Abstract
Hepatitis A virus (HAV) has previously been reported to agglutinate human red blood cells at acidic pHs. Treatment of erythrocytes with different enzymes and chemical reagents indicated that HAV attachment is mediated through an interaction with sialylglycoproteins. HAV hemagglutination could be blocked by incubating the virus with glycophorin A, indicating that this sialylglycoprotein is the erythrocyte receptor. The number of receptors used was estimated to be around 500 per cell. At the same time, HAV-induced hemagglutination could also be blocked by either monoclonal antibody H7C27 or an anti-VP3(102-121) ascitic fluid, indicating that lysine 221 of VP1 and the surrounding VP3 residues lining the capsid pit are involved in HAV binding to erythrocytes.
Hepatitis A virus (HAV) is a nonenveloped, positive-strand RNA virus classified as the type species of the genus Hepatovirus within the family Picornaviridae (45). Three types of intact antigenic HAV particles have been described: (i) RNA-containing capsids composed of proteins VP1, VP2, VP3, and possibly VP4 (virions); (ii) RNA-containing capsids composed of proteins VP1, VP0, and VP3 (provirions); and (iii) empty capsids with the same capsid composition as provirions but lacking RNA (procapsids) (8).
HAV has a limited number of antigenic sites. The immunodominant site, composed of closely clustered epitopes, is defined by two major groups of escape mutants that include residues 70, 71, and 74 of VP3 and residues 102, 171, and 176 of VP1 (29, 30). There is another, apparently distinct antigenic site represented by mutants at residue 221 of VP1, and an additional and still undefined third antigenic site to which no escape mutant has so far been isolated (29, 30). Several monoclonal antibodies (MAbs), such as K34C8, K24F2, and B5B3, are directed toward the immunodominant site, while MAbs H7C27 and MAK-4E7 are directed against the other two antigenic sites, respectively. Some of the epitopes contained in the immunodominant site, such as those defined by MAb K24F2, are detected in the first stages of capsid formation on the pentameric subunits, while others, such as those defined by MAb K34C8, are formed by structural changes during the assembly of pentamers into intact particles (40).
A class I integral membrane glycoprotein of unknown natural function has been independently isolated by two groups from AGMK cells (22) and from a hybrid marmoset-Vero cell line (2) and has been characterized as a receptor for HAV (havcr-1). Additionally, a human homologue of this HAV receptor (huhavcr-1) has been isolated from human liver and kidney cells (15), and although nothing is known about its natural biological function, it has been reported to play a major role in T-cell helper differentiation and as an asthma determinant gene (27, 28). On the other hand, like other picornaviruses, HAV binds to the surfaces of human erythrocytes, causing hemagglutination (13, 14). This hemagglutination is optimally observed at pH 5.5.
In the present work, we describe the nature of the receptor and the virus residues involved in the attachment of HAV to the erythrocyte surface.
MATERIALS AND METHODS
Virus and cells.
The cytopathogenic HM-175 strain of HAV (courtesy of T. Cromeans, Centers for Disease Control, Atlanta, Ga.) (11) was propagated in FRhK-4 cell monolayers. Concentrated viral stocks were obtained as previously described (9). Briefly, at 5 to 6 days postinfection, cells from a T-175 cm2 flask were harvested by trypsin treatment, collected by centrifugation, resuspended in 500 μl of NT buffer (0.1 M NaCl, 10 mM Tris-HCl, 1% NP-40 [pH 7.4]), and incubated for 30 min at room temperature. These lysed cell suspensions were centrifuged at 1,700 × g for 5 min, and the supernatants were again centrifuged at 13,000 × g for 5 min. Viruses recovered in the supernatants were submitted to three sonication cycles of 30 s at 60 W in the presence of 0.4% sodium dodecyl sulfate. Five hundred microliters of these concentrated viral stocks was layered onto a 5 to 45% sucrose gradient in TNMg buffer (20 mM Tris-HCl, 10 mM NaCl, 50 mM MgCl2 [pH 6.7]) and spun at 205,000 × g for 165 min. Fractions containing infectious virus (150S) and empty particles (70S) were identified both by determination of the refraction index and by a sandwich enzyme-linked immunosorbent assay (ELISA) (33) consisting of HAV capture by a convalescent-phase serum (HCS-2) followed by detection with MAb K24F2 (for information concerning antibodies, see below). Positive fractions from six gradients were pooled (150S and 70S particles were pooled separately) and dialyzed against water to remove sucrose. Finally, both pooled samples were concentrated by ultracentrifugation at 229,600 × g for 4 h and resuspension in a final volume of 500 μl.
Infectious fractions (150S) were quantified by determining the most probable number of cytopathogenic units per milliliter by infecting cell monolayers grown in 96-well microtiter plates (31).
Antibodies.
Several HAV-specific MAbs were used: K34C8 and K24F2 (Commonwealth Serum Laboratories, Victoria, Australia); B5B3, generously provided by B. Ferns (University College London Medical School, London, United Kingdom); H7C27, generously provided by R. Decker (Abbot Laboratories, North Chicago, Ill.); and MAK-4E7, generously provided by B. Flehmig (University of Tübingen, Tübingen, Germany). A convalescent-phase serum, HCS-2, generously provided by R. Lluna (Hospital Militar, Barcelona, Spain) and the polyclonal ascitic antibody anti-VP3(102-121), raised against the synthetic peptide comprising residues 102 to 121 of VP3 (32), were also used.
Erythrocyte purification.
Human type-O erythrocytes (provided by the blood bank of the Hospital Vall d'Hebron, Barcelona, Spain) were purified on preformed self-generated gradients of Percoll (Pharmacia Biotech) according to the manufacturer's specifications. The gradient was formed by spinning 10 ml of a 70% solution of Percoll in 150 mM NaCl at 2,000 × g for 15 min. Two milliliters of gradient material was removed from the bottom of the tube, and 2 ml of 50% heparinized blood in 150 mM NaCl was layered on top of the gradient. The sample was centrifuged for 5 min at 400 × g, the platelets on the top of the gradient were removed and replaced by saline, and the gradient was centrifuged again at 800 × g for 15 min. After separation of the erythrocyte-containing fraction, the cells were washed three times in Alsever's solution (2.05% [wt/vol] glucose, 0.8% sodium citrate, 0.055% citric acid, 0.42% NaCl [pH 6.7]), collected by centrifugation at 500 × g for 5 min, and resuspended in the same solution. Before the hemagglutination assays, erythrocytes were again washed three times in phosphate-buffered saline (PBS) (0.137 M NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4) at pH 5.5 or 7.2.
Number of HAV receptors on erythrocytes.
The number of HAV receptors on the erythrocyte membrane was estimated by flow cytometry. Briefly, 105 erythrocytes were incubated with different amounts of 150S particles for 2 h at 4°C in PBS (pH 5.5). Unbound viruses were removed by three washes with PBS (pH 7.2) containing 5% skim milk, and bound viruses were detected by incubation with two anti-HAV MAbs (K34C8 and K24F2, diluted 1:50,000 and 1:5,000, respectively, in PBS [pH 7.2]) for 2 h at 4°C. After three washes with PBS-5% skim milk, the erythrocytes were incubated with a fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G for 45 min at 4°C. Finally, PBS-washed cells were analyzed in a flow cytometer (Beckman Coulter XL). The forward-angle light scatter was used to select cell size, and the side-angle light scatter was used to selected shape and structure; consequently, only the subpopulation of intact erythrocytes was used for the fluorescence measurements. Cursor M, including the labeled erythrocytes, was defined. For the establishment of this cursor, erythrocytes incubated with the antibodies but without viruses were used as negative controls.
The multiplicity of adsorbed virus per erythrocyte (MOA) was calculated as the ratio between the number of infectious viruses (150S) added and the number of total erythrocytes. The assayed MOA values were 10, 40, and 200. The estimated number of HAV receptors per erythrocyte was calculated by dividing the number of erythrocytes included in cursor M by the number of infectious viruses added.
Statistical differences in the mean number of receptors and the mean percentage of positive erythrocytes obtained after incubation with different virus inocula were determined by analysis of variance.
Hemagglutination assay.
Hemagglutination assays were performed in 96-well U-bottom plates. Serial twofold dilutions of 150S or 70S particles were made in PBS at pH 5.5 or 7.2. Twenty microliters of diluted particles was dispensed to each well, and 20 μl of a 1% erythrocyte suspension at pH 5.5. or 7.2 was added, mixed, and incubated for 1 h at room temperature. Negative controls were also assayed by incubating 20 μl of PBS with the same volume of the erythrocyte suspension under the same conditions. The hemagglutination titer was expressed as the reciprocal of the greatest dilution of HAV that caused complete agglutination of the erythrocytes (end-point dilution) in comparison with the negative controls. A hemagglutination unit was defined as the amount of particles present at the hemagglutination end-point dilution.
Enzymatic treatments of erythrocytes.
One percent erythrocyte suspensions in PBS, at pH 7.2, were treated with agitation for 1 h at 37°C with different concentrations of the following enzymes: 0.05 g of trypsin (1:250, from porcine pancreas; Difco)/ml, 0.1 mg of chymotrypsin (type I-S from bovine pancreas; Sigma)/ml, and 0.1 and 0.05 U of neuraminidase (type V from Clostridium perfringens; Sigma)/ml. Additionally, enzymatic treatments with 1.5, 0.5, and 0.05 mU of phospholipase C (type I from C. perfringens; Sigma)/ml and with 0.625 and 0.125 U of phosphatidylinositol-specific phospholipase C (from Bacillus cereus; Sigma)/ml were performed with agitation for 15 min at 37°C. After incubation, erythrocytes were washed three times in PBS at pH 5.5 and were used in hemagglutination assays as described above.
All hemagglutination titers were obtained in comparison to PBS-treated erythrocytes.
Periodate treatment of erythrocytes.
Sodium periodate at final concentrations of 0.05 and 0.025 mM was added to 1% erythrocyte suspensions at pH 7.2 and incubated with agitation for 15 min at 37°C. An equal volume of 1% (vol/vol) glycerol was added at the end of the treatments to neutralize periodate, followed by three washes of the erythrocytes with PBS at pH 5.5. These treated erythrocytes were used in hemagglutination assays in comparison with PBS-treated erythrocytes supplemented with glycerol.
Incubation of HAV particles with heparan sulfate, chondroitin sulfate, and glycophorin A.
Four and 8 hemagglutination units of HAV 150S and 70S particles were incubated with the glycosaminoglycans heparan sulfate and chondroitin sulfate, at concentrations of 0.2 and 0.02 mg/ml. Additionally, the same amounts of HAV particles were incubated with 250 and 25 μg of the glycoprotein glycophorin A (type MN; Sigma)/ml. All incubations were performed with agitation for 2 h at 37°C at pH 5.5 and 7.2, and hemagglutination assays were performed at pH 5.5, with PBS-treated HAV particles as controls.
Incubation of HAV particles with different antibodies.
HAV particles were incubated with several MAbs (K34C8, K24F2, B5B3, H7C27,and MAK-4E7) and with the anti-VP3(102-121) ascitic antibody. All incubations were performed with agitation at 37°C for 2 h, at pH 7.2, by using either 4 or 8 hemagglutination units, and subsequently, the antibody-blocked particles were used in hemagglutination assays at pH 5.5.
All antibodies were used at the highest dilution yielding maximum HAV recognition. Nonimmune ascitic fluids and the convalescent-phase HCS-2 serum were used as negative and positive controls, respectively.
In addition, in some experiments, the virus was incubated with 100 μg of glycophorin A/ml prior to antibody recognition.
ELISAs.
A sandwich ELISA for recognition of 150S HAV particles was performed (10). Particles were captured by the HCS-2 convalescent-phase serum and detected with either MAb H7C27 or MAb K34C8. Mock-infected FRhK-4 cell extracts were used as negative controls.
Alternatively, a competitive ELISA in which the anti-VP3(102-121) antibody was preincubated with the virus for 2 h at 37°C prior to being added to a microplate coated with the synthetic peptide VP3(102-121) was used to evaluate viral recognition by this antibody. A significant decrease in peptide recognition was indicative of viral recognition (10). Viruses were incubated with glycophorin A prior to being recognized by anti-VP3(102-121) antibody. Under these conditions, a decrease in peptide recognition was indicative of a nonblocking effect of glycophorin A, while a lack of decrease in peptide recognition was indicative of a blockage of antibody binding by glycophorin A.
RESULTS
Hemagglutination of human erythrocytes by different HAV antigenic particles.
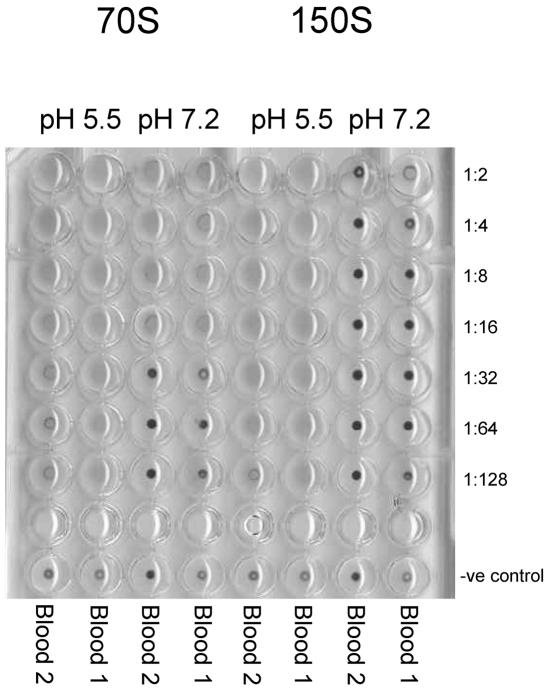
To study the hemagglutination capacities of the different antigenic HAV particles, sucrose-purified HAV fractions corresponding to infectious virus (150S virions and provirions), and empty particles (70S procapsids) were tested in hemagglutination assays at either a neutral (7.2) or an acidic (5.5) pH (Fig. 1). While 150S particles exhibited hemagglutination only at pH 5.5, 70S particles induced hemagglutination at both neutral and acidic pHs, although more efficiently in the latter case.
FIG. 1.
Effect of pH on HAV hemagglutination. Two different human type-O erythrocytes were assayed with twofold dilutions of 150S or 70S HAV particles at pH 7.2 and 5.5.
Number of HAV receptors on the erythrocyte surface.
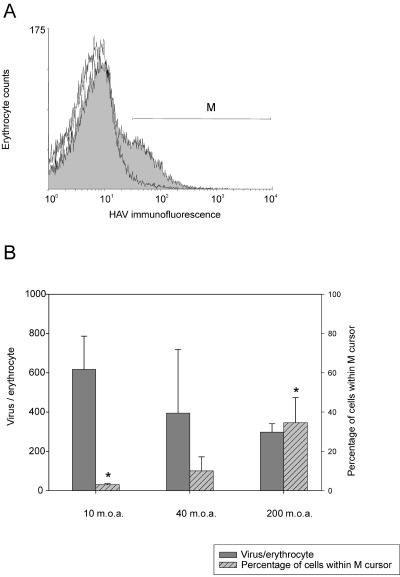
In order to estimate the number of HAV receptors per erythrocyte, a fixed number of erythrocytes was incubated with different amounts of purified infectious virus particles (150S) and detected by an indirect immunofluorescence assay with MAbs K34C8 and K24F2, combined with cytometric quantitation. The immunolabeled cell population contained in cursor M (Fig. 2A) was used to determine the number of viral receptors per erythrocyte, which was around 500 (430 ± 241).
FIG. 2.
Immuno-flow cytometry detection of adsorbed HAV 150S particles on human erythrocytes. (A) Typical immunodetection experiment. Two curves are superimposed. The open curve on the left corresponds to erythrocytes in the absence of viruses but immunoassayed for HAV; the shaded curve on the right corresponds to erythrocytes with adsorbed viruses that were immunoassayed for HAV. Cursor M includes the subpopulation of erythrocytes with the highest fluorescence labeling. (B) The number of HAV receptors on the erythrocyte membrane and the percentage of erythrocytes with adsorbed HAV particles are plotted versus the viral inoculum added, or MOA. Asterisks indicate mean values that are significantly different (P < 0.05).
Analysis of several experiments using different theoretical inocula, i.e., MOA values of 10, 40, and 200, did not reveal significant differences in the number of adsorbed viruses per erythrocyte (Fig. 2B). However, statistically significant (P < 0.05) differences were detected in the percentage of cells in the M region, with a direct positive relation between the virus inoculum and the proportion of labeled cells.
Effects of different erythrocyte treatments on HAV hemagglutination.
To characterize the nature of the receptor(s) to which HAV particles attach, erythrocytes were subjected to different chemical treatments such as trypsin digestion (which cleaves proteins at the carboxy peptide bonds of arginine and lysine residues), α-chymotrypsin digestion (which cleaves proteins at the carboxy peptide bonds of aromatic residues), phospholipase C digestion (which hydrolyzes the phosphate bond on phosphatidylcholine, phosphatidylinositol, and other glycerophospholipids), sodium periodate oxidation (which strongly oxidates carbohydrates), and neuraminidase digestion (which liberates N-acetylneuraminic acid). Treated erythrocytes were then tested in hemagglutination assays in comparison to nontreated cells.
Treatment of erythrocytes with either 50 mg of trypsin/ml or 1 mg of α-chymotrypsin/ml led to different levels of hemagglutination inhibition, ranging from 0 to 100%, with regard to nontreated erythrocytes; this high degree of variability was associated with the blood batch tested. Thus, after trypsin treatment, the hemagglutination induced by 150S or 70S particles was not inhibited in 33 or 25% of the blood samples assayed, respectively, while the rest of the blood batches tested showed a mean inhibition of 79 or 73%, respectively. Regarding the treatment with α-chymotrypsin, inhibition of the hemagglutination induced by both types of particles was observed, with means of 75 and 50%, respectively.
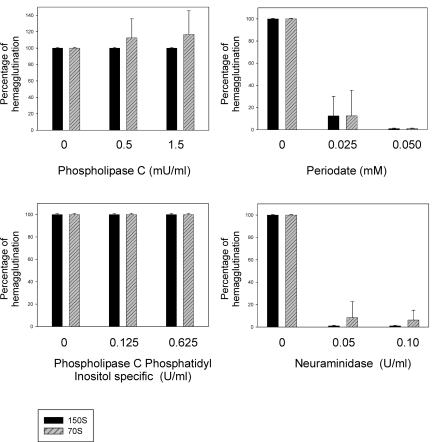
HAV-induced hemagglutination was dramatically inhibited (more than 90% reduction [Fig. 3]) after treatment of erythrocytes with sodium periodate, indicating a carbohydrate nature of the receptor(s). Three cell surface carbohydrates to which viruses commonly bind are sialic acid, heparan sulfate, and chondroitin sulfate. To assay whether the carbohydrate-dependent HAV hemagglutination required sialic acid, erythrocytes were treated with neuraminidase, which removes α-2,3- and α-2,6-linked sialic acid residues on the cell surface. Again, hemagglutination was severely decreased (Fig. 3). Interestingly, although procapsid-induced hemagglutination was very sensitive to the neuraminidase digestion of erythrocytes, it was remarkably more resistant than virion-induced hemagglutination. From these results it may be concluded that a sialylglycoprotein is involved in HAV hemagglutination.
FIG. 3.
Effects of several enzymatic and oxidation treatments of human erythrocytes on HAV-induced hemagglutination.
Treatment of erythrocytes with the general phospholipase C (active against all glycerophospholipids) (Fig. 3) had no inhibitory effects on virion-induced hemagglutination, but in contrast, surprisingly, an increase in procapsid-induced hemagglutination was observed. Similarly, the phosphatidylinositol-specific phospholipase C showed no inhibitory effect (Fig. 3). These results exclude involvement in HAV hemagglutination of the decay-accelerating factor (DAF/CD55) or any other sialylglycoproteins anchored to the cell membrane through a glycosylphosphatidylinositol.
Effects of different virus preincubations on HAV hemagglutination.
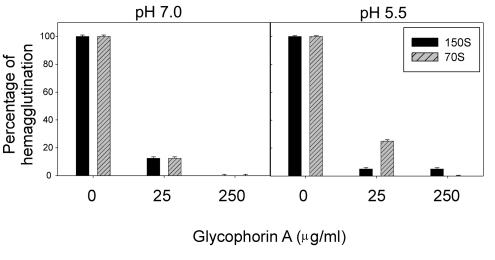
One of the sialylglycoproteins most frequently used by different viruses as an erythrocyte receptor is glycophorin A. To ascertain this possibility in the case of HAV, 4 or 8 hemagglutinating units of either virions or procapsids was preincubated with two different concentrations of glycophorin A, at either pH 5.5 or pH 7.2, just prior to the agglutination assay. Treatment with the highest concentration (250 μg/ml) abolished the hemagglutination capacities of both viruses and procapsids (Fig. 4), independently of the concentration of virus particles used and the pH tested. At the lower concentration of glycophorin A (25 μg/ml), hemagglutination was also severely decreased in all cases, although residual activity was detected (Fig. 4). These results indicate that glycophorin A may act as an erythrocyte receptor for HAV.
FIG. 4.
Effect of glycophorin A virus-blocking treatments on HAV-induced hemagglutination (and hemagglutination units).
In order to exclude any role of heparan sulfate and chondroitin sulfate as HAV receptors on the erythrocyte membrane, again 4 or 8 hemagglutinating units of either virions or procapsids was preincubated, at both pH 5.5 and pH 7.2, with each of these two glycosaminoglycans just before the agglutination assay. None of these pretreatments inhibited HAV hemagglutination.
Mapping of the erythrocyte-interacting HAV residues.
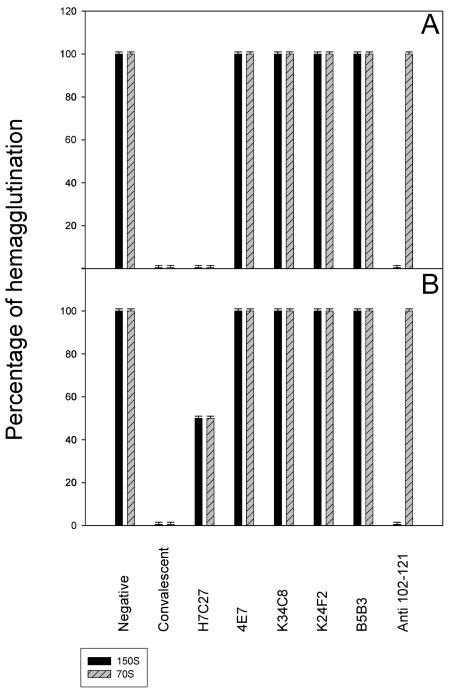
A panel of different MAbs was tested in order to elucidate the HAV residues involved in the erythrocyte-HAV interaction(s). Some MAbs directed against the HAV immunodominant site, such as K24F2 (defined by Asp 70 and Ser 71 of VP3 [29, 30]), K34C8 (defined by Val 72 of VP3 [37]), and B5B3 (defined by Asp 70 of VP3 and Ser 102 of VP1 [30]), did not reduce the hemagglutination titer (Fig. 5). MAb MAK-4E7, whose defining residues are unknown (29, 30), also failed to affect HAV hemagglutination. In contrast, significant inhibition of hemagglutination was observed with MAb H7C27, which is defined by residue Lys 221 of VP1 (29, 30); the level of inhibition at this antibody dilution was proportional to the number of virus particles (Fig. 5). Additionally, a similar degree of inhibition was observed after the virus was blocked with a convalescent-phase antiserum. The same results were obtained with the virion and procapsid HAV fractions (Fig. 5).
FIG. 5.
Effects of several antibody blocking treatments on HAV-induced hemagglutination. Experiments used 4 (A) or 8 (B) hemagglutination units.
Since MAb H7C27 significantly inhibits (100%) HAV recognition by an antipeptide antibody against a linear B-T epitope defined by amino acids 102 to 121 of VP3 (10, 36), a new set of hemagglutination inhibition experiments was performed using an anti-VP3(102-121) antibody (32). Interestingly, this antibody could inhibit only virion-induced, not procapsid-induced, hemagglutination (Fig. 5), again suggesting the existence of differences in surface conformation between virions and procapsids.
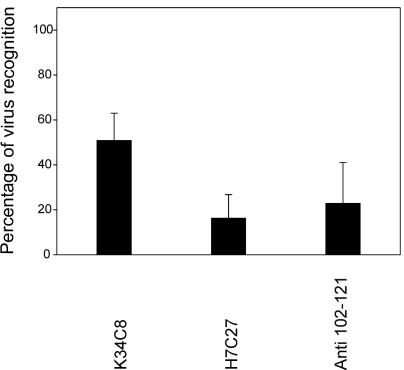
To ascertain whether the HAV residues identified were actually interacting with glycophorin A, 4 hemagglutination units of 150S HAV particles were preincubated with soluble glycophorin A at 100 μg/ml and subsequently tested for their recognition by MAbs H7C27 and K34C8 or the anti-VP3(102-121) antibody. A reduction in the recognition of HAV by the MAbs in glycophorin A-incubated particles relative to that in PBS-incubated particles was indicative of a close location of the residues involved in glycophorin A binding and MAb binding. A significant reduction in recognition by MAb H7C27 (around 84%) was observed after incubation with glycophorin A (Fig. 6). Preincubation of the virus with glycophorin A caused a ∼77% inhibition of anti-VP3(102-121) binding to virus (Fig. 6).
FIG. 6.
Reduction of antibody virus recognition by glycophorin A. Four hemagglutination units of 150S HAV particles was preincubated with soluble glycophorin A at 100 μg/ml and subsequently tested for recognition by MAbs H7C27 and K34C8 or the anti-VP3(102-121) antibody.
DISCUSSION
HAV can bind and agglutinate human type-O and -A erythrocytes (13, 14, 41); however, its erythrocyte receptor remains to be elucidated. HAV binding capacity was totally lost after treatment of erythrocytes with periodate. In some blood batches, digestion with trypsin or chymotrypsin completely prevented hemagglutination, too, although in other blood batches, hemagglutination was still present. This striking behavior is likely to be due, on the one hand, to the different levels of glycosylation or the type of glucid added, which could alter protease accessibility, and, on the other, to the use of different sialic acid molecules, located before or after protease cleavage sites (1, 18). Nevertheless, overall, our data indicate that the HAV binding erythrocyte receptor is a glycoprotein.
Many viruses that bind to carbohydrates interact with sialic acid, heparan sulfate, or chondroitin sulfate. Among picornaviruses, enterovirus 70 (44), encephalomyocarditis virus (42), mengovirus (26), rhinovirus 87 (43), and persistent Theiler’s virus (21, 46) use sialylglycoproteins as erythrocyte receptors, and foot-and-mouth disease virus (FMDV) (20) and neurovirulent Theiler's virus (35) use heparan sulfate as a receptor in several cells. In the case of HAV, since hemagglutination was mostly inhibited by neuraminidase treatment of erythrocytes and was not affected by incubation of viruses with either heparan sulfate or chondroitin sulfate, it could be concluded that the receptor is a sialylglycoprotein. Sialic acid residues are required in cellular receptors by many different viruses and may play either a direct role, as an integral part of the virus binding site on the receptor, or an indirect role, interacting with positively charged amino acid residues and holding the receptor in the required configuration for virus recognition.
Since HAV-induced hemagglutination was not inhibited by treatment of erythrocytes with phosphatidylinositol-specific phospholipase C, sialylglycoproteins anchored to the cell membrane through a glycosylphosphatidylinositol may be ruled out, and hence the involvement of DAF, one of the most frequently used sialylglycoprotein receptors in picornavirus infection and hemagglutination (5), was excluded. Another sialylglycoprotein frequently employed by hemagglutinating viruses is glycophorin A (26, 42). The hemagglutination inhibition induced by preincubations of HAV particles with soluble glycophorin A confirmed that HAV also uses this sialylglycoprotein as a receptor on human erythrocytes.
Since the number of adsorbed viruses per cell was not significantly enhanced by increasing the multiplicity of added viruses, it may be assumed that the number of receptors used per cell is rather low. This figure was estimated to be around 500. This value is quite similar to that reported for mengovirus (368) and fivefold lower than that calculated for enterovirus 70 (2,688), with glycophorin A used as an erythrocyte receptor by both picornaviruses (44).
Although HAV interacted with soluble glycophorin A at both neutral and acidic pHs, the results described here revealed a critical role of pH in the HAV-erythrocyte interaction. As a matter of fact, several HAV capsid modifications associated with low pH have been observed, such as the conversion of immature to mature virions, exposure of hydrophobic domains on the capsid surface, and cleavage of VP0 to VP2 (6, 7). Interestingly, virion-induced hemagglutination was more pH dependent than procapsid-induced hemagglutination, suggesting the occurrence of conformational differences between 150S and 70S particles in the capsid region involved in erythrocyte attachment. No actual crystallographic data exist on the structure of HAV, and only deduced models are available (25; M. Luo, personal communication). However, X-ray crystallography studies of several picornaviruses, such as poliovirus (3) and FMDV (12), revealed that the structural differences between empty capsids and mature viruses are mostly confined to the interior of the capsid. In poliovirus, the only significant differences described for the outer surface occur at positions 171, 233, and 244 of VP1 and at the carboxy terminus of VP3, where three amino acid residues are lost in the mature capsids (3). Additionally, it has been reported that lowering the pH to 4.6 causes substantial conformational changes to the pit area of mengovirus virions (23), including movements of the GH loop in VP1, the GH loop in VP3, and the carboxy-terminal region of VP2.
In order to map the capsid region involved in attachment to erythrocytes, several MAbs and a polyclonal anti-VP3(102-121) antibody were assayed for their abilities to inhibit HAV hemagglutination. MAb H7C27 could significantly inhibit the hemagglutination induced by both virions and procapsids. This MAb is directed against a region in the vicinity of residue Lys 221 of VP1, which is presumably located at the VP1 GH loop. Remarkably, this VP1 GH loop in poliovirus contains Ser 233, which aligns with Thr 222 of HAV, located contiguously to Lys 221 of HAV, and whose α-carbon position is deviant in procapsid particles relative to the mature virion (3). As mentioned above, this loop undergoes a conformational change under acidic conditions in mengovirus. Assuming a similar phenomenon in HAV, it may be suggested that the acidic conformation is optimal for the virus-erythrocyte interaction, although the previously mentioned potential deviation of Thr 222 in empty capsids could enable its interaction with the receptor, even at a neutral pH. In the case of FMDV, this VP1 GH loop contains the RGD sequence, which is responsible for cellular attachment through an integrin interaction (16), and in poliovirus this loop contains residues 213 to 214 and 222 to 236, which interact with the poliovirus receptor (4). It is also noteworthy that only virion-induced hemagglutination was inhibited by the anti-VP3(102-121) antibody. Several residues of the amino-terminal and central regions of the sequence comprising amino acids 102 to 121 of VP3 are located close to both Thr 222 of VP1 and the carboxy terminus of VP3, whose conformation is likely to differ in procapsids and virions. Encephalomyocarditis virus and mengovirus bind to the sialic acid anchored to the glycophorin A of erythrocytes (26, 42) through the HI loop of VP1, which is located at the icosahedral fivefold axis. In the case of HAV, the region involved in erythrocyte attachment seems to be located at or near the predicted pit (25).
One important unsolved question is the biological rationale of HAV attachment to human erythrocytes. Hemagglutination has been helpful in identifying cell-binding receptors for many viruses (19, 24, 34), and in the present case, glycophorin A has been identified as a binding receptor for HAV. However, glycophorin A seems to be found exclusively on the erythrocyte membrane, and thus its actual role on HAV pathogenesis is unclear, since the low pH of blood should be fatal under physiological conditions. Nevertheless, since HAV binds to soluble glycophorin A at both neutral and acidic pHs, a suboptimal attachment of HAV to erythrocytes may be expected to take place at a neutral pH, too. Additionally, capsid conformation “breathing” required for HAV attachment to erythrocytes may result from other conditions besides acidic pHs.
It has been suggested that erythrocyte glycoproteins may function as decoy receptors, attracting pathogens to the erythrocyte and keeping them away from target tissues (17). In this context, escaping from erythrocyte attachment may constitute an advantage for a viremic infectious agent whose target organ is the liver.
The receptors described for HAV (15, 22) are class I integral membrane glycoproteins comprising an extracellular domain, containing an N-terminal immunoglobulin-like cysteine-rich region (Cys-rich), followed by a threonine-, serine-, and proline-rich region (TSP-rich) (22). The Cys-rich region displays homology to members of the immunoglobulin superfamily, and the TSP-rich region has the characteristics of mucin-like glycoproteins (15). Although the Cys-rich region is necessary and sufficient for HAV binding (38), the TSP-rich region seems to have a major function in the virus-receptor interaction, leading to uncoating of the viral genome. It has been suggested that this mucin-like region may serve as a scaffolding for the Cys-rich region presentation or may interact directly with the viral particles (39). Assuming the latter situation and keeping in mind that glycophorin A is also a mucin-like glycoprotein, a similar interaction through sialic acid molecules could be postulated for the HAV-TSP-rich region interaction.
Acknowledgments
We acknowledge the technical expertise of the Serveis Científic-Tècnics of the University of Barcelona and the generosity of J. M. Hernandez of the Blood Bank of the Hospital Vall d'Hebron for providing us with blood samples.
This study was supported in part by grant BIO99-0455 from the CICYT, Madrid, Spain, grant 2001/SGR/00098 from the Generalitat de Catalunya, and the Centre de Biotecnologia de Catalunya (CeRBa) of the Generalitat de Catalunya.
REFERENCES
- 1.Allaway, G. P., and A. T. H. Burness. 1986. Site of attachment of encephalomyocarditis virus on human erythrocytes. J. Virol. 59:768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashida, M., and C. Hamada. 1997. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J. Gen. Virol. 78:1565-1569. [DOI] [PubMed] [Google Scholar]
- 3.Basavappa, R., R. Syed, O. Flore, J. P. Icenogle, D. J. Filman, and J. M. Hogle. 1994. Role and mechanism of the maturation cleavage of VP0 in poliovirus assembly: structure of the empty capsid assembly intermediate at 2.9 Å resolution. Protein Sci. 3:1651-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belnap, D. M., B. M. McDermott, D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelsson, J. M., M. Chan, K. R. Salomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosyl-phosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop, N. E. 1999. Conformational changes in the hepatitis A virus capsid in response to acidic conditions. J. Med. Microbiol. 48:443-450. [DOI] [PubMed] [Google Scholar]
- 7.Bishop, N. E. 1999. Effect of low pH on the hepatitis A virus maturation cleavage. Acta Virol. 43:291-296. [PubMed] [Google Scholar]
- 8.Bishop, N. E., and D. A. Anderson. 2000. Uncoating kinetics of hepatitis A virus virions and provirions. J. Virol. 74:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop, N. E., D. L. Hugo, S. V. Borovec, and D. A. Anderson. 1994. Rapid and efficient purification of hepatitis A virus from cell culture. J. Virol. Methods 47:203-216. [DOI] [PubMed] [Google Scholar]
- 10.Bosch, A., J. F. González-Dankaart, I. Haro, R. Gajardo, J. A. Pérez, and R. M. Pintó. 1998. A new continuous epitope of hepatitis A virus. J. Med. Virol. 54:95-102. [DOI] [PubMed] [Google Scholar]
- 11.Cromeans, T., M. D. Sobsey, and H. A. Fields. 1987. Development of a plaque assay for a cytopathogenic, rapidly replicating isolate of a hepatitis A virus. J. Med. Virol. 22:45-56. [DOI] [PubMed] [Google Scholar]
- 12.Curry, S., E. Fry, W. Blakemore, R. Abu-Ghazaleh, T. Jackson, A., King, S. Lea, J. Newman, and D. Stuart. 1997. Dissecting the roles of VP0 cleavage and RNA packaging in picornavirus capsid stabilization: the structure of empty capsids of foot-and-mouth disease virus. J. Virol. 71:9743-9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois, D. R., L. N. Binn, P. L. Summers, R. L. Timchak, D. A. Barvir, R. H. Marchwicki, and K. H. Eckels. 1990. Preparation of non-infectious hepatitis A virus hemagglutinin for detecting hemagglutination inhibition antibodies. J. Virol. Methods 28:299-304. [DOI] [PubMed] [Google Scholar]
- 14.Eckels, K. H., P. L. Summers, and D. R. Dubois. 1989. Hepatitis A virus hemagglutination and a test for hemagglutination inhibition antibodies. J. Clin. Microbiol. 27:1375-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feigelstock, D., P. Thompson, P. Mattoo, Y. Zhang, and G. G. Kaplan. 1998. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 72:6621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70:625-637. [DOI] [PubMed] [Google Scholar]
- 17.Gagneux, P., and A. Varki. 1999. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9:747-755. [DOI] [PubMed] [Google Scholar]
- 18.Gardner, B., S. F. Parsons, A. H. Merry, and D. J. Anstee. 1989. Epitopes on sialoglycoprotein alpha: evidence for heterogeneity in the molecule. Immunology 68:283-289. [PMC free article] [PubMed] [Google Scholar]
- 19.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J. Virol. 77:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. I. Newman, and A. M. Q. King. 1996. Efficient infection of cells in culture by type-O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jnaoui, K., M. Minet, and T. Michiels. 2002. Mutations that affect the tropism of DA and GDVII strains of Theiler's virus in vitro influence sialic acid binding and pathogenicity. J. Virol. 76:8138-8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan, G., A. Totsuka, P. Thompson, T. Akatsuka, Y. Moritsugu, and S. M. Feinstone. 1996. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15:4282-4296. [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, S., U. Boege, S. Krishnaswamy, I. Minor, T. J. Smith, M. Luo, D. G. Scraba, and M. G. Rossmann. 1990. Conformational variability of a picornavirus capsid: pH-dependent structural changes of Mengo virus related to its host receptor attachment site and disassembly. Virology 175:176-190. [DOI] [PubMed] [Google Scholar]
- 24.Krempl, C., M.-L. Ballesteros, G. Zimmer, L. Enjuanes, H.-D. Klenk, and G. Herrler. 2000. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 81:489-496. [DOI] [PubMed] [Google Scholar]
- 25.Luo, M., M. G. Rossmann, and A. C. Palmenberg. 1988. Prediction of three-dimensional models for foot-and-mouth disease virus and hepatitis A virus. Virology 166:503-514. [DOI] [PubMed] [Google Scholar]
- 26.Mann, L. M., K. Anderson, M. Luo, and C. W. Bond. 1992. Molecular and structural basis of hemagglutination in mengovirus. Virology 190:337-345. [DOI] [PubMed] [Google Scholar]
- 27.McIntire, J., D. T. Umetsu, and R. H. DeKruyff. 2004. TIM-1, a novel allergy and asthma susceptibility gene. Semin. Immunopathol. 25:335-348. [DOI] [PubMed] [Google Scholar]
- 28.McIntire, J., S. E. Umetsu, A. Omid, M. Potter, V. K. Kuchroo, G. S. Barsh, G. J. Freeman, D. T. Umetsu, and R. H. DeKruyff. 2001. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2:1109-1116. [DOI] [PubMed] [Google Scholar]
- 29.Nainan, O. V., M. A. Brinton, and H. S. Margolis. 1992. Identification of amino acids located in the antibody binding sites of human hepatitis A virus. Virology 191:984-987. [DOI] [PubMed] [Google Scholar]
- 30.Ping, L. H., and S. M. Lemon. 1992. Antigenic structure of human hepatitis A virus defined by analysis of escape mutants selected against murine monoclonal antibodies. J. Virol. 66:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintó, R. M., J. M. Diez, and A. Bosch. 1994. Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses. J. Med. Virol. 44:310-315. [DOI] [PubMed] [Google Scholar]
- 32.Pintó, R. M., J. F. Gonzalez-Dankaart, G. Sánchez, S. Guix, M. J. Gómara, M. García, I. Haro, and A. Bosch. 1998. Enhancement of the immunogenicity of a synthetic peptide bearing a VP3 epitope of hepatitis A virus. FEBS Lett. 438:106-110. [DOI] [PubMed] [Google Scholar]
- 33.Pintó, R. M., S. Guix, J. F. González-Dankaart, S. Caballero, G. Sánchez, K. J. Guo, E. Ribes, and A. Bosch. 2002. Hepatitis A virus polyprotein processing by Escherichia coli proteases. J. Gen. Virol. 83:359-368. [DOI] [PubMed] [Google Scholar]
- 34.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enterovirus: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed]
- 35.Reddi, H. V., and H. L. Lipton. 2002. Heparan sulfate mediates infection of high-neurovirulence Theiler's viruses. J. Virol. 76:8400-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez, G., R. M. Pintó, and A. Bosch. 2004. A novel CD4+ T-helper lymphocyte epitope in the VP3 protein of hepatitis A virus. J. Med. Virol. 72:525-532. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez, G., R. M. Pintó, H. Vanaclocha, and A. Bosch. 2002. Molecular characterization of hepatitis A virus isolates from a transcontinental shellfish-borne outbreak. J. Clin. Microbiol. 40:4148-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silberstein, E., G. Dveksler, and G. G. Kaplan. 2001. Neutralization of hepatitis A virus (HAV) by an immunoadhesin containing the cysteine-rich region of HAV cellular receptor-1. J. Virol. 75:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silberstein, E., L. Xing, W. van de Beek, J. Lu, H. Cheng, and G. G. Kaplan. 2003. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobulin- and mucin-like regions. J. Virol. 77:8765-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton, J. T., V. Raina, P. L. Winokur, K. Walters, D. Klinzman, E. Rosen, and J. H. McLinden. 1993. Antigenic and immunogenic properties of recombinant hepatitis A virus 14S and 70S subviral particles. J. Virol. 67:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summers, P. L., D. R. Dubois, W. Houston Cohen, P. O. Macarthy, L. N. Binn, M. H. Sjogren, R. Snitbhan, B. L. Innis, and K. H. Eckels. 1993. Solid-phase antibody capture hemadsorption assay for detection of hepatitis A virus immunoglobulin M antibodies. J. Clin. Microbiol. 31:1299-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavakkol, A., and A. T. H. Burness. 1990. Evidence for a direct role for sialic acid in the attachment of encephalomyocarditis virus to human erythrocytes. Biochemistry 29:10684-10690. [DOI] [PubMed] [Google Scholar]
- 43.Uncapher, C. R., C. M. DeWitt, and R. J. Colonno. 1991. The major and minor group receptor families contain all but one human rhinovirus serotype. Virology 180:814-817. [DOI] [PubMed] [Google Scholar]
- 44.Utagawa, E. T., K. Miyamura, A. Mukoyama, and R. Kono. 1982. Neuraminidase-sensitive erythrocyte receptor for enterovirus type 70. J. Gen. Virol. 63:141-148. [DOI] [PubMed] [Google Scholar]
- 45.van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.). 2000. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 46.Zhou, L., Y. Luo, Y. Wu, and M. Luo. 2000. Sialylation of the host receptor may modulate entry of demyelinating persistent Theiler's virus. J. Virol. 74:1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]