Abstract
Background
The separate value of endoscopic ultrasonography (EUS), multidetector computed tomography (CT), and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in the optimal sequence in staging esophageal cancer has not been investigated adequately.
Methods
The staging records of 216 consecutive operable patients with esophageal cancer were reviewed blindly. Different staging strategies were analyzed, and the likelihood ratio (LR) of each module was calculated conditionally on individual patient characteristics. A logistic regression approach was used to determine the most favorable staging strategy.
Results
Initial EUS results were not significantly related to the LRs of initial CT and FDG-PET results. The positive LR (LR+) of EUS-fine-needle aspiration (FNA) was 4, irrespective of CT and FDG-PET outcomes. The LR+ of FDG-PET varied from 13 (negative CT) to 6 (positive CT). The LR+ of CT ranged from 3–4 (negative FDG-PET) to 2–3 (positive FDG-PET). Age, histology, and tumor length had no significant impact on the LRs of the three diagnostic tests.
Conclusions
This study argues in favor of PET/CT rather than EUS as a predictor of curative resectability in esophageal cancer. EUS does not correspond with either CT or FDG-PET. LRs of FDG-PET were substantially different between subgroups of negative and positive CT results and vice versa.
Accurate preoperative staging in esophageal cancer is important in the choice of treatment, preventing unnecessary toxic preoperative chemoradiation and/or surgical explorations. Moreover, it is essential to determine optimal treatment and to monitor treatment response after neoadjuvant therapy.1–3 Radical surgery with curative intent is only possible if distant metastases (M1) and infiltration of the primary tumor into adjacent vital structures (T4b) are absent. If present, primary (chemo)radiation, brachytherapy or stent placement are more adequate and less invasive alternatives as palliative treatment.4–7
Currently, preoperative staging of esophageal cancer includes endoscopic ultrasonography (EUS) with or without fine-needle aspiration (FNA) of suspicious lymph nodes, 16–64 multidetector/slice computed tomography (CT), external ultrasound (US) of the cervical region, and bronchoscopic examination, if indicated, in mid/upper thoracic tumors. To detect distant nodal and systemic metastases, whole-body positron emission tomography with 18F-fluordeoxyglucose (FDG-PET) or PET/CT is widely used.3 These staging methods are used in different sequences, according to the guidelines employed. Despite these dedicated staging techniques, surgical resection is still abandoned in 10–50% of all cases due to excessive locoregional tumor extent or presence of distant metastases.3,8
Assessment of resectability is based on both local and distant criteria. Imaging techniques are more or less complementary, but outcome may also depend on the sequence of the preoperative workup. Furthermore, a recent study showed significant but small differences in perceived patient burden between PET and CT compared with EUS.9 Therefore, it is important to know the adequate sequences of these different diagnostic methods and when to use PET/CT or only CT (upfront), followed by EUS, and vice versa. Several studies found that FDG-PET combined with EUS-FNA improved preoperative staging of esophageal cancer.3,10–13 Fusion of FDG-PET and CT images also provided an increase in preoperative management from 6 to 25%.14–16 The optimal staging strategy, however, remains unclear, and the additional value of combined PET/CT has not been determined adequately yet.
Therefore, we used a logistic regression approach to determine the extent to which the individual value of each diagnostic staging technique depends on the order in which the procedure is applied and to determine if this staging method adds useful information to what is already known, either because of individual characteristics or on the basis of preliminary staging results.17 Three routine diagnostic staging techniques (EUS, CT, and FDG-PET) were tested in terms of curatively intended resectability of esophageal cancer. For this purpose, we compared the likelihood ratios (LRs) in different staging strategies, calculated at the level of the individual patient.
Patients and Methods
Study Design
The medical records from a multicenter (Academic Medical Center, Amsterdam and University Medical Center, Groningen) prospective cohort staging improvement study lasting from October 2002 to October 2004 were used.18 The study consisted of 258 consecutive patients with biopsy-proven cancer of the thoracic esophagus or gastroesophageal junction (GEJ). Exclusion criteria were age <18 years, inability to undergo major surgery, pregnancy, and history of another malignancy in the previous 5 years. According to the above-mentioned criteria, 216 operable patients were eligible for participation. Informed consent was obtained from all 216 patients who formed our study population. Patient and tumor characteristics are listed in Table 1. All these patients underwent thoracic and abdominal CT, EUS with FNA on indication, and whole-body FDG-PET within a time period of 6 weeks. All PET/CT and EUS were performed and reviewed independently by well-trained and experienced investigators in both highly qualified centers. Interpretation of each modality was blinded, and investigators were unaware of other clinical or diagnostic data.18 Resectability was determined by local tumor invasion of vital structures, excluding non-curatively resectable group of unresectable tumor (T4b), unresectable conglomerate of nonregional nodal disease, or distant metastases (M1). Distant metastases included lymph node metastases in the cervical area or at the celiac axis depending upon primary tumor location or hematogenous metastases, usually to liver and lungs and bone metastasis. To exclude pathological cervical lymph nodes, external ultrasonography of the neck with FNA was performed on indication. All potential sites of incurable disease were confirmed pathologically or were followed with additional imaging during at least 12 months. All records, including histology achieved from biopsy, surgical explorations, and resections were registered and available for analysis. Pathological confirmation or any progression of unconfirmed suspicious lesion during 6-month follow-up was considered as gold standard.
Table 1.
Clinicopathological characteristics and univariate analysis of coefficients
| N = 216 | % | Resectable (n = 150) | Unresectable (n = 66) | p-Value | |
|---|---|---|---|---|---|
| Gender | 0.60 | ||||
| Male | 181 | 83.8 | 84.7% | 81.8% | |
| Female | 35 | 16.2 | |||
| Age (years) | 0.11 | ||||
| Median (range) | 63 | 29–82 | 63.44 (9.26) | 61.17 (10.05) | |
| Localizationa | 0.14 | ||||
| High | 23 | 10.6 | 12 (8.0%) | 11 (16.7%) | |
| Low | 139 | 64.4 | 101 (67.3%) | 38 (57.6%) | |
| GEJ | 54 | 25.0 | 37 (24.7%) | 17 (25.8%) | |
| Tumor length (cm) | 0.001 | ||||
| Median (range) | 5.0 | 0–18 | 5.47 | 7.36 | |
| Histological type | 0.058 | ||||
| AC | 168 | 77.8 | 122 (81.3%) | 46 (69.7%) | |
| SCC | 48 | 22.2 | 28 (18.7%) | 20 (30.3%) | |
| Test outcomes | |||||
| EUS outcome | Unresectable | 2 (1.3%) | 8 (12.1%) | n.a. | |
| CT outcome | Unresectable | 9 (6.0%) | 26 (39.4%) | n.a. | |
| FDG-PET outcome | Unresectable | 5 (3.3%) | 30 (45.5) | n.a. | |
| Clinical stage | |||||
| T1 | 9 | 4.2 | |||
| T2 | 22 | 10.4 | |||
| T3 | 171 | 80.7 | |||
| T4 | 10 | 4.7 | |||
| Missing value | 4 | – | |||
Staging based on total staging (EUS-FNA, CT, FDG-PET, and additional investigations, such as external sonography of the neck and bronchoscopy)
GEJ gastroesophageal junction, tumor length length of the tumor on EUS, AC adenocarcinoma, SCC squamous cell carcinoma, MWU Mann–Whitney U-test, χ 2 Pearson chi-square test, grouping variable: irresectability
aHigh, above the carina, Low, below the carina
Computed Tomography
A 16 or 64 multidetector row spiral CT scanner (Philips MX 8000; Philips Medical Systems, Best, The Netherlands or Somatom Sensation; Siemens Medical Systems, Erlangen, Germany) was used for CT imaging. CT scans (collimation 16 × 1.5 mm) were performed with both intravenous and oral contrast fluid and achieved in craniocaudal direction from the neck to the upper abdomen including the liver. Images had 3 mm reconstructed slice thickness with 1.5 mm effective section thickness. Round lymph nodes with low attenuation and lymph nodes with a size cutoff of 10 mm in smallest diameter were suspected to be pathologic.
Endoscopic Ultrasound
EUS was performed with a radial scanner (GF-UM 130 or GF-UM160, 5–20 MHz; Olympus Medical Systems, Tokyo, Japan), and EUS-guided FNA of suspected lymph nodes was obtained via a separate linear-array echoendoscope (GIF-UC140P; Olympus Medical Systems, Tokyo, Japan or FGUX-36, 5–7.5 MHz; Pentax, Benelux, Breda, The Netherlands). A 22-gauge needle was used for aspiration (Echo tip; Wilson-Cook Medical Inc., Winston-Salem, NC). If passage of a standard echoendoscope was not feasible because of stenosis, a small-caliber probe (MH-908, 7.5 MHz; Olympus Medical Systems, Tokyo, Japan) was used in an attempt to pass the tumor. EUS was performed with the patient in left decubitus position under sedation using 2.5–10 mg midazolam intravenously.
Positron Emission Tomography with 18F-Fluorodeoxyglucose
All patients were fasted for at least 4 h before FDG-PET imaging. FDG-PET was performed with an ECAT 951/31 or an ECAT HR+ positron camera (Siemens/CTI, Knoxville, TN, USA). Depending on body weight, a mean dose of 400–580 MBq FDG was administered intravenously. Data acquisition started in whole-body mode 90 min after injection, for 5 min per bed position from the skull to the mid femur.
Statistical Analyses
The results of EUS, CT, and FDG-PET together with the results of surgical exploration and pathological evaluation of the resection specimen were converted into a final gold-standard dichotomous outcome: resectable with curative intent (“resectable” hereinafter), or incurable/unresectable. Baseline variables were divided into three groups: (1) individual patient characteristics including age and gender, (2) tumor characteristics of the primary tumor including histological type, location, and length measured on EUS, and (3) staging characteristics including EUS outcome, CT outcome, and FDG-PET outcome. Resectable and unresectable tumors were compared by using the Pearson chi-square test (χ 2) for ordinal/nominal variables and the Mann–Whitney U-test (MWU) for continuous variables.
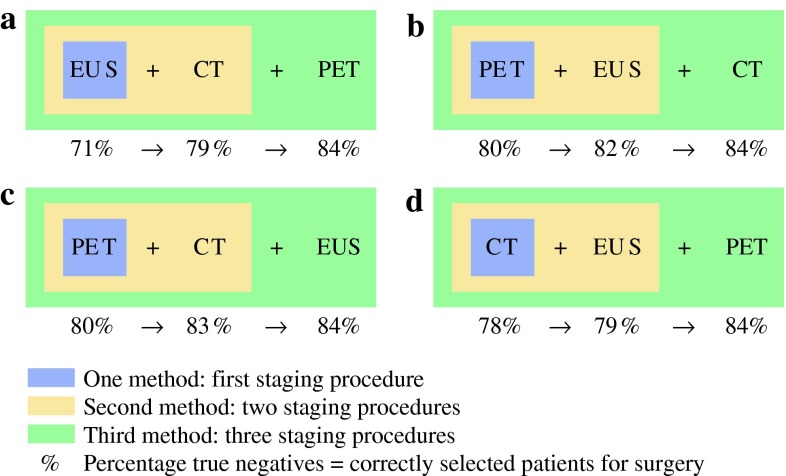
To estimate the probability of curative surgery (resectable) versus palliative treatment (unresectable), logistic regression analyses were used according to a recently developed regression approach.17 In this approach, the regression equation for the likelihood ratio (LR) of the test results (logistic regression model) is obtained by taking the difference in coefficients between prior and posterior odds. The prior odds model included all covariates that were significantly related to the resectability of the esophageal malignancy. The posterior odds model included all variables from the prior odds model plus the results of one or two of the additional imaging tests. In this way, the LR of a resectable tumor is calculated for individual risk profiles. In the conventional log-odds formulation of the Bayes rule, the natural logarithm (ln) of LR is the difference between ln(posterior odds) and ln(prior odds).17,19 Although we performed regression analyses for the three diagnostic imaging modalities in various sequences, only the four scenarios which are clinically relevant are presented (scenarios A–D, Fig. 1).
Fig. 1.
Models A–D: Four different staging scenarios and the number of true-negative test outcomes of each one-, two-, and three-step procedure (in percentages)
Descriptive statistics were obtained using SPSS 14.0 for Windows. The multivariable logistic regression analyses for prior and posterior odds models and the LR were programmed in S-PLUS (V6; Insightful Corp., Seattle, WA). Values of p less than 0.05 were considered statistically significant.
Results
The length of the tumor was a statistically significant risk factor for irresectability (p = 0.001). Not surprisingly, all staging characteristics were also significantly related with curative resectability. Age, gender, tumor location, and histological type were not significantly correlated with curative resection (Table 1). For each scenario (Fig. 1, models A–D), the coefficients of the ln(prior odds), ln(posterior odds), and ln(LR) regression models are presented in Table 2. Age, histological type, and tumor length did not significantly contribute to the LRs of the involved diagnostic tests in any of the models (p > 0.05).
Table 2.
Logistic regression models for the likelihood ratio of CT, FDG-PET, and EUS conditional on age, tumor length, and histological type
| Stage | Test | Covariate | Logistic regression | Likelihood ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | p-Value | Coeff. | SE | p-Value | |||
| Order A: 1. EUS, 2. CT, 3. FDG-PET | ||||||||
| I | EUS | – | 1.79 | 0.86 | 0.04 | – | – | – |
| II | EUS | 1.42 | 0.97 | 0.14 | −0.37 | 0.53 | 0.49 | |
| CT | 2.42 | 0.46 | <0.001 | 2.42 | 0.60 | <0.001 | ||
| III | EUS | 1.48 | 1.01 | 0.14 | 0.06 | 0.53 | 0.91 | |
| CT | 1.69 | 0.53 | 0.001 | −0.73 | 0.33 | 0.03 | ||
| FDG-PET | 2.91 | 0.57 | <0.001 | 2.91 | 0.92 | 0.001 | ||
| Order B: 1. FDG-PET, 2. EUS, 3. CT | ||||||||
| I | FDG-PET | – | 3.37 | 0.55 | <0.001 | – | – | – |
| II | FDG-PET | 3.36 | 0.55 | <0.001 | −0.01 | 0.07 | 0.95 | |
| EUS | 1.81 | 0.93 | 0.05 | 1.81 | 2.73 | 0.51 | ||
| III | FDG-PET | 2.91 | 0.57 | <0.001 | −0.45 | 0.19 | 0.02 | |
| EUS | 1.48 | 1.01 | 0.14 | −0.33 | 0.49 | 0.50 | ||
| CT | 1.69 | 0.53 | 0.001 | 1.69 | 0.65 | 0.01 | ||
| Order C: 1. FDG-PET, 2. CT, 3. EUS | ||||||||
| I | FDG-PET | – | 3.37 | 0.55 | <0.001 | – | – | – |
| II | FDG-PET | 2.90 | 0.57 | <0.001 | −0.46 | 0.19 | 0.01 | |
| CT | 1.78 | 0.52 | 0.001 | 1.78 | 0.54 | 0.001 | ||
| III | FDG-PET | 2.91 | 0.57 | <0.001 | 0.01 | 0.10 | 0.92 | |
| CT | 1.69 | 0.53 | 0.001 | −0.08 | 0.11 | 0.45 | ||
| EUS | 1.48 | 1.01 | 0.14 | 1.48 | 2.62 | 0.57 | ||
| Order D: 1. CT, 2. EUS, 3. FDG-PET | ||||||||
| I | CT | – | 2.47 | 0.45 | <0.001 | – | – | – |
| II | CT | 2.42 | 0.46 | <0.001 | 0.05 | 0.06 | 0.44 | |
| EUS | 1.42 | 0.97 | 0.14 | 1.42 | 2.61 | 0.59 | ||
| III | CT | 1.69 | 0.53 | 0.03 | −0.73 | 0.33 | 0.03 | |
| EUS | 1.48 | 1.01 | 0.14 | 0.06 | 0.53 | 0.91 | ||
| FDG-PET | 2.91 | 0.92 | 0.001 | 2.91 | 0.92 | 0.001 | ||
Coeff. coefficient, SE standard error, I one and first staging procedure, II second method/two staging procedures, III third method/three staging procedures
Impact of Different Staging Tests in Different Staging Scenarios
Figure 2 illustrates the difference a test outcome (Fig. 2a–c) and histological type (Fig. 2d) made on the LR+ of a following test. We chose tumor length as the X-variable only to spread out our dot plot rather than because of its correlation with the outcomes of the investigated modalities. In Fig. 2a, b, there is a significant difference between negative and positive outcomes of the preceding test. In Fig. 2c, the effect of EUS after PET or CT is not significant, although there is a tendency towards a visually apparent clustering into four groups. This is based on the combination of positive/negative CT results and histological type (adenocarcinoma/squamous cell carcinoma; Fig. 2d).
Fig. 2.
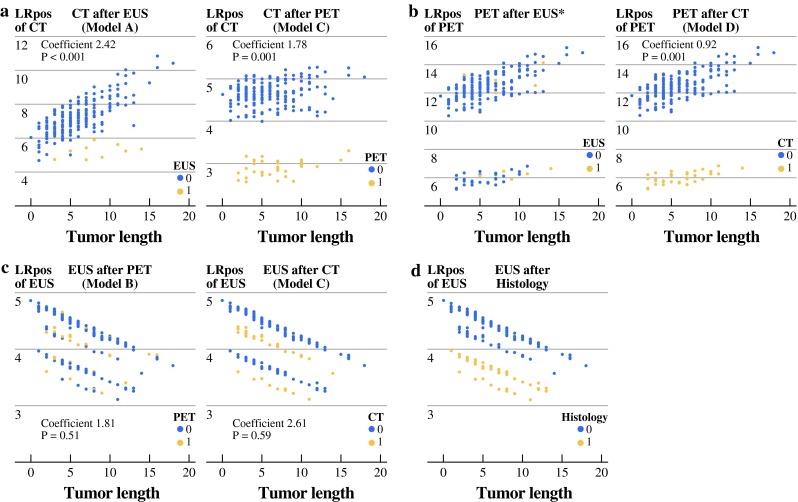
Positive likelihood ratio of CT, FDG-PET, and EUS conditional on tumor length (a–c) and on histological type (d) stratified by negative and positive test results of both other tests
In Table 2, the outcomes of EUS were not significantly related with either the LRs of the CT results or with the LRs of the FDG-PET results (model A; p = 0.49 and p = 0.91, respectively). CT results were strongly related to the LRs of the FDG-PET results (Table 2, model A; p = 0.03). The negative regression coefficient for CT (Table 2, model D; coeff. = −0.73) indicates that LR+ and LR− of FDG-PET were lower when CT was also positive compared with negative CT findings. Visa versa, FDG-PET results were strongly related with the LRs of CT (Table 2, model C; p = 0.01). EUS had no impact as a test for incurability, if it was performed after FDG-PET and CT in the staging workup (Table 2, model C; p = 0.57), nor was there a significant relation between FDG-PET + CT results and the LRs of EUS (Table 2, model C; p = 0.92 and p = 0.45, respectively). There was also no significant relation between FDG-PET imaging and the LRs of EUS-FNA (Table 2, model B; p = 0.95). However, in the workup with FDG-PET and EUS-FNA, PET was strongly related to the LRs of CT imaging, but EUS-FNA was not (Table 2, model B; p = 0.02 and p = 0.50, respectively).
Optimal Preoperative Workup
In Fig. 1 it is already obvious that a staging scenario with a PET scan upfront followed by CT and EUS will yield the highest ratio of true-negative test outcomes of all one- and two-step strategies.
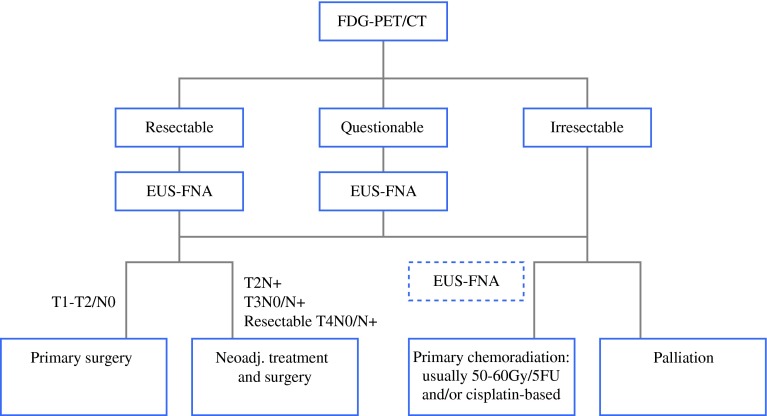
According to all of the above-described models, we composed an idealized protocol for optimal staging workup using EUS, CT, and FDG-PET on split levels for patients with clearly resectable, questionably resectable, and irresectable esophageal tumors (Fig. 3). In this flowchart, we recommend performance of PET/CT upfront in every patient, followed by EUS in those with clearly resectable disease to identify patients with locally advanced disease, as they may benefit from neoadjuvant chemoradiation before surgery. When there is disagreement about resectability with curative intent based on the location of suspect lymph nodes or because of tumor depth, we advise EUS-FNA for pathological examination of FDG-avid sites and/or suspicious lesions on PET/CT imaging in advanced, questionably resectable disease. In patients with primary irresectable disease that could possibly be managed curatively by definitive chemoradiation, EUS-FNA should be performed on indication. However, EUS can be omitted in patients with clearly incurable disease, so they can be referred immediately for palliative treatment.
Fig. 3.
Flowchart illustrating optimal staging protocol for patients with esophageal cancer on split levels for clearly resectable, questionably resectable, and irresectable esophageal tumors. 5FU 5-fluorouracil
Discussion
In this study a validated reformulated logistic regression approach was used to calculate the likelihood ratios of CT, FDG-PET, and EUS in order to determine the resectability with curative intent for different patient, tumor, and staging characteristics.17 Given the outcomes of one or two diagnostic tests, we were able to determine the value added by each test in the staging workup of esophageal cancer patients in predicting a dichotomous outcome (curative resectability versus irresectability). It was not possible to make subdivisions based on age, histological type, or tumor length in deciding whether to perform a test or not.
According to the results of this logistic regression approach, PET/CT has to be recommended as the first staging procedure, reserving EUS for limited cases and candidates with curable disease (Fig. 3). CT and FDG-PET outcomes strongly overlap and strengthen each other. FDG-PET reaches the highest LR+ when CT is negative (LR+ = 12.5–13.0), and vice versa the LR+ of CT reaches 4.4. EUS is insensitive with respect to resectability, with nonsignificant LRs when the results are expressed as a dichotomous outcome. This finding is not in line with the generally allotted role of EUS in esophageal cancer staging. One explanation might be that criteria based on nodal status and depth of tumor invasion alone are not strong enough to preclude surgical resection. Even though EUS is a powerful test for detecting lymph node metastases and tumor depth, these outcomes have almost no influence on decision-making when incurability/irresectability is the only parameter to be assessed. Only when EUS clearly identifies patients with a T4b tumor or cytologically proven nonregional nodes is it helpful for the exclusion of patients from potentially curative surgery. In the current study, only ten tumors (10/216; 5%) were considered as not curatively resectable on EUS as they were staged as T4b tumors. Usually the endoscopist will stage a tumor as T4 if he or she is clearly convinced of tumor invasion into surrounding structures precluding radical surgery. However, if invasion is not clear or is doubtful, the tumor will probably be staged as T3 and the patient, in most cases, will be offered neoadjuvant therapy as standard treatment. Based on these results, EUS has limited impact beyond PET/CT on staging advanced esophageal tumors in terms of curative resectability. EUS seems to be more valuable as an additional long-term prognostic factor rather than a potential predictor of irresectability at time of diagnosis.
Currently, neoadjuvant chemoradiation is being increasingly applied in the treatment of locally advanced esophageal cancer in an effort to improve microscopic radical resectability and survival by downstaging the tumor process and reducing local recurrence rates. In this way, staging has major consequences on treatment selection and also when comparing outcomes between studies and institutes. Furthermore, EUS is an invasive diagnostic procedure and not always applicable because of stenosis or use of a less accurate miniprobe in up to 30% of cases, which may lead to inadequate assessment of depth invasion and nodal staging.20 Moreover, in a previous study the perceived patient burden of EUS in assessment of the preoperative tumor stage was relatively high compared with CT and or PET/CT. Both EUS and FDG-PET have relatively good accuracy in restaging esophageal cancer after neoadjuvant therapy. Although both imaging methods have their limitations in assessing response to neoadjuvant chemoradiation, the accuracy rate of CT alone is poor.21
The more tests used in a preoperative staging program, the higher the chance of a correct outcome. However, one must balance likelihoods and certitude against costs, radiation, and inconvenience for the patient. Difficulties arise when test outcomes are contradictory. This study offers a new perspective on the performance of current diagnostic tests in the staging workup for esophageal cancer patients. It indicates the individual impact of each test on medical decision-making and the congruence between them. These results strongly argue for use of PET/CT as the first staging procedure, reserving EUS-FNA for those cases with uncertainty or disagreement about the location of positive lymph nodes (regional versus nonregional nodes) or tumor depth, which may affect curative resectability. Biopsies of FDG-avid sites at time of EUS will actually increase the yield of pathological proof from initial EUS without scheduling a separate EUS to prove irresectable disease.
Acknowledgment
This study was supported by a ZonMw program for Health Care Efficiency Research. We also thank Dr J. van Bastelaar for reviewing the final manuscript grammatically.
Conflict of interest
There is no conflict of interests and no financial benefit has been obtained from any company.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Messa C, Bettinardi V, Picchio M, et al. PET/CT in diagnostic oncology. Q J Nucl Med Mol Imaging. 2004;48:66–75. [PubMed] [Google Scholar]
- 2.Reinartz P, Wieres FJ, Schneider W, Schur A, Buell U. Side-by-side reading of PET and CT scans in oncology: which patients might profit from integrated PET/CT? Eur J Nucl Med Mol Imaging. 2004;31:1456–1461. doi: 10.1007/s00259-004-1593-y. [DOI] [PubMed] [Google Scholar]
- 3.Van Westreenen HL, Heeren PAM, van Dullemen HM, et al. Positron emission tomography with F-18-fluorodeoxyglucose in a combined staging strategy of esophageal cancer prevents unnecessary surgical explorations. J Gastrointest Surg. 2005;9:54–61. doi: 10.1016/j.gassur.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 4.Pera M, Pera M. Recent changes in the epidemiology of esophageal cancer. Surg Oncol. 2001;10:81–90. doi: 10.1016/S0960-7404(01)00025-1. [DOI] [PubMed] [Google Scholar]
- 5.Pera M, Manterola C, Vidal O, Grande L. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 6.Siesling S, van Dijck JA, Visser O, Coebergh JW. Working Group of The Netherlands Cancer Registry. Trends in incidence of and mortality from cancer in The Netherlands in the period 1989–1998. Eur J Cancer. 2003;39:2521–2530. doi: 10.1016/S0959-8049(03)00622-1. [DOI] [PubMed] [Google Scholar]
- 7.Steyerberg EW, Homs MYV, Stokvis A, Essink-Bot ML, Siersema PD. Stent placement or brachytherapy for palliation of dysphagia from esophageal cancer: a prognostic model to guide treatment selection. Gastrointest Endosc. 2005;62:333–340. doi: 10.1016/S0016-5107(05)01587-7. [DOI] [PubMed] [Google Scholar]
- 8.Clements DM, Bowrey DJ, Havard TJ. The role of staging investigations for oesophago-gastric carcinoma. Eur J Surg Oncol. 2004;30:309–312. doi: 10.1016/j.ejso.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Westerterp M, van Westreenen HL, Deutekom M, et al. Patients’ perception of diagnostic tests in the preoperative assessment of esophageal cancer. Patient Prefer Adherence. 2008;2:157–162. [PMC free article] [PubMed] [Google Scholar]
- 10.Flamen P, Lerut A, van Custem E, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg. 2000;120:1085–1092. doi: 10.1067/mtc.2000.110464. [DOI] [PubMed] [Google Scholar]
- 11.Heeren PA, Jager PL, Bongaerts F, van Dullemen H, Sluiter W, Plukker JT. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med. 2004;45:980–987. [PubMed] [Google Scholar]
- 12.Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133–1136. doi: 10.1016/S0003-4975(99)00974-1. [DOI] [PubMed] [Google Scholar]
- 13.van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–3812. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Shalom R, Guralnik L, Tsalic M, et al. The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2005;32:918–924. doi: 10.1007/s00259-005-1795-y. [DOI] [PubMed] [Google Scholar]
- 15.Jadvar H, Henderson RW, Conti PS. 2-Deoxy-2-[F−18]fluoro-d-glucose-positron emission tomography/computed tomography imaging evaluation of esophageal cancer. Mol Imaging Biol. 2006;8:193–200. doi: 10.1007/s11307-006-0036-5. [DOI] [PubMed] [Google Scholar]
- 16.Munden RF, Macapinlac HA, Erasmus JJ. Esophageal cancer: The role of integrated CT-PET in initial staging and response assessment after preoperative therapy. J Thorac Imaging. 2006;21:137–145. doi: 10.1097/00005382-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Janssens AC, Deng Y, Borsboom GJ, Eijkemans MJ, Habbema JD, Steyerberg EW. A new logistic regression approach for the evaluation of diagnostic test results. Med Decis Making. 2005;25:168–177. doi: 10.1177/0272989X05275154. [DOI] [PubMed] [Google Scholar]
- 18.Van Westreenen HL, Westerterp M, Sloof GW, et al. Limited additional value of positron emission tomography in staging oesophageal cancer. Br J Surg. 2007;94:1515–1520. doi: 10.1002/bjs.5708. [DOI] [PubMed] [Google Scholar]
- 19.Janssens AC, Steyerberg EW, Jiang Y, Habbema JD, Van Duijn CM, Criswell LA. Value of the HLA-DRB1 shared epitope for predicting radiographic damage in rheumatoid arthritis depends on the individual patient risk profile. J Rheumatol. 2006;33:2383–2389. [PubMed] [Google Scholar]
- 20.Wallace MB, Hawes RH, Sahai AV, Van Velse A, Hoffman BJ. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc. 2000;51:309–313. doi: 10.1016/S0016-5107(00)70360-9. [DOI] [PubMed] [Google Scholar]
- 21.Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy—systematic review. Radiology. 2005;236:841–851. doi: 10.1148/radiol.2363041042. [DOI] [PubMed] [Google Scholar]