK kinase activity. PAK1 binds Nischarin via its kinase domain and the interaction is enhanced when PAK1 is in its activated or ‘open' conformation, as in the presence of activated Rac. However, the interaction of Nischarin with PAK1 is not dependent on kinase activity per se, but rather on PAK1 being in the open configuration. There are a number of other proteins that bind to PAKs and affect their function. Most of these proteins interact with the PAK regulatory domain and alter PAK's conformation or cellular localization (Bokoch et al, 1996; Galisteo et al, 1996; Manser et al, 1998; Puto et al, 2003); however, the PAK kinase domain can also bind proteins, including the Raf-1 and LIM kinases (Edwards et al, 1999; Zang et al, 2002).
In addition to the autoinhibitory role of the PAK N-terminus (Parrini et al, 2002), several other mechanisms for negative regulation of PAK kinase activity are known. Thus various kinases, such as PKA, can inactivate PAKs by phosphorylation of key residues (Howe and Juliano, 2000), while certain phosphatases can inactivate PAKs through dephosphorylation (Koh et al, 2002). The mechanism underlying Nischarin's inhibition of PAK activity has not yet been worked out in detail. However, our initial results suggest that Nischarin can hinder the autophosphorylation and activation of PAK1 and interrupt PAK's ability to phosphorylate substrates. In contrast, Nischarin does not seem to be a very good substrate for PAK, and thus it is less likely that Nischarin blocks PAK1 by simply competing with other substrates for phosphorylation.
Nischarin's ability to inhibit PAK kinase activity is closely correlated with its effects on cell migration. Thus, although the N-terminal domain of Nischarin binds well to PAK1, it has no effect on either cell migration or kinase activity, nor does the isolated C-terminal domain; full-length Nischarin is required to inhibit in both instances. Reduction of endogenous Nischarin levels using RNA interference reinforces the concept that Nischarin regulates cell migration, most likely by affecting PAK activity. Thus, while overexpression of Nischarin inhibits PAK kinase activity and cell migration, siRNA-mediated reduction of endogenous Nischarin levels enhances migration, as well as increasing the activity of endogenous PAK.
It is interesting that neither the full N-terminus (1–802) nor the previously identified integrin-binding domain (434–581) of Nischarin (Alahari et al, 2000) is able to inhibit cell migration significantly. This emphasizes that Nischarin's effects on migration are primarily mediated through inhibiting PAK rather than through binding to integrins and blocking their function. However, by associating both with integrins and with PAKs, Nischarin might allow a focusing of PAK inhibition in certain regions of the cell.
Since Nischarin regulates PAK effects on cell migration, one would expect these molecules to colocalize in membrane protrusions involved in motility. Thus, in actively spreading fibroblasts, PAK1 and Nischarin colocalize in membrane ruffles, structures known to be involved in cell migration (Sastry and Burridge, 2000). Nischarin shows stronger membrane colocalization with a constitutively active PAK1 mutant than with WT PAK1, and conversely Nischarin does not display membrane colocalization with kinase-dead PAK1. Interestingly, examination at higher power suggests that the area of PAK/Nischarin overlap is behind the leading edge of the membrane protrusion, while PAK alone is enriched in the edge. This observation is consistent with the idea that Nischarin binds to and inhibits PAK after PAK has been activated in sites of membrane protrusive activity. As we described previously, although Nischarin binds the α5β1 integrin, Nischarin does not localize to focal adhesions (Alahari et al, 2000). However, certain other proteins that strongly interact with integrins do not localize in focal adhesions (Hemler, 2001; Degani et al, 2002) and Nischarin seems to follow this pattern, localizing instead in more dynamic membrane protrusions. Nonetheless, the presence of the α5β1 integrin affects the binding of Nischarin to PAKs, suggesting a functional interconnection between Nischarin's ability to bind integrins and its association with PAKs.
While it is clear that Nischarin plays a key role in the regulation of PAK activity and the control of cell migration, the precise mechanisms involved are not yet fully understood. One hypothesis that is consistent with current data is as follows. In migrating cells active PAK is recruited to membrane protrusions (ruffles/lamellipodia/filopodia) where it contributes to enhanced actin filament formation and reduced contractility. However, in order for stable adhesions to form, contractility must increase (Sastry and Burridge, 2000), and thus PAK activity must be turned off. We hypothesize that Nischarin (perhaps in association with α5β1) is also recruited to membrane protrusions. There, Nischarin can bind active PAK, reducing its kinase activity, permitting increased contractility and allowing adhesive contacts to form. As mature adhesive contacts develop, PAK is inactivated and the Nischarin is released, allowing the process to recycle. Thus, Nischarin may be a key physiological regulator of a biochemical cycle that plays an important role in cell migration.
Materials and methods
Vectors
Full-length mouse Nischarin cDNA, all Nischarin deletion mutants and β-galactosidase were expressed from pcDNA3.1 B+ Myc/His (Invitrogen). Nischarin was also expressed from pEGFP-N1 vector (Clontech). Full-length human PAK1 (provided by J Chernoff), its regulatory domain (1–248), its kinase domain (248–545), the T423E, K299R, L107F and K299R/L107F mutants, the autoinhibitory domain (AID) and β-galactosidase were expressed from pcDNA3.1 B+ V5/His (Invitrogen). For the in vitro binding studies, GST-tagged PAK1, PAK1-K299R and GST-PAK1-248-545-K299R were expressed from the bacterial expression vector pGEX-6P-3 (Amersham Biosciences). For expression in a bacterial system, the cDNA for the Nischarin N-terminus was inserted into the pET30a (+) vector (Novagen). As a control, a fusion protein (TGV-6xHis) containing the GAL4 DNA-binding domain was used (Ye and Juliano, 2002). Myc-tagged PAK4 and PAK5 (provided by A Minden) were expressed from the vectors pSRa3 and pCAN-Myc1, respectively (Dan et al, 2002). HA-tagged Rac1Q61L and HA-Rac1Q61L/40C (provided by C Der) were expressed from the pCGN vector.
Immunoprecipitation
For Nischarin/PAK binding, Cos-7 cells were transfected with 1 μg each of Nischarin and PAK1 plasmids using FuGENE 6 (Roche). After 48 h, the cells were lysed in an NP-40 lysis buffer (50 mM Tris–HCl pH 7.5, 250 mM NaCl, 5 mM EDTA, 0.1% NP-40) with protease and phosphatase inhibitors. The lysates were incubated with anti-Myc antibody 9E10 (Covance), anti-V5 antibody (Stratagene) or anti-PAK1 (N-20) (Santa Cruz Biotechnology) at 4°C for 2 h. The immune complexes were precipitated with protein G–Sepharose® and the precipitates were resolved on a 4–20% gradient gel. The immunoprecipitated proteins were detected with the antibodies used for immunoprecipitation or anti-HA antibody HA.11 (Covance). To examine the interaction of endogenous Nischarin and PAK1, PC12 cells were lysed and 15 mg of the cleared supernatant was incubated with 100 μg of agarose-conjugated anti-PAK1 antibody (N20) or agarose-conjugated control rabbit IgG overnight at 4°C. The immunoprecipitates were immunoblotted as above for endogenous Nischarin with a mouse anti-Nischarin monoclonal antibody supernatant (gift of Dr R Campos, Becton-Dickinson Inc.) or control mouse IgG, and for endogenous PAK with N20.
In vitro expression and binding of Nischarin
Nischarin N-terminus-6xHis or TGV-6xHis were produced in the Expressway™ (Invitrogen) bacterial cell-free system, and the cleared supernatants were used for binding studies. The rabbit reticulocyte-based TnT® Quick Coupled Transcription/Translation System (Promega) was used to express the N-terminus of Nischarin in a eukaryotic cell-free system. The DH5α E. coli strain transformed with pGEX-6P-3 vectors was used for expression of PAK1-GST fusion proteins. Only the kinase-dead form of the PAK1 kinase domain was used because the active kinase is toxic to E. coli. A 10 μg portion of the GST fusion proteins was incubated with 50 μl generated from either the TnT® System or the Expressway™ System brought to a total volume of 300 μl with 0.1% NP-40 lysis buffer. The samples were incubated for 1 h at 30°C at 500 rpm and the proteins were pulled down with glutathione–Sepharose 4B.
Kinase assays
For evaluating PAK activity, Cos-7 cells were transfected with V5-PAK1 and various Myc-Nischarin constructs, or with Myc-β-gal. After 24 h of incubation in DMEM containing 10% FBS, the medium was replaced with serum-free DMEM for another 24 h. Cells were stimulated with 10% FBS for 15 min, and lysed in modified RIPA buffer with protease inhibitors. For immunoprecipitation, about 900 μg of lysate was incubated with 9 μg of anti-V5 antibody (Invitrogen) for 2 h at 4°C, then precipitated with protein A–Sepharose. Kinase assays were carried out for 5 or 30 min at 30°C in 50 μl of kinase reaction buffer containing 5–15 μg of myelin basic protein, 1 mM ATP and 10 μCi [32P]ATP. The reaction was stopped with 6 × sample buffer. The samples were electrophoresed on 15% SDS–PAGE gels, stained with Coomassie blue, dried and exposed to X-ray films. In some experiments, constitutively active V5-PAK-T423E and Myc-Nischarin or Myc-β-gal were used; the cells expressing these constructs were maintained in 0.5% FBS-containing medium for 48 h, and kinase assays were performed as described above. As a negative control, kinase-dead V5-PAK-K299R was used. Similar assays were used to measure the activity in immunoprecipitates of endogenous PAK from PC12 cells. Activity of JNK was evaluated as described (Alahari, 2003).
Transwell cell migration assay
Haptotactic cell migration assays were performed essentially as described (Alahari et al, 2000). Briefly, CHO B2α27 cells were transiently transfected with constitutively active PAK (V5-PAK-T423E) or with V5-PAK-K299R, plus full-length or truncated forms of Nischarin. A pRC β-gal plasmid (Stratagene) (1 μg) was used as a marker. The underside of the transwell was coated with fibronectin and 200 000 cells were added to the upper surface. The β-galactosidase-positive cells that migrated through the membrane during an overnight incubation were counted by staining for β-gal. The ratio of migrant transfected cells to total transfected cells was normalized to the vector control. PC12 cells were transfected with β-gal plasmid and 150 or 300 nM of rat Nischarin siRNA or control siRNA, using Lipofectamine 2000. In some cases, 1 μg of a plasmid expressing the PAK1 autoinhibitory domain (AID) was also transfected. The underside of the transwell was coated with collagen and 300 000 cells were added to the upper surface. The migrant fraction of transfected cells was evaluated as described above.
Confocal microscopy
The basic procedures have been described previously (Alahari et al, 2000). Briefly, rat embryonic fibroblasts were transiently transfected with 0.2 μg of GFP or GFP-Nischarin along with 1 μg of Myc-PAK1 or mutated versions of PAK1. After 24 h of transfection, cells were serum starved overnight in 1% BSA-containing DMEM. Cells were trypsinized and replated on fibronectin-coated coverslips for various times in the presence of 10% FBS-containing DMEM. The cells were washed with PBS, fixed in 0.37% formaldehyde, permeabilized in 1% Triton X-100 and blocked in BSA for 1 h at room temperature. The coverslips were stained with anti-Myc antibody for 1 h at room temperature followed by incubation with TRITC-conjugated secondary antibody. The coverslips were observed on an Olympus Confocal FV300 fluorescent microscope with 60 × oil immersion objective; images were acquired by using Olympus Fluoview software.
Reduction of endogenous Nischarin by siRNA
A 21 base pair siRNA for rat Nischarin, rCrCUrCrGUrGrCrArCrCUUrGrArCrCUrGTT, as well as a ‘scrambled' siRNA and an siRNA for human MDR-1, both used as controls, were synthesized by Proligo Inc. To determine the effectiveness of the Nischarin siRNA, rat PC12 cells were transfected using Lipofectamine 2000 with the plasmid pcDNA-CD4 and with siRNA at a concentration of 150 nM. At 48 h after transfection, cells were detached with trypsin–EDTA and anti-CD4-coated magnetic beads (Dynal) were used to enrich the transfected cells by magnetic selection. The cells were lysed in RIPA buffer and the soluble supernatants were used for immunoblotting. Nischarin levels were determined with an anti-Nischarin antibody. Cell migration experiments were carried out after similar transfection with Nischarin siRNA or control siRNAs (see above) with β-gal as a marker. Experiments involving the effect of siRNA on protein kinase activity did not involve any enrichment procedure.
Supplementary Material
Supplementary Material
Acknowledgments
This work was supported by NIH grants GM26165 and HL4500 to RLJ. PJR was supported by ACS Fellowship PF-01-061-01-CSM. We thank David Rinker for expert editorial assistance.
References
- Abbi S, Ueda H, Zheng C, Cooper LA, Zhao J, Christopher R, Guan JL (2002) Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol Biol Cell 13: 3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahari SK (2003) Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res 288: 415–424 [DOI] [PubMed] [Google Scholar]
- Alahari SK, Lee JW, Juliano RL (2000) Nischarin, a novel protein that interacts with the integrin alpha5 subunit and inhibits cell migration. J Cell Biol 151: 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahari SK, Reddig PJ, Juliano RL (2002) Biological aspects of signal transduction by cell adhesion receptors. Int Rev Cytol 220: 145–184 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Cerione RA (1999) Pak to the future. Trends Cell Biol 9: 350–355 [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348: 241–255 [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM (2003) Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781 [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG (1996) Interaction of the Nck adapter protein with p21-activated kinase (PAK1). J Biol Chem 271: 25746–25749 [DOI] [PubMed] [Google Scholar]
- Brown MC, West KA, Turner CE (2002) Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell 13: 1550–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, Meyer HE, Scheffzek K, Wittinghofer A (2001) Conformational switch and role of phosphorylation in PAK activation. Mol Cell Biol 21: 5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Ressad F, Pantaloni D (1999) Control of actin dynamics in cell motility. Role of ADF/cofilin. J Biol Chem 274: 33827–33830 [DOI] [PubMed] [Google Scholar]
- Chong C, Tan L, Lim L, Manser E (2001) The mechanism of PAK activation. Autophosphorylation events in both regulatory and kinase domains control activity. J Biol Chem 276: 17347–17353 [DOI] [PubMed] [Google Scholar]
- Dan C, Nath N, Liberto M, Minden A (2002) PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol 22: 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degani S, Balzac F, Brancaccio M, Guazzone S, Retta SF, Silengo L, Eva A, Tarone G (2002) The integrin cytoplasmic domain-associated protein ICAP-1 binds and regulates Rho family GTPases during cell spreading. J Cell Biol 156: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1: 253–259 [DOI] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W (2002) Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem 277: 5644–5650 [DOI] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J (1996) The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem 271: 20997–21000 [DOI] [PubMed] [Google Scholar]
- Hemler ME (2001) Specific tetraspanin functions. J Cell Biol 155: 1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA (2002) Role of integrins in cell invasion and migration. Nat Rev Cancer 2: 91–100 [DOI] [PubMed] [Google Scholar]
- Howe AK, Juliano RL (2000) Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol 2: 593–600 [DOI] [PubMed] [Google Scholar]
- Juliano RL (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42: 283–323 [DOI] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM (2000) p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1). J Biol Chem 275: 41201–41209 [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T (2003) Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 12: 841–849 [DOI] [PubMed] [Google Scholar]
- Koh CG, Tan EJ, Manser E, Lim L (2002) The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol 12: 317–321 [DOI] [PubMed] [Google Scholar]
- Kumar R, Vadlamudi RK (2002) Emerging functions of p21-activated kinases in human cancer cells. J Cell Physiol 193: 133–144 [DOI] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC (2000) Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell 102: 387–397 [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH (2000) Integrin cytoplasmic domain-binding proteins. J Cell Sci 113: 3563–3571 [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192 [DOI] [PubMed] [Google Scholar]
- Parrini MC, Lei M, Harrison SC, Mayer BJ (2002) Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol Cell 9: 73–83 [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465 [DOI] [PubMed] [Google Scholar]
- Puto LA, Pestonjamasp K, King CC, Bokoch GM (2003) p21-activated kinase 1 (PAK1) interacts with the Grb2 adapter protein to couple to growth factor signaling. J Biol Chem 278: 9388–9393 [DOI] [PubMed] [Google Scholar]
- Ridley AJ (2001) Rho GTPases and cell migration. J Cell Sci 114: 2713–2722 [DOI] [PubMed] [Google Scholar]
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P (1999) Inhibition of myosin light chain kinase by p21-activated kinase. Science 283: 2083–2085 [DOI] [PubMed] [Google Scholar]
- Sano H, Liu SC, Lane WS, Piletz JE, Lienhard GE (2002) Insulin receptor substrate 4 associates with the protein IRAS. J Biol Chem 277: 19439–19447 [DOI] [PubMed] [Google Scholar]
- Sastry SK, Burridge K (2000) Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res 261: 25–36 [DOI] [PubMed] [Google Scholar]
- Sells MA, Boyd JT, Chernoff J (1999) p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol 145: 837–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J (1997) Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol 7: 202–210 [DOI] [PubMed] [Google Scholar]
- Sells MA, Pfaff A, Chernoff J (2000) Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol 151: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R (2002) Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 4: 681–690 [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R (2004) p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 5: 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ (1997) Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol 17: 1324–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M (2001) Regulation of the p21-activated kinase (PAK) by a human Gbeta-like WD-repeat protein, hPIP1. Proc Natl Acad Sci USA 98: 6174–6179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Juliano R (2002) Evaluation of strategies for the intracellular delivery of proteins. Pharm Res 19: 1302–1309 [DOI] [PubMed] [Google Scholar]
- Zang M, Hayne C, Luo Z (2002) Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem 277: 4395–4405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


