Abstract
The specificity determinants for susceptibility to resistance by the Fv1 n and b alleles map to amino acid 110 of the murine leukemia virus CA protein. To study the interaction between Fv1 and CA, we examined changes in CA resulting in the loss of susceptibility to Fv1 resistance in naturally occurring NB- and NR-tropic viruses. A variety of amino acid changes affecting Fv1 tropism were identified, at CA positions 82, 92 to 95, 105, 114, and 117, and they all were mapped to the apparent exterior of virion-associated CA. These amino acids may form a binding surface for Fv1.
The Fv1 gene is one of a series of mouse genes, originally described in the early 1970s, that control the susceptibility of mice to murine leukemia virus (MLV) infection (32, 34). There are two major alleles of Fv1, Fv1n and Fv1b, which are defined by their ability to block specific subclasses of MLV (17). Fv1n, originally described for NIH Swiss mice, is able to block the replication of B-tropic MLV while allowing the replication of N-tropic virus, and Fv1b, found in BALB/c mice, does the reverse. A further subclass of MLV replicates equally well in mice carrying either allele. Such viruses are called NB tropic. A third allele of Fv1, Fv1nr, found in a few inbred strains of mice and apparently also in some wild mice, restricts B-tropic MLV and some, but not all, N-tropic viruses (28, 48). In this paper, we will refer to N-tropic viruses that are not restricted by Fv1nr as being NR tropic.
Fv1 acts in a cell-autonomous manner to restrict virus replication (44), but the precise mechanism for restriction is unclear. It has been shown that viral replication is blocked at a stage after virus entry into the cell and before the integration of newly synthesized viral DNA into the host genome (22, 41). The block to infection is not absolute in vitro, but the number of infected cells is reduced by a factor of 50 to 1,000 (17). When expressed at natural levels, e.g., in mouse fibroblast lines, neither Fv1n nor Fv1b shows significant in vitro restriction of NB-tropic MLV.
Genetic studies initially suggested that the target for Fv1 restriction is the MLV capsid (CA) protein (20, 43). Subsequent studies indicated that viral tropism is determined by a pair of adjacent amino acids, at positions 109 and 110, in CA (10, 40). A more recent study has shown that position 110 is the most important residue for N and B tropism (29). N-tropic MLV has an Arg residue at this position, and B-tropic MLV has a Glu residue. The determinants for NB and NR tropism have not been fully characterized.
The Fv1 gene was cloned a few years ago (3) and was found to have sequence similarity to a family of endogenous retroviruses called HERV-L (60% identity over 1.3 kb) or MuERV-L (1, 3). Based on its position within the element and the presence of a major homology region (3), Fv1 apparently encodes a Gag-related protein. Gag proteins bind tightly to each other via interaction domains during virus assembly (35), which suggests a possible mechanism for Fv1's action on MLV CA (16). To date, however, there is no direct evidence for the binding of Fv1 to CA. Biochemical analyses are greatly complicated by the extremely low natural expression levels of Fv1 in vivo. We have therefore taken a genetic approach to analyzing the viral determinants of NB and NR tropism, using a rapid fluorescence-activated cell sorting (FACS)-based approach for Fv1 testing (5), in an attempt to delineate the region(s) of CA that interacts with Fv1.
MATERIALS AND METHODS
Cells and viruses.
Mus dunni, NIH 3T3, BALB-3T3, and HEK-293T cells were cultivated in Dulbecco's modified Eagle medium containing 10% fetal calf serum and antibiotics. Viruses were generated by simultaneous CaPO4-mediated transient transfections of 293T cells with three plasmids providing vector, gag-pol, and env functions (4, 5). Virus-containing supernatants were filtered, frozen in aliquots at −70°C, and titrated on Fv1-null M. dunni cells.
Fv1 assay.
Transduction assays for Fv1 function were performed as previously described (4, 5). M. dunni cells were first transduced with the Fv1 gene via a delivery virus and then were infected with a virus carrying the CA gene to be examined (the tester virus), and in both cases sufficient virus was added to infect 35 to 40% of the cells. The effect of Fv1 was then assessed by two-color FACS analysis. Alternatively, analyses were performed on NIH 3T3 and BALB-3T3 cells with just the tester virus.
Recombinant DNA.
All recombinant DNA work was done by established techniques (45). Plasmids encoding infectious molecular clones from Gross passage A (pGN104) (6) and Friend murine leukemia virus (F-MLV) clone 57 (39) were provided by L. Boone and R. Friedrich, respectively. Delivery plasmids encoding Fv1n (pLFv1nIEG) and Fv1b (pLFv1bIEG) have been described previously (5). An analogous construct encoding Fv1nr (pLFv1nrIEG) was prepared from pLFv1nIEG by changing the Ser at Fv1 residue 352 to Phe. Gag-Pol expression plasmids for N-tropic AKV (endogenous ecotropic MLV from AKR mice) (pCIG3N), B-tropic AKV (pCIG3B), and NB-tropic Moloney MLV (Mo-MLV) (pHIT60) have been described previously (5). A similar plasmid for NB-tropic F-MLV (pczFLV57gp) was prepared as follows. An 8.4-kb gel-purified EcoRI fragment from pF-MuLV57 containing a permuted copy of F-MLV was ligated by the use of T4 DNA ligase (NEB). The resulting product was ethanol precipitated and used as a template for a PCR with the Expand long template PCR system (Roche) and primers MB21 5′-GGCCGCGGCCGCTGAAAACATGGGCCAGAC-3′ and MB22 5′-GGCCGCGGCCGCGGGGATTAGGAGGTCCCGC-3′ (NotI sites are underlined) according to the supplier's instructions. The NotI-digested PCR product (5,420 bp) was cloned into the NotI site of pcDNA3.1/zeo(+) (Invitrogen), yielding a cytomegalovirus immediate early promoter-driven gag-pol expression construct. Site-directed mutagenesis was performed with a QuikChange site-directed mutagenesis kit (Stratagene). Sequences of the oligonucleotide primers used are available upon request. The structure of each prepared plasmid was verified by restriction mapping and/or sequencing prior to use. All DNA preparations were purified on Qiagen columns prior to transfection. CA mutations in these plasmids are designated in the text by the virus of origin (indicated by N- [N-AKV], B- [B-AKV], Mo, or F), the original residue, the position, and the new residue. For example, N-N82D has an Asp at CA position 82 replacing the Asn found in N-tropic AKV.
Fv1 sequencing.
High-molecular-weight DNA was prepared from the spleen of a 129SvEv mouse obtained from the Biological Services division of the National Institute for Medical Research. Genomic DNAs from NZB/B1NJ, NZW/LacJ, and RF/J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). The Fv1 open reading frame was PCR amplified with primers GT16 (5′CAA AAA GAT CTA GAT GAA TTT CCC ACG TGC G3′) and GT17 (5′TGG ATA GTC GAC ATC TAT ACT ATC TTG GTG AG3′), which lie at the 5′ and 3′ ends of Fv1, respectively, subcloned into M13mp18 at the BglII and Sal sites present in the primers, and sequenced. Thirty-five-cycle PCRs of 25 μl contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, a 10 mM concentration of each deoxynucleoside triphosphate, 10 pmol of each primer, 2 mM MgCl2, 100 ng of template DNA, 1.25 U of Taq DNA polymerase, and 0.09 U of Pfu DNA polymerase. PCRs were carried out in a PTC-100 thermal cycler under the following conditions: denaturation at 96°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 2 min.
DNA sequence accession numbers.
The sequence of the Fv1nr allele has been deposited in GenBank under accession number AY294331. The sequence of the CA gene of the Gross passage A virus was determined from pGN104 DNA (accession number AY294332).
RESULTS
The study of viral escape mutants has shed considerable light on the relationships between viruses and their hosts (38, 54). MLV variants that exhibit NR or NB tropism can be considered escape mutants. The identification of the sequence changes responsible for escape from Fv1 restriction might therefore be expected to provide valuable information on the interaction between Fv1 and its target on CA. With this consideration in mind, we set out to identify the specific changes allowing the generation of NR- or NB-tropic viruses.
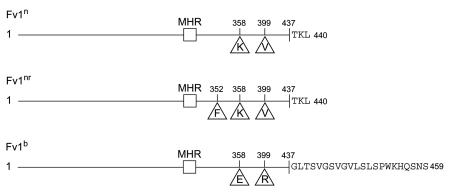
Prior to starting this analysis, it was important to develop an assay for NR-tropic viruses. The Fv1 gene from Fv1nr 129 mice was cloned by PCR and then sequenced. The Fv1nr gene has a single nucleotide change compared to Fv1n, resulting in a single Ser→Phe change at amino acid 352 of Fv1. DNAs from three other strains that were previously typed as Fv1nr, namely NZB/B1NJ, NZW/LacJ, and RF/J (W. P. Rowe and J. W. Hartley [cited in reference 48]), had the identical substitution (12). These observations are consistent with the proposition that the Ser→Phe mutation converts the Fv1n genotype to Fv1nr. Differences in the predicted protein products of these three alleles of Fv1 are shown in Fig. 1.
FIG. 1.
Differences between predicted open reading frames of the n, nr, and b alleles of Fv1. MHR, position of the major homology region. Differences between the proteins are indicated with the single letter amino acid code. Fv1n, GenBank accession no. X97720; Fv1b, GenBank accession no. X97719; Fv1nr, GenBank accession no. AY294332.
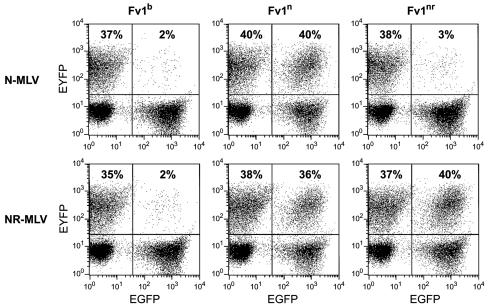
To confirm that the phenotypic differences associated with the nr allele resulted from the substitution at position 352, we engineered this change into the Fv1n delivery virus (see Materials and Methods). Figure 2 shows the restriction profiles associated with the three alleles of the Fv1 gene for N- and NR-tropic MLV. As expected, the Fv1nr allele showed a clear-cut effect, restricting N-AKV but not affecting the replication of an otherwise identical virus carrying the N-N114H change that is characteristic of NR-tropic AKR-L1 (24).
FIG. 2.
Restriction profiles of products of n, b, and nr alleles of Fv1. Fv1-null M. dunni cells were transduced with vectors encoding the different Fv1 alleles followed by testing with N-AKV and NR-tropic virus (N-AKV with an N114H CA change) and subsequent FACS analysis. Fv1-expressing cells were identified by the expression of enhanced green fluorescent protein, and successful infections with tester viruses were identified by the expression of enhanced yellow fluorescent protein. The effect of Fv1 can be gauged by comparing the percentages of infected cells in the absence or presence of Fv1.
CA sequence of naturally occurring viruses.
It is well established that the primary viral determinants of N and B tropism map to amino acid 110 of CA (29). However, a previous study showed that the introduction of the corresponding amino acid (Ala) from NB-tropic Mo-MLV did not give rise to an NB-tropic virus (41). In contrast, genetic studies pointed to the involvement of a linked amino acid at position 105 (11, 43). Similarly, the only NR-tropic virus investigated thus far showed a modification at position 114 (24). As a starting point for identifying amino acids that are responsible for changes in Fv1 tropism, we compared the sequences of four NB-tropic (Moloney, Rauscher, Friend, and AKR-B2NB) and two NR-tropic (Gross and AKR-L1) MLVs with N-tropic (N-AKV) and B-tropic (B-AKV and MCF1233) viruses (Fig. 3). These sequences have all been published (see the legend to Fig. 3 for references), with the exception of that of Gross virus. The Gross virus sequence was determined by sequencing the CA region of plasmid pGN104 (6). An inspection of the data shown in Fig. 3 allowed us to draw several conclusions. First, this comparison confirms that changes at position 110 are not necessary for NR or NB tropism since both NR-tropic isolates and three of the four NB-tropic viruses are identical to N-AKV or B-AKV at this position. Second, it is clear that both NB and NR tropism can arise in multiple ways since no two isolates showed the same changes. Third, various isolates showed several changes in the vicinity of amino acid 110, raising the possibility that more than one CA determinant may play a role in the generation of NB-tropic viruses. To examine these effects in more detail, we introduced several of these changes, either singly or in combination, into tester viruses and examined their effects on Fv1 restriction.
FIG. 3.
CA protein sequences of natural N-, B-, NR-, and NB-tropic MLV isolates. The predicted CA protein sequence of N-tropic AKV (6, 10, 19) is compared to those of nine other MLV CA proteins. AKR-L1 (24) and Gross virus (GenBank accession no. AY294332) are NR tropic; B-AKV (6, 10) and MCF1233 (47) are B tropic; and AKR-B2NB (11), Mo-MLV (46), R-MLV (25), and F-MLV (GenBank accession no. X02794) are NB tropic. Identities are indicated with dots, and differences are indicated with the single letter amino acid code. Bold characters indicate the primary Fv1 specificity determinants at position 110.
CA determinants of NR tropism.
The NR tropism of the AKR-L1 virus is specified by amino acid 114 of CA. However, a second NR isolate, Gross virus, does not have this substitution but rather carries an N82D change (Fig. 3). We introduced this substitution into N-AKV and confirmed that the resulting construct specified NR tropism (data not shown). We conclude that a substitution at amino acid 82 or 114 could convert N-tropic MLV to NR tropism. However, as discussed below, these are not the only changes that can have this effect.
CA determinants of NB tropism.
Conventional in vitro Fv1 assays rely on the comparison of virus titers obtained on different cell lines carrying the appropriate alleles of Fv1. Such assays do not show any effect of Fv1n or Fv1b on NB-tropic MLV replication. However, it was shown that the b allele of Fv1 (but not the n allele) is capable of inhibiting Mo-MLV expression when it is overexpressed (5). We decided to test whether our assay, which can accurately measure relatively small differences in virus restriction (4, 5), would reveal any phenotypic differences between NB-tropic viruses from different sources. We made tester viruses by incorporating Mo-MLV or F-MLV CA and B-AKV CA with a mutation at position 105 to yield a CA identical to that of AKR-B2NB. These viruses were tested on M. dunni cells transduced with Fv1n, Fv1b, and Fv1nr. Despite the sequence differences in their CA proteins, Mo-MLV and F-MLV yielded the same results, encountering no resistance with Fv1n or Fv1nr but partial inhibition with Fv1b. In contrast, AKR-B2NB was not restricted by Fv1b, and although it was unaffected by Fv1n, it was fully restricted by Fv1nr. Interestingly, attempts to introduce the same change at position 105 in N-AKV yielded little or no infectious virus, even though reverse transcriptase (RT)-positive virions were produced (data not shown).
We next wanted to identify the amino acids responsible for NB tropism in Mo-MLV. We set out to exchange amino acids that differed in the CA proteins of N-AKV and Mo-MLV, focusing on the region surrounding amino acid 110, with the aim of exchanging Fv1 tropism. The data for these constructs are summarized in Fig. 4. In order to convert Mo-MLV to N tropism, we had to exchange three amino acids, at positions 82, 110, and 117. Reciprocal changes at the same positions in N-AKV yielded a virus that was similar, but not identical, to Mo-MLV. Single or double changes involving residues 82 and 117 tended to yield NR-tropic viruses, providing yet another way of converting N tropism to NR tropism. The results with constructs involving an Ala residue at position 110 were more complex, sometimes yielding a virus capable of being restricted by additional Fv1 alleles in a manner reminiscent of Ala substitutions at position 358 of Fv1 (4). The addition of the position 82 change, but not the position 117 change, abolished this affect. Parallel experiments were performed to introduce Mo-MLV residues into B-AKV, with similar overall results, namely that although individual changes could give changes in the restriction pattern, changes at all three positions were required to give a Mo-MLV-like virus (Fig. 4).
FIG. 4.
Map of NB tropism determinants of Mo-MLV. The figure shows (from left to right) the names of the different constructs tested, the amino acid changes introduced, restriction by Fv1, and the tropisms of the different constructs. The symbols are the same as those used in other work (4, 5): +, full restriction, equivalent to a reduction from 35% infection of Fv1-negative cells to 2% infection of Fv1-positive cells; (+), slightly less restriction; ±, only half the percentage of Fv1+ cells were infected compared to the percentage of infected cells in the Fv1-negative population; (−), slight inhibition; and −, there was no difference in the numbers of infected cells between the Fv1+ and Fv1-negative populations, and therefore there was no restriction. Mo-D82N/H117L yielded a very-low-titer virus; data for this construct were therefore excluded from this figure.
The amino acids determining the NB tropism of F-MLV were next addressed in a similar manner (Fig. 5). The CA protein of F-MLV differs from that of N-AKV at positions 82, 92 to 95, 107, and 117 within the important region, as judged from the Mo-MLV studies. It carries the N-tropism-determining amino acid Arg at position 110. By themselves, the F-MLV-specific changes at positions 82 and 92 reduced restriction by the nr allele without affecting restriction by the b allele. The introduction of a Glu residue at position 82 had a lesser effect than did that of an Asp residue at position 92. Changing residues 93 to 95 created an NR-tropic virus. Combining the changes at positions 92 and 93 to 95 gave rise to a virus with an identical tropism to that of F-MLV, but the change at position 82 had no effect on the substitutions at positions 93 to 95. Interestingly, it proved possible to generate a virus showing no restriction by any of the three Fv1 alleles by introducing the position 117 change in addition to changes at positions 92 to 95.
FIG. 5.
Map of NB tropism determinants of F-MLV. See the legend to Fig. 4 for further details.
Positioning tropism determinants in a CA model.
Our results and those of previous workers have identified several CA amino acids that can influence the interaction between Fv1 and incoming virions. Further interpretation of these data would be greatly facilitated by the existence of a crystal structure for a gammaretrovirus CA molecule. Unfortunately, although crystal structures of CA proteins from viruses representing three genera of retrovirus have been determined (8, 21, 26, 37), the gammaretroviruses are not represented on this list. We therefore decided to model the Mo-MLV CA structure based on available structures and detailed sequence comparisons of different CA proteins.
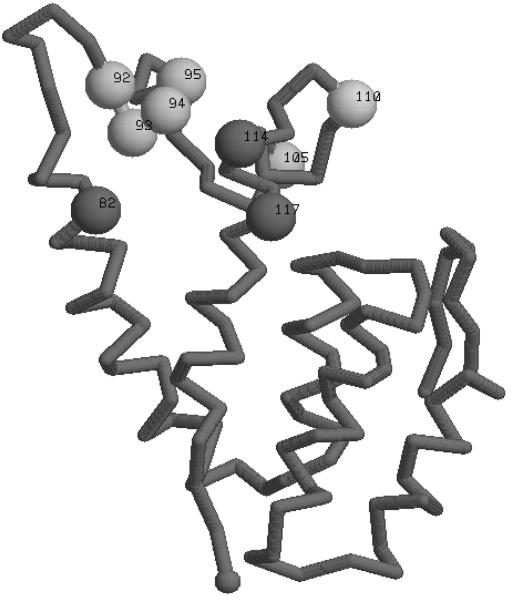
A panel of related but clearly distinct gammaretroviral CAs were identified with the search program Quest (50) and aligned for secondary structure prediction with PsiPred3 (23). The resulting alignment was then compared to the known CA structures by use of a multiple sequence threading program (51), generating an α-carbon molecular model for MLV CA (52). The predicted structure for the N-terminal domain of N-AKV CA is shown in Fig. 6. The positions of the amino acids which were shown to influence the interaction of Fv1 and CA (82, 92 to 95, 105, 110, 114, and 117) are indicated with arrows in Fig. 6. They lie close to one another on the predicted exterior of CA, apparently defining a surface with which Fv1 might interact.
FIG. 6.
Map of Fv1-allele-determining amino acids on the CA protein. An α-carbon model for the structure of the N-terminal domain of AKV CA (amino acids 11 to 135) is shown (52). Amino acids affecting Fv1 tropism are indicated with numbered spheres; the C-terminal end is marked with an unnumbered gray sphere.
DISCUSSION
In this paper we have (i) molecularly characterized the Fv1nr allele, (ii) identified several different amino acids in CA that affect the interaction with Fv1, (iii) shown that there are multiple routes to NR and NB tropism, and (iv) identified a potential Fv1 binding pocket in the N-terminal domain of CA. These observations will now be discussed in terms of the evolution of resistance to Fv1 restriction as well as the implications for the interaction between CA and Fv1-related molecules.
Previous studies have shown that a forced passage of B-tropic MLV through Fv1n cells in vitro rapidly leads to the isolation of an NB-tropic virus, whereas the passage of N-tropic MLV in Fv1b cells does not (20, 34). No reversal of tropism, i.e., N→B or B→N, was observed. The new virus has a characteristic change in its RNA fingerprint (13) resulting from a G-to-A substitution in T1 oligonucleotide B (43). This change yielded the NB-tropic B-D105N virus (11). The failure to isolate an NB-tropic virus from N-MLV can now be rationalized by our observation that N-D105N has little or no biological activity. It should also be noted that the Q110E change to interconvert N- and B-tropic viruses requires two simultaneous nucleotide changes in order to give resistance to restriction, an event that would be rare compared to the single base change required for the D105N change. The failure to isolate other mutations in forced passage experiments (13) is perhaps surprising. It may well be that few, if any, single amino acid changes in CA can give rise to a virus that is both Fv1 unrestricted and fully replication competent. However, a detailed analysis of the effect of single amino acid changes in this region of CA on the growth rate is outside the scope of this paper.
This point is underscored by the structure of NB-tropic Mo-MLV, F-MLV, and Rauscher MLV (R-MLV). The three viruses were generated independently by the in vivo passage of cells or (presumably) N-tropic virus in Fv1b mice (33, 36, 42). Each has several amino acid changes compared to AKV. Did these changes arise by sequential point mutations or by recombination with endogenous proviruses? The near identity of the F-MLV and R-MLV CA sequences despite their independent origins is most simply explained by recombination with a single endogenous provirus. Sequence studies of cloned endogenous MLVs (A. Stevens and J. P. Stoye, unpublished data) as well as BLAST searches of the NCBI mouse genome assembly (release 32, February 2004) indicated that most, if not all, endogenous nonecotropic MLV proviruses carry determinants of B tropism. This analysis (J. P. Stoye, unpublished data) identified a potential donor on chromosome 5 for the B-tropic sequences of MCF1233, suggesting that tropism changes can occur in this way. No endogenous MLVs with CA sequences identical to those of Mo-MLV, F-MLV, or R-MLV CA were found, suggesting their derivation from AKV by a series of point mutations. However, there is considerable MLV polymorphism (14) between BALB/c mice, the strain in which the NB-tropic forms of both F-MLV and R-MLV are likely to have arisen (33, 42), and sequenced C56BL/6 mice. Thus, recombination with an as yet unidentified provirus cannot be excluded.
We have defined a series of amino acids in CA that affect Fv1 restriction. Based on our model of CA and by analogy with solved CA structures, these amino acids appear to lie close to one another in a region that is surface exposed and does not participate in CA-CA contacts (7, 30, 31). It thus might represent a potential binding domain for Fv1, with specificity imposed by the various combinations of amino acids lining the pocket. Alternatively, these alterations might influence the structure of the exposed loops on the very surface of the CA molecule. In any event, some of the CA amino acids seem to play a major role in determining specificity. For example, no virus carrying an Arg residue at position 110 is restricted by Fv1n unless the Lys at position 358 of Fv1 is changed to Ala (4). Similarly, the introduction of an Ala residue at CA position 110 can render MLV susceptible to Fv1n restriction (Fig. 4), provided that position 82 is not occupied by an Asp. It appears that the presence of positively charged amino acids at both CA position 110 and Fv1 position 358 is incompatible with a stable binding interaction. In contrast, other changes have no effect by themselves but have an effect in combination with other specific changes. Thus, the E92D change has no effect on MLV tropism unless it is accompanied by the VDA93IND alteration. A further explanation of such effects is dependent on obtaining detailed structures of Fv1 and MLV CA, which are subjects of intensive on-going investigation.
There is one major caveat to the foregoing discussion—it assumes a direct and specific interaction between Fv1 and CA. To date, we have been unable to demonstrate a direct and specific interaction. For example, yeast two-hybrid experiments have consistently failed to show an interaction between CA and Fv1 (unpublished data). However, the range of specific interactions that we have shown seems incompatible with a series of adapter molecules. We therefore assume that the precise structure of CA in a subviral particle is crucial in determining whether it can serve as a target for Fv1 restriction. Recent studies (M. Dodding, M. Bock, M. W. Yap, and J. P. Stoye, unpublished data) indicate that recognition by Fv1 is dependent on proper CA processing, and most probably, assembly.
It is tempting to speculate which regions of the CA proteins of other viruses interact with restricting molecules such as Lv1, which has phenotypic similarity to Fv1 (2, 9, 18, 49). The region of CA affecting Fv1 binding runs from the end of helix 4 (amino acid 82) through the start of helix 7 (amino acid 117). We would therefore predict that Lv1 restriction of human immunodeficiency virus type 1 might be influenced by changes in CA from about amino acids 85 through 122. This region includes the cyclophilin binding domain (15), which has already been implicated in Lv1 binding (27, 53), but it also extends about 25 amino acids downstream. Experiments to test the importance of these residues are under way.
Acknowledgments
We thank Larry Boone and Roland Friedrich for providing cloned Gross and Friend MLV samples.
This work was supported by the UK Medical Research Council.
REFERENCES
- 1.Bénit, L., N. de Parseval, J.-F. Casella, I. Callebaut, A. Cordonnier, and T. Heidmann. 1997. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and a gag coding sequence closely related to the Fv1 restriction gene. J. Virol. 71:5652-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, K. N., M. Bock, G. Towers, and J. P. Stoye. 2001. Identification of the regions of Fv1 necessary for MLV restriction. J. Virol. 75:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone, L. R., F. E. Myer, D. M. Yang, C.-Y. Ou, C. K. Koh, L. E. Roberson, R. W. Tennant, and W. K. Yang. 1983. Reversal of Fv-1 host range by in vitro restriction endonuclease fragment exchange between molecular clones of N-tropic and B-tropic murine leukemia virus genomes. J. Virol. 48:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs, J. A. G., T. Wilk, R. Welker, H.-G. Kräusslich, and S. D. Fuller. 2003. Structural organization of authentic, mature HIV-1 virions and cores. EMBO J. 22:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos-Olivas, R., J. L. Newman, and M. F. Summers. 2000. Solution structure and dynamics of the Rous sarcoma virus capsid protein and comparison with capsid proteins of other retroviruses. J. Mol. Biol. 296:633-649. [DOI] [PubMed] [Google Scholar]
- 9.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Musing, H. G. Gorrlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DesGroseillers, L., and P. Jolicoeur. 1983. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J. Virol. 48:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi, K., A. Kawana, A. Iwamoto, H. Yoshikura, and T. Odawara. 1997. One base change is sufficient for host range conversion of murine leukemia virus from B to NB tropism. Arch. Virol. 142:1889-1894. [DOI] [PubMed] [Google Scholar]
- 12.Ellis, S. A. 2000. Evolutionary and functional studies of the mouse retroviral restriction gene, Fv1. Ph.D. thesis. University of London, London, United Kingdom.
- 13.Faller, D. V., and N. Hopkins. 1978. T1 oligonucleotide maps of N-, B-, and B→NB-tropic murine leukemia viruses derived from BALB/c. J. Virol. 26:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel, W. N., J. P. Stoye, B. A. Taylor, and J. M. Coffin. 1990. A genetic linkage map of endogenous murine leukemia viruses. Genetics 124:221-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamble, T. R., F. F. Vajdos, S. Yoo, D. K. Worthylake, M. Houseweart, W. I. Sundquist, and C. P. Hill. 1996. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell 87:1285-1294. [DOI] [PubMed] [Google Scholar]
- 16.Goff, S. P. 1996. Operating under a gag order: a block against incoming virus by the Fv1 gene. Cell 86:691-693. [DOI] [PubMed] [Google Scholar]
- 17.Hartley, J. W., W. P. Rowe, and R. J. Huebner. 1970. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J. Virol. 5:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. J. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herr, W. 1984. Nucleotide sequence of AKV murine leukemia virus. J. Virol. 49:471-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins, N., J. Schindler, and R. Hynes. 1977. Six NB-tropic leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J. Virol. 21:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin, Z., L. Jin, D. L. Peterson, and C. L. Lawson. 1999. Model for lentiviral capsid core assembly based on crystal dimers of EIAV p26. J. Mol. Biol. 286:83-93. [DOI] [PubMed] [Google Scholar]
- 22.Jolicoeur, P. 1979. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr. Top. Microbiol. Immunol. 86:67-122. [DOI] [PubMed] [Google Scholar]
- 23.Jones, D. T. 2000. The Psipred protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 24.Jung, Y. T., and C. A. Kozak. 2000. A single amino acid change in the murine leukemia virus capsid gene responsible for the Fv1nr phenotype. J. Virol. 74:5385-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khimani, A. H., M. Lim, T. G. Graf, T. F. Smith, and R. M. Ruprecht. 1997. Phylogenetic relationship of the complete Rauscher murine leukemia virus genome with other murine leukemia virus genomes. Virology 238:64-67. [DOI] [PubMed] [Google Scholar]
- 26.Khorasanizadeh, S., R. Campos-Olivas, and M. F. Summers. 1999. Solution structure of the capsid protein from the human T-cell leukemia virus type-1. J. Mol. Biol. 291:491-505. [DOI] [PubMed] [Google Scholar]
- 27.Kootstra, N. A., C. Münk, N. Tonnu, N. R. Lander, and I. M. Verma. 2003. Abrogation of postentry restriction of HIV-1-based lentiviral vector transduction in simian cells. Proc. Natl. Acad. Sci. USA 100:1298-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozak, C. A. 1985. Analysis of wild-derived mice for Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J. Virol. 55:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target for Fv1 resistance. Virology 225:300-306. [DOI] [PubMed] [Google Scholar]
- 30.Langford, G. A., N. Yannoutsos, E. Cozzi, R. Lancaster, K. Elsome, P. Chen, A. Richards, and D. J. G. White. 1994. Production of pigs transgenic for human decay accelerating factor. Transplant. Proc. 26:1400-1401. [PubMed] [Google Scholar]
- 31.Li, S., C. P. Hill, W. I. Sundquist, and J. T. Finch. 2000. Image reconstruction of helical assemblies of the HIV-1 CA protein. Nature 407:409-413. [DOI] [PubMed] [Google Scholar]
- 32.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45:163-169. [PubMed] [Google Scholar]
- 33.Lilly, F. 1967. Susceptibility to two strains of Friend leukemia virus in mice. Science 155:461-462. [DOI] [PubMed] [Google Scholar]
- 34.Lilly, F., and T. Pincus. 1973. Genetic control of murine viral leukemogenesis. Adv. Cancer Res. 17:231-277. [Google Scholar]
- 35.Luban, J., K. B. Alin, K. L. Bossolt, T. Humaran, and S. P. Goff. 1992. Genetic assay for multimerization of retroviral gag polyproteins. J. Virol. 66:5157-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moloney, J. B. 1960. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J. Natl. Cancer Inst. 24:933-951. [PubMed] [Google Scholar]
- 37.Momany, C., L. C. Kovari, A. J. Prongay, W. Keller, R. K. Gitti, B. M. Lee, A. E. Gorbalenya, L. Tong, J. McClure, L. S. Ehrlich, M. F. Summers, C. Carter, and M. G. Rossmann. 1996. Crystal structure of dimeric HIV-1 capsid protein. Nat. Struct. Biol. 3:763-770. [DOI] [PubMed] [Google Scholar]
- 38.Oldstone, M. B. A. 1997. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology 234:179-185. [DOI] [PubMed] [Google Scholar]
- 39.Oliff, A. I., G. L. Hager, E. H. Chang, E. M. Scolnick, H. W. Chan, and D. R. Lowy. 1980. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J. Virol. 33:475-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou, C.-Y., L. R. Boone, C.-K. Koh, R. W. Tennant, and W. K. Yang. 1983. Nucleotide sequences of the gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J. Virol. 48:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pryciak, P. M., and H. E. Varmus. 1992. Fv-1 restriction and its effects on murine leukemia virus integration in vivo and in vitro. J. Virol. 66:5959-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauscher, F. J. 1962. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J. Natl. Cancer Inst. 29:515-543. [PubMed] [Google Scholar]
- 43.Rommelaere, J., H. Donis-Keller, and N. Hopkins. 1979. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell 16:43-50. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg, N., and P. Jolicoeur. 1997. Retroviral pathogenesis, p. 475-585. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Press, Cold Spring Harbor, N.Y. [PubMed]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Shinnick, T. M., R. A. Lerner, and J. G. Sutcliffe. 1981. Nucleotide sequence of Moloney murine leukemia virus. Nature 293:543-548. [DOI] [PubMed] [Google Scholar]
- 47.Sijts, E. J. A. M., C. J. M. Leupers, E. A. M. Mengedé, W. A. M. Loenen, P. J. van den Elsen, and C. J. M. Melief. 1994. Cloning of the MCF1233 murine leukemia virus and identification of sequences involved in viral tropism, oncogenicity and T cell epitope formation. Virus Res. 34:339-349. [DOI] [PubMed] [Google Scholar]
- 48.Steeves, R., and F. Lilly. 1977. Interactions between host and viral genomes in mouse leukemia. Annu. Rev. Genet. 11:277-296. [DOI] [PubMed] [Google Scholar]
- 49.Stoye, J. P. 2002. An intracellular block to primate lentivirus replication. Proc. Natl. Acad. Sci. USA 99:11549-11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor, W. R. 1998. Dynamic database searching with templates and multiple alignment. J. Mol. Biol. 280:375-406. [DOI] [PubMed] [Google Scholar]
- 51.Taylor, W. R. 1997. Multiple sequence threading: an analysis of alignment quality and stability. J. Mol. Biol. 269:902-943. [DOI] [PubMed] [Google Scholar]
- 52.Taylor, W. R., and J. P. Stoye. 2004. Consensus structural models for the amino terminal domain of the retrovirus restriction gene Fv1 and the murine leukaemia virus capsid proteins. BMC Struct. Biol. 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towers, G. J., T. Hatziioannou, S. Cowan, S. P. Goff, J. Luban, and P. D. Bieniasz. 2003. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 9:1138-1143. [DOI] [PubMed] [Google Scholar]
- 54.Webster, R. G., and W. G. Laver. 1980. Determination of the number of nonoverlapping antigenic areas on the Hong Kong (H3N2) influenza hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology 104:139-148. [DOI] [PubMed] [Google Scholar]