Abstract
We analyzed human immunodeficiency virus type 1 (HIV-1) Nef variants to further evaluate the functional relevance of the R71T substitution previously proposed to attenuate viral replication (Fackler et al., Curr. Biol. 11:1294-1299, 2001). Our results demonstrate that this variation in the proline-rich region does not significantly affect the functional activity of Nef or HIV-1 infectivity or replication.
The human immunodeficiency type 1 (HIV-1) nef gene is an important pathogenesis factor. Among other functions, Nef enhances viral replication and down-modulates cell surface expression of CD4, the primary receptor of HIV-1 (5, 8, 17, 25, 26). Both Nef activities correlate significantly (10, 12, 16) and might be mechanistically linked, because reduced surface levels of CD4 promote virion release, enhance Env incorporation, and might prevent viral superinfection (2, 3, 15, 21). However, the relevance of Nef-mediated CD4 down-regulation for the enhanced replicative capacity of HIV-1 remains controversial (6, 22). Recently, it has been proposed that a naturally occurring R71T substitution in the otherwise well-conserved proline-rich motif (P69xR/T71PxxPxxPxRP78) impairs the ability of Nef to enhance HIV-1 replication without having an effect on CD4 down-modulation (7). We were surprised by this finding, because, e.g., the HIV-1 NL4-3 Nef containing a T at position 71 is commonly used as a positive control for the ability of Nef to stimulate viral replication. Furthermore, the R71T variation in Nef apparently does not prevent efficient viral replication and AIDS progression in vivo because it is found in both asymptomatic and immunodeficient HIV-1-infected individuals (13).
To further investigate the functional relevance of the R71T variation we generated five pairs of Nef variants. T71 was changed to R in the NL4-3, 012wm-93(1) and 167rw-95(1) Nefs; the reciprocal R71T change was introduced into the 001gh-93(1) and 057dr-94(1) Nefs. Two (001gh and 012wm) of the four primary nef alleles were derived from nonprogressors, and two (057dr and 167rw) were from AIDS patients (13). The original Nef sequences can be retrieved from GenBank with accession numbers AAB60579, AF129333, AF129340, AF129356, AF129381 and AF129385. Substitutions were introduced by PCR using mutagenic internal oligonucleotides essentially as described elsewhere (12). Sequence analysis confirmed that all constructs differed only by the predicted R71T change or vice versa in the PxxP motif. First, all 10 nef alleles were inserted into a bicistronic vector coexpressing Nef and green fluorescent protein (GFP) (11) to compare the ability of R71- and T7-Nefs to modulate the cell surface expression of various human receptor molecules (8, 23, 24, 27, 28). Quantitative fluorescence-activated cell sorter (FACS) analysis was performed as described previously (4, 23) and revealed that both groups of Nef proteins modulated cell surface expression of CD4, CD28, major histocompatibility complex class I (MHC-I) and MHC-II, and the MHC-II-associated invariant chain (Ii) with similar efficiency levels (Fig. 1).
FIG. 1.
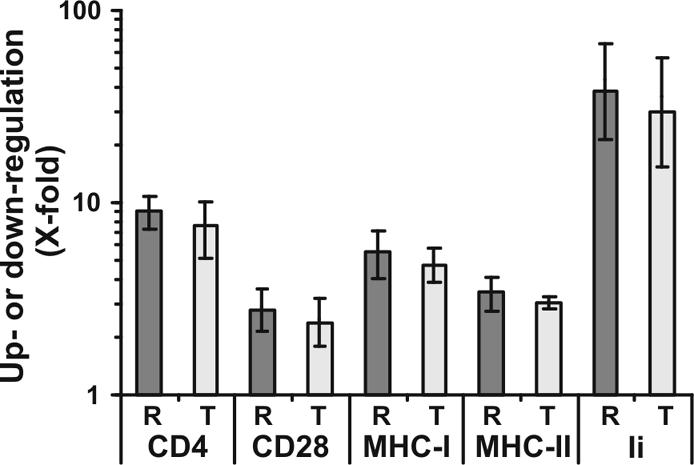
The R71T variation in Nef does not alter its ability to modulate cell surface expression of various human receptor molecules. HeLa-CIITA cells (27) and Jurkat cells were transfected with a bicistronic vector coexpressing GFP and the R71 (R) or T71 (T) forms of the NL4-3, 012wm-93(1), BT-94(1) 167rw-95(1), 001gh-93(1), and 057dr-94(1) Nef proteins. Values were determined as described previously (4, 23) and represent severalfold (X-fold) down-modulation of CD4, CD28, MHC-I, and MHC-II or up-regulation of Ii on cells expressing high levels of GFP and hence Nef. Shown are average values ± standard deviations derived from one representative transfection with the five pairs of R71- and T71-Nefs. Similar results were obtained in an independent experiment.
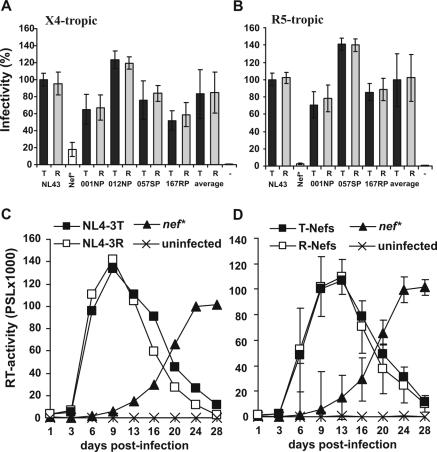
To assess the effect of the R71T variation on viral infectivity and replication we generated R71- and T7-Nef variants of the proviral CXCR4-tropic HIV-1 NL4-3 molecular clone (1) and a CCR5-tropic derivative of NL4-3 containing the 005pf135 V3 loop region (18). R5-tropic NL4-3 clones expressing the 012NP R71 Nef contained undesired point mutations and were excluded from the analysis. All other constructs differed exclusively by the predicted R71T variation in their nef sequences. Virus stocks of X4- and R5-tropic HIV-1 NL4-3 T71 and R71 Nef variants were generated by transient transfection of 293T cells and used to infect P4-CCR5 indicator cells expressing both CCR5 and CXCR4 as described previously (18). The results demonstrated that the R71T variation did not significantly affect the ability of Nef to enhance virion infectivity independently of the viral coreceptor tropism (Fig. 2A and B). Similarly, R71- and T7-Nefs enhanced viral replication of both X4- and R5-tropic HIV-1 NL4-3 variants in peripheral blood mononuclear cells (PBMC) with indistinguishable efficiency characteristics (Fig. 2C and D and data not shown).
FIG. 2.
The R71T variations does not affect the ability of Nef to enhance viral infectivity or replication in human PBMC. (A and B) P4-CCR5 cells were infected with X4-tropic (A) or R5-tropic (B) HIV-1 NL4-3 variants expressing the indicated R71 (R)- or T71 (T)-Nefs or containing a premature stop codon in the nef gene (Nef*). Infections were performed in triplicate with three independent virus stocks of the X4- or R5-tropic HIV-1 NL4-3 variants containing 2.5 ng of p24 antigen. The average values of the nine measurements ± standard deviations are shown. Infectivity is shown relative to that of the respective NL4-3 variants expressing the wild type T71-Nef. −, uninfected cells. (C) Unstimulated PBMC were infected in triplicate with X4-tropic HIV-1 expressing the original NL4-3 T71-Nef or the R71 mutant Nef immediately after isolation, stimulated with phytohemagglutinin (Murex, Burgwedel, Germany) (4 μg/ml) for 48 h 3 days later, and cultured for 28 days. As a control, cells were also infected with a nef-defective NL4-3 mutant virus. Virus production was monitored by reverse transcriptase (RT) assay as described previously (19). Results shown in panel A represent average values derived from a triple infection of PBMC derived from a single blood donor. (D) Average RT activity ± standard deviations measured after infection of PBMC with X4-tropic HIV-1 variants expressing the R71 or T71 forms of the NL4-3, 012wm-93(1), 167rw-95(1), 001gh-93(1), and 057dr-94(1) Nef proteins. Similar results were obtained in independent experiments using different virus stocks, the R5-tropic form of HIV-1 NL4-3, and PBMC from different donors. PSL, photon-stimulated light emission.
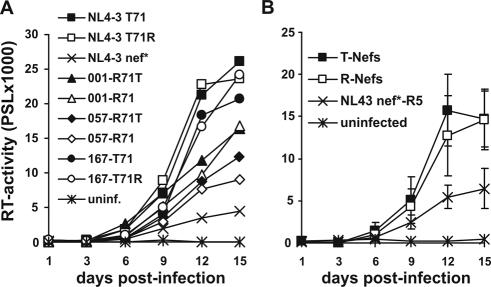
An attenuating effect of T71 in Nef on HIV-1 replication has been previously shown in cocultures of immature dendritic cells (imDCs) and PBMC (7). Therefore, we next investigated the effect of the R71T variation on HIV-1 replication in imDC/PBMC cocultures. DCs were generated essentially as described previously (14). Briefly, PBMC were isolated from leukapheresis preparations by density gradient separation using Lymphoprep (Nycomed Pharma AS, Oslo, Norway). Cells were cultured in RPMI 1640 (BioWhittaker, Verviers, Belgium)-1% glutamine (Sigma-Aldrich, Deisenhofen, Germany)-1% penicillin-streptomycin (Sigma-Aldrich)-1% HEPES (N-2-hydroexyethylpiperazine-N′-2-ethanesulfonic acid) (Gibco BRL, Karlsruhe, Germany)-1% heat-inactivated (56°C, 30 min) human plasma. After 1 h of incubation the nonadherent cell fraction was removed and the adherent cells were further incubated for 24 h. Afterwards, cells were fed with granulocyte-macrophage colony-stimulating factor (GM-CSF) (Amgen GmbH, Munich, Germany) (800 U/ml) and interleukin-4 (IL-4) (Strathmann, Hamburg, Germany) (500 U/ml) and incubated for a further 48 h. Then, cells were fed again with GM-CSF (400 U/ml) and IL-4 (500 U/ml) and incubated for further 48 h. On day 5 the cells were immature dendritic cells. They were collected and counted, and a part was used for experiments. The remaining cells were treated with a maturation cocktail composed of GM-CSF (40 U/ml), IL-4 (200 U/ml), IL-1β (Sigma-Aldrich) (1 ng/ml), prostaglandin E2 (Cayman Chemicals, Ann Arbor, Mich.) (0.5 μg/ml), and tumor necrosis factor (Boehringer Ingelheim, Vienna, Austria) (1.25 ng/ml). FACS analysis revealed that mature DCs (maDCs) showed increased expression of CD80, CD83, CD86, and MHC-II and reduced expression of CD14 compared to imDCs (data not shown). Our results demonstrated that all matching pairs of R71- and T7-Nefs did not differ significantly in their abilities to enhance HIV-1 replication in cocultures of imDCs with nonactivated autologous PBMC (Fig. 3A). On average, both groups of nef alleles were equally efficient in this assay (Fig. 3B). In comparison, cultures of maDCs and imDCs alone or of unstimulated PBMC did not yield significant levels of HIV-1 replication (data not shown).
FIG. 3.
Replication of HIV-1 R71- and T71-Nef variants in cocultures of imDCs and autologous PBMC. (A) Average RT activities obtained after triplicate infection with the indicated HIV-1 Nef variants. (B) Average RT values ± standard deviations for the T71 and R71 groups of HIV-1 Nef variants. ImDCs (5 × 104) were isolated as described (14) and incubated with the R5-tropic HIV-1 NL4-3 005pf135 V3 Env variant expressing the indicated R71- or T71-Nefs for 90 min. The cells showed the characteristic features of imDCs, e.g., high expression of MHC-I and MHC-II, moderate expression of CD14, and relatively low expression of CD80, CD83 and CD86 compared to maDCs. Subsequently, the cells were washed twice with fresh medium to remove unbound virus and incubated overnight. One day after infection 2 × 105 nonactivated autologous PBMC were added to the cultures. Infections were performed in triplicate with virus stocks containing 2.5 ng p24 antigen. Virus production in the culture supernatant was determined by RT assay (19). The results were confirmed in an independent experiment.
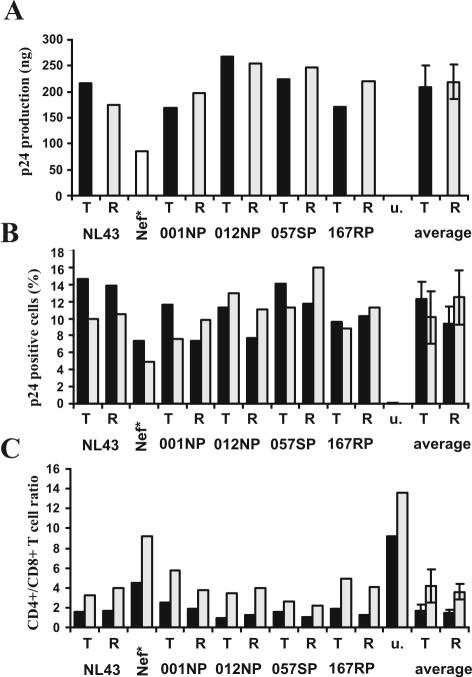
Nef also increases HIV-1 replication in ex vivo human lymphoid tissue (HLT) (9). This experimental system is of high physiological relevance, because HLT maintains its original complexity of cell populations and cytoarchitecture and does not require exogenous activation to support productive HIV infection and viral spread. As expected, NL4-3 nef-open virus replicated more efficiently than the nef-defective form (nef*) (Fig. 4A). The R71T variation in Nef, however, did not significantly affect virus production. Furthermore, the frequencies of p24-positive HIV-1-infected cells did not differ significantly between HLT infected with R71- and T7-Nef variants (Fig. 4B). CD4+ T-cell depletion in HLT correlates with the efficiency of viral replication (10). Concordant with this, we found that the CD4+/CD8+ T-cell ratio was high in uninfected tissues and only moderately reduced in HLT infected with the nef-defective HIV-1 variant (Fig. 4C). In contrast, all HIV-1 R71- and T7-Nef variants resulted in a strong decline in the CD4+/CD8+ T-cell ratio. Our observation that the R71T Nef variation does not impair the ability of HIV-1 to replicate efficiently and to cause CD4+ T-cell depletion in HLT is consistent with its presence in some AIDS patients with high viral loads and low CD4+ T-cell counts (13).
FIG. 4.
The R71T variation in Nef does not attenuate HIV-1 replication and CD4+ T-cell depletion in HLT. (A) Cummulative p24 production over 15 days of HLT infection with the indicated X4-tropic HIV-1 NL4-3 Nef variants. Infections were performed without adding exogenous IL-2 with virus stocks containing 10 ng of p24 antigen and cummulative virus production was monitored at 3, 6, 9, 12 and 15 days postinfection. u, uninfected tissue. (B) Frequency of HIV-1 infected p24+ cells and (C) CD4+/CD8+ T-cell ration in the tissue blocks (black bars) and cells that migrated in the gel foams (grey bars) at the end of culture at 15 days postinfection. Tissue culture, infections and FACS analysis were performed as described previously (9, 10). Similar results were obtained in two independent experiments using tissues derived from different donors.
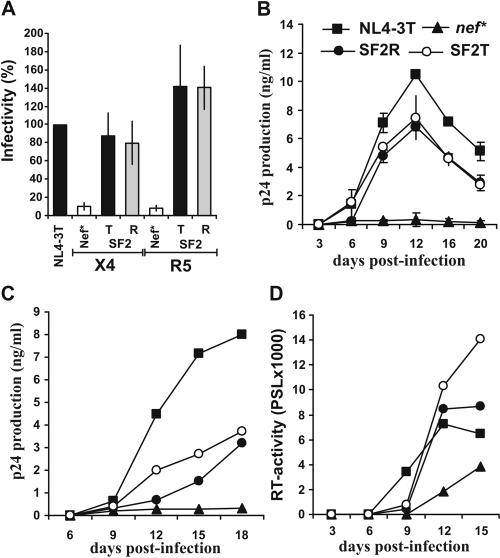
Our results demonstrate that the R71T variation has no significant effect on the functional activity of primary Nef proteins. Furthermore, the matching R71- and T71-Nefs showed only modest or no appreciable differences in their capacities to interact with PAK2 (20) (K. Saksela and G. H. Renkema, personal communication). In contrast, a previous report suggested that the R71T conversion alters the ability of Nef to bind cellular partners, impairs its activity in enhancing HIV-1 replication, and might help the virus to establish a latent reservoir (7). The effects of the R71T variation could be context dependent, and this change might attenuate the function of some nef alleles, such as SF2 (7). To evaluate this possibility we generated SF2-Nefs containing the corresponding R75T variation. We found that the R75- and T75-SF2-Nefs modulate the cell surface expression of various human receptors with undistinguishable efficiency (data not shown). More importantly, however, the R75T variation consistently did not reduce the functional activity of the SF2-Nef in enhancing virion infectivity or in stimulating HIV-1 replication in PBMC, imDCs-PBMC cocultures, and ex vivo infected HLT (Fig. 5).
FIG. 5.
The R75T variation does not impair HIV-1 SF2-Nef function. (A) P4-CCR5 cells were infected in triplicate with two independent virus stocks of the indicated X4- or R5-tropic HIV-1 Nef variants containing 1.0 ng p24 antigen. Shown are average values of the 6 measurements ± standard deviations. Infectivity is shown relative to the respective HIV-1 NL4-3 variant expressing the wild type T71-Nef. (B) Unstimulated PBMC were infected in duplicate with the X4-tropic HIV-1 Nef variants and cultured as described in the legend to Fig. 2. Similar results were obtained with an independent virus stock and with PBMC derived from two different donors. (C) Replication of R5-tropic HIV-1 SF2 R75- and T75 Nef variants in cocultures of imDCs and autologous PBMC. Cells were isolated, characterized and infected as described in the legend to Fig. 3. The results were confirmed in two independent infections. (D) Replication of X4-tropic HIV-1 NL4-3 Nef variants in ex vivo HLT. Infections were performed as described in the legend to Fig. 4 and virus production was monitored by RT assay.
In summary, our results clearly argue against a relevant role of the R71T or R75T variation for Nef function or for viral pathogenesis, respectively, in HIV-1-infected individuals. We could not confirm that this change separates the ability of Nef to down-modulate CD4 and to stimulate viral replication. Most likely both CD4 down-modulation and cellular activation contribute to the accelerated replication of HIV-1 variants expressing functional Nef. For example, it has been shown that the HIV-1 Nef intersects the macrophage CD40L signaling pathway to promote resting-cell infection (29). The relative contributions of both CD4 down-modulation and effects on cellular signaling pathways for viral spread in primary human cells need further investigation. Furthermore, it will be of interest to determine whether other naturally occurring sequence variations in Nef might affect these activities.
Acknowledgments
We thank N. Bailer and S. Aftring for excellent technical assistance, T. Mertens for constant encouragement, and I. Bennett for critical reading.
We thank the Wilhelm-Sander Foundation and the Deutsche Forschungsgemeinschaft for financial support.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arganaraz, E. R., M. Schindler, F. Kirchhoff, M. J. Cortes, and J. Lama. 2003. Enhanced CD4 down-modulation by late-stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 278:33912-33919. [DOI] [PubMed] [Google Scholar]
- 3.Benson, R. E., A. Sanfridson, J. S. Ottinger, C. Doyle, and B. R. Cullen. 1993. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus nef prevents viral super infection. J. Exp. Med. 177:1561-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 7.Fackler, O. T., D. Wolf, H. O. Weber, B. Laffert, P. D'Aloja, B. Schuler-Thurner, R. Geffin, K. Saksela, M. Geyer, B. M. Peterlin, G. Schuler, and A. S. Baur. 2001. A natural variability in the proline-rich motif of Nef modulates HIV-1 replication in primary T cells. Curr. Biol. 11:1294-1299. [DOI] [PubMed] [Google Scholar]
- 8.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 9.Glushakova, S., J.-C. Grivel, K. Suryanarayana, P. Meylan, J. D. Lifson, R. Desrosiers, and L. Margolis. 1999. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J. Virol. 73:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iafrate, A. J., S. Carl, S. Bronson, C. Stahl-Hennig, T. Swigut, J. Skowronski, and F. Kirchhoff. 2000. Disrupting surfaces of Nef required for down-regulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J. Virol. 74:9836-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhoff, F., P. J. Easterbrook, N. Douglas, M. Troop, T. C. Greenough, J. Weber, S. Carl, J. L. Sullivan, and R. D. Daniels. 1999. Sequence variations in human immunodeficiency virus type 1 Nef are associated with different stages of disease. J. Virol. 73:5497-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 16.Lundquist, C. A., M. Tobiume, J. Zhou, D. Unutmaz, and C. Aiken. 2002. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J. Virol. 76:4625-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papkalla, A., J. Munch, C. Otto, and F. Kirchhoff. 2002. Nef enhances human immunodeficiency virus type 1 infectivity and replication independently of viral coreceptor tropism. J. Virol. 76:8455-8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potts, B.J. 1990. “Mini” reverse transcriptase (RT) assay, pp. 103-106. In A. Aldovini, and B.D. Walker (ed.), Techniques in HIV research. Stockton Press, New York, N.Y.
- 20.Renkema, G. H., A. Manninen, D. A. Mann, M. Harris, and K. Saksela. 1999. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 9:1407-1410. [DOI] [PubMed] [Google Scholar]
- 21.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 22.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler, M., S. Wuerfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Münch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 25.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol. 64:453-663. [DOI] [PubMed] [Google Scholar]
- 26.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C., Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swigut, T., N. Shody, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swingler, S., B. Brichacek, J. M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]