Abstract
Elevated levels of prostaglandins (PGs), products of cyclooxygenases (COXs), are found in the plasma and stool of rotavirus-infected children. We sought to determine the role of COXs, PGs, and the signal transduction pathways involved in rotavirus infection to elucidate possible new targets for antiviral therapy. Human intestinal Caco-2 cells were infected with human rotavirus Wa or simian rotavirus SA-11. COX-2 mRNA expression and secreted PGE2 levels were determined at different time points postinfection, and the effect of COX inhibitors on rotavirus infection was studied by an immunofluorescence assay (IFA). To reveal the signal transduction pathways involved, the effect of MEK, protein kinase A (PKA), p38 mitogen-activated protein kinase (MAPK), and NF-κB inhibitors on rotavirus infection was analyzed. In infected Caco-2 cells, increased COX-2 mRNA expression and secreted PGE2 levels were detected. Indomethacin (inhibiting both COX-1 and COX-2) and specific COX-1 and COX-2 inhibitors reduced rotavirus infection by 85 and 50%, respectively, as measured by an IFA. Indomethacin reduced virus infection at a postbinding step early in the infection cycle, inhibiting virus protein synthesis. Indomethacin did not seem to affect viral RNA synthesis. Inhibitors of MEK, PKA, p38 MAPK, and NF-κB decreased rotavirus infection by at least 40%. PGE2 counteracted the effect of the COX and PKA inhibitors but not of the MEK, p38 MAPK, and NF-κB inhibitors. Conclusively, COXs and PGE2 are important mediators of rotavirus infection at a postbinding step. The ERK1/2 pathway mediated by PKA is involved in COX induction by rotavirus infection. MAPK and NF-κB pathways are involved in rotavirus infection but in a PGE2-independent manner. This report offers new perspectives in the search for therapeutic agents in treatment of severe rotavirus-mediated diarrhea in children.
Rotavirus—a member of the Reoviridae family—is a nonenveloped, double-stranded RNA virus. It is the single most important cause of severe, and sometimes life-threatening, viral gastroenteritis and dehydrating diarrhea in young children worldwide. Each year, rotavirus causes approximately 111 million episodes of gastroenteritis requiring only home care, 25 million clinic visits, 2 million hospitalizations, and 352,000 to 592,000 deaths (median, 440,000 deaths) in children below 5 years of age. By age 5, nearly every child worldwide will have had an episode of rotavirus gastroenteritis, 1 in 5 will visit a clinic, 1 in 65 will be hospitalized, and approximately 1 in 293 will die as result of the infection. Children in underdeveloped countries account for 82% of rotavirus deaths (reference 44 and references therein).
Rotavirus generally replicates in mature enterocytes of the small intestine, leading to induction of virus gene expression and a variety of inflammatory cytokines, reduction of enterocyte gene expression, and vacuolization (6, 8, 48). Recently, it has been reported that rotavirus can enter the body's interior in infected children, resulting in antigenemia and possible viremia (5). This finding is important for the understanding of the pathogenesis of rotavirus infection, which, despite its prevalence and extensive studies in different animal models, is only incompletely understood.
Previously, elevated levels of the prostaglandins (PGs) PGE2 and PGF2 in the plasma and stool of rotavirus-infected children have been reported (66), indicating that cyclooxygenases (COXs) and PGs might be involved in rotavirus pathogenesis. COXs are essential enzymes in the biosynthesis of PGs. They convert arachidonic acid, released from membrane glycerophospholipids by phospholipase A2, to PGH2. Specific isomerases then transform PGH2 to biologically active PGs such as PGE2 and PGF2 (12, 22).
Two distinct genes, COX-1 and COX-2, encode two respective COXs. COX-1 is expressed constitutively in most cells, including intestinal crypt cells. Recently, novel splice variants of COX-1 (PCOX1a, PCOX1b, and COX-3) have been identified and were found to be highly expressed in the brain and heart (9). COX-2 expression is inducible in a variety of cells such as epithelial cells and macrophages (15, 26, 31, 55). The expression of COX-2 appears to be highly regulated by a number of mitogen-activated protein kinases (MAPKs) and transcription factors, in particular, NF-κB (3, 17, 41, 49, 57). In addition, infection with many viruses, including herpes viruses (29, 33, 34, 59, 67), poxviruses (43), human T-cell leukemia virus (37), and bovine leukemia virus (BLV) (47), has been associated with the modulation of COX-2 expression and PG production.
PGs serve as second messengers that elicit a wide range of physiological responses in cells and tissues. Particularly, PGs of the E series are known to have immunomodulatory properties. In addition to mediating inflammatory symptoms, PG may exert anti-inflammatory effects. For example, PGE2 inhibits the secretion of gamma interferon, a cytokine that has antiviral activity (23), and switches the immune response toward a Th2-type cytokine profile (interleukin-4 and interleukin-5), being less effective in developing an antiviral response (4). In addition, PGE2 has a stimulating effect on the replication of viruses, including herpes viruses (1, 29, 59, 60, 68) and BLV (47). In contrast, PGE2 is known to inhibit human immunodeficiency virus type 1 (HIV-1) replication in macrophages (24) and is associated with sustained loss of viral replication in chronic hepatitis B patients (61).
Primary PGs, PGE1 and PGE2, can be converted to the cyclopentenone PGs (cyPGs) PGA1 and PGA2, respectively (42). It has been shown that cyPGs have biological activities different from those of the primary PGs. CyPGs inhibit the replication of a variety of viruses, including both DNA and RNA viruses (51). Interestingly, rotavirus infection is inhibited by PGA1 (58).
At present, no effective rotavirus vaccine is available. The live attenuated rhesus rotavirus-tetravalent vaccine Rotashield, the first rotavirus vaccine licensed, was withdrawn from the world market in late 1999 due to an increase in the incidence of intussusception (16, 39). This, together with the various levels of efficacy associated with the use of some live-attenuated rotaviruses developed for oral administration (2, 28), points to the need for developing alternative strategies for the prevention and/or treatment of rotavirus diarrheal disease. In this study, we determined whether rotavirus infection of Caco-2 cells influenced COX-2 mRNA expression and PGE2 secretion. In addition, the effect of COX inhibitors and PGE2 on the rotavirus infection of Caco-2 cells was studied. Furthermore, the effect of inhibitors of pathways known to mediate regulation of COX activity on rotavirus infection of Caco-2 was analyzed. The results clearly show that COXs and PGE2 are important early mediators of rotavirus infection and that inhibition of COX activity or PG synthesis blocks rotavirus infection at a postbinding step, as measured by an immunofluorescence assay (IFA). These findings might lead to new treatment strategies for rotaviral diarrheal disease.
MATERIALS AND METHODS
Cells, culture media, viruses, and reagents.
Caco-2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; GibcoBRL, Paisly, Scotland) containing 10% (vol/vol) fetal calf serum (FCS; Integro, Dieren, The Netherlands), 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 1% (vol/vol) nonessential amino acids (BioWhittaker, Verviers, Belgium) at 37°C and 5% CO2.
The human rotavirus strain Wa, the simian rotavirus strain SA-11, and the polyclonal antiserum (K3ppIV) against the simian rotavirus strain SA-11 were kindly provided by M. Koopmans (Bilthoven, The Netherlands). The serum was prepared by inoculating rabbits with partially purified (using ultracentrifugation) SA-11 rotaviruses originating from infected MA-104 cells. The inoculum used is known to contain NSP4 protein; therefore, this serum also recognizes the NSP4 protein. In addition, the antiserum cross-reacts with the human rotavirus strain Wa used in this study. The monoclonal anti-β-tubulin antibody and indomethacin, NS-398, KT-5720, PD09859, U0126, PGE2, pyrrolidine dithiocarbamate (PDTC), and SB203580 were purchased from Sigma-Aldrich (St. Louis, Mo.). SC-560 was obtained from Calbiochem (San Diego, Calif.) Possible cytotoxic effects of the inhibitors and their solvents were tested, using cell proliferation reagent WST-1 and lactate dehydrogenase cytotoxicity detection kit (Roche Diagnostics, Mannheim, Germany) assays according to the manufacturer's protocol. All inhibitors were used at concentrations that were not toxic to the cells.
Infection-inhibition studies.
To test the effect of the different specific signal pathway inhibitors on rotavirus infection, 1.5 × 104 Caco-2 cells per well were plated on heavy Teflon-coated microscope slides (Cel-line/Erie Scientific, Portsmouth, N.H.) (7-mm diameter) 1 day before starting the experiment. Cells were rinsed three times with culture medium without FCS (DMEM-FCS) and incubated with different concentrations of the inhibitors for 1 h at 37°C prior to infection. Simultaneously, rotavirus was activated for 1 h at 37°C with 10 μg of trypsin/ml diluted in DMEM-FCS. Subsequently, Caco-2 cells were inoculated with 100 focus-forming units (ffu) of rotavirus in the presence or absence of the inhibitors. At 15 h postinoculation (p.i.), cells were fixed in ice-cold methanol at −20°C for 10 min and stored in phosphate-buffered saline (PBS). The infection was monitored by an indirect IFA. For this purpose, cells were incubated for 90 min at room temperature with the polyclonal antirotavirus (K3ppIV) serum diluted in PBS (1:1,600), rinsed four times with PBS, and stained for 60 min with goat anti-rabbit Texas Red-conjugated immunoglobulin (IgG) (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) diluted in PBS (1:200). Finally, cells were washed extensively and mounted in Mowiol solution (25) containing 2.5% (wt/vol) DABCO (1,4-diazabicyclo[2.2.2]octane) and 0.5 μg of DAPI (4′,6-diamidino-2-phenylindole dihydrochloride:hydrate; Sigma-Aldrich)/ml. Fluorescence was viewed with a Nikon Eclipse E800 microscope. The numbers of infected cells in the drug-treated and nontreated cells were expressed as a percentage of the average number of infected cells in the nontreated cell cultures.
RNA isolation and RT-PCR.
Total RNA from mock- or rotavirus-infected Caco-2 cells at different time points p.i. was isolated with an RNeasy RNA isolation kit according to the manufacturer's protocol (QIAGEN, Hilden, Germany). Single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) were isolated using the method described by Chen et al. (10). Random and oligo(dT)15 primers (ratio of 2:1) were used to synthesize cDNA by the use of Moloney murine leukemia virus reverse transcriptase according to the protocol of the manufacturer (Promega, Madison, Wis.). To amplify a 305-bp fragment of COX-2, oligonucleotides p338 (5′-TTC AAA TGA GAT TGT GGG AAA AT-3′), identical to nucleotides (nt) 574 to 596 (18), and p339 (5′-AGA TCA TCT CTG CCT GAG TAT CTT-3′), reverse complement of nt 855 to 878 (18), and a temperature-cycling protocol that consisted of 10 min of preheating at 96°C followed by 40 cycles of 1 min of denaturation at 96°C, 1 min of primer annealing at 60°C, and 1 min of primer extension at 72°C were used. cDNA was amplified by PCR as previously described (32).
The efficiency of the reverse transcriptase-PCR (RT-PCR) was determined by amplifying 18S RNA by the use of oligonucleotide 5′ 18S (5′-TCC TGC CAG TAG CAT ATG CTT G-3′) and oligonucleotide 3′ 18S (5′-AGA GGA GCG AGC GAC CAA AGG-3′) or by amplifying human β-actin by the use of oligonucleotide p68 (5′-CAA GGC CAA CCG CGA GAA G-3′) and oligonucleotide p69 (5′-CAG GGT ACA TGG TGG TGC C-3′), resulting in a 587-bp PCR product, as described previously (62). The temperature-cycling protocol for these PCRs consisted of 10 min of preheating at 96°C followed by 30 cycles of 1 min of denaturation at 96°C, 1 min of primer annealing at 60°C, and 1 min of primer extension at 72°C. Rotaviral RNA was reverse transcribed as described above, with the exception that 7% (vol/vol) dimethyl sulfoxide (DMSO) was added to the cDNA synthesis reaction mixture. Rotavirus Wa NSP4 cDNA was amplified in the presence of 7% (vol/vol) DMSO by the use of oligonucleotide p87 (GGA ACC ATG GAA AAG CTT ACC GAC CTC, identical to nt 46 to 62 [7] containing an NcoI site) and oligonucleotide p88 (TCC CCC GGG TCA CAT TAA GAC CGT TCC T, reverse complement of nt 730 to 750 [7] containing a SmaI site). The temperature-cycling protocol used consisted of 10 min of preheating at 96°C followed by 25 cycles of 1 min of denaturation at 96°C, 2 min of primer annealing at 53°C, and 2 min of primer extension at 72°C.
In addition, rotavirus Wa and SA-11 VP4 cDNA was amplified in the presence of 7% (vol/vol) DMSO by the use of oligonucleotide p90 (TAT ACC ATG GCT TCA CTC ATT TAT AGA C, identical to nt 7 to 28 [39] containing an NcoI site) and oligonucleotide p91S (TTG AGG ATC CTA TGC CTT ATA TGA TAT TTC, reverse complement of nt 883 to 900 [39] containing a stop codon and a BamHI site). For this PCR, the temperature-cycling protocol consisted of 10 min of preheating at 96°C followed by 25 cycles of 1 min of denaturation at 96°C, 2 min of primer annealing at 51°C, and 2 min of primer extension at 72°C. PCR products were visualized and quantified using a Gel Doc 2000 system and Multi-Analyst software version 1.1 (Bio-Rad Laboratories, Inc., Hercules, Calif.).
EIAs for PGE2.
The amount of secreted PGE2 in the supernatants of mock- or rotavirus-infected (multiplicity of infection [MOI] = 1) Caco-2 cells was determined at the indicated time points with a competitive enzyme immunoassay (EIA; Amersham Pharmacia Biotech, Freiburg, Germany) according to the manufacturer's protocol.
Western blot analyses.
Caco-2 cells were mock infected or infected with rotavirus (MOI = 1). At the indicated time points, cells were lysed in lysis buffer (20 mM Tris, 1 mM EDTA, and 0.75% [vol/vol] Triton X-100 containing 0.1 mg of soybean trypsin inhibitor/ml, 0.01 mg of pepstatin A/ml, 1% [vol/vol] aprotinin, 0.01 mg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride) by three cycles of freezing and thawing. Cell lysates were cleared by centrifugation at 10,000 × g for 5 min at 4°C. Aliquots of the protein samples were separated in a sodium dodecyl sulfate-15% polyacrylamide gel and transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) (0.1 μM) as previously described (63). Subsequently, the membranes were incubated in blot buffer (50 mM Tris-HCl [pH 7.8], 2 mM CaCl2, 5% [wt/vol] dry milk, 0.01% [vol/vol] antifoam, 0.05% [vol/vol] Triton X-100) for 1 h to block nonspecific binding and incubated with antirotavirus serum K3ppIV diluted in blot buffer (1:1,000) for 15 h at 4°C or with the monoclonal anti-β-tubulin antibody diluted in blot buffer (1:400) for 1 h at room temperature. Membranes were washed three times with blot buffer and incubated for 1 h with peroxidase-labeled goat anti-rabbit IgG (1:2,000) diluted in blot buffer or with peroxidase-labeled rabbit anti-mouse IgG (1:1,000) diluted in blot buffer. The membranes were extensively washed with PBS before proteins were visualized using 0.05% (wt/vol) diaminobenzidine in 50 mM Tris-HCl (pH 7.5)-0.013% (vol/vol) H2O2. Blots were scanned and analyzed using ImageQuant TL software (Amersham Biosciences, Buckinghamshire, England).
Virus titration.
Viral infectivity in culture media and cells was determined at 15 h p.i. by a quantitative assay on Caco-2 cells. Caco-2 cells grown on 10-well heavy Teflon-coated microscope slides were inoculated with serial dilutions of the medium samples combined with the cleared cell lysates from infected cells made in culture medium. Subsequently, the amount of ffu was determined using an indirect IFA as described above.
Statistical analyses.
Each assay was carried out at least in duplicate, and each experiment was repeated at least once. Data are presented as means ± standard errors of the means. For statistical analysis, one-way analysis of variance was performed using a Tukey-Kramer test and GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, Calif.). In all tests, P < 0.05 was considered statistically significant.
RESULTS
Rotavirus infection of Caco-2 cells induces COX2 mRNA expression and PGE2 secretion.
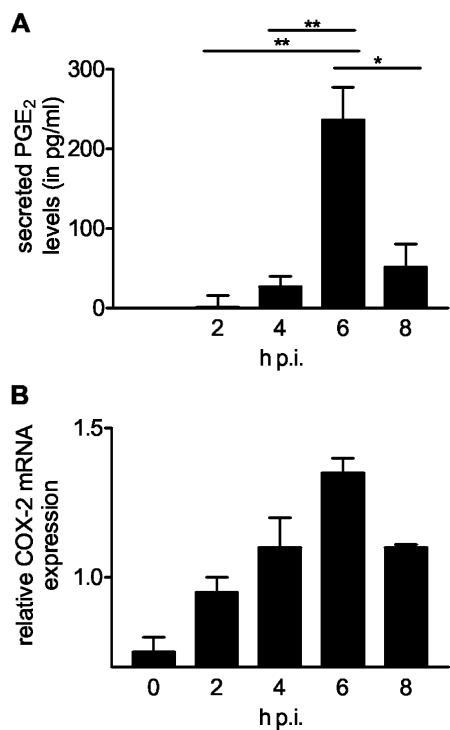
To determine whether rotavirus infection of epithelial cells induces PGE2 secretion, we used a competitive PGE2 EIA to monitor the secretion of PGE2 by enterocyte-like Caco-2 cells infected with human rotavirus Wa. Increased levels of secreted PGE2 were found in culture media of rotavirus-infected cells from 4 h p.i. (Fig. 1A). At that time viral RNA and protein synthesis could first be detected by RT-PCR and Western blotting, respectively (data not shown). The amount of secreted PGE2 reached its maximum at 6 h p.i. and then decreased to significantly lower levels at 8 h p.i. (Fig. 1A). COX-1 and COX-2 are key enzymes in the biosynthesis of PGE2 in epithelial cells. Because only COX-2 is inducible, we determined whether mRNA expression of this enzyme increased during rotavirus infection. Semiquantitative RT-PCR analysis showed a gradual increase in COX-2 mRNA expression from 0 to 6 h p.i. The increase in COX-2 mRNA expression peaked at 6 h p.i., showing twofold induction compared to the results seen at 0 h p.i., and decreased thereafter (Fig. 1B). Therefore, the levels of secreted PGE2 and COX-2 mRNA expression show similar temporal patterns.
FIG. 1.
Rotavirus infection of Caco-2 cells induced COX-2 mRNA expression and PGE2 secretion. Caco-2 cells were infected with rotavirus Wa (MOI = 1) or mock infected. Media and RNA were collected at different time points p.i. (A) The amount of secreted PGE2 in the media was determined using a competitive enzyme immunoassay. The amount of PGE2 secreted by mock-infected cells was subtracted from PGE2 levels secreted by infected cells at the different time points. The significant differences (*, P < 0.01; **, P < 0.001) between samples at different time points p.i are indicated. Error bars indicate the standard errors of the means (n = 4). (B) The level of COX-2 mRNA expression as determined by a semiquantitative RT-PCR using COX-2-specific primers in infected cells relative to that found in noninfected cells at each time point. Error bars indicate the standard errors of the means (n = 2).
Inhibition of COXs affects rotavirus infection.
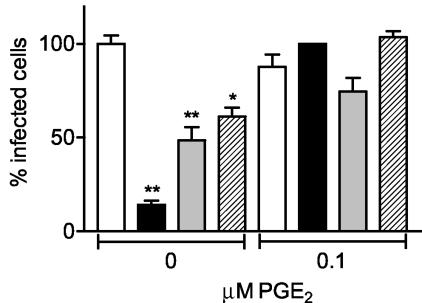
The increased PGE2 secretion and COX-2 mRNA expression found in rotavirus-infected Caco-2 cells could either be a defense mechanism of the cell against the virus or may be induced by the virus to its own benefit. To investigate whether COX activity is essential during rotavirus infection, Caco-2 cells were infected with rotavirus in the presence of COX inhibitors. Caco-2 cells were incubated with inhibitors starting 1 h prior to inoculation and until 15 h p.i. The nonspecific COX inhibitor indomethacin (inhibiting both COX-1 and COX-2) reduced rotavirus Wa infection of Caco-2 cells by 85% at a concentration of 17 μM (Fig. 2). The inhibition appeared to be concentration dependent (data not shown). Unfortunately, concentrations of indomethacin higher than 17 μM could not be used because it appeared that the inhibitor's solvent (DMSO) had a negative effect on the infection and/or was toxic for the cells at concentrations of indomethacin higher than 17 μM (determined by using a cell proliferation and a lactate dehydrogenase cytotoxicity detection assay as described in Materials and Methods; data not shown).
FIG. 2.
Inhibition of COX activity blocked rotavirus infection in a PGE2-dependent way. Caco-2 cells were incubated with culture medium (containing a concentration of DMSO similar to that present in the inhibitor solutions) (open bars), the nonspecific COX inhibitor indomethacin (17 μM; n = 8) (black bars), the COX-1-specific inhibitor SC-560 (1 μM; n = 5) (gray bars), or the COX-2-specific inhibitor NS-398 (0.055 μM; n = 4) (hatched bars) in the absence or presence of 0.1 μM PGE2. Caco-2 cells were incubated with inhibitors with or without PGE2 1 h prior to rotavirus Wa inoculation (100 ffu) and during the entire experiment until cells were fixed at 15 h p.i. in ice-cold methanol. Subsequently, the number of infected cells was determined by an indirect IFA using the polyclonal antirotavirus serum K3ppIV. The numbers of infected cells in the drug-treated and nontreated cells were expressed as a percentage of the average number of infected cells in the nontreated cell cultures. The significant differences (*, P < 0.01; **, P < 0.001) in the numbers of infected cells found between cells incubated with a COX inhibitor and cells incubated with culture medium are indicated. Note that the concentration of each inhibitor was nontoxic to the cells. Error bars indicate the standard errors of the means.
Rotaviruses display different serotypes (G1 to G9), human rotavirus Wa being a G1 serotype virus. To determine whether inhibition of rotavirus infection by indomethacin was serotype dependent, the effect of indomethacin on infection of Caco-2 cells with the G3 serotype simian rotavirus SA-11 was analyzed. The infection of Caco-2 cells with this rotavirus was reduced more than 80% by the presence of indomethacin (data not shown), indicating that the observed effect of indomethacin on rotavirus infection was not restricted to one specific serotype of the virus.
Several drugs are available that specifically inhibit either COX-1 or COX-2. These specific inhibitors of COX-1 (SC-560; the inhibitory concentration at which 50% of the enzyme activity is blocked [IC50] is 90 nM) and COX-2 (NS-398; IC50 = 1.77 μM) were able to reduce rotavirus Wa infection of Caco-2 cells by 50 and 40%, respectively, at concentrations slightly higher than (COX-1) or far below (COX-2) their IC50 values (Fig. 2). Thus, the activity of both enzymes is required to establish a rotavirus infection in Caco-2 cells. Again no higher concentrations of the inhibitors could be used due to the effects of their solvent (DMSO) on virus infection and cell viability (data not shown).
To determine whether the effect of COX inhibitors on rotavirus infection was caused by their effect on PGE2 biosynthesis, we repeated the inhibition experiments in the presence of PGE2. Addition of PGE2 appeared to restore the number of infected cells to levels comparable to those seen with the control but did not have any effect on the number of infected cells in the absence of the COX inhibitors (Fig. 2). These results clearly indicate that PGE2 is essential for rotavirus infection of Caco-2 cells.
Kinetics of the inhibition of rotavirus infection.
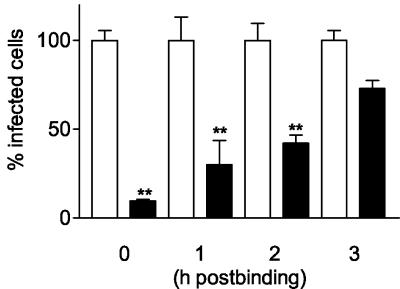
In the experiments described so far, the inhibitors were added 1 h prior to and during virus inoculation. To determine the kinetics of inhibition of rotavirus infection by blocking COX activity in more detail, Caco-2 cells were inoculated with rotavirus (1,000 ffu) for 2 h at 4°C. This allows virus binding to cells without entry. After removing any unbound virus, cells were placed at 37°C to allow virus entry and indomethacin was added immediately or after 1, 2 or 3 h at 37°C. We found that indomethacin inhibits rotavirus infection significantly when added at up to 2 h after the cells were placed at 37°C (Fig. 3). The maximum inhibitory effect (>90% inhibition) of indomethacin on rotavirus infection was reached when indomethacin was added immediately after the cells were placed at 37°C. Inhibition by indomethacin was still highly significant (>55% inhibition) when indomethacin was added after 2 h. No significant inhibition of the infection was observed when indomethacin was added 3 h after the cells were placed at 37°C. This demonstrates that COX activity and PGE2 play an essential role early in the virus infection cycle but after virus binding to its host cell.
FIG. 3.
Indomethacin inhibits rotavirus infection at a postbinding step. Caco-2 cells were inoculated with rotavirus (1,000 ffu) for 2 h at 4°C to allow virus binding but not virus entry. After all unbound virus was removed, cells were placed at 37°C to allow virus entry (t = 0 h postbinding) and indomethacin (17 μM) (black bars) or culture medium (containing a concentration of DMSO similar to that present in the inhibitor solution) (open bars) was added immediately or after the indicated times until cells were fixed at 15 h postbinding. The number of infected cells was determined by an indirect IFA using the polyclonal antirotavirus serum K3ppIV. The numbers of infected cells in the indomethacin-treated and nontreated cells were expressed as a percentage of the average number of infected cells in the nontreated cell cultures. The significant differences (**, P < 0.001) in the numbers of infected cells between cells incubated with indomethacin and cells incubated with culture medium are indicated. Error bars indicate the standard errors of the means (n = 4).
Effect of indomethacin on synthesis of viral RNA, proteins, and progeny.
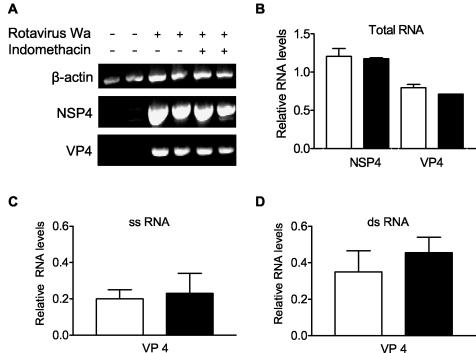
To determine at which level of the virus infection cycle indomethacin acts, Caco-2 cells were inoculated with rotavirus Wa or SA-11 (MOI = 1) for 2 h at 4°C. Virus inoculate was removed, cells were thoroughly washed before indomethacin was added, and the cells were placed at 37°C. Total RNA, dsRNA, and ssRNA were extracted 15 h after infection, and viral RNA production was determined by a semiquantitative RT-PCR of rotavirus NSP4 and VP4 RNA (Fig. 4). In addition, viral protein synthesis was analyzed by Western blotting using the antirotavirus serum K3ppIV and production of infectious virus was monitored by determining the amount of ffu in cell lysates combined with the culture media. Indomethacin had no effect on the production of total RNA derived from rotavirus NSP4 and VP4 RNA (Fig. 4A and B) or on the ssRNA and dsRNA derived from VP4 (Fig. 4C and D).
FIG. 4.
Indomethacin had no effect on VP4 and NSP4 RNA expression. Caco-2 cells were infected with rotavirus Wa (MOI = 1) (A and B) or SA-11 (MOI = 1) (C and D) or were mock infected (A) in the absence (open bars) or presence (closed bars) of 17 μM indomethacin. Caco-2 cells were incubated with indomethacin 1 h prior to rotavirus inoculation and during the rest of the experiment. At 15 h p.i. cells were harvested to isolate total RNA (A and B), ssRNA (C), or dsRNA (D). (A) RT-PCR products of the isolated total RNA obtained using β-actin-, rotavirus Wa NSP4-, or rotavirus Wa VP4-specific primers. (B) Semiquantitative analysis of the PCR products shown in panel A. (C) Semiquantitative analysis of the RT-PCR products of the isolated ssRNA performed using rotavirus VP4-specific primers. (D) Semiquantitative analysis of the RT-PCR products of the isolated dsRNA performed using rotavirus VP4-specific primers. Error bars indicate the standard errors of the means. Viral RNA levels are expressed relative to the β-actin mRNA (B) or 18S RNA (C and D) levels. Note that in the absence of indomethacin, cells were incubated with culture medium containing a concentration of DMSO similar to that present in the inhibitor solution.
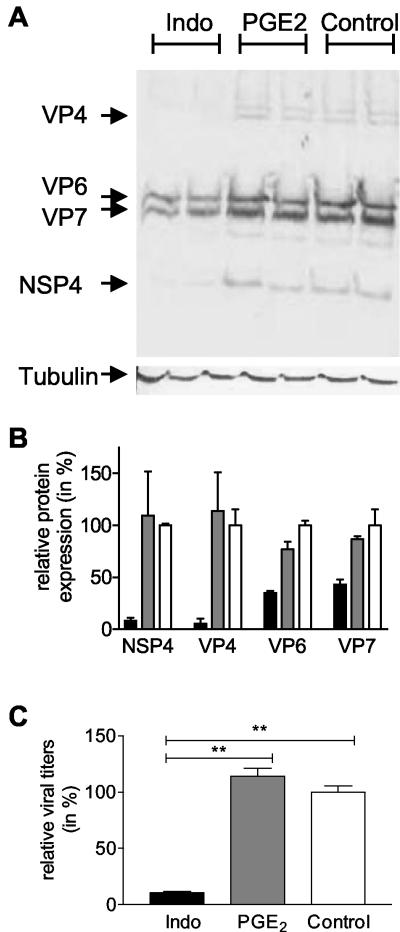
In contrast to the effect of indomethacin on RNA synthesis, indomethacin clearly inhibited viral protein synthesis (Fig. 5A), confirming our results obtained with the IFAs. Expression levels of the NSP4, VP4, VP6, and VP7 proteins were reduced 92, 95, 66, and 57%, respectively, in the presence of indomethacin (Fig. 5B). In addition, inhibition of COX by indomethacin resulted in a 10-fold decrease in the yield of infectious viral particles (Fig. 5C). PGE2 alone did not alter viral RNA expression (data not shown), protein synthesis (Fig. 5B), or production of infectious viral particles (Fig. 5C). These results show that inhibition of rotavirus infection by blocking COX activity is most likely due to an effect on viral protein synthesis and consequently on infectious particle formation.
FIG. 5.
Indomethacin inhibits viral protein synthesis and production of infectious viral particles. Caco-2 cells were infected with rotavirus Wa (MOI = 1) in the absence or presence of 17 μM indomethacin (Indo) or in the presence of 1 μM PGE2. Caco-2 cells were incubated with indomethacin or PGE2 1 h prior to rotavirus Wa inoculation and during the entire experiment. At 15 h p.i. cells were harvested to isolate proteins. In parallel, cells and media were collected and combined for analysis of the production of infectious viral particles. (A) Protein samples were analyzed on a sodium dodecyl sulfate-15% polyacrylamide gel followed by Western blotting using the polyclonal antirotavirus serum K3ppIV or the monoclonal anti-β-tubulin antibody. The positions of the rotavirus structural proteins VP4, VP6, and VP7 and the nonstructural protein NSP4 are indicated. In the bottom panel, the results seen with β-tubulin, the loading control, are shown. (B) The Western blot shown in panel A was used to quantify viral protein synthesis in cells treated with culture medium (open bars), PGE2 (gray bars), or indomethacin (black bars). Indomethacin inhibited NSP4, VP4, VP6, and VP7 protein synthesis 92, 95, 65, and 56%, respectively. Viral protein levels were expressed relative to β-tubulin levels. Error bars indicate the standard errors of the means. (C) Media and cells from control cultures (open bars) and indomethacin-treated (black bars) and PGE2-treated (gray bars) cell cultures were collected, and Caco-2 cells were inoculated with serial dilutions of the medium samples combined with the cleared cell lysates. The amount of ffu in the samples was determined using an indirect IFA. The amounts of ffu produced in indomethacin-treated and nontreated cells were expressed as a percentage of the average amount of ffu produced in the nontreated cell cultures. The significant differences (**, P < 0.001) are indicated. Error bars indicate the standard errors of the means (n = 5). Please note that in the absence of indomethacin (= nontreated control cells), cells were incubated with culture medium containing a concentration of DMSO similar to that present in the medium of indomethacin-treated cells.
Inhibition of BMK, ERK1/2, p38 MAPK, and NF-κB decreases rotavirus infection.
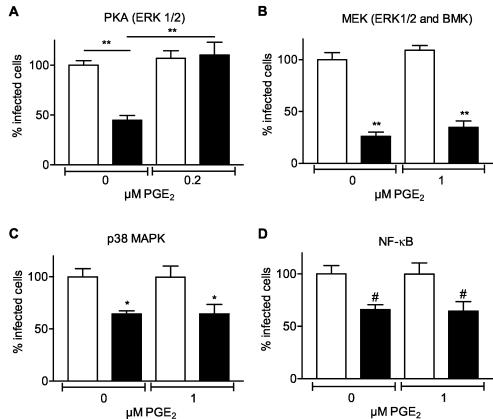
As mentioned before, the expression of COX-2 is highly regulated by a number of MAPKs and NF-κB. Therefore, we investigated whether inhibition of these pathways influenced rotavirus infection of Caco-2 cells. Four groups of MAPK cascades have been described: extracellular signal-related kinase (ERK1/2), Jun N-terminal kinase (JNK), p38 MAPK, and big MAPK (BMK, ERK5) (35, 54). First we studied the effect of inhibiting the cyclic AMP (cAMP)-dependent protein kinase A (PKA), activating the ERK1/2 pathway through the activation of a second signaling cascade involving Rap1 and B-raf (20, 53, 64), on rotavirus infection. Inhibition of PKA resulted in a 50% decrease in the number of cells that became infected by rotavirus (Fig. 6A). The observed effect was concentration dependent (data not shown) and could be neutralized by adding PGE2 (Fig. 6A). In addition, inhibition of both the ERK1/2 and BMK pathways by U0126 reduced the amount of infected cells by 75% (Fig. 6B). Interestingly, this inhibitory effect of U0126 could not be counteracted by adding PGE2. Also, the p38 MAPK inhibitor SB203580 inhibited rotavirus infection in a concentration-dependent manner (data not shown) up to about 40% (Fig. 6C). Adding PGE2 did not overcome the effect of the p38 MAPK inhibitor S203580 (Fig. 6C). Finally, the effect of inhibiting the activation of NF-κB by PDTC on the rotavirus infection was analyzed. A 40% decrease in the number of infected cells was observed. Exogenous PGE2 did not counteract the effect of NF-κB inhibition on the rotavirus infection (Fig. 6D). Please note that for all inhibitors, no higher concentrations could be used due to the effects of their solvent (DMSO) on virus infection and cell viability (data not shown). Conclusively, the ERK1/2, BMK, p38 MAPK, and NF-κB pathways are involved in rotavirus infection because inhibition of these pathways resulted in a reduction of rotavirus infection. However, only the ERK1/2 pathway affects rotavirus infection in a COX- and PGE2-dependent manner. The BMK, p38 MAPK, and NF-κB pathways seem to affect rotavirus infection in a COX- and PGE2-independent manner.
FIG. 6.
Inhibition of MAPK and NF-κB pathways affects rotavirus infection. Caco-2 cells were incubated with culture medium (open bars) or inhibitors of MAPK or NF-κB pathways (black bars) 1 h prior to virus inoculation (100 ffu) and during the entire experiment in the absence or presence of the indicated concentrations of PGE2. Cells were fixed at 15 h p.i. in ice-cold methanol, and the number of infected cells was determined by an indirect IFA using the polyclonal antirotavirus serum K3ppIV. The numbers of infected cells in the drug-treated and nontreated cells were expressed as a percentage of the average number of infected cells in the nontreated cell cultures. (A) KT-5720 (50 μM); (B) U0126 (50 μM); (C) SB203580 (50 μM); (D) PDTC (1 μM). The significant differences (#, P < 0.05; *, P < 0.01; **, P < 0.001) in the percentages of infected cells between cells incubated with the inhibitor and cells incubated with culture medium are indicated. Error bars indicate the standard errors of the means (n ≥ 5). Please note that in the absence of inhibitors (= nontreated control cells), cells were incubated with culture medium containing a concentration of DMSO similar to that present in the medium of cells treated with the specific inhibitors.
DISCUSSION
The high morbidity and mortality levels associated with rotavirus, as well as the economic burden, point to the urgent need for the development of new methods for the treatment or prevention of rotavirus diarrheal disease. In this study, we explored the possible role of COXs and PGs in rotavirus infection to gain more insight into rotavirus pathogenesis and to possibly obtain new targets for antiviral therapy.
In rotavirus-infected enterocyte-like Caco-2 cells, an increase in PGE2 secretion peaking at 6 h p.i., which coincided with the pattern of COX-2 mRNA expression, was found early in the infection cycle. Thus, the increase in the secretion of PGE2 seems due to the induction of COX-2 mRNA expression by rotavirus infection. After 6 h PGE2 and COX2 mRNA levels decrease, which is most likely due to the negative feedback mechanism, as has been reported by Poligone and Baldwin (46).
Our results are in agreement with other studies in which elevated levels of PGE2 and PGF2 were detected in the plasma and stool of rotavirus-infected children (66) and elevated intestinal PGE2 concentrations were found in piglets early after rotavirus infection (69). In addition, many other viruses are known to modify COX-2 expression and PG production (29, 33, 34, 37, 43, 47, 59, 67). In many cases PGE2 has a stimulating effect on the replication of viruses, including herpes viruses (1, 29, 59, 60, 68) and BLV (47). However, it inhibits HIV-1 replication in macrophages (24) and hepatitis B virus replication in chronically infected patients (61).
To study whether COX activity is required for rotavirus infection in vitro, the infection was studied in the presence of COX inhibitors. Indeed, rotavirus infection was inhibited by the nonspecific COX inhibitor indomethacin as well as by the COX-1-specific inhibitor SC-560 and the COX-2-specific inhibitor NS-398, indicating that both enzymes are essential for efficient rotavirus infection. The specific inhibitors of COX-1 and COX-2 were used at concentrations slightly higher than and far below their IC50 values, respectively. Higher concentrations of the inhibitors resulted in a higher concentration of their solvent (DMSO) that appeared to be toxic for the cells and to inhibit rotavirus infection by itself. Therefore, no conclusions can be drawn on the efficiency of blocking rotavirus infection by the COX-1 and COX-2 inhibitors.
COX inhibitors blocked not only G1 serotype human rotavirus Wa infection but also the G3 serotype simian rotavirus SA-11 infection of Caco-2 cells. This indicates that COX inhibitors might have a broad effect on rotavirus infection, blocking rotaviruses of different serotypes and with different species specificity. In addition, our findings are in agreement with the observations that the duration of rotavirus diarrheal illness in young children is reduced after oral administration of aspirin, a nonspecific COX inhibitor similar to indomethacin (19, 56, 66), and that indomethacin abolished rotavirus-induced secretion of potassium ions in infected piglet jejunum (65).
The observation that inhibition of COXs efficiently inhibits rotavirus infection points towards a key role for PGs in rotavirus infection. Support for this hypothesis was obtained from experiments in which PGE2 was added during the infection-inhibition studies. The addition of PGE2 counteracted the effect of the COX inhibitors on rotavirus infection, indicating the importance of PGE2 in rotavirus infection. However, when PGE2 was added in the absence of COX inhibitors it did not have any effect on the infection. This confirmed reports from earlier studies that no effect of PGE and PGF2 alpha on the growth of rotavirus in cell culture was observed (21).
To get more insight into the mechanism by which PGE2 modulates rotavirus infection we determined the time point of maximum inhibition by indomethacin. The maximum inhibitory effect (>90% inhibition) on rotavirus infection was reached when indomethacin was added immediately after virus binding to the cells and rapidly decreased to 25% inhibition when indomethacin was added 3 h after virus binding. Therefore, PGE2 plays a role in an early step of the virus infection cycle. PGE2 has been shown to activate several viral promoters, including the human cytomegalovirus major immediate-early promoter (34, 68), the human T-cell leukemia virus type 1 long terminal repeat promoter (38), and the HIV-1 long terminal repeat promoter (13, 14). In addition, PGE2 was shown to increase the production of multiple murine gammaherpesvirus gene products (59) and BLV pol and tax mRNA levels (47). However, here we show that in rotavirus-infected cells the levels of viral RNA encoding NSP4 and VP4 were not affected by the COX inhibitor. In contrast to the viral RNA levels, rotavirus protein levels, in particular those of NSP4 and VP4, as well as the production of viral progeny were decreased in indomethacin-treated cells. This latter finding is most likely a secondary effect caused by the inhibition of viral protein synthesis. The observation that levels of viral RNA are not modified by indomethacin in contrast to effects on viral protein synthesis may seem contradictory. However, it has been shown before that the synthesis of several rotaviral genes is (partially) independent of (both cellular and viral) protein synthesis (30). Second, it is known that the rotavirus RNA polymerase is efficiently recycled following (negative) strand synthesis and catalyzes RNA synthesis in a nearly linear manner for several hours (11). Third, since there is still a residual amount of viral protein synthesis observed in the presence of indomethacin, this may be sufficient to synthesize viral RNA at (nearly) normal levels. Fourth, whereas in the absence of indomethacin viral dsRNA is transported out of the cell (in viral particles), this does not occur (or occurs to a lesser extent) in indomethacin-treated cells. Therefore, one or a combination of the abovementioned phenomena may explain the observation that intracellular rotaviral RNA levels do not differ between indomethacin-treated and nontreated cells.
Our observations suggest an important role of PGE2 in rotavirus protein synthesis rather than in viral RNA synthesis. PGE2 exerts its function by interacting with the PGE2 receptors (EPs). These receptors are coupled to G proteins and activate or inhibit second messenger systems inside the cell. Depending on the cell type this results in an influx of Ca2+, activation of PKC and/or PKA, and increased or decreased cellular levels of cAMP (40). One or several of these events may influence rotavirus protein synthesis. Interestingly, in this paper we have shown that inhibition of PKA also resulted in the inhibition of the rotavirus infection.
Previously, it was reported that PGA1, a conversion product of PGE1, potently inhibited rotavirus SA-11 replication in monkey kidney MA104 cells. Whereas it did not affect virus adsorption or penetration, PGA1 partially inhibited VP4 and VP7 synthesis, selectively reduced glucosamine incorporation into the NSP4 viral enterotoxin, and impaired virus maturation (58). This result seems to contrast with our observations with PGE2. However, it had been shown earlier that cyPGs such as PGA1 and PGA2 have biological activities with respect to virus replication different from those of the primary PGs (51). Since cyPGs are derived from PGE, this might indicate that virus replication is first mediated by the primary PGs and subsequently shut off by the cyPGs that are formed.
It has been shown previously that rotavirus activates NF-κB, known to regulate COX activity, within 2 h p.i (48) through binding of VP4 to tumor necrosis factor receptor-associated factor 2 (36). It is tempting to speculate that this may be at least one pathway through which rotavirus activates COX activity and induces PGE2 secretion. Therefore, we studied whether inhibition of NF-κB had an effect on rotavirus infection. Although inhibition of NF-κB pathways resulted in a significant decrease of rotavirus infection of Caco-2 cells, this effect could not be overcome by the addition of PGE2, suggesting an effect of NF-κB on rotavirus infection independent of that of COX-2 and PGE2. NF-κB activation during virus infection initially has been interpreted as a protective response of the host to the viral pathogen (reviewed in reference 52). However, more and more evidence indicates that NF-κB activation could be a strategy adopted by different viruses to block apoptosis and prolong survival of the host cell to gain time for replication and increase viral progeny production. This has been shown for several viruses, including HIV, herpesviruses, and encephalomyocarditis virus (reviewed in reference 50). Whether this is also the case for rotaviruses is not known, but it could be an explanation for our results observed using the NF-κB inhibitor.
In addition to NF-κB, a number of MAPKs are known to regulate the expression of COX-2 (3, 17, 41, 49, 57). Therefore, we also investigated whether inhibition of the MAPK pathways ERK1/2, BMK, and p38 MAPK influenced rotavirus infection of Caco-2 cells. Inhibiting the ERK1/2 pathway by inhibiting the cAMP-dependent PKA resulted in a significant decrease in the amount of rotavirus-infected Caco-2 cells. Exogenous PGE2 counteracted this effect, indicating that it occurs in a PGE2-dependent manner. The inhibitor U0126 was used in this study to inhibit both the ERK1/2 and BMK pathways. U0126 resulted in a strong inhibition of rotavirus infection. Moreover, the effect on rotavirus infection could not be overcome by adding PGE2. Also, inhibition of the p38 MAPK pathway resulted in a PGE2-independent decrease of rotavirus infection of Caco-2 cells. Exactly how inhibition of MAPK pathways decreases rotavirus infection is not clear, but it has been reported previously that MAPK pathways also contribute to the replication of other viruses such as, e.g., herpes simplex virus type 2 (55), influenza virus (45), and encephalomyocarditis virus (27). The precise role of MAPK in these virus infections has not been elucidated, but MAPKs are most likely involved at the level of translation-transcription of the viral genome (27).
In conclusion, this paper shows that secretion of PGE2 and COX-2 mRNA expression are increased in rotavirus-infected cells. Moreover, COX activity and PGE2 appeared to be essential to establish a rotavirus infection in cultured cells. PGE2 is necessary early in the infection cycle and is most likely involved in viral protein synthesis and production of virus progeny rather than in viral RNA production. The ERK1/2 pathway seemed to be involved in COX-2 induction by rotavirus infection, since PGE2 could restore rotavirus infection in the presence of a specific ERK1/2 pathway inhibitor. Additionally, other MAPK and NF-κB pathways are involved in rotavirus infection but in a PGE2-independent manner. The precise mechanism of these pathways in relation to the rotavirus infection will be a subject for further research. The results obtained in this study offer new perspectives in the search for therapeutic agents in treatment of severe rotavirus-mediated diarrhea in infants and children.
Acknowledgments
We are grateful to Marion Koopmans and Erwin Duizer (RIVM, Bilthoven, The Netherlands) for providing us with the rotavirus strains Wa and SA-11 and the polyclonal antirotavirus serum and to Jan Dekker (Rudolf Magnus Institute, Utrecht, The Netherlands) for critical reading of the manuscript and helpful suggestions.
This work was supported by grants from the Sophia Foundation for Medical Research, Rotterdam, The Netherlands, and The Netherlands Organization for Scientific Research.
Footnotes
This paper is dedicated to Bram Rossen.
REFERENCES
- 1.Baker, D. A., J. Thomas, J. Epstein, D. Possilico, and M. L. Stone. 1982. The effect of prostaglandins on the multiplication and cell-to-cell spread of herpes simplex virus type 2 in vitro. Am. J. Obstet. Gynecol. 144:346-349. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, G. L., J. S. Lund, S. V. Mitchell, L. De Bruyn, L. Piggford, A. L. Smith, J. Furmedge, P. J. Masendycz, H. C. Bugg, N. Bogdanovic-Sakran, J. B. Carlin, and R. F. Bishop. 2002. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine 20:2950-2956. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, S. R., R. Sawdy, and G. E. Mann. 1999. Induction of cyclooxygenase-2 expression in human myometrial smooth muscle cells by interleukin-1beta: involvement of p38 mitogen-activated protein kinase. J. Physiol. 520:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betz, M., and B. S. Fox. 1991. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 146:108-113. [PubMed] [Google Scholar]
- 5.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 6.Boshuizen, J. A., J. H. Reimerink, A. M. Korteland-van Male, V. J. van Ham, M. P. Koopmans, H. A. Büller, J. Dekker, and A. W. Einerhand. 2003. Changes in small intestinal homeostasis, morphology, and gene expression during rotavirus infection of infant mice. J. Virol. 77:13005-13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Both, G. W., J. S. Mattick, and A. R. Bellamy. 1983. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc. Natl. Acad. Sci. USA 80:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casola, A., M. K. Estes, S. E. Crawford, P. L. Ogra, P. B. Ernst, R. P. Garofalo, and S. E. Crowe. 1998. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology 114:947-955. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekharan, N. V., H. Dai, K. L. Roos, N. K. Evanson, J. Tomsik, T. S. Elton, and D. L. Simmons. 2002. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 99:13926-13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, D., J. L. Gombold, and R. F. Ramig. 1990. Intracellular RNA synthesis directed by temperature-sensitive mutants of simian rotavirus SA11. Virology 178:143-151. [DOI] [PubMed] [Google Scholar]
- 11.Chen, D., and J. T. Patton. 2000. De novo synthesis of minus strand RNA by the rotavirus RNA polymerase in a cell-free system involves a novel mechanism of initiation. RNA 6:1455-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, N., and C. S. Reis. 2002. Distinct roles of eicosanoids in the immune response to viral encephalitis: or why you should take NSAIDS. Viral Immunol. 15:133-146. [DOI] [PubMed] [Google Scholar]
- 13.Dumais, N., B. Barbeau, M. Olivier, and M. J. Tremblay. 1998. Prostaglandin E2 up-regulates HIV-1 long terminal repeat-driven gene activity in T cells via NF-kappaB-dependent and -independent signaling pathways. J. Biol. Chem. 273:27306-27314. [DOI] [PubMed] [Google Scholar]
- 14.Dumais, N., S. Bounou, M. Olivier, and M. J. Tremblay. 2002. Prostaglandin E(2)-mediated activation of HIV-1 long terminal repeat transcription in human T cells necessitates CCAAT/enhancer binding protein (C/EBP) binding sites in addition to cooperative interactions between C/EBPbeta and cyclic adenosine 5′-monophosphate response element binding protein. J. Immunol. 168:274-282. [DOI] [PubMed] [Google Scholar]
- 15.Fiebich, B. L., B. Mueksch, M. Boehringer, and M. Hull. 2000. Interleukin-1beta induces cyclooxygenase-2 and prostaglandin E(2) synthesis in human neuroblastoma cells: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB. J. Neurochem. 75:2020-2028. [DOI] [PubMed] [Google Scholar]
- 16.Franco, M. A., and H. B. Greenberg. 2001. Challenges for rotavirus vaccines. Virology 281:153-155. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 18.Goel, A., C. R. Boland, and D. P. Chauhan. 2001. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 172:111-118. [DOI] [PubMed] [Google Scholar]
- 19.Gracey, M., M. A. Phadke, V. Burke, S. K. Raut, and B. Singh. 1984. Aspirin in acute gastroenteritis: a clinical and microbiological study. J. Pediatr. Gastroenterol. Nutr. 3:692-695. [DOI] [PubMed] [Google Scholar]
- 20.Grewal, S. S., A. M. Horgan, R. D. York, G. S. Withers, G. A. Banker, and P. J. Stork. 2000. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase. J. Biol. Chem. 275:3722-3728. [DOI] [PubMed] [Google Scholar]
- 21.Grover, M., O. Giouzeppos, R. D. Schnagl, and J. T. May. 1997. Effect of human milk prostaglandins and lactoferrin on respiratory syncytial virus and rotavirus. Acta Paediatr. 86:315-316. [DOI] [PubMed] [Google Scholar]
- 22.Harris, S. G., J. Padilla, L. Koumas, D. Ray, and R. P. Phipps. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23:144-150. [DOI] [PubMed] [Google Scholar]
- 23.Hasler, F., H. G. Bluestein, N. J. Zvaifler, and L. B. Epstein. 1983. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J. Immunol. 131:768-772. [PubMed] [Google Scholar]
- 24.Hayes, M. M., B. R. Lane, S. R. King, D. M. Markovitz, and M. J. Coffey. 2002. Prostaglandin E(2) inhibits replication of HIV-1 in macrophages through activation of protein kinase A. Cell Immunol. 215:61-71. [DOI] [PubMed] [Google Scholar]
- 25.Heimer, G. V., and C. E. Taylor. 1974. Improved mountant for immunofluorescence preparations. J. Clin. Pathol. 27:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herschman, H. R. 1999. Function and regulation of prostaglandin synthase 2. Adv. Exp. Med. Biol. 469:3-8. [DOI] [PubMed] [Google Scholar]
- 27.Hirasawa, K., A. Kim, H.-S. Han, J. Han, H.-S. Jun, and J.-W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iosef, C., T. Van Nguyen, K. Jeong, K. Bengtsson, B. Morein, Y. Kim, K. O. Chang, M. S. Azevedo, L. Yuan, P. Nielsen, and L. J. Saif. 2002. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine 20:1741-1753. [DOI] [PubMed] [Google Scholar]
- 29.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, M. A., and M. A. McCrae. 1989. Molecular biology of rotaviruses. VIII. Quantitative analysis of regulation of gene expression during virus replication. J. Virol. 63:2048-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katori, M., and M. Majima. 2000. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm. Res. 49:367-392. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki, E. S., and A. M. Wang. 1989. Detection of gene expression, p. 89-97. In H. A. Erlich (ed.), PCR technology: principles and applications for DNA amplification. Stockton Press, New York, N.Y.
- 33.Khyatti, M., and J. Menezes. 1990. The effect of indomethacin, prostaglandin E2 and interferon on the multiplication of herpes simplex virus type 1 in human lymphoid cells. Antivir. Res. 14:161-172. [DOI] [PubMed] [Google Scholar]
- 34.Kline, J. N., G. M. Hunninghake, B. He, M. M. Monick, and G. W. Hunninghake. 1998. Synergistic activation of the human cytomegalovirus major immediate early promoter by prostaglandin E2 and cytokines. Exp. Lung Res. 24:3-14. [DOI] [PubMed] [Google Scholar]
- 35.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351(Pt. 2):289-305. [PMC free article] [PubMed] [Google Scholar]
- 36.LaMonica, R., S. S. Kocer, J. Nazarova, W. Dowling, E. Geimonen, R. D. Shaw, and E. R. Mackow. 2001. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. J. Biol. Chem. 276:19889-19896. [DOI] [PubMed] [Google Scholar]
- 37.Mori, N., H. Inoue, T. Yoshida, T. Tanabe, and N. Yamamoto. 2001. Constitutive expression of the cyclooxygenase-2 gene in T-cell lines infected with human T cell leukemia virus type I. Int. J. Cancer. 94:813-819. [DOI] [PubMed] [Google Scholar]
- 38.Moriuchi, M., H. Inoue, and H. Moriuchi. 2001. Reciprocal interactions between human T-lymphotropic virus type 1 and prostaglandins: implications for viral transmission. J. Virol. 75:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy, T. V., P. M. Gargiullo, M. S. Massoudi, D. B. Nelson, A. O. Jumaan, C. A. Okoro, L. R. Zanardi, S. Setia, E. Fair, C. W. LeBaron, M. Wharton, J. R. Livengood, and J. R. Livingood. 2001. Intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344:564-572. [DOI] [PubMed] [Google Scholar]
- 40.Negishi, M., Y. Sugimoto, and A. Ichikawa. 1995. Molecular mechanisms of diverse actions of prostanoid receptors. Biochim. Biophys. Acta 1259:109-119. [DOI] [PubMed] [Google Scholar]
- 41.Newton, R., L. M. Kuitert, M. Bergmann, I. M. Adcock, and P. J. Barnes. 1997. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem. Biophys. Res. Commun. 237:28-32. [DOI] [PubMed] [Google Scholar]
- 42.Ohno, K., M. Fujiwara, M. Fukushima, and S. Narumiya. 1986. Metabolic dehydration of prostaglandin E2 and cellular uptake of the dehydration product: correlation with prostaglandin E2-induced growth inhibition. Biochem. Biophys. Res. Commun. 139:808-815. [DOI] [PubMed] [Google Scholar]
- 43.Palumbo, G. J., W. C. Glasgow, and R. M. Buller. 1993. Poxvirus-induced alteration of arachidonate metabolism. Proc. Natl. Acad. Sci. USA 90:2020-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parashar, U. D., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 46.Poligone, B., and A. S. Baldwin. 2001. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J. Biol. Chem. 276:38658-38664. [DOI] [PubMed] [Google Scholar]
- 47.Pyeon, D., F. J. Diaz, and G. A. Splitter. 2000. Prostaglandin E2 increases bovine leukemia virus tax and pol mRNA levels via cyclooxygenase 2: regulation by interleukin-2, interleukin-10, and bovine leukemia virus. J. Virol. 74:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rollo, E. E., K. P. Kumar, N. C. Reich, J. Cohen, J. Angel, H. B. Greenberg, R. Sheth, J. Anderson, B. Oh, S. J. Hempson, E. R. Mackow, and R. D. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 49.Roshak, A. K., J. R. Jackson, K. McGough, M. Chabot-Fletcher, E. Mochan, and L. A. Marshall. 1996. Manipulation of distinct NFkappaB proteins alters interleukin-1beta-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J. Biol. Chem. 271:31496-31501. [DOI] [PubMed] [Google Scholar]
- 50.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 51.Santoro, M. G. 1997. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 5:276-281. [DOI] [PubMed] [Google Scholar]
- 52.Santoro, M. G., A. Rossi, and C. Amici. 2003. NF-kappaB and virus infection: who controls whom. EMBO J. 22:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt, J. M., and P. J. Stork. 2000. beta 2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J. Biol. Chem. 275:25342-25350. [DOI] [PubMed] [Google Scholar]
- 54.Shi, Y., and M. Gaestel. 2002. In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol. Chem. 383:1519-1536. [DOI] [PubMed] [Google Scholar]
- 55.Smith, W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 56.Soriano-Brucher, H., P. Avendano, M. O'Ryan, S. D. Braun, M. D. Manhart, T. K. Balm, and H. A. Soriano. 1991. Bismuth subsalicylate in the treatment of acute diarrhea in children: a clinical study. Pediatrics 87:18-27. [PubMed] [Google Scholar]
- 57.Subbaramaiah, K., J. C. Hart, L. Norton, and A. J. Dannenberg. 2000. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 275:14838-14845. [DOI] [PubMed] [Google Scholar]
- 58.Superti, F., C. Amici, A. Tinari, G. Donelli, and M. G. Santoro. 1998. Inhibition of rotavirus replication by prostaglandin A: evidence for a block of virus maturation. J. Infect. Dis. 178:564-568. [DOI] [PubMed] [Google Scholar]
- 59.Symensma, T. L., D. Martinez-Guzman, Q. Jia, E. Bortz, T. T. Wu, N. Rudra-Ganguly, S. Cole, H. Herschman, and R. Sun. 2003. COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J. Virol. 77:12753-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiry, E., B. Mignon, F. Thalasso, and P. P. Pastoret. 1988. Effect of prostaglandins PGE2 and PGF alpha 2 on the mean plaque size of bovine herpesvirus 1. Ann. Rech. Vet. 19:291-293. [PubMed] [Google Scholar]
- 61.Thivierge, M., C. Le Gouill, M. J. Tremblay, J. Stankova, and M. Rola-Pleszczynski. 1998. Prostaglandin E2 induces resistance to human immunodeficiency virus-1 infection in monocyte-derived macrophages: downregulation of CCR5 expression by cyclic adenosine monophosphate. Blood 92:40-45. [PubMed] [Google Scholar]
- 62.van Klinken, B. J., J. Dekker, S. A. van Gool, J. van Marle, H. A. Buller, and A. W. Einerhand. 1998. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am. J. Physiol. 274:G871-G878. [DOI] [PubMed] [Google Scholar]
- 63.Verburg, M., I. B. Renes, D. J. Van Nispen, S. Ferdinandusse, M. Jorritsma, H. A. Buller, A. W. Einerhand, and J. Dekker. 2002. Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J. Histochem. Cytochem. 50:1525-1536. [DOI] [PubMed] [Google Scholar]
- 64.Vossler, M. R., H. Yao, R. D. York, M. G. Pan, C. S. Rim, and P. J. Stork. 1997. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89:73-82. [DOI] [PubMed] [Google Scholar]
- 65.Woodard, J. P., W. Chen, E. O. Keku, S. C. Liu, J. G. Lecce, and J. M. Rhoads. 1993. Altered jejunal potassium (Rb+) transport in piglet rotavirus enteritis. Am. J. Physiol. 265:G388-G393. [DOI] [PubMed] [Google Scholar]
- 66.Yamashiro, Y., T. Shimizu, S. Oguchi, and M. Sato. 1989. Prostaglandins in the plasma and stool of children with rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 9:322-327. [DOI] [PubMed] [Google Scholar]
- 67.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zijlstra, R. T., B. A. McCracken, J. Odle, S. M. Donovan, H. B. Gelberg, B. W. Petschow, F. A. Zuckermann, and H. R. Gaskins. 1999. Malnutrition modifies pig small intestinal inflammatory responses to rotavirus. J. Nutr. 129:838-843. [DOI] [PubMed] [Google Scholar]