Abstract
At least four members of the integrin family of receptors, αVβ1, αVβ3, αVβ6, and αVβ8, have been identified as receptors for foot-and-mouth disease virus (FMDV) in vitro. Our investigators have recently shown that the efficiency of receptor usage appears to be related to the viral serotype and may be influenced by structural differences on the viral surface (H. Duque and B. Baxt, J. Virol. 77:2500-2511, 2003). To further examine these differences, we generated soluble αVβ3 and αVβ6 integrins. cDNA plasmids encoding the individual complete integrin αV, β3, and β6 subunits were used to amplify sequences encoding the subunits' signal peptide and ectodomain, resulting in subunits lacking transmembrane and cytoplasmic domains. COS-1 cells were transfected with plasmids encoding the soluble αV subunit and either the soluble β3 or β6 subunit and labeled with [35S]methionine-cysteine. Complete subunit heterodimeric integrins were secreted into the medium, as determined by radioimmunoprecipitation with specific monoclonal and polyclonal antibodies. For the examination of the integrins' biological activities, stable cell lines producing the soluble integrins were generated in HEK 293A cells. In the presence of divalent cations, soluble αVβ6 bound to representatives of type A or O viruses, immobilized on plastic dishes, and significantly inhibited viral replication, as determined by plaque reduction assays. In contrast, soluble αVβ3 was unable to bind to immobilized virus of either serotype; however, virus bound to the immobilized integrin, suggesting that FMDV binding to αVβ3 is a low-affinity interaction. In addition, soluble αVβ3 did not neutralize virus infectivity. Incubation of soluble αVβ6 with labeled type A12 or O1 resulted in a significant inhibition of virus adsorption to BHK cells, while soluble αVβ3 caused a low (20 to 30%), but consistent, inhibition of virus adsorption. Virus incubated with soluble αVβ6 had a lower sedimentation rate than native virus on sucrose density gradients, but the particles retained all of their structural proteins and still contained bound integrin and, therefore, were not exhibiting characteristics of a picornavirus A particle.
Foot-and-mouth disease virus (FMDV), the etiologic agent of foot-and-mouth disease (FMD), is the type species of the Aphthovirus genus of the Picornaviridae. FMD is a devastating disease of livestock which results in severe economic consequences to affected countries (3, 85). The wide range of species which are affected by the disease, as well as differences in the severity of symptoms between species, has focused attention on host and viral factors involved in viral pathogenesis. In addition, the recent “reemergence” of the disease in developed countries, along with an inability to effectively control the disease in developing countries, highlights the need to explore new technologies involving both vaccines and antiviral agents.
Of the possible host factors involved in picornaviral pathogenesis, the viral receptor plays a major role in both host and tissue tropism (21, 74, 79). FMDV binds to cells through a conserved Arg-Gly-Asp (RGD) sequence located within a flexible external loop between the βG and βH strands (G-H loop) of viral protein 1 (VP1) (11, 23, 49, 57, 59). The RGD tripeptide is a recognition sequence for a number of members of the integrin family of cell surface receptors (77), and FMDV utilizes four members of the αV subgroup of integrins (αVβ1, αVβ3, αVβ6, and αVβ8) as viral receptors in vitro (16, 20, 39, 41, 43, 64, 65). Integrins are heterodimeric type I membrane proteins, consisting of two subunits (α and β), which dimerize noncovalently at the cell surface (35-37). Of the 24 known integrins, only 8 (αVβ1, αVβ3, αVβ5, αVβ6, αVβ8, α5β1, α8β1, and αIIbβ3) utilize the RGD recognition sequence to interact with their natural ligands (77).
Soluble viral receptors have played a pivotal role in studies of picornavirus early virus-cell interactions due to their ability to interact with virus directly in the absence of the cell membrane. Soluble poliovirus (PV) receptor was first shown to interact with and neutralize PV infectivity in vitro (45, 46). Since these early studies, the structures of receptor-bound PV (14, 28, 31), major and minor group human rhinoviruses (HRV) complexed with intercellular adhesion molecule 1 and the very low density lipoprotein receptor, respectively, have been solved (32, 47, 92). In addition the structures of virus-receptor complexes of swine vesicular disease virus with the coxsackievirus-adenovirus receptor (24), coxsackievirus B3 (CBV3) with the coxsackievirus-adenovirus receptor (29), and echovirus 7 with decay accelerating factor (30) have also been reported. Soluble picornavirus receptors have allowed detailed examination of receptor affinities among different groups of viruses (30, 48, 58, 93) and functional domains of viral receptors (13, 17, 52, 53, 69, 82) and also allowed analysis of picornaviral receptor-dependent conformational alterations that occur during internalization and uncoating (2, 26, 67, 83, 90).
In addition to FMDV, the parechoviruses echovirus 9 and coxsackievirus A9 (CAV9) utilize αV integrin receptors to infect cells via exposed RGD sequences on the viral capsid (44, 66, 70, 75, 88, 89), and echoviruses 1 and 8 utilize a non-RGD-recognizing integrin (α2β1) to infect cells (15). However, even though soluble forms of the human homologs of αVβ3 and αVβ6 have been shown to possess binding activities for their natural ligands (61, 91), there have been no studies of the interactions of the αV integrin-recognizing picornaviruses with soluble integrin heterodimers. It has been demonstrated that FMDV binds to isolated purified αVβ3 and α5β1 integrins (38, 42); however, the latter integrin is not a viral receptor in vitro (65). The recently reported crystallographic three-dimensional structure of soluble human αVβ3 (94, 95) suggests that structural studies of FMDV-integrin interactions are feasible. In addition, since the virus appears to be able to utilize at least four different αV integrins (see above), it would be useful to examine the affinities of different serotypes and clinical isolates for each of the integrins to determine their roles in infection of susceptible hosts and to determine the role (if any) of these receptors in viral uncoating. In this work, we have generated soluble secreted forms of the bovine αVβ3 and αVβ6 integrins and examined their interactions with representatives of two FMDV serotypes. Surprisingly, we found that even though the viruses can utilize both receptors to mediate infection of cells in culture, they appear to interact very poorly with the soluble secreted αVβ3 integrin, while the αVβ6 integrin is able to bind to virus and neutralize infectivity.
MATERIALS AND METHODS
Viruses, cells, and antibodies.
FMDV type A12 strain 119ab (A12) was derived from the infectious cDNA clone pRMC35 (73). A derivative of this virus with an NP sequence inserted in place of the GVRGDF sequence in the G-H loop (A12-RGD−), which can neither infect cells in culture nor cause disease in susceptible animals, has been described previously (59). FMDV type A24 Cruziero (A24) was recovered in vesicular fluid from a foot lesion of a steer experimentally inoculated with virus intradermally into the tongue. The virus was used directly and not passaged in tissue culture. Two type O1 Campos (O1Camp) variants, derived from infectious cDNAs containing capsid sequences represented in a vaccine seed stock from Brazil, have been described (78). These viruses are designated O1C3056H (referred to as vCRM8 in reference 78), a highly cattle-virulent virus which is unable to bind to heparin-Sepharose (78) and utilizes integrin receptors to infect cells (20, 64, 65), and O1C3056R (referred to as vCRM4 in reference 78), a cattle-avirulent virus which binds to heparin-Sepharose (78) and infects cells expressing heparan sulfate (HS) on the membrane in the absence of integrin receptors (65). A derivative of O1C3056R with an RGD→KGE mutation in the G-H loop, O1C3056R-KGE (referred to as vCRM48-KGE in reference 65) was derived by site-directed mutation and has been described elsewhere (65). This virus can also infect cells expressing HS in the absence of integrin receptors (65). A genetically engineered type A12 virus chimera containing the G-H loop from type O1BFS (A/O) has been described previously (71). COS-1 cells were maintained in Dulbecco's minimal essential medium (MEM) containing 10% fetal bovine serum and an additional 2 mM l-glutamine and 1 mM sodium pyruvate. Human embryonic kidney (HEK) 293A cells were maintained in Dulbecco's MEM containing 10% fetal bovine serum (defined; HyClone) with an additional 2 mM l-glutamine and 1 mM sodium pyruvate. BHK-21 cells were maintained in MEM containing 10% bovine sera and 10% tryptose phosphate broth. BHK3 cells are a derivative of BHK-21 cells which have been adapted to grow in either monolayer or suspension culture. They were obtained from the Empresa Colombiana de Productos Veterinarios in Bogotá, Colombia, and maintained in monolayer culture in the same medium as BHK-21 cells. A stable BHK3 cell line expressing the bovine αVβ6 integrin (BHK3αVβ6) was generated by cotransfecting cDNAs encoding the bovine αV (64) and β6 (20) subunits, using FuGENE6 (Roche Molecular Biochemicals) as described elsewhere (20), and selecting cells resistant to G418 and Zeocin (Invitrogen). Cells were cloned by single-cell cloning, and integrin expression was monitored by immunohistochemistry (63).
The monoclonal antibodies (MAbs) LM609 (MAB1976) and 23C6 (CBL544), which react with the αVβ3 heterodimer (19, 34), CSβ6 (MAB2076), which reacts with the β6 subunit (91), and 10D5 (MAB2077), which reacts with the αVβ6 heterodimer (62), were purchased from Chemicon International Inc. MAbs LM609 and CSβ6 have been shown to cross-react with the bovine homologues of their respective integrins (20, 63, 64) (see Fig. 1, below). A rabbit antiserum prepared against purified human αVβ3 was a gift from Merja Roivainen, National Public Health Institute, Helsinki, Finland.
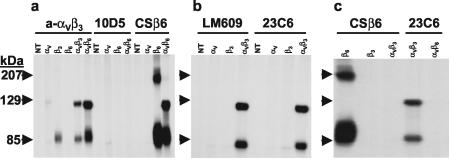
FIG. 1.
Analysis of soluble integrins secreted from transfected cells. COS-1 cells were transfected with soluble integrin subunits, as denoted in the figure, and labeled with [35S]methionine-cysteine between 24 and 72 h after transfection as described in Materials and Methods. The medium was collected, and the presence of soluble integrins or integrin subunits was determined by RIP, using the antibodies denoted in the figure followed by nonreducing SDS-PAGE and autoradiography. NT, nontransfected cells. The specificities of the antibodies were as follows: rabbit polyclonal anti-αVβ3 antibody (anti-αVβ3); monoclonal anti-αVβ6 antibody (10D5); monoclonal anti-β6 antibody (CSβ6); monoclonal anti-αVβ3 antibodies (LM609 and 23C6).
Generation of plasmids encoding soluble bovine integrin subunits.
cDNA encoding the signal peptide and ectodomain of the bovine αV integrin subunit was PCR amplified from the plasmid pBovαVZEO (64) using Pfu polymerase and primers 101 and 102 (Table 1), which insert a stop codon before the coding sequences for the transmembrane and cytoplasmic domains. The resulting amplicon was cloned into pCR-BluntII-TOPO (Invitrogen), and the cloning orientation was determined by sequencing. The amplicon was cut from the plasmid with XhoI and KpnI and cloned into pcDNA3.1(-) (Invitrogen) digested with the same enzymes and containing a neomycin selection marker to produce pBovssαVNEO.
TABLE 1.
Primers used in the cloning of the bovine integrin subunit ectodomainsa
| Primer | Sequence (5′→3′) | Subunit | Orientation |
|---|---|---|---|
| 101 | GGGCTAGCATGGCTTTTCCGCCGCGG | αv | Forward |
| 102 | GGGGTACCTTATCAAGGAACAGGCATGGGTGC | αv | Reverse |
| 103 | AACTCGAGATGCGAGCGCGGCCGCGG | β3 | Forward |
| 104 | GCTCTAGATTATCAGTCAGGGCCCTTGGGACAC | β3 | Reverse |
| 161 | TCTAGACTCGAGCTGAAACGGATGGGGATT | β6 | Forward |
| 197 | TCTAGATCTCTAATTTGGAGGTTTTGGACA | β6 | Reverse |
Initiating ATGs and stop codons are shown in bold. Restriction sites built into the primers and used during the cloning are underlined. They are Kpnl (primer 102) and Xhol (primers 103 and 161). Other restriction sites used in the cloning correspond to those found in the multiple cloning site of pCR-Bluntll-TOPO (Invitrogen).
An identical strategy was employed to clone the cDNAs encoding the signal peptides and ectodomains of the bovine β3 subunit from the plasmid pBovβ3ZEO (64), using primers 103 and 104 (Table 1), and the bovine β6 subunit from the plasmid pBovβ6 (20), using primers 161 and 197 (Table 1). The amplicon encoding the signal peptide and ectodomain of the β3 subunit was cut from the plasmid with XhoI and PstI and inserted into pcDNA3.1/zeo(-) (Invitrogen) to generate pBovssβ3ZEO. Similarly, the amplicon encoding the signal peptide and ectodomain of the β6 subunit was cut from the plasmid with XhoI and KpnI and inserted into pcDNA3.1/zeo(-) (Invitrogen) to generate pBovssβ6ZEO.
Production of soluble secreted bovine integrins.
To characterize soluble bovine integrins, the subunits were transiently expressed in COS-1 cells as described previously (20, 64). Twenty-four hours after transfection, the medium was exchanged for MEM containing 1/20 the normal amount of methionine and [35S]methionine-cysteine (50 μCi). After an additional 48 h of incubation, the overlay medium was removed and analyzed for the production of soluble integrins by radioimmunoprecipitation (RIP) and nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using specific anti-integrin polyclonal antibodies and MAbs.
To produce large amounts of unlabeled soluble bovine integrins, stable cell lines were generated expressing either soluble αVβ3 or soluble αVβ6. Briefly, HEK 293A cells were cotransfected with pBOVssαVNEO and either pBOVssβ3ZEO or pBOVssβ6ZEO as described above. After selecting cells resistant to both G418 and Zeocin, colonies were isolated by single-cell cloning and analyzed for soluble integrin production by labeling with [35S]methionine-cysteine and RIP as described above. Cell cultures producing the soluble integrins were seeded into roller bottles. After incubation overnight, the medium was exchanged for serum-free medium and the cells were incubated for an additional 48 h. The medium was removed, clarified by centrifugation, and concentrated by ultrafiltration through Centricon Plus-80 concentrators with a PL-30 membrane (Amicon).
Enzyme-linked immunosorbent assay (ELISA) quantitation of soluble bovine integrins.
Serial dilutions of concentrated culture medium containing soluble integrins were prepared in 0.05 M carbonate-bicarbonate buffer, pH 9.6 (coating buffer; carbonate-bicarbonate buffer capsules; Sigma Chemical Co.). A 100-μl aliquot of each dilution was added to plastic 96-well dishes (Immulon 2HB; Dynatech Laboratories Inc.) and incubated at 4°C overnight. The plates were washed with phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBS-Tween) and blocked with 100 μl of 1% nonfat dry milk in PBS containing 0.5 mM CaCl2 and 0.9 mM MgCl2 (blocking buffer) for 1 h at room temperature. Plates were washed with PBS-Tween, and 100 μl of primary antibody was added to each well. The primary antibody for soluble αVβ3 was a rabbit anti-human αVβ3 antiserum, diluted 1:7,000 in blocking buffer, and the primary antibody for soluble αVβ6 was MAb CSβ6, diluted 1:1,000 in blocking buffer. The plates were incubated for 1 h at room temperature and washed with PBS-Tween, and 100 μl of either horseradish peroxidase (HRP)-conjugated donkey anti-rabbit immunoglobulin G (IgG; Pierce Chemical Co.) or HRP-conjugated goat anti-mouse IgG (Pierce Chemical Co.) (1:1,000 in blocking buffer) was added and incubated for 0.5 h at room temperature. After washing with PBS-Tween, substrate (tetramethyl benzidine; microwell peroxidase substrate system; KPL Chemicals) was added and incubated for 5 to 15 min at room temperature. The reaction was stopped with 1 M H2SO4, and the plates were read at 405 nm. Standard curves were generated using purified human soluble αVβ6 (a gift from Dean Sheppard, University of California, San Francisco) or purified human αVβ3 (Chemicon Chemical Co.).
Analysis of soluble integrin interaction with FMDV.
Immulon 2HB 96-well plates were incubated overnight at 4°C with a hyperimmune bovine anti-FMDV antiserum diluted 1:3,000 in coating buffer. The plates were washed with PBS-Tween and blocked with blocking buffer for 1 h at room temperature. Virus (1 μg), diluted in Tris-buffered saline (TBS; 25 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing 0.1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, and 0.1% bovine serum albumin (BSA) (TBS+) was added and the plates were incubated overnight at 4°C. The plates were washed with PBS-Tween, soluble αVβ3 or αVβ6 (500 ng) diluted in TBS+ was added, and the plates were incubated for 1 h at room temperature. The presence of bound integrin was determined as described above.
An alternative assay, as described by Jackson et al. (42), was also performed. Briefly, soluble integrin (1 μg/ml) in TBS+ without BSA was bound to Immulon wells overnight at 4°C. After blocking the plates as described above, virus (1 μg) in TBS+ was added and incubated at room temperature for 1 h. Virus binding to the integrin was detected using specific antiviral MAbs.
Neutralization assays.
Virus and soluble integrins were diluted in TBS+ and incubated at 37°C for 1 h. The virus-integrin mixture (250 μl), containing approximately 40 to 60 PFU of virus, was inoculated onto BHK-21 cells in 35-mm tissue culture dishes and incubated for 1 h at 37°C. The inoculum was removed, and the cells were overlayed with 0.6% gum tracacanth in MEM. After 48 h of incubation, the cells were stained with crystal violet-HistoChoice (Amresco Co. Inc.) and plaques were counted. The results are given as the concentration of soluble integrin that reduced the number of plaques by 50% (IC50).
Virus binding assays.
Types A12 and O1C3056H were grown in BHK-21 cells in the presence of [35S]methionine and purified as described elsewhere (56). The number of virus particles per milliliter was determined spectrophotometrically (4). Labeled virus was diluted in TBS+ and incubated with soluble αVβ3 or αVβ6, diluted in the same buffer, at 37°C for 1 h. Binding assays were performed using BHK3αVβ6 cells, diluted to 2.5 × 106 cells/ml in TBS+, and virus at 3,000 particles/cell as previously described (12).
RESULTS
Analysis of soluble bovine αVβ3 and αVβ6.
COS-1 cells were transfected with the individual truncated αV, β3, or β6 subunit cDNAs or cotransfected with the truncated αV and either the truncated β3 or β6 subunit cDNAs. The cells were labeled between 24 and 72 h after transfection with [35S]methionine-cysteine, and the culture medium was analyzed for the presence of secreted integrins by RIP as described in Materials and Methods. The results are shown in Fig. 1. A rabbit antiserum prepared against human αVβ3 immunoprecipitated proteins of the expected molecular weights from the medium of cells transfected with the truncated αV, β3, and αVβ3 subunit cDNAs but did not react with any proteins in the media of cells transfected with the β6 subunit cDNA alone (Fig. 1a). This antiserum, however, immunoprecipitated both the αV and β6 subunits secreted from cells cotransfected with both subunits (Fig. 1a), suggesting that a complete soluble αVβ6 integrin heterodimer was secreted into the medium. In support of this, we utilized the MAb CSβ6, which only reacts with the β6 subunit (62). This MAb immunoprecipitated a protein of the expected molecular weight in the medium of cells transfected with the β6 subunit cDNA alone, but it did not react with the truncated αV subunit (Fig. 1a). The identity of the high-molecular-weight protein immunoprecipitated by this MAb from cells transfected with the β6 subunit cDNA (Fig. 1a and c) is unknown, but it might be either a highly glycosylated form of the subunit or a dimer. CSβ6, however, immunoprecipitated both the αV and β6 subunits secreted from cells cotransfected with both subunit cDNAs, confirming the results with the polyclonal antibody, that the soluble αVβ6 is a heterodimer. The high-molecular-weight protein present in the β6-transfected cells was not detected in cotransfected cells. The MAb 10D5, which reacts with the complete human αVβ6 integrin (62) and inhibits FMDV infection mediated by the human αVβ6 integrin in vitro (43), did not react with the soluble bovine αVβ6 integrin (Fig. 1a).
MAbs LM609 (19) and 23C6 (34), which react only with the complete integrin heterodimer, were used to analyze the soluble αVβ3 integrin. Both antibodies immunoprecipitated the αV and β3 subunits only from the medium of cotransfected cells (Fig. 1b), indicating that αVβ3 is also secreted as an intact heterodimer. The CSβ6 antibody only reacted with secreted β6 or αVβ6 subunits (Fig. 1a and c) but did not react with either the soluble αV or αVβ3 (Fig. 1c). Similarly, the 23C6 antibody did not react with soluble αVβ6 (Fig. 1c).
Interactions of soluble bovine integrins with FMDV.
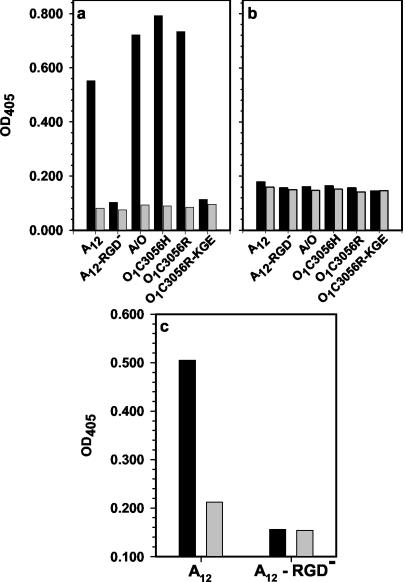
The ability of the soluble integrins to bind to FMDV was analyzed in a capture ELISA-type format, as described in Materials and Methods, and compared with conditioned media from nontransfected HEK 293A cells. We examined integrin binding to type A12 virus, a type A12 virus with RGD deleted (A12-RGD−) (59), two variants of type O1Camp (78), and a type A12 virus containing the G-H loop from type O1BFS (A/O) (71) (Fig. 2). Soluble αVβ6 bound strongly to types A12, O1Camp, and A/O (Fig. 2a). The binding was dependent on divalent cations, as it was inhibited by EDTA (data not shown), and also dependent on the RGD sequence, since there was no binding to either a type A12 virus with RGD deleted or an O1Camp with an RGD→KGE mutation (Fig. 2a). The integrin was able to bind to virus containing the RGD sequence regardless of whether the virus utilized the integrin to mediate infection, as evidenced by the fact that there was no difference in integrin binding between the bovine-virulent O1Camp variant (O1C3056H) that required integrin expression to infect cells (65, 78) and the bovine-avirulent variant (O1C3056R) that only utilized membrane-expressed HS to bind and enter cells (65, 78).
FIG. 2.
Analysis of binding of soluble αVβ3 and αVβ6 to FMDV. (a and b) Virus (1 μg) was captured on antibody-coated plastic wells as described in Materials and Methods. Culture medium from nontransfected HEK 293A cells (grey bars) or from transfected cells containing either soluble αVβ6 (a) or αVβ3 (b) (0.5 μg/well; black bars) was incubated with the virus, and binding of integrin was determined using either rabbit anti-αVβ3 (a) or MAb CSβ6 (b) as described in Materials and Methods. (c) Wells were coated with either medium from nontransfected cells (grey bars) or from transfected cells containing soluble αVβ3 (1 μg/ml; black bars) as described in Materials and Methods. Virus (1 μg) was incubated with the integrin, and the binding of virus was determined using an antiviral MAb.
In contrast, soluble αVβ3 surprisingly exhibited little or no binding to any of the viruses used (Fig. 2b), even though the soluble integrin was able to bind to its natural ligand, vitronectin, in a similar assay (results not shown). It has been previously shown by Jackson and colleagues (42) that FMDV was able to interact with purified human αVβ3 containing both the transmembrane and cytoplasmic domains when the integrin was immobilized first and virus was then allowed to bind to it. In the assay shown in Fig. 2b, the soluble integrin was bound to immobilized virus (see Materials and Methods). To examine whether the difference in our results might be due to the assay system, we performed the assay as described by Jackson et al. (42). The results (for type A only), shown in Fig. 2c, indicated that virus can interact with the soluble αVβ3 and that the type of assay, and not the absence of the transmembrane and cytoplasmic domains, seems to account for the differences. A similar result was found for the type O virus (data not shown).
Effect of soluble bovine αVβ3 and αVβ6 on FMDV infectivity.
To determine whether the binding of soluble integrins to virus affects the ability of FMDV to infect cells, we performed plaque reduction neutralization assays as described in Materials and Methods. The results are shown in Table 2 and are represented as the integrin concentration required to inhibit plaque formation by 50%. Soluble αVβ3 was unable to inhibit infectivity of any of the viruses used, a result consistent with its inability to interact with the viruses (Fig. 2b). In contrast, soluble αVβ6 neutralized both type A and O virus infectivity. The IC50 values for types A24, A/O, and O1C3056H were similar, between 11 and 20 ng/ml; however, about 100-fold more soluble integrin was required to neutralize types A12 and O1C3056R (Table 2). The specificity of the integrin inhibition was shown by the inability of the soluble αVβ6 to neutralize the O1C3056R-KGE virus, which can only grow in cells expressing HS but cannot utilize an integrin receptor (65) (Table 2).
TABLE 2.
Neutralization of FMDV infectivity by soluble bovine integrinsa
| Virus | IC50 (ng/ml)b
|
|
|---|---|---|
| αvβ3 | αvβ6 | |
| A12 | >5,500 | 1,325 |
| A24 | >5,500 | 14 |
| A/O | >5,500 | 11 |
| O1C3056H | >5,500 | 20 |
| O1C3056R | >5,500 | 2,600 |
| O1C3056R-KGE | >5,500 | >15,500 |
Neutralization was determined by plaque reduction as described in Materials and Methods.
The concentration of integrin which reduced the number of plaques by 50%.
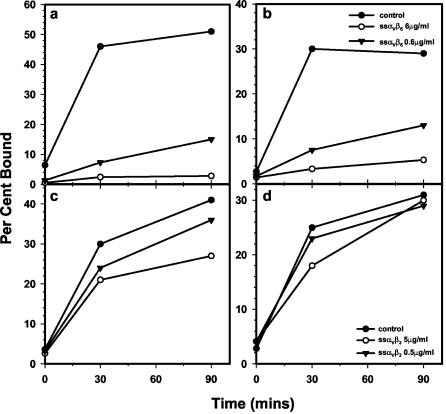
To examine the mechanism of neutralization by the soluble integrins, we examined the binding of radiolabeled types A12 and O1C3056H to BHK3αVβ6 cells. We utilized this cell line because binding of virus was much improved over that observed in the wild-type BHK3 and BHK-21 cells (data not shown), allowing us to compare virus adsorption under different conditions. The results in Fig. 3 show that the adsorption of both type A12 (Fig. 3a) and O1C3056H (Fig. 3b) were markedly inhibited by the soluble αVβ6 integrin in a concentration-dependent manner. Surprisingly, we also found that soluble αVβ3 caused a slight, but repeatable, inhibition of binding of both viruses to cells (Fig. 3c and d). When we tried to interfere with the αVβ3 inhibition using the function-blocking MAb LM609, we found that an antibody concentration of 10 μg/ml had no effect on the integrin-induced inhibition, but the antibody itself inhibited binding at higher concentrations (data not shown), probably as a result of interaction with hamster integrins.
FIG. 3.
Effect of soluble integrins on the adsorption of FMDV to cells. FMDV types A12 and O1C3056H, labeled with [35S]methionine and purified as described in Materials and Methods, were incubated either with soluble αVβ3 or αVβ6 at the concentrations indicated in the figure or with medium from nontransfected HEK 293A cells. Binding assays were performed in BHK3αVβ6 cells at 4°C, using a virus concentration of 3,000 particles/cell, as described in Materials and Methods. (a and b) Soluble αVβ6 incubated with either type A12 (a) or O1C3056H (b). (c and d) Soluble αVβ3 incubated with either type A12 (c) or O1C3056H (d).
Analysis of the virus-integrin complex.
The labeled viruses, incubated with soluble αVβ6 and used in the experiment shown in Fig. 3, were analyzed on sucrose density gradients. Both the labeled type A12 (Fig. 4a) and O1C3056H (Fig. 4b) migrated slower in the gradient than virus incubated with the medium from nontransfected HEK 293A cells. A similar experiment, using viruses incubated with soluble αVβ3, revealed no difference in viral migration in the gradients (data not shown). The viruses in the peak fractions of the gradients, shown in Fig. 4, were analyzed by RIP and PAGE. In both cases, the virus- soluble αVβ6 complexes could not be immunoprecipitated with an anti-FMDV polyclonal serum but were immunoprecipitated with the anti-β6 antibody, CSβ6 (data not shown), indicating that the virus still contained bound integrin. In addition, both viruses contained the complete complement of viral proteins, VP1 to -4 (data not shown).
FIG. 4.
Analysis of the virus-soluble αVβ6 complex. The samples used for these binding assays were centrifuged in an SW41 rotor through a 10-to-50% (wt/vol) sucrose gradient in TBS+ at 17,000 rpm for 18 h at 4°C. The gradients were fractionated into 0.4-ml fractions, and 0.1-ml samples were counted. (a) [35S]methionine-labeled type A12 incubated with either medium from nontransfected cells (•) or soluble αVβ6 (6 μg/ml) (○). (b) [35S]methionine-labeled type O1C3056H incubated with either medium from nontransfected cells (•) or soluble αVβ6 (6 μg/ml) (○). Samples were run on parallel gradients but are represented on the same graph for clarity.
DISCUSSION
FMDV is unique among the Picornaviridae in that it can utilize four different integrin receptors to mediate infection in cell culture (16, 20, 39, 41, 43, 64, 65), yet the roles these individual receptors play in the pathogenesis of the disease are not known. In addition, as a result of tissue culture adaptation, the virus acquires the ability to use HS as a receptor (5-7, 40, 55, 65), although this trait is associated with loss of virulence in susceptible animals (78). Finally, there is evidence for a third, as-yet-unidentified receptor which may be associated with virulence (6, 7, 86, 87, 96). These facts, coupled with a recent study showing that different virus serotypes appear to utilize the integrin receptors with varying efficiencies (20), have complicated studies on early virus-host interactions and viral pathogenesis. Recent outbreaks in developed countries have also highlighted the need to develop antiviral technologies which target specific host or viral factors involved in disease.
In this study, we have generated soluble heterodimers of two of the four known FMDV receptors, αVβ3 and αVβ6, and analyzed their interactions with representatives of serotypes A and O. Analysis of the integrins secreted by transient expression of subunit cDNAs in COS-1 cells showed that, in both cases, the subunits were secreted as complete integrin heterodimers (Fig. 1). Using a MAb that only reacts with the β6 subunit (91), proteins of the expected size of the soluble αV and β6 subunits were immunoprecipitated from the medium of transfected cells (Fig. 1a). Similarly, a polyclonal antibody generated against human αVβ3 reacted with the αV, but not the β6 subunit, yet immunoprecipitated both the αV and β6 subunits from cotransfected cells (Fig. 1a). We also utilized a MAb which reacts with the complete αVβ6 heterodimer (62) and has been shown to inhibit FMDV infection mediated by the human αVβ6 integrin (43). Interestingly, the antibody did not react with the soluble bovine αVβ6 integrin (Fig. 1a) and was also unable to prevent viral infection mediated by the bovine αVβ6 homolog (data not shown). The medium of cells cotransfected with the soluble αV and β3 subunits also contains the integrin as a complete heterodimer, as evidenced by immunoprecipitation of both subunits with two MAbs which only react with the intact integrin (19, 34) (Fig. 1b). The anti-β6 MAb did not react with the αVβ3 heterodimer, and the anti-αVβ3 MAb did not react with the αVβ6 heterodimer (Fig. 1c), indicating that the correct specific integrins are being synthesized.
To examine the ability of representative viruses to bind to soluble integrins, we utilized a capture ELISA-type assay, using the soluble integrins to bind to immobilized virus on plastic dishes (Fig. 2). Soluble αVβ6 bound specifically to each virus which contained an RGD sequence in the G-H loop (Fig. 2b). The integrin was not able to bind to a type A12 virus with an RGD deletion in the loop or to a type O virus with an RGD→KGE mutation. The latter virus is still capable of replicating in non-integrin-expressing cells, since it has an HS binding site in VP3 (65). In contrast, soluble αVβ3 integrin did not bind to any of the viruses we tested in this assay. This result was surprising, since the A12, A/O, and O1C3056H viruses have been shown to be able to utilize this integrin to mediate infection (20, 64, 65). The O1C3056R virus does not require integrins to infect cells (65); however, the presence of the RGD recognition sequence in the G-H loop should have allowed recognition of the αVβ3 integrin, just as it was recognized by the αVβ6 integrin (Fig. 2b).
Since the results with soluble αVβ3 contrast with those reported by Jackson and colleagues (42), who analyzed the binding of each of the seven FMDV serotypes to purified human αVβ3 immobilized on plastic, we performed the assay in a similar format as theirs. The results in Fig. 2c show that virus can interact with the soluble αVβ3 in an RGD-dependent manner. The binding of integrins in solution to immobilized ligands is a function of affinity (84), while the binding of ligands to immobilized integrins is a measure of avidity (80). Thus, these results suggest that αVβ6 can act as a high-affinity receptor for FMDV, while αVβ3 can function as a viral receptor but at much lower affinity. It is interesting to speculate that the role of the high-affinity αVβ6 may be to capture virus and help in the initial replication within the susceptible animal, while the low-affinity αVβ3 plays a role in disseminating the virus within the animal to distant sites of replication.
The interaction of soluble αVβ6 with FMDV results in neutralization of viral infectivity (Table 2). The results clearly show that the integrin is responsible for the neutralization, as the type O virus with KGE in place of the RGD in the G-H loop, which can still replicate by binding to the HS receptor (O1C3056R-KGE), is not neutralized by the integrin, while the same virus with the RGD sequence in the loop (O1C3056R) is neutralized by the integrin. The integrin IC50 for this virus, however, is about 100-fold greater than that for the same virus without the HS binding site, which requires that the integrin mediate infection (O1C3056H). Thus, neutralization of the HS binding virus is probably the result of steric hindrance of the HS binding site by the bound integrin. The finding that the integrin IC50 was about 100-fold greater for the A12 virus than for either the type A24 or A/O virus was surprising and, at this point, we are not sure why this virus should be that much less sensitive to integrin neutralization. One possible explanation is that this virus has been extensively passaged in BHK-21 cells and may have become more adapted to hamster integrins than the other viruses used in this study. We have shown that the type A viruses, in contrast to the type O viruses, utilize the αVβ3 integrin with an equal or greater efficiency than αVβ6, and exchanging the type A12 G-H loop with a type O loop did not affect the efficiency of integrin utilization (20). Yet, the A/O virus was as sensitive to integrin-mediated neutralization as type A24 and more sensitive than the type A12 parent. As expected, the soluble αVβ3 integrin was not able to neutralize any of the viruses tested (Table 2).
The mechanism of soluble αVβ6-mediated neutralization appears to be by inhibiting the binding of virus to cells (Fig. 3a and b) via soluble integrin binding to the receptor binding site on the virus (57, 72). The inhibition of binding of the type A12 (Fig. 3a) or the type O1C3056H (Fig. 3b) virus by the soluble integrin was about the same, even though the integrin IC50 was quite different for each virus (Table 2). We also found that the soluble αVβ3 integrin caused a low-level inhibition of adsorption of both viruses (Fig. 3c and d). This inhibition appeared to be concentration dependent and contrasted with the results in the virus neutralization assays (Table 2). Thus, it is possible that the low-affinity interaction of the integrin with virus in solution is manifest at the level of virus adsorption.
Interaction of PV with soluble PV receptor results in a conformational alteration of the capsid, generating a particle with a lower sedimentation rate in sucrose gradients and the loss of VP4, called an A particle (2, 26, 90). This particle is identical to the one generated during the natural infection of cells by PV (22, 51). The interaction of soluble intercellular adhesion molecule 1 with major group HRVs 3 and 14 results in quick release of the RNA from the capsid (27, 33, 92, 93), while the interaction of another major group HRV (HRV 16) is more stable and the conformational alterations occur more slowly (47, 68). In contrast, the interaction of the soluble low-density lipoprotein receptor with minor group HRVs does not result in virion conformational change and neutralizes virus infectivity by particle aggregation (54). The main difference between the interactions of PV and major group HRVs with their receptors as opposed to the minor group HRV-receptor interactions is that the receptors of the former group bind into a surface canyon on the virion (14, 28, 47, 92, 93), while receptors of the latter group bind around the fivefold axis of the particle and not in a canyon (32). Like the minor group HRV, integrin receptors interact with FMDV on the surface via the G-H loop (1, 50, 57), and it has been demonstrated that uncoating of FMDV in infected cells proceeds as a single step in acidic endosomes, resulting in the generation of pentameric subunits and RNA, without any apparent intermediates (8-10, 18). The results of this work support these conclusions, since interaction of virus with soluble αVβ6 did not result in the generation of any conformational changes to the virion (Fig. 4). Analysis of the soluble αVβ6-virus complex showed that the virions still retained bound receptor and also had a full complement of viral proteins. The slower sedimentation rate of virus which has been incubated with soluble αVβ6 is probably the result of the particles still retaining bound receptor and not the result of generation of an A particle or other structural intermediate.
A number of antiviral strategies for picornaviruses which target viral attachment and entry events have been developed (see references 60 and 76 and references therein). In addition, the importance of integrins in a number of human pathologies has resulted in the synthesis of small-molecule integrin antagonists and mimetics (25, 81). The results in this work show that soluble receptors can interfere with FMDV infection in vitro, and they provide a basis for further work to analyze both soluble receptors and anti-integrin compounds for use in FMD control strategies.
Acknowledgments
We thank Elizabeth Rieder for helpful comments on the manuscript. We also thank Dean Sheppard and Amha Atakilit, University of California San Francisco, for providing antibodies and soluble human integrins, and Merja Roivainen, National Public Health Institute, Helsinki, Finland, for providing us with high-titer rabbit antisera against human αVβ3.
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature 337:709-716. [DOI] [PubMed] [Google Scholar]
- 2.Arita, M., S. Koike, J. Aoki, H. Horie, and A. Nomoto. 1998. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J. Virol. 72:3578-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrach, H. L. 1968. Foot-and-mouth disease. Annu. Rev. Microbiol. 22:201-244. [DOI] [PubMed] [Google Scholar]
- 4.Bachrach, H. L., R. Trautman, and S. S. Breese. 1964. Chemical and physical properties of virtually pure foot-and-mouth disease virus. Am. J. Vet. Res. 25:333-342. [PubMed] [Google Scholar]
- 5.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 6.Baranowski, E., C. M. Ruiz-Jarabo, F. Lim, and E. Domingo. 2001. Foot-and-mouth disease virus lacking the VP1 G-H loop: the mutant spectrum uncovers interactions among antigenic sites for fitness gain. Virology 288:192-202. [DOI] [PubMed] [Google Scholar]
- 7.Baranowski, E., C. M. Ruiz-Jarabo, N. Sevilla, D. Andreu, E. Beck, and E. Domingo. 2000. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J. Virol. 74:1641-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxt, B. 1987. Effect of lysosomotropic compounds on early events in foot-and-mouth disease virus replication. Virus Res. 7:257-271. [DOI] [PubMed] [Google Scholar]
- 9.Baxt, B., and H. L. Bachrach. 1982. The adsorption and degradation of foot-and-mouth disease virus by isolated BHK-21 cell plasma membranes. Virology 116:391-405. [DOI] [PubMed] [Google Scholar]
- 10.Baxt, B., and H. L. Bachrach. 1980. Early interactions of foot-and-mouth disease virus with cultured cells. Virology 104:42-55. [DOI] [PubMed] [Google Scholar]
- 11.Baxt, B., and Y. Becker. 1990. The effect of peptides containing the arginine-glycine-aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes. 4:73-83. [DOI] [PubMed] [Google Scholar]
- 12.Baxt, B., D. O. Morgan, B. H. Robertson, and C. A. Timpone. 1984. Epitopes on foot-and-mouth disease virus outer capsid protein VP1 involved in neutralization and cell attachment. J. Virol. 51:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bella, J., P. R. Kolatkar, C. W. Marlor, J. M. Greve, and M. G. Rossmann. 1998. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc. Natl. Acad. Sci. USA 95:4140-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belnap, D. M., B. M. McDermott, Jr., D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergelson, J. M., N. St. John, S. Kawaguchi, M. Chan, H. Stubdal, J. Modlin, and R. W. Finberg. 1993. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J. Virol. 67:6847-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αVβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casasnovas, J. M., J. K. Bickford, and T. A. Springer. 1998. The domain structure of ICAM-1 and the kinetics of binding to rhinovirus. J. Virol. 72:6244-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavanagh, D., D. J. Rowlands, and F. Brown. 1978. Early events in the interaction between foot-and mouth disease virus and primary pig kidney cells. J. Gen. Virol. 41:255-264. [DOI] [PubMed] [Google Scholar]
- 19.Cheresh, D. A., and R. C. Spiro. 1987. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 262:17703-17711. [PubMed] [Google Scholar]
- 20.Duque, H., and B. Baxt. 2003. Foot-and-mouth disease virus receptors: comparison of bovine αV integrin utilization by type A and O viruses. J. Virol. 77:2500-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 22.Everaert, L., R. Vrijsen, and A. Boeye. 1989. Eclipse products of poliovirus after cold-synchronized infection of HeLa cells. Virology 171:76-82. [DOI] [PubMed] [Google Scholar]
- 23.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70:625-637. [DOI] [PubMed] [Google Scholar]
- 24.Fry, E. E., N. J. Knowles, J. W. Newman, G. Wilsden, Z. Rao, A. M. King, and D. I. Stuart. 2003. Crystal structure of swine vesicular disease virus and implications for host adaptation. J. Virol. 77:5475-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gadek, T. R., and R. S. McDowell. 2003. Discovery of small molecule leads in a biotechnology datastream. Drug Discov. Today 8:545-550. [DOI] [PubMed] [Google Scholar]
- 26.Gomez Yafal, A., G. Kaplan, V. R. Racaniello, and J. M. Hogle. 1993. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology 197:501-505. [DOI] [PubMed] [Google Scholar]
- 27.Greve, J. M., C. P. Forte, C. W. Marlor, A. M. Meyer, H. Hoover-Litty, D. Wunderlich, and A. McClelland. 1991. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J. Virol. 65:6015-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, Y., V. D. Bowman, S. Mueller, C. M. Bator, J. Bella, X. Peng, T. S. Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2000. Interaction of the poliovirus receptor with poliovirus. Proc. Natl. Acad. Sci. USA 97:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He, Y., P. R. Chipman, J. Howitt, C. M. Bator, M. A. Whitt, T. S. Baker, R. J. Kuhn, C. W. Anderson, P. Freimuth, and M. G. Rossmann. 2001. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat. Struct. Biol. 8:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He, Y., F. Lin, P. R. Chipman, C. M. Bator, T. S. Baker, M. Shoham, R. J. Kuhn, M. E. Medof, and M. G. Rossmann. 2002. Structure of decay-accelerating factor bound to echovirus 7: a virus-receptor complex. Proc. Natl. Acad. Sci. USA 99:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He, Y., S. Mueller, P. R. Chipman, C. M. Bator, X. Peng, V. D. Bowman, S. Mukhopadhyay, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2003. Complexes of poliovirus serotypes with their common cellular receptor, CD155. J. Virol. 77:4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewat, E. A., E. Neumann, J. F. Conway, R. Moser, B. Ronacher, T. C. Marlovits, and D. Blaas. 2000. The cellular receptor to human rhinovirus 2 binds around the 5-fold axis and not in the canyon: a structural view. EMBO J. 19:6317-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoover-Litty, H., and J. M. Greve. 1993. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J. Virol. 67:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horton, M. A., D. Lewis, K. McNulty, J. A. Pringle, and T. J. Chambers. 1985. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 45:5663-5669. [PubMed] [Google Scholar]
- 35.Hynes, R. O. 1999. Cell adhesion: old and new questions. Trends Cell Biol. 9:M33-M37. [PubMed] [Google Scholar]
- 36.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 37.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 38.Jackson, T., W. Blakemore, J. W. Newman, N. J. Knowles, A. P. Mould, M. J. Humphries, and A. M. King. 2000. Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin α5β1: influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J. Gen. Virol. 81:1383-1391. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, T., S. Clark, S. Berryman, A. Burman, S. Cambier, D. Mu, S. Nishimura, and A. M. King. 2004. Integrin αVβ8 functions as a receptor for foot-and-mouth disease virus: role of the β-chain cytodomain in integrin-mediated infection. J. Virol. 78:4533-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson, T., A. P. Mould, D. Sheppard, and A. M. King. 2002. Integrin αVβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson, T., A. Sharma, R. A. Ghazaleh, W. E. Blakemore, F. M. Ellard, D. L. Simmons, J. W. Newman, D. I. Stuart, and A. M. King. 1997. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth disease viruses to the purified integrin αVβ3 in vitro. J. Virol. 71:8357-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson, T., D. Sheppard, M. Denyer, W. Blakemore, and A. M. King. 2000. The epithelial integrin αVβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan, G., M. S. Freistadt, and V. R. Racaniello. 1990. Neutralization of poliovirus by cell receptors expressed in insect cells. J. Virol. 64:4697-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan, G., D. Peters, and V. R. Racaniello. 1990. Poliovirus mutants resistant to neutralization with soluble cell receptors. Science 250:1596-1599. [DOI] [PubMed] [Google Scholar]
- 47.Kolatkar, P. R., J. Bella, N. H. Olson, C. M. Bator, T. S. Baker, and M. G. Rossmann. 1999. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 18:6249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lea, S. M., R. M. Powell, T. McKee, D. J. Evans, D. Brown, D. I. Stuart, and P. A. van der Merwe. 1998. Determination of the affinity and kinetic constants for the interaction between the human virus echovirus 11 and its cellular receptor, CD55. J. Biol. Chem. 273:30443-30447. [DOI] [PubMed] [Google Scholar]
- 49.Leippert, M., E. Beck, F. Weiland, and E. Pfaff. 1997. Point mutations within the βG-βH loop of foot-and-mouth disease virus O1K affect virus attachment to target cells. J. Virol. 71:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logan, D., R. Abu-Ghazaleh, W. Blakemore, S. Curry, T. Jackson, A. King, S. Lea, R. Lewis, J. Newman, N. Parry, D. Rowlands, D. Stuart, and E. Fry. 1993. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature 362:566-568. [DOI] [PubMed] [Google Scholar]
- 51.Lonberg-Holm, K., L. B. Gosser, and J. C. Kauer. 1975. Early alteration of poliovirus in infected cells and its specific inhibition. J. Gen. Virol. 27:329-342. [DOI] [PubMed] [Google Scholar]
- 52.Marlovits, T. C., C. Abrahamsberg, and D. Blaas. 1998. Soluble LDL minireceptors. Minimal structure requirements for recognition of minor group human rhinovirus. J. Biol. Chem. 273:33835-33840. [DOI] [PubMed] [Google Scholar]
- 53.Marlovits, T. C., C. Abrahamsberg, and D. Blaas. 1998. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J. Virol. 72:10246-10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marlovits, T. C., T. Zechmeister, M. Gruenberger, B. Ronacher, H. Schwihla, and D. Blaas. 1998. Recombinant soluble low density lipoprotein receptor fragment inhibits minor group rhinovirus infection in vitro. FASEB J. 12:695-703. [DOI] [PubMed] [Google Scholar]
- 55.Martinez, M. A., N. Verdaguer, M. G. Mateu, and E. Domingo. 1997. Evolution subverting essentiality: dispensability of the cell attachment Arg-Gly-Asp motif in multiply passaged foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 94:6798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mason, P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect normally insusceptible cells via the Fc receptor. Virology 192:568-577. [DOI] [PubMed] [Google Scholar]
- 57.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 91:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDermott, B. M., Jr., A. H. Rux, R. J. Eisenberg, G. H. Cohen, and V. R. Racaniello. 2000. Two distinct binding affinities of poliovirus for its cellular receptor. J. Biol. Chem. 275:23089-23096. [DOI] [PubMed] [Google Scholar]
- 59.McKenna, T. S., J. Lubroth, E. Rieder, B. Baxt, and P. W. Mason. 1995. Receptor binding site-deleted foot-and-mouth disease (FMD) virus protects cattle from FMD. J. Virol. 69:5787-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McKinlay, M. A. 2001. Recent advances in the treatment of rhinovirus infections. Curr. Opin. Pharmacol. 1:477-481. [DOI] [PubMed] [Google Scholar]
- 61.Mehta, R. J., B. Diefenbach, A. Brown, E. Cullen, A. Jonczyk, D. Gussow, G. A. Luckenbach, and S. L. Goodman. 1998. Transmembrane-truncated αVβ3 integrin retains high affinity for ligand binding: evidence for an “inside-out” suppressor? Biochem. J. 330:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munger, J. S., X. Huang, H. Kawakatsu, M. J. Griffiths, S. L. Dalton, J. Wu, J. F. Pittet, N. Kaminski, C. Garat, M. A. Matthay, D. B. Rifkin, and D. Sheppard. 1999. The integrin αVβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319-328. [DOI] [PubMed] [Google Scholar]
- 63.Neff, S., and B. Baxt. 2001. The ability of integrin αVβ3 to function as a receptor for foot-and-mouth disease virus is not dependent on the presence of complete subunit cytoplasmic domains. J. Virol. 75:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neff, S., P. W. Mason, and B. Baxt. 2000. High-efficiency utilization of the bovine integrin αVβ3 as a receptor for foot-and-mouth disease virus is dependent on the bovine β3 subunit. J. Virol. 74:7298-7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αVβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelsen-Salz, B., H. J. Eggers, and H. Zimmermann. 1999. Integrin αVβ3 (vitronectin receptor) is a candidate receptor for the virulent echovirus 9 strain Barty. J. Gen. Virol. 80:2311-2313. [DOI] [PubMed] [Google Scholar]
- 67.Nurani, G., B. Lindqvist, and J. M. Casasnovas. 2003. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J. Virol. 77:11985-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson, N. H., P. R. Kolatkar, M. A. Oliveira, R. H. Cheng, J. M. Greve, A. McClelland, T. S. Baker, and M. G. Rossmann. 1993. Structure of a human rhinovirus complexed with its receptor molecule. Proc. Natl. Acad. Sci. USA 90:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 70.Pulli, T., E. Koivunen, and T. Hyypia. 1997. Cell-surface interactions of echovirus 22. J. Biol. Chem. 272:21176-21180. [DOI] [PubMed] [Google Scholar]
- 71.Rieder, E., B. Baxt, J. Lubroth, and P. W. Mason. 1994. Vaccines prepared from chimeras of foot-and-mouth disease virus (FMDV) induce neutralizing antibodies and protective immunity to multiple serotypes of FMDV. J. Virol. 68:7092-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rieder, E., A. Berinstein, B. Baxt, A. Kang, and P. W. Mason. 1996. Propagation of an attenuated virus by design: engineering a novel receptor for a noninfectious foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 93:10428-10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rieder, E., T. Bunch, F. Brown, and P. W. Mason. 1993. Genetically engineered foot-and-mouth disease viruses with poly(C) tracts of two nucleotides are virulent in mice. J. Virol. 67:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rieder, E., and E. Wimmer. 2002. Cellular receptors of picornaviruses: an overview, p. 61-70. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 75.Roivainen, M., L. Piirainen, T. Hovi, I. Virtanen, T. Riikonen, J. Heino, and T. Hyypia. 1994. Entry of coxsackievirus A9 into host cells: specific interactions with αVβ3 integrin, the vitronectin receptor. Virology 203:357-365. [DOI] [PubMed] [Google Scholar]
- 76.Rotbart, H. A. 2002. Treatment of picornavirus infections. Antivir. Res. 53:83-98. [DOI] [PubMed] [Google Scholar]
- 77.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 78.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413-1429. [DOI] [PubMed] [Google Scholar]
- 80.Seow, K. T., J. P. Xiong, M. A. Arnaout, J. Welge, F. Rippmann, and S. L. Goodman. 2002. Divalent cations and the relationship between αA and βA domains in integrins. Biochem. Pharmacol. 64:805-812. [DOI] [PubMed] [Google Scholar]
- 81.Shimaoka, M., and T. A. Springer. 2003. Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2:703-716. [DOI] [PubMed] [Google Scholar]
- 82.Silberstein, E., G. Dveksler, and G. G. Kaplan. 2001. Neutralization of hepatitis A virus (HAV) by an immunoadhesin containing the cysteine-rich region of HAV cellular receptor 1. J. Virol. 75:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silberstein, E., L. Xing, W. van de Beek, J. Lu, H. Cheng, and G. G. Kaplan. 2003. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin- and mucin-like regions. J. Virol. 77:8765-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith, J. W., R. S. Piotrowicz, and D. Mathis. 1994. A mechanism for divalent cation regulation of β3-integrins. J. Biol. Chem. 269:960-967. [PubMed] [Google Scholar]
- 85.Sutmoller, P., S. S. Barteling, R. C. Olascoaga, and K. J. Sumption. 2003. Control and eradication of foot-and-mouth disease. Virus Res. 91:101-144. [DOI] [PubMed] [Google Scholar]
- 86.Taboga, O., C. Tami, E. Carrillo, J. I. Nunez, A. Rodriguez, J. C. Saiz, E. Blanco, M. L. Valero, X. Roig, J. A. Camarero, D. Andreu, M. G. Mateu, E. Giralt, E. Domingo, F. Sobrino, and E. L. Palma. 1997. A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J. Virol. 71:2606-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tami, C., O. Taboga, A. Berinstein, J. I. Nunez, E. L. Palma, E. Domingo, F. Sobrino, and E. Carrillo. 2003. Evidence of the coevolution of antigenicity and host cell tropism of foot-and-mouth disease virus in vivo. J. Virol. 77:1219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Triantafilou, K., M. Triantafilou, Y. Takada, and N. Fernandez. 2000. Human parechovirus 1 utilizes integrins αVβ3 and αVβ1 as receptors. J. Virol. 74:5856-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Triantafilou, M., K. Triantafilou, K. M. Wilson, Y. Takada, and N. Fernandez. 2000. High affinity interactions of coxsackievirus A9 with integrin αVβ3 (CD51/61) require the CYDMKTTC sequence of β3, but do not require the RGD sequence of the CAV-9 VP1 protein. Hum. Immunol. 61:453-459. [DOI] [PubMed] [Google Scholar]
- 90.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin αVβ6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J. Biol. Chem. 269:6940-6948. [PubMed] [Google Scholar]
- 92.Xing, L., J. M. Casasnovas, and R. H. Cheng. 2003. Structural analysis of human rhinovirus complexed with ICAM-1 reveals the dynamics of receptor-mediated virus uncoating. J. Virol. 77:6101-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xing, L., K. Tjarnlund, B. Lindqvist, G. G. Kaplan, D. Feigelstock, R. H. Cheng, and J. M. Casasnovas. 2000. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO J. 19:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiong, J. P., T. Stehle, B. Diefenbach, R. Zhang, R. Dunker, D. L. Scott, A. Joachimiak, S. L. Goodman, and M. A. Arnaout. 2001. Crystal structure of the extracellular segment of integrin αVβ3. Science 294:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiong, J. P., T. Stehle, R. Zhang, A. Joachimiak, M. Frech, S. L. Goodman, and M. A. Arnaout. 2002. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296:151-155. [DOI] [PubMed] [Google Scholar]
- 96.Zhao, Q., J. M. Pacheco, and P. W. Mason. 2003. Evaluation of genetically engineered derivatives of a Chinese strain of foot-and-mouth disease virus reveals a novel cell-binding site which functions in cell culture and in animals. J. Virol. 77:3269-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]