Abstract
Previous reports have suggested that herpes simplex virus type 1 (HSV-1) immediate-early regulatory protein ICP0 stabilizes cyclins D1 and D3 during infection by inducing the degradation of cdc34, the E2-conjugating enzyme that is responsible for regulating the stability of these cyclins. Since ICP0 has complex effects on the progress of viral infection that vary greatly with cell type and viral dose, it can be difficult to distinguish between direct effects caused by ICP0 itself and indirect effects caused by the rate of the progression of infection in the absence of ICP0 at the chosen multiplicity of infection. This report describes the fates of cdc34 and cyclins D1 and D3 during HSV-1 infection under conditions that ensured that viral infection and gene expression were proceeding at equivalent rates in the presence and absence of ICP0. It was confirmed that both D-type cyclins were unstable during HSV-1 infection of a variety of cell types, but no effect on cdc34 was observed, even when high levels of ICP0 were expressed. Furthermore, there was no evidence that ICP0 protected either cyclin D1 or cyclin D3 from degradation. Reconstruction of the conditions of the experiments in the previous studies, using the stated cell type and multiplicities of infection, indicated that the original results could be explained by differences in the rate of progression of infection rather than by the presence or absence of ICP0. The data presented in this report are incompatible with the hypothesis that ICP0 induces the degradation of cdc34 and thereby stabilizes cyclins D1 and D3 during HSV-1 infection.
It has been established by several extensive and varied studies that herpes simplex virus type 1 (HSV-1) immediate-early (IE) protein ICP0 plays an important role in the regulation of the balance between lytic and latent viral infection (reviewed in references 2, 7, 10, and 11). If ICP0 is not expressed or carries a mutation in key functional domains, HSV-1 has a much reduced probability of initiating a lytic infection and instead frequently establishes infections that become stalled at the IE phase or are quiescent (see reference 3 and references therein). The presence of active ICP0 greatly increases commitment to lytic infection and can lead to reactivation of quiescent or latent viral genomes (reviewed in reference 10). Therefore, ICP0 is involved in the regulation of a property that is of critical importance for the biology and clinical impact of HSV-1. This insight has stimulated many studies into the molecular mechanisms by which ICP0 influences the outcome of HSV-1 infection. These investigations have revealed an increasingly complex picture of the interactions of ICP0 with viral and cellular proteins and the effects of ICP0 on cellular pathways, structures, and proteins. These issues have been discussed in detail in a number of recent papers (3, 4, 8).
While the potential biochemical properties of ICP0 have been studied in some depth, their consequences and relevance for the biology of HSV-1 infections have remained more elusive. One problem lies in the multiplicity of infection (MOI) and cell type dependence of the phenotype of ICP0-null mutant HSV-1 viruses, such as dl1403 (14). It has long been known that the defect of dl1403 and similar viruses can be overcome by using a high MOI and that the extent of the defect varies between cell types. For example, ICP0 is not required at all for efficient infection of U2OS cells (18), while dl1403 forms plaques about 1,000-fold less efficiently than wild-type viral strains in human fibroblast cells passaged a limited number of times. In a recent detailed study, it was observed that viral gene expression and replication of dl1403 were subject to a broad threshold of input genome load, such that at input MOIs below the threshold, dl1403 had a high probability of establishing a stalled or quiescent infection in human fibroblast cells, while dl1403 infections initiated above the threshold produced levels of viral gene expression and progeny virus that were equivalent to a wild-type virus infection with the same number of input viral genomes (3). In other words, above the threshold, the infection is ICP0 independent, and the rate of progression of infection depends on viral genome load, while below the threshold, the infection becomes ICP0 dependent, and wild-type and ICP0-negative viral infections with equivalent viral genome loads do not have the same outcome.
This broad threshold MOI for the dependence on ICP0 for the efficiency of HSV-1 infection varies with cell type, and its apparent value is dependent on the cell type in which the titer of the ICP0-null mutant virus was determined. Since ICP0 mutant viruses do not exhibit a defect in U2OS cells, titers of wild-type and ICP0-null mutant viruses in this cell type give very similar particle-to-PFU (or viral genome-to-PFU) ratios (3, 18). Thus, if infections of other cell types are initiated on the basis of viral titers determined in U2OS cells, input viral genome numbers of wild-type and ICP0-null mutant viruses will be equivalent. If such infections are designed on the basis of virus titers determined in other cell types, input viral genome numbers in wild-type and ICP0-null mutant virus infections may differ substantially, with corresponding differences in the rates of progression of infection and effects on the cell.
This paper reports the fates of cyclin D1 and cyclin D3 and the cellular ubiquitin-conjugating enzyme cdc34 (UbcH3) during wild-type and ICP0-null mutant virus infections under conditions of equalized input viral genome numbers and equivalent rates of progression of infection. The background to this study is the original observation that ICP0 interacts with cyclin D3 (9). On the basis that cyclin D3 was degraded apparently more rapidly in ICP0-null mutant HSV-1 infections than in wild-type HSV-1 infections, it was concluded that cyclin D3 was stabilized through its interaction with ICP0, an observation supported by the properties of a mutant virus that expresses a form of ICP0 that fails to bind to cyclin D3 (16). Later it was reported that cyclin D1 was also more stable in ICP0 mutant HSV-1 infections than in wild-type HSV-1 infections, although in this case cyclin D1 did not interact with ICP0 (17). An explanation for the stabilization of both of these D-type cyclins while only one interacted with ICP0 was proposed after it was found that a glutathione S-transferase (GST) fusion protein containing a portion of ICP0 from near its C-terminal end stimulated autoubiquitination of the E2 ubiquitin-conjugating enzyme cdc34 in vitro (15) and that cdc34 was apparently less stable in HSV-1-infected cells (6, 8). Since one role of cdc34 is to ubiquitinate and therefore control the stability of cyclins D1 and D3, ICP0-induced loss of cdc34 would lead to stabilization of these cyclins. However, the relative rates at which cellular proteins are degraded during wild-type and ICP0-null mutant HSV-1 infections will depend on the proportion of cells that are infected and the rate of development of these infections. The present study was initiated in order to test the hypotheses that the rates of degradation of cyclins D1 and D3 were affected directly by ICP0, and not indirectly by its role in stimulating viral infection, and that any degradation of cdc34 was concordant with an effect on stability of cyclins D1 and D3. The fact that other laboratories have shown that cyclins D1 and D3 are efficiently degraded during HSV-1 infection, even in the presence of ICP0 (1, 13), creating an apparent conflict in the literature, is pertinent.
MATERIALS AND METHODS
Viruses and cells.
Parental virus HSV-1 strain 17 syn+ and ICP0 deletion mutant dl1403 (14) were grown in BHK cells and titrated in U2OS cells; both viruses were used to infect other cell types at the stated MOIs based on their titers in U2OS cells. Virus in1411 has an insertion mutation that inactivates expression of ICP4 (12). Viral stocks were prepared from BHK cells infected at low MOIs or from the complementing BHK M49 cell line in the case of in1411. When the cells exhibited extensive cytopathic effect, the medium was harvested, clarified by low-speed centrifugation, and stored at 4°C, and the titer of the virus was determined regularly. Virus stocks prepared in this manner were stable for several weeks. Analysis of several independent stocks of strain 17 and dl1403 prepared by this method gave reproducibly similar particle-to-PFU ratios (calculated on the basis of PFU titers in U2OS cells), with the averages being 58 and 32, respectively (3). These values are consistent with the results of others (18). The dl1403 stock used in these experiments had a particle-to-PFU ratio of 20, a value essentially identical to those quoted above, given the experimental variability in these determinations. BHK cells were grown in Glasgow modified Eagle's medium containing 100 U of penicillin per ml and 100 μg of streptomycin per ml and supplemented with 10% newborn calf serum and 10% tryptose phosphate broth. Vero and U2OS cells were grown in Glasgow modified Eagle's medium or Dulbecco's modified Eagle's medium, respectively, supplemented with 10% fetal calf serum and antibiotics as described above. HEp-2 cells were grown in the same medium as U2OS cells. Human fetal foreskin fibroblast cells (HFFF-2; European Collection of Cell Cultures) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 1% glutamine, and antibiotics as described above.
FACS analysis.
Cells in 35-mm-diameter dishes were infected at the appropriate MOI and then harvested for fluorescence-activated cell sorter (FACS) analysis 6 h after virus absorption. The method used and the sources of the antibodies to detect ICP4 and ICP0 were as described previously (3).
Infections and Western blots.
Whole-cell extracts of transfected cells were prepared by washing cell monolayers with phosphate-buffered saline (PBS) and then lysing the cells in an appropriate volume of sodium dodecyl sulfate-polyacrylamide gel boiling mix. After the mixture was boiled, the proteins were separated by electrophoresis on sodium dodecyl sulfate-7.5% polyacrylamide gels and electrophoretically transferred to nitrocellulose filters, which were then divided into three segments containing proteins in the apparent molecular mass ranges of below 46, 46 to 95, and greater than 95 kDa. The filters were blocked overnight in PBS containing 0.1% Tween 20 and 5% dried milk and then incubated with primary antibodies for 2 h in the same buffer. After extensive washing of the filters, they were incubated with horseradish peroxidase-conjugated secondary antibody in a solution containing PBS, 0.1% Tween 20, and 2% dried milk for 2 h and then washed again extensively before detection of bound antibody by enhanced chemiluminescence (NEN) and exposure to film. The low-molecular-weight portion of the filter was probed sequentially for cdc34, cyclin D3, and cyclin D1. The middle portion was probed for viral DNA replication protein UL42, and the high-molecular-weight portion was probed sequentially for viral IE proteins ICP4 and ICP0. ICP4, ICP0, and UL42 were detected using monoclonal antibodies 10176, 11060, and Z1F11, respectively (3). Anti-cyclin D1 monoclonal (sc-20044) and anti-cyclin D3 rabbit polyclonal antibodies (sc-182) were obtained from Santa Cruz Biotechnology. Anti-cdc34 monoclonal antibody C25820 was obtained from BD Transduction Laboratories.
RESULTS AND DISCUSSION
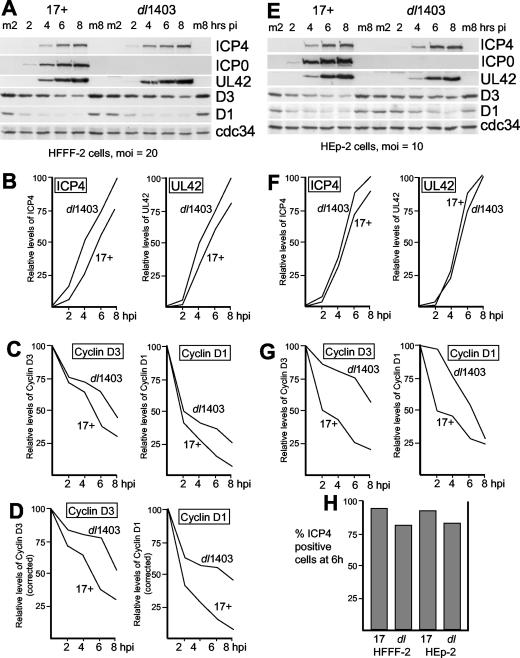
To assess the effect of ICP0 on the rate of degradation of cyclins D1 and D3 during HSV-1 infection, stocks of wild-type HSV-1 strain 17 and the ICP0-null mutant dl1403 were titrated in U2OS cells and then used to infect HFFF-2 human fibroblasts. A MOI of 20 PFU per cell was selected, as this is above the threshold required for dl1403 to initiate a productive infection as efficiently as wild-type virus in this cell type (3). Because strain 17 and dl1403 have similar particle-to-PFU ratios in U2OS cells, the viral genome loads applied in the two infections were similar. Parallel samples were harvested at 2, 4, 6, and 8 h after a 1-h absorption period, with mock-infected samples prepared at the 2-h and 8-h time points. FACS analysis of parallel cultures indicated that 93 and 80% of the cells were infected and expressing ICP4 in HSV-1 strain 17- and dl1403-infected cells, respectively (Fig. 1H). Western blot analysis of the cell extracts (Fig. 1A) illustrated that the kinetics of expression of ICP4 and UL42 were very similar in strain 17 and dl1403 infections, confirming that the infections were developing at equivalent rates (Fig. 1B).
FIG. 1.
Effects of HSV-1 infection on the levels of cdc34 and cyclins D1 and D3 in HFFF-2 and HEp-2 cells. (A) Samples harvested at 2, 4, 6, and 8 h after infection of HFFF-2 cells with HSV-1 strain 17 and dl1403 at a MOI of 20 (based on U2OS cell titers). Total cell proteins were separated by electrophoresis and transferred to nitrocellulose filters, and then the levels of viral proteins ICP4, ICP0, and UL42, and cellular proteins cyclin D3, cyclin D1, and cdc34 were detected using appropriate antibodies. Mock-infected cell samples harvested at the 2-h and 8-h time points were analyzed in parallel (m2 and m8, respectively). pi, postinfection. (B) The band intensities of viral proteins ICP4 and UL42 in cells infected with HSV strain 17 and dl1403 were determined by densitometry. The intensity of the ICP4 band at 8 h in dl1403-infected cells was set at a value of 100; the relative levels of ICP4 in cells infected with strain 17 and dl1403 at all four time points were determined in relation to this value. The same procedure was used to determine the relative levels of UL42 expression. hpi, hours postinfection. (C) The average intensities of the bands from the mock-infected cyclin D1 and cyclin D3 lanes were calculated; the levels of the cyclins were calculated as a percentage of this value. (D) The percentage levels of cyclins D3 and D1 in panel C were recalculated taking into account the slightly greater levels of ICP4 and UL42 expressed in dl1403-infected cells. (E) Same as panel A, except that HEp-2 cells and a MOI of 10 PFU per cell were used. (F and G) Same as panels B and C except the results presented in panel E are analyzed. (H) The percentages of cells that were infected and expressing ICP4 in all four infection conditions were determined by FACS analysis of parallel samples harvested at 6 h. 17, HSV-1 strain 17; dl, dl1403.
Probing the relevant portion of the same filter for cyclin D3 and cyclin D1 revealed that the two cyclins were indeed degraded during HSV-1 infection, with cyclin D1 being less stable (Fig. 1A), but under these conditions, their rates of degradation were greater in cells infected with strain 17 than in cells infected with dl1403 (Fig. 1C). These data indicate that ICP0 does not stabilize the two cyclins during HSV-1 infection of HFFF-2 cells. Indeed, if the data were corrected for the slightly greater rate at which this particular dl1403 infection was progressing compared to that of strain 17 (Fig. 1B), the two cyclins appeared to be degraded at approximately twice the rate in the presence of ICP0 than in its absence (Fig. 1D). The levels of cdc34 remained stable throughout the experiment in both infections (Fig. 1A).
To determine whether these results were due to a property of ICP0 expressed by HSV-1 strain F (the strain used in the previous studies) that was not replicated by ICP0 of strain 17, the experiments were repeated to compare the fate of the cellular proteins during strain 17 and strain F infections of HFFF-2 cells, using the conditions described above. The results demonstrated that the infections with the two strains were essentially identical in terms of rates of expression of viral proteins, degradation of cyclins D1 and D3, and lack of effect on cdc34 (data not shown).
To determine whether this result was a cell type-specific phenomenon, the experiment was repeated in HEp-2 cells (MOI of 10), with very similar results (Fig. 1E to G). In these parallel infections, the kinetics of viral gene expression were similar (Fig. 1F), and again cyclins D1 and D3 were less stable in HSV-1 strain 17 infection than in dl1403 infection (Fig. 1G). As in HFFF-2 cells, the levels of cdc34 were not significantly affected in either infection (Fig. 1E). HFFF-2 and HEp-2 cells were not unusual with respect to the stability of cdc34 and the lack of stabilization of cyclins D3 and D1 by ICP0 during HSV-1 infection, since similar results were obtained in equalized high-multiplicity infections of U2OS and Vero cells (data not shown).
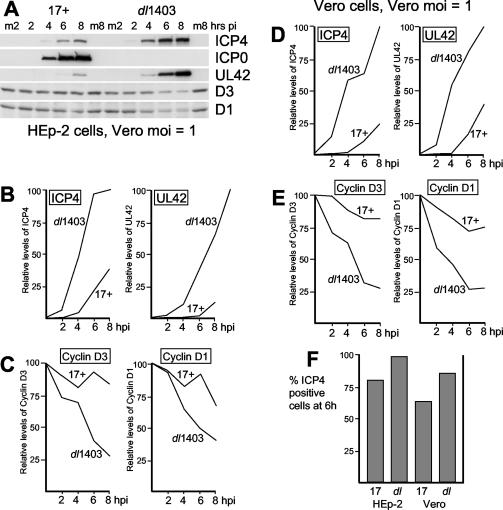
That the results in Fig. 1 lead to a conclusion so opposed to that in previous studies poses the question of how such a situation could arise. In the original study (9), Vero cells were infected with a wild-type virus and an ICP0-null mutant virus at a MOI of 0.5 PFU per cell, with the titers of both viruses determined in Vero cells (9). Under these conditions, cyclin D3 was lost from the cells infected by the ICP0-null mutant virus more rapidly than in the wild-type virus infection. This MOI would be insufficient for the wild-type virus to infect all the cells in the population. However, since the ICP0-null mutant has a 20- to 30-fold reduction in plaque-forming efficiency in Vero cells compared to that in U2OS cells (3, 18), the actual MOI of the mutant would have been around 10 to 15 PFU per cell, based on U2OS cell titers, whereas the wild-type viral MOI would have been around 0.25 U2OS PFU per cell (because wild-type HSV-1 plates approximately twofold more efficiently on Vero cells than on U2OS cells [3]). This amount of an ICP0-null mutant virus is far above the threshold MOI at which an ICP0-null mutant virus infects Vero cells efficiently (3), and the viral genome load per infected cell would have been much higher than that in the parallel wild-type HSV-1 infection. Therefore, it would be expected that the mutant virus infection would proceed more efficiently than the wild-type control under these conditions. A similar consideration applies to later studies on both cyclin D1 and cyclin D3 (16, 17). None of these three earlier studies presented data on the relative progress of the infections by detection of viral early proteins.
To reconcile the differences between the present and previous studies, infections were performed by reconstructing the conditions used in the earlier work. Vero and HEp-2 cells were infected with HSV-1 strain 17 and dl1403 at a MOI of 1 based on their titers in Vero cells. FACS analysis indicated that 6 h after infection, 63% of the HEp-2 cells in the cell population infected by HSV-1 strain 17 expressed ICP4, compared to 84% of cells in the dl1403-infected sample (Fig. 2F), and Western blot analysis showed that ICP4 and UL42 were accumulating at much higher rates in the dl1403-infected cells than in strain 17-infected cells (Fig. 2A and B). Concomitantly, the rates of degradation of cyclins D1 and D3 were now much lower in cells infected with strain 17 than in cells infected with dl1403 (Fig. 2C). Very similar results were obtained in Vero cells infected by the same regimen (Fig. 2D to F). These data clearly show that the results of the original studies can be reproduced using similar infection protocols but that the apparent effect of ICP0 in stabilizing cyclins D1 and D3 is an artifact caused by the unequal rates of progression of infection that result from input viruses being equalized on the basis of their titers in Vero cells. We conclude that cyclins D1 and D3 are degraded in response to HSV-1 infection but that ICP0 does not stabilize either of them.
FIG. 2.
Effects of HSV-1 infection on the levels of cdc34 and cyclins D1 and D3 in HEp-2 cells and Vero cells, infected at MOIs of strain 17 and dl1403 based on their titers in Vero cells. (A) Samples harvested at 2, 4, 6, and 8 h after infection of HEp-2 cells with HSV-1 strain 17 and dl1403 at a MOI of 1 (based on Vero cell titers). Total cell proteins were separated by electrophoresis and transferred to nitrocellulose filters, and then the levels of viral proteins ICP4, ICP0, and UL42, and cellular proteins cyclin D3 and cyclin D1 were detected using appropriate antibodies. Mock-infected cell samples harvested at the 2-h and 8-h time points were analyzed in parallel (m2 and m8, respectively). pi, postinfection. (B) The band intensities of viral proteins ICP4 and UL42 in cells infected with strain 17 and dl1403 were determined by densitometry. The intensity of the ICP4 band at 8 h in dl1403-infected cells was set at a value of 100; the relative levels of ICP4 in cells infected with strain 17 and dl1403 at all four time points were determined in relation to this value. The same procedure was used to determine the relative levels of UL42 expression. hpi, hours postinfection. (C) The average intensity of the bands from the mock-infected cyclin D1 and cyclin D3 lanes was calculated; the levels of the cyclins were calculated as a percentage of this value. (D and E) Same as panels B and C, except that Vero cells were infected. (F) The percentages of cells that were infected and expressing ICP4 in all four infection conditions were determined by FACS analysis of parallel samples harvested at 6 h. 17, HSV-1 strain 17; dl, dl1403.
In a more recent study from the laboratory that conducted the original experiments, the ICP0 mutant virus was used at MOIs based on its U2OS titer, and the wild-type virus was used at MOIs based on its Vero cell titer (6). Because wild-type virus forms plaques approximately twofold more efficiently on Vero cells than on U2OS cells (3), the effective MOI of the ICP0 mutant virus would have been approximately twice that of the wild-type virus. Used at a MOI of 0.5 in Vero cells (which is close to the threshold MOI above which an ICP0-null mutant virus replicates efficiently in this cell type [3]), the wild-type and ICP0 deletion viruses appeared to cause similar losses of cyclin D3 6 h postinfection (6), consistent with the analysis described above. It is true that these researchers found differences in the retained cyclin D3 in the cells infected with wild-type and ICP0-null mutant virus 10 h postinfection, but in the experiment presented, the levels of cyclin D3 did not vary linearly with time in the wild-type virus-infected cells; the cyclin D3 level 10 h after infection was much higher than that at 6 h after infection (6).
The results of the present study indicating that the levels of cdc34 remain stable during HSV-1 infection of HFFF-2 and HEp-2 cells are also relevant to this analysis. A GST fusion protein containing a segment of ICP0 from the C-terminal third of the protein was shown to stimulate autoubiquitination of cdc34 in vitro (15), and later it was proposed that this activity led to a decrease in cdc34 levels during HSV-1 infection (8). Degradation of cdc34 could result in stabilization of cyclins D1 and D3, since cdc34 is involved in the normal turnover of these cyclins. Examination of the previously published data shows that, while cdc34 levels were higher in human fibroblast cells infected with wild-type virus in the presence of the proteasome inhibitor MG132 than in the absence of MG132, in the absence of the drug the total levels of cdc34 in infected and mock-infected cells were similar (8). This result is consistent with the data presented here, showing no evidence of a reduction in the amounts of cdc34 during normal HSV-1 infection of the cell types we have tested. Although a later study reported that cdc34 was lost in an ICP0-dependent manner during infection of SK-N-SH cells at a MOI of 10 (6), this does not necessarily reflect the situation in other cell types, particularly since the same laboratory found that SK-N-SH cells express exceptional levels of ICP0, even in very low-multiplicity infections (5).
A relevant point is that if the stabilities of cdc34, cyclin D1, and cyclin D3 are linked during HSV-1 infection, then loss of cdc34 should precede or at least be coordinate with any difference in the stabilities of cyclins D1 and D3 in wild-type and ICP0-null mutant viral infections, analyzed in the same samples from infections of the same cell type. The previous studies did not conduct the type of parallel analysis presented here that monitors the levels of all relevant proteins in the same samples. Furthermore, the observation that cdc34 remained stable during HSV-1 infection of HFFF-2 and HEp-2 cells (Fig. 1) was reproduced in both U2OS and Vero cells (data not shown); no evidence for HSV-1-induced loss or modification of cdc34 was observed in the presence or absence of ICP0 in any of the four cell types.
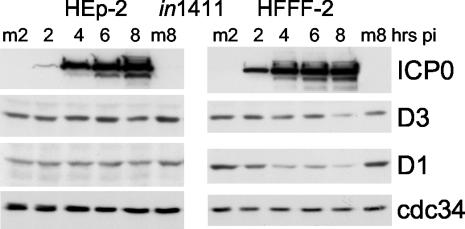
To determine whether the amounts of ICP0 expressed in HFFF-2 and HEp-2 cells during a normal infection with HSV-1 strain 17 are insufficient to induce degradation of cdc34, these cell types were infected with ICP4-null mutant virus in1411 at a MOI that gave 93 and 88% ICP0-positive infected cells, respectively, in the two cell types. Densitometry of the ICP0 bands in cells infected with in1411 compared to cells infected with strain 17 analyzed in parallel (in which the percentages of infected cells were similar) indicated that at least five times more ICP0 was expressed in the cells infected with in1411 than in the cells infected with strain 17 (data not shown), a result of the absence of ICP4 leading to overexpression of the other viral IE proteins.
Despite the very high levels of ICP0 being expressed, there was no evidence for degradation of cdc34 in either cell type (Fig. 3), a result inconsistent with the hypothesis that ICP0 promotes degradation of cdc34 (6, 8). The results with cyclins D1 and D3 in this experiment differed between the two cell types. While cyclin D3 levels decreased slightly during in1411 infection of HFFF-2 cells and the levels of cyclin D1 decreased to a greater extent in the parallel infected HEp-2 cells, both proteins remained stable, despite equivalent levels of ICP0 being expressed (Fig. 3). Therefore, the greater rates of degradation of cyclins D1 and D3 during strain 17 infection of HEp-2 cells, compared to those in the dl1403 infection (Fig. 1E and G) are unlikely to be due directly to an activity of ICP0 itself. Since the results presented in Fig. 1 have established that ICP0 stabilizes neither cyclin D1 nor cyclin D3, comparison of strain 17, dl1403, and in1411 infections (Fig. 1 and 3) demonstrates that the rate of degradation of these cyclins is increased in cells that are productively infected and does not correlate with ICP0 expression levels.
FIG. 3.
Effects of HSV-1 infection in the absence of ICP4 on the levels of cdc34 and cyclins D1 and D3 in HEp-2 cells and HFFF-2 cells. Samples harvested at 2, 4, 6, and 8 h after the indicated cell type was infected with in1411. Total cell proteins were separated by electrophoresis and transferred to nitrocellulose filters, and then the levels of viral protein ICP0 and cellular proteins cdc34, cyclin D3, and cyclin D1 were detected using appropriate antibodies. Reprobing of the filter demonstrated that neither ICP4 nor UL42 were expressed in these infections (not shown). Mock-infected cell samples harvested at the 2-h and 8-h time points were analyzed in parallel (m2 and m8, respectively). pi, postinfection.
In conclusion, the results presented here do not confirm the previous observations and suggested implications for the effects of ICP0 on cdc34, cyclin D1, and cyclin D3 during HSV-1 infection. Levels of cdc34 were unaffected by even high levels of ICP0 in several cell types, and even long exposures of the filters of Fig. 1 and 3 did not detect the appearance of the modified forms of cdc34 during infection that were predicted from in vitro studies using an ICP0 segment fused to GST (8, 15). While the possible fate of cdc34 in SK-N-SH cells (6) was not addressed here, the levels of cdc34 remained stable in HFFF-2, HEp-2, Vero, and U2OS cells (Fig. 1 and data not shown). The data are not consistent with the hypothesis that ICP0 stabilizes cyclins D1 and D3 by any mechanism during HSV-1 infection, but an explanation has been provided to account for the published discrepancies. The fact that the defect of ICP0-null HSV-1 viruses varies in a nonlinear way between different cell types and that experimental outcomes depend so crucially on how input MOIs are determined and selected creates opportunities for artifacts that may have been overlooked in all good faith in certain earlier studies. The approach described here may prove useful in exploring the biological relevance of other reported activities of ICP0.
Acknowledgments
This work was supported by the Medical Research Council.
REFERENCES
- 1.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 267:335-349. [DOI] [PubMed] [Google Scholar]
- 2.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 3.Everett, R. D., C. Boutell, and A. Orr. 2004. The phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 78:1763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everett, R. D., G. Sourvinos, C. Leiper, J. B. Clements, and A. Orr. 2004. The formation of nuclear foci of herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes. J. Virol. 78:1903-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagglund, R., and B. Roizman. 2003. Herpes simplex virus 1 mutant in which the ICP0 HUL-1 E3 ubiquitin ligase site is disrupted stabilizes cdc34 but degrades D-type cyclins and exhibits diminished neurotoxicity. J. Virol. 77:13194-13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 78:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 11.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa. [Google Scholar]
- 12.Russell, J., E. C. Stow, N. D. Stow, and C. M. Preston. 1987. Abnormal forms of the herpes simplex virus immediate early polypeptide Vmw175 induce the cellular stress response. J. Gen. Virol. 68:2397-2406. [DOI] [PubMed] [Google Scholar]
- 13.Song, B., J. J. Liu, K. C. Yeh, and D. M. Knipe. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326-334. [DOI] [PubMed] [Google Scholar]
- 14.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 15.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Sant, C., Y. Kawaguchi, and B. Roizman. 1999. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc. Natl. Acad. Sci. USA 96:8184-8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Sant, C., P. Lopez, S. J. Advani, and B. Roizman. 2001. Role of cyclin D3 in the biology of herpes simplex virus 1 ICP0. J. Virol. 75:1888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]