Abstract
The γ134.5 gene product is important for the resistance of herpes simplex virus type 1 (HSV-1) to interferon. However, since the inhibition of protein synthesis observed in cells infected with a γ134.5 mutant virus results from the combined loss of the γ134.5 gene product and the failure to translate the late Us11 mRNA, we sought to characterize the relative interferon sensitivity of mutants unable to produce either the Us11 or the γ134.5 polypeptide. We now demonstrate that primary human cells infected with a Us11 mutant virus are hypersensitive to alpha interferon, arresting translation upon entry into the late phase of the viral life cycle. Furthermore, immediate-early expression of Us11 by a γ134.5 deletion mutant is sufficient to render translation resistant to alpha interferon. Finally, we establish that the Us11 gene product is required for wild-type levels of replication in alpha interferon-treated cells and, along with the γ134.5 gene, is an HSV-1-encoded interferon resistance determinant.
At least two herpes simplex virus gene products have been implicated in the virus's ability to resist the pleiotropic effects of interferon (IFN). One of these is the product of the ICP0 gene, a multifunctional polypeptide produced very early in the viral life cycle that transactivates viral gene expression (reviewed in reference 11). ICP0-deficient mutants are hypersensitive to IFN, as viral mRNAs do not accumulate in IFN-treated Vero cells whereas cellular mRNAs encoding IFN-induced gene products increase in abundance (10, 16, 17, 20). This particular IFN antiviral effect requires the cellular PML gene product, and it has been proposed that the disassembly of PML bodies observed in herpes simplex virus type 1 (HSV-1)-infected cells, which requires the ubiquitin E3 ligase activity of ICP0 (1, 24), enables the virus to prevent the induction of IFN-responsive genes (6). While the ICP0 polypeptide prevents the transcriptional induction of cellular IFN-responsive genes, the γ134.5 gene, a second HSV-1 genetic determinant which when altered results in an IFN-hypersensitive virus, encodes a product that operates by preventing host defenses from inactivating the critical translation initiation factor eIF-2 (4, 7, 17).
Upon binding the catalytic subunit of protein phosphatase 1α (PP1α), the γ134.5-PP1α holoenzyme prevents the accumulation of phosphorylated, inactive eIF-2α (the α subunit of eukaryotic initiation factor 2) in infected cells, preserving viral translation rates (12). However, in many established human cell lines infected with a γ134.5 mutant virus, the onset of viral DNA synthesis and the accumulation of γ2 late viral mRNA transcripts are accompanied by the complete cessation of cellular and viral protein synthesis (9). Thus, not only are γ134.5 mutants deficient in functions intrinsic to the γ134.5 gene product, but by failing to translate the viral γ2 mRNAs, they are also deficient in all the activities encoded by this entire class of genes as well. Importantly, when dealing with phenotypes ascribed to a deficiency in the γ134.5 gene, it is fair to question whether the failure to translate these late γ2 viral mRNAs contributes to the observed phenotype. One of these late γ2 mRNAs encodes the Us11 polypeptide, a double-stranded RNA (dsRNA) binding (13), ribosome-associated protein (23) that physically associates with PKR (3, 22) and can prevent PKR activation in response to dsRNA and PACT, a cellular protein that can activate PKR in an RNA-independent manner (21). Furthermore, Us11 can preclude the premature cessation of protein synthesis observed in cells infected with a γ134.5 mutant when it is expressed at immediate-early times, as opposed to late times, postinfection (14, 18). Recently, we demonstrated that the premature cessation of translation observed in cells infected with a γ134.5 mutant actually results from the combined loss of γ134.5 function and failure to translate the Us11 mRNA, establishing that HSV-1 utilizes different mechanisms to regulate eIF-2α phosphorylation at discrete phases of the viral life cycle (19). These findings prompted us to evaluate the contribution made by the Us11 gene product to the overall IFN-resistant phenotype of HSV-1, which up until this point remained unexplored.
To ascertain the importance of the Us11 gene product in the ability of HSV-1 to replicate in alpha IFN (IFN-α)-treated cells, we made use of a panel of mutant viruses that we had previously constructed (19) and first compared their ability to sustain late viral protein synthesis following infection of cells in either the presence or absence of IFN-α. After primary human foreskin fibroblasts (FS4 cells, a gift from Jan Vilcek, NYU School of Medicine) were either mock treated or treated with recombinant human IFN-α (a gift from Roche Pharmaceuticals, Nutley, N.J.) overnight, they were mock infected or infected (multiplicity of infection [MOI] = 5) with either wild-type (WT) HSV-1, the γ134.5 null mutant Δ34.5, a γ134.5 null mutant that expresses Us11 as an immediate-early protein as opposed to a γ2 late protein [Δ34.5-(IE)Us11], a Us11 null mutant (pAUs11), or a virus in which the Us11 mutation was repaired (pAUs11-Rep). Late in the viral life cycle, the cultures were metabolically labeled for 1 h with 35S-labeled amino acids and total protein was subsequently solubilized in sodium dodecyl sulfate (SDS)-sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 0.7 M β-mercaptoethanol) prior to fractionation by SDS-polyacrylamide gel electrophoresis. Figure 1 establishes the fidelity of our experimental system, demonstrating that late viral protein synthesis is resistant to IFN-α treatment in cells infected with WT virus but IFN-α sensitive in cells infected with a γ134.5 mutant virus. While others have reported that translation in cells infected with a γ134.5 mutant is sensitive to IFN (4, 7), this is the first time the IFN sensitivity of this mutant has been examined in primary human cells. Further examination of the data immediately revealed that translation ongoing in cells infected with the Us11 null mutant pAUs11 is extremely sensitive to IFN-α, while protein synthesis in cells infected with either WT virus, Δ34.5-(IE)Us11, or pAUs11-Rep continues relatively unaffected by the IFN treatment. The observation that translation in cells infected with pAUs11-Rep is resistant to IFN-α is particularly important, as it proves that correcting the mutant Us11 allele in the IFN-sensitive pAUs11 mutant restores the IFN-resistant phenotype, ruling out the possibility that the IFN-sensitive phenotype of pAUs11 is due to an adventitious mutation that occurred in the construction of this recombinant.
FIG. 1.
The Us11 gene product is required for translation of viral proteins in primary human fibroblasts treated with IFN-α. FS4 cells untreated or treated overnight with 500 U of recombinant human IFN-α/ml were mock infected or infected (MOI = 5) with either a γ134.5 deletion mutant (Δ34.5), a Δ34.5 strain that expressed Us11 as an IE protein [Δ34.5-(IE)Us11], a Us11 null mutant (pAUs11), a virus in which the Us11 mutant allele was repaired (pAUs11-Rep), or WT HSV-1 (WT). At 18 h postinfection, the cultures were metabolically labeled with 35S-amino acids for 1 h. Total protein was isolated and fractionated by SDS-polyacrylamide gel electrophoresis. An exposure of the fixed, dried gel is shown. The bottom panel shows an immunoblot of the samples that was probed with a polyclonal antibody raised against the γ134.5 protein. The arrowhead to the right of the blot denotes the position of the full-length γ134.5 polypeptide encoded by the HSV-1 Patton strain. The migration of molecular mass standards (in kilodaltons) appears to the left of each panel.
The apparent resistance of rates of translation to IFN-α in cells infected with Δ34.5-(IE)Us11 is particularly striking, as this virus contains a complete deletion of both copies of the γ134.5 gene, implying that the artificial expression of Us11 as an IE polypeptide can indeed compensate for the loss of the γ134.5 protein and maintain viral translation in IFN-α-treated cells. Moreover, this finding supports the observation that translation in cells infected with a Us11 null mutant is hypersensitive to IFN and is consistent with the hypothesis that the dramatic inhibition of translation observed in IFN-treated cells infected with a γ134.5 mutant is due to the combined loss of both γ134.5 and Us11. The important contribution of Us11 is further strengthened by the extreme IFN-α sensitivity of translation in cells infected with the pAUs11 mutant, as this virus carries two WT copies of the γ134.5 gene and expresses WT levels of the γ134.5 protein (Fig. 1) (19), suggesting that the γ134.5 polypeptide on its own is not sufficient to prevent the shutdown of translation in HSV-1-infected primary human cells that have been treated with IFN-α.
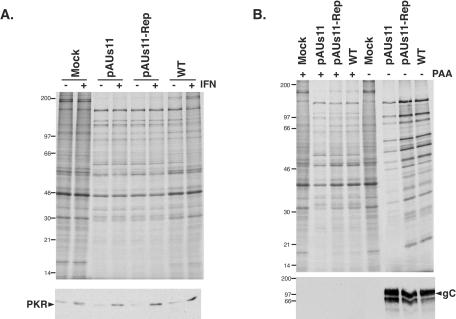
Previously, we established that the premature cessation of translation observed in cells infected with an HSV-1 γ134.5 mutant actually results from the absence of the γ134.5 gene product and the failure to translate the γ2 late mRNA which encodes Us11. While viral DNA replication, or a temporally linked event such as the accumulation of late mRNA transcripts, is required for this translational arrest, the reduction of translation rates described here in IFN-treated cells infected with a Us11 mutant, which produces WT levels of the γ134.5 protein, may or may not occur with the same kinetics. In particular, overall levels of PKR are greater in IFN-treated cells, possibly increasing the responsiveness of this cellular sensor to dsRNA, a molecular indicator of viral infection, and potentially inhibiting translation before the onset of viral DNA replication. To learn the point in the viral life cycle at which translation is inhibited in IFN-treated cells infected with pAUs11, the labeling experiments were performed with cells treated with both IFN-α and phosphonoacetic acid (PAA), an inhibitor of viral DNA synthesis that prevents the life cycle from advancing into the late phase. The inclusion of IFN-α along with PAA in the culture media suppressed the sensitivity of viral translation to IFN-α, establishing that the IFN-induced translational block observed in cells infected with the pAUs11 mutant virus requires either viral DNA synthesis or a tightly linked event such as the accumulation of true late γ2 transcripts (Fig. 2A). As a control to verify that the IFN-α treatment was indeed effective in the PAA-containing media, we measured the steady-state level of PKR, a cellular gene product known to accumulate in response to IFN treatment, by immunoblotting lysates from untreated and IFN-treated cells. The increased abundance of PKR in response to IFN-α corroborates the effectiveness of this treatment (Fig. 2A). To confirm that PAA successfully inhibited true late gene expression, polypeptides synthesized in the presence and absence of PAA were examined. Figure 2B demonstrates that the pattern of viral proteins synthesized in infected, PAA-treated cells differs markedly from those detected in untreated cells; in addition, PAA prevents the reduction in translation rates observed in cells infected with a Us11 mutant virus. Finally, PAA also blocks the accumulation of gC, a known γ2 or true late polypeptide (Fig. 2B).
FIG. 2.
The inhibition of translation in IFN-treated cells requires entry into the late phase of the viral life cycle. (A) FS4 cells treated with PAA in the presence and absence of IFN-α were infected with the indicated viruses, metabolically labeled with 35S-amino acids for 1 h at 14 h postinfection, and processed as described in the legend to Fig. 1. In the lower panel, samples were analyzed by immunoblotting with anti-PKR antisera. (B) FS4 cells, either untreated or treated with PAA, were infected with the indicated viruses and processed as described in the legend to Fig. 1. The lower panel shows an immunoblot of the samples that was probed with an anti-gC polyclonal antibody.
Prior to evaluating the impact of IFN-α treatment on the replication of our mutant viruses in primary FS4 cells, we observed that commonly used concentrations of IFN-α (1,000 U/ml) completely inhibited replication of WT HSV-1 in our FS4 cell strain despite the synthesis of large quantities of viral proteins. Similar findings were reported previously by others working with different strains of fibroblasts and HSV-1, who noted that treatment with recombinant IFN-α or IFN-β blocks virus morphogenesis at a late stage and inhibits the release of particles (5). To overcome this obstacle, we first identified an IFN-α concentration (250 U/ml) that allowed the completion of the WT HSV-1 life cycle with the hope that an IFN-sensitive mutant might retain this phenotype under these conditions. After overnight treatment with 250 U of IFN-α/ml, FS4 cells were infected (MOI = 10−3) with either WT HSV-1, Δ34.5, Δ34.5-(IE)Us11, pAUs11, or pAUs11-Rep and the incubation continued for 5 days, at which point the cultures were lysed by freeze-thawing and the titer of the virus produced was determined in Vero cells. Under these conditions, while the genotypically WT viruses (WT and pAUs11-Rep) demonstrate only 3- to 4.7-fold reduction in viral growth following IFN-α treatment, the same treatment results in a 62,000-fold reduction in the growth of the γ134.5 null mutant Δ34.5 (Fig. 3). It is likely that the magnitude of this difference reflects the failure to translate the true late γ2 Us11 mRNA along with the absence of the γ134.5 gene product in IFN-α-treated cells infected with the Δ34.5 mutant. Cells infected with the Us11 null mutant pAUs11 exhibited a 44-fold reduction in viral replication following IFN-α treatment, establishing that loss of the Us11 gene significantly impairs viral replication in IFN-α-treated cells. Importantly, pAUs11 still retains the ability to express the γ134.5 gene product, preventing further reduction in replication under these conditions. In contrast, cultures infected with Δ34.5-(IE)Us11, a mutant that does not contain a γ134.5 gene, can only express Us11 as an IE protein to prevent host defenses from inhibiting translation. Here, a 939-fold reduction in replication of Δ34.5-(IE)Us11 was detected in IFN-α-treated cells. This finding allows us to conclude that the expression of Us11 in the absence of the γ134.5 gene product supports substantial levels of viral replication in IFN-α-treated cells, resulting in a 66-fold increase in the overall amount of virus produced relative to that for IFN-α-treated cells infected with Δ34.5. In addition, the fact that Δ34.5-(IE)Us11 is not as resistant to IFN-α as viruses that express the γ134.5 protein (Fig. 3) but directs similar rates of translation in primary human cells (Fig. 1) and remains neuroattenuated in animal models (15) is consistent with the proposal that the γ134.5 protein performs additional functions besides regulating eIF-2α phosphorylation (2, 8).
FIG. 3.
The Us11 gene product is important for WT levels of resistance to IFN-α. FS4 cells, either untreated or treated overnight with 250 U of IFN-α/ml, were infected with the indicated viruses (MOI = 10−3). After 5 days, cell-free lysates were prepared by freeze-thawing the cultures, and the amount of infectious virus produced was quantified by performing a plaque assay with Vero cells.
Taken together, our data suggest that the extreme IFN-α-sensitive viral replication observed in primary human fibroblasts infected with Δ34.5 results from the absence of both the viral γ134.5 and the viral Us11 gene products. By comparing replication of pAUs11, which is a Us11 null mutant with a WT γ134.5 gene, with that of Δ34.5-(IE)Us11, which is a γ134.5 null mutant that expresses Us11 as an IE gene, we have demonstrated that both of these gene products contribute significantly to the ability of HSV-1 to replicate in IFN-α-treated cells and that both are required for WT levels of replication in IFN-treated primary human cells. This is the first demonstration that the Us11 gene product contributes to HSV-1 replication in IFN-treated cells, raising the possibility that it might also perform a similar role in viral pathogenesis.
Acknowledgments
We thank Ed Mocarski for his thoughtful comments and Roz Eisenberg along with Gary Cohen for generously providing the anti-gC antibody.
This work was supported by a grant from the NIH to I.M. M.M. was supported in part by NIH training grants to the Department of Microbiology, NYU School of Medicine (T32 AI0718021), and a joint institutional training award in virus-host interactions to Mount Sinai School of Medicine and NYU School of Medicine (T32 AI07647).
Footnotes
For Bernard Roizman, il miglior fabbro, on the occasion of his 75th year.
REFERENCES
- 1.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, S. M., A. R. MacLean, J. D. Aitken, and J. Harland. 1994. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J. Gen. Virol. 75:3679-3686. [DOI] [PubMed] [Google Scholar]
- 3.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 Us11 protein interacts with protein kinase R in infected cells and requires a 30 amino acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerveny, M., S. Hessefort, K. Yang, G. Cheng, M. Gross, and B. He. 2003. Amino acid substitutions in the effector domain of the γ134.5 protein of herpes simplex virus 1 have differential effects on viral response to interferon-α. Virology 307:290-300. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee, S., E. Hunter, and R. Whitley. 1985. Effect of cloned human interferons on protein synthesis and morphogenesis of herpes simplex virus. J. Virol. 56:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, G., M. E. Brett, and B. He. 2001. Val193 and Phe195 of the γ134.5 protein of herpes simplex virus 1 are required for viral resistance to interferon α/β. Virology 290:115-120. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, G., K. Yang, and B. He. 2003. Dephosphorylation of eIF-2α mediated by the γ134.5 protein of herpes simplex virus type 1 is required for viral response to interferon but is not sufficient for efficient viral replication. J. Virol. 77:10154-10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, J., and B. Roizman. 1992. The γ34.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 76:2180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22:761-770. [DOI] [PubMed] [Google Scholar]
- 12.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of eukaryotic initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoo, D., C. Perez, and I. Mohr. 2002. Characterization of RNA determinants recognized by the arginine- and proline-rich region of Us11, a herpes simplex virus type 1-encoded double-stranded RNA binding protein that prevents PKR activation. J. Virol. 76:11971-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 15.Mohr, I., D. Sternberg, S. Ward, D. Leib, M. Mulvey, and Y. Gluzman. 2001. An HSV-1 γ34.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J. Virol. 75:5189-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulvey, M., J. Poppers, A. Ladd, and I. Mohr. 1999. A herpesvirus ribosome associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73:3375-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulvey, M., J. Poppers, D. Sternberg, and I. Mohr. 2003. Regulation of eIF2α phosphorylation by different functions that act during discrete phases in the HSV-1 life cycle. J. Virol. 77:10917-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 21.Peters, G. A., D. Khoo, I. Mohr, and G. C. Sen. 2002. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 76:11054-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poppers, J., M. Mulvey, C. Perez, D. Khoo, and I. Mohr. 2003. Identification of a lytic-cycle Epstein-Barr virus gene product that can regulate PKR activation. J. Virol. 77:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein Us11 is a virion component and associates with 60S ribosomal subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]