Abstract
Poliovirus and some other picornaviruses trigger relocation of certain nuclear proteins into the cytoplasm. Here, by using a protein changing its fluorescence color with time and containing a nuclear localization signal (NLS), we demonstrate that the poliovirus-triggered relocation is largely due to the exit of presynthesized nuclear protein into the cytoplasm. The leakiness of the nuclear envelope was also documented by the inability of nuclei from digitonin-permeabilized, virus-infected (but not mock-infected) cells to retain an NLS-containing derivative of green fluorescent protein (GFP). The cytoplasm-to-nucleus traffic was also facilitated during infection, as evidenced by experiments with GAPDH (glyceraldehyde-3-phosphate dehydrogenase), cyclin B1, and an NLS-lacking derivative of GFP, which are predominantly cytoplasmic in uninfected cells. Electron microscopy demonstrated that a bar-like barrier structure in the channel of the nuclear pores, seen in uninfected cells, was missing in the infected cells, giving the impression of fully open pores. Transient expression of poliovirus 2A protease also resulted in relocation of the nuclear proteins. Lysates from poliovirus-infected or 2A-expressing cells induced efflux of 3×EGFP-NLS from the nuclei of permeabilized uninfected cells. This activity was inhibited by the elastase inhibitors elastatinal and N-(methoxysuccinyl)-l-alanyl-l-alanyl-l-prolyl-l-valine chloromethylketone (drugs known also to be inhibitors of poliovirus protease 2A), a caspase inhibitor zVAD(OMe), fmk, and some other protease inhibitors. These data suggest that 2A elicited nuclear efflux, possibly in cooperation with a zVAD(OMe).fmk-sensitive protease. However, poliovirus infection facilitated nuclear protein efflux also in cells deficient in caspase-3 and caspase-9, suggesting that the efflux may occur without the involvement of these enzymes. The biological relevance of nucleocytoplasmic traffic alterations in infected cells is discussed.
Picornaviruses (61) are cytoplasmic viruses. All essential steps of their reproduction, such as translation and replication of the viral RNA and maturation of virions, are confined to the cytoplasm. Not surprisingly, picornaviruses, such as poliovirus, echovirus, and encephalomyocarditis virus, are known to produce infectious progeny in nucleus-free cytoplasts (24, 54) or cytoplasmic extracts (5, 48, 66). This does not mean, however, that the nucleus or its components have no role in picornavirus reproduction. Indeed, the nucleus-free cytoplasts generated much less virus than did intact cells, and the former, in contrast to the latter, failed to support viral reproduction after infection with double-stranded replicative form RNA of poliovirus (14). Also, some cellular proteins known to have predominantly nuclear localization appear to relocate into the cytoplasm and to stimulate viral RNA translation (46, 37) or replication (45, 71). On the other hand, the entry of virus-specific (and possibly of some cellular) cytoplasmic proteins into the nucleus of picornavirus-infected cell exerts important effects on the course of viral reproduction and the cell fate. Thus, viral proteases 2A and 3C were reported to target several transcription factors (56, 73, 78-80) and histones (21). Accumulation of virus-specific proteins in the nuclei of poliovirus-infected (8, 22) and encephalomyocarditis virus-infected (3, 4) cells was directly observed. Nuclear alterations developing upon different forms of picornavirus-induced apoptosis (7, 28, 35, 40, 50, 69) also require entry of certain proapoptotic host proteins (e.g., effector caspases and DNases) into the nuclei (cf., references 20 and 55).
Nucleocytoplasmic protein exchange is a sophisticated, tightly regulated process ensuring accurate control of gene expression and other cellular functions (43, 44, 74). The nucleus is surrounded by an envelope (10, 58) consisting of the outer and inner protein-containing lipid membranes and an underlying meshwork-like proteinaceous lamina. The major gates for the nucleocytoplasmic exchange are represented by nuclear pores, a complex structure of ∼125 MDa composed of dozens of proteins termed nucleoporins or Nups (18, 57). The nuclear pore complex (NPC) exhibits a highly conserved architecture with an eightfold rotational symmetry that includes a central aqueous cylindrical channel surrounded with numerous specialized appendages. Relatively small molecules may go out of and into the nucleus by simple diffusion (e.g., proteins <30 kDa diffuse through the NPC relatively rapidly) but the nucleocytoplasmic traffic of macromolecules is generally energy dependent, specific, and largely accomplished by dedicated carriers, importins and exportins, recognizing appropriate motifs on the cargoes named nuclear localization signals (NLS) and nuclear export signals (NES), respectively (25, 26, 39, 65). A GTPase called Ran is a major controller of the cargo loading and/or unloading, as well as of directionality of the traffic (64). The inner nuclear membrane and the lamina are also intimately associated, through bridges involving integral proteins, with protein and DNA moieties of the peripheral chromatin (10). This association, as well as the interaction of certain chromosome-bound proteins with components of the nucleocytoplasmic transport machinery, is involved in the transcriptional control through affecting heterochromatinization and silencing (23, 36).
Recently, we have revealed a marked modification of the nucleocytoplasmic traffic early upon infection with two picornaviruses, poliovirus and coxsackievirus B3, as well as a negative-strand RNA genome virus, vesicular stomatitis virus (6). Derivatives of green fluorescent protein (GFP), including its trimeric form, fused to NLS from simian virus 40 (SV40) T antigen were, as expected, confined to nuclei in uninfected cells but could be readily detected in the cytoplasm at early steps of infection (6). Independently, redistribution of a variety of nuclear proteins into the cytoplasm after infection with poliovirus and rhinovirus was observed also by Gustin and Sarnow (33, 34). In principle, such nucleus-to-cytoplasm relocation of proteins may be accomplished through different mechanisms. The simplest one may involve the loss of an NLS. Indeed, the exit of autoantigen La from nuclei of poliovirus-infected cells (46) may, at least in part, be promoted by its truncation by the viral 3C protease, resulting in the loss of NLS (62). In many cases, however, relocating proteins do retain their NLS (6, 33, 34). Two not mutually exclusive hypothesis have been put forward to explain such cases: the alleviation of the barrier functions of nuclear envelope (6) and the impairment of active nuclear import resulting in the cytoplasmic accumulation of newly synthesized proteins (32-34).
The present study aims at further characterization of alterations of nucleocytoplasmic traffic upon poliovirus infection. Our data demonstrate not only that some nuclear proteins go relatively freely out into the cytoplasm but that the movement in the opposite direction is also nonspecifically facilitated. Functional leakiness of the nuclear envelope in the infected cells is accompanied by, and is likely due to, structural alterations of NPC, which can be documented by electron microscopy. These effects appear to be at least in part due to the 2A protease of poliovirus since the expression of the prototeolytically active form of the enzyme results in a similar protein relocation. The implications of alterations of the nucleocytoplasmic transport machinery for both viral reproduction and development of cytopathic effect are also discussed.
MATERIALS AND METHODS
Viruses and cells.
HeLa-B cells (69) were cultivated on Dulbecco modified Eagle medium with 10% bovine serum, whereas MCF-C9DN (7), Hep-2, and 293 cells were grown on Dulbecco modified Eagle medium with 10% fetal bovine serum. The cells grown on petri dishes were washed with serum-free medium. If appropriate, cells were preincubated with inhibitors for 10 min at 37°C and infected with 50 to 100 PFU/cell (if not indicated otherwise) of Mahoney strain of poliovirus type 1 (a relatively high input multiplicity of infection [MOI] was used because in some experiments poliovirus reproduction was suppressed by inhibitors in order to understand whether replication of the viral RNA was required for the alteration of nucleocytoplasmic traffic, or translation of the input RNA was sufficient to trigger the effect). After a 30-min adsorption with agitation at 18°C, the cells were washed again and incubated with 5% CO2 in the appropriate serum-free medium at 37°C for indicated time intervals with inhibitors where indicated.
Plasmids.
Plasmids coding for enhanced GFP (EGFP) fused to the NLS of SV40 (pEGFP-NLS) or three in-frame copies of GFP fused to the same NLSs were described previously (6). Plasmid pTimer-NLS was constructed by inserting the NLS-containing XhoI-BamHI fragment of pEGFP-NLS into the plasmid encoding Timer-NA (81), the nonaggregating form of the Timer fluorescent protein (68). Plasmid pEGFP-GFP5 encoding five in-frame copies of EGFP (20) was kindly donated by Y. Lazebnik. Cyclin B-EGFP encoding vector was a gift of D. Bulavin (National Cancer Institute). Vectors encoding the poliovirus 2A wild-type and H20N mutant protease under the control of cytomegalovirus (CMV) promoter were constructed by insertion of PCR fragments corresponding to the 2A sequences from plasmids pEP2A or pEP2A(H20N) (82), kindly donated by R. Lloyd (Baylor College of Medicine), into the EcoRI-BamHI site of pUHC. There was an AUG in an optimal Kozak context upstream of the 2A-coding sequence, and two in-frame stop codons downstream of it. pUHC was derived from pUHD10-3 by replacing the XhoI-EcoRI fragment containing a modified tetracycline-dependent form of CMV promoter with XhoI-EcoRI fragment containing the fully active CMV promoter from pUHD15-1. Both pUHD10-3 and pUHD15-1 were kindly donated by H. Bujard (Heidelberg University Molecular Biology Center). The 2A-coding region of pUHC-2A and pUHC-2A(H20N) was checked by sequencing. Plasmid pMIV-3×GFP-NLS used for retrovirus-mediated cell transformation was made by inserting NheI-BamHI fragment of pEGFP-NLS-2×GFP with the Klenow-filled NheI end (6) into pMarxIV vector harboring the neomycin resistance gene (20).
Transient transfection. For transfection, Lipofectamine 2000 (Invitrogen) was used essentially according to the manufacturer's recommendations. HeLa and MCF-7-derived cells were transfected with appropriate plasmids, infected with poliovirus, or directly observed after the indicated time intervals.
Stable cell transformation. Retrovirus-mediated cell transformation was performed essentially as described previously (20) by using pMIV-3×GFP-NLS vector encoding three copies of GFP fused in frame with SV40 NLS, which proved to be stable in healthy and poliovirus-infected HeLa cells. After 2 weeks of selection at 500 μg of G418 (Sigma)/ml, the cells were plated onto 96-well plates at a concentration of approximately one cell per two wells. The resulting clones were screened for fluorescence, and the best of them was designated HeLa-3E and chosen for future experiments.
Preparation of cell extracts.
HeLa-B cells grown on 10 roller bottles (∼108 cells/per bottle) until near confluence were infected with poliovirus at an input MOI of ∼100 PFU/cell and incubated for 3 h at 37°C. After treatment with EDTA and pelleting at 300 × g for 15 min at 4°C, the cells were resuspended in 50 ml (about 10 pellet volumes) of the extract preparation buffer (50 mM PIPES, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 10 μg of cytochalasin B/ml; pH 7) and centrifuged at 300 × g for 5 min at 4 °C. The supernatant was discarded, and the cells were lysed by three cycles of freezing and thawing. The lysate was centrifuged for 1 h at 100,000 × g. The resulting supernatant (S100; protein concentration of 15 to 29 mg/ml) was dialyzed against 100 volumes of the permeabilization buffer (50 mM PIPES, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol; pH 7) for 6 h and frozen as small aliquots. Prior to the use, S100 extracts were diluted, usually 10-fold, with the buffer described above.
For the preparation of extracts (S15) from the plasmid-transfected cells, the cells (∼106) grown on a 60-mm petri dish were detached and pelleted at 300 × g as described above, washed with 500 μl of the extract preparation buffer, and resuspended in 200 μl of the same buffer. After lysis by freezing and thawing, the final centrifugation was performed at 15,000 × g (simply for technical reasons [due to a small volume of the sample]). The lysates were dialyzed as described above. For the experiments, the S15 lysates were diluted, usually twofold, with the permeabilization buffer.
Cell permeabilization.
Cell permeabilization of HeLa-3E cells was achieved by treatment with digitonin (1). To get a monolayer of permeabilized cells, the cells grown on coverslips were twice washed with cold phosphate-buffered saline and once with the permeabilization buffer. The cells were then treated with 40 μg of digitonin/ml supplemented with Hoechst 33258 in the permeabilization buffer for 5 min on ice, followed by washing with the buffer. This kind of Hoechst dye does not penetrate the plasma membrane and therefore stains permeabilized cells only.
Nucleus isolation.
Nucleus isolation was performed as described previously (17). Briefly, cells were detached with EDTA, washed with phosphate-buffered saline, and resuspended in ice-cold STM buffer (50 mM Tris-HCl [pH 7.4], 0.25 M sucrose, 5 mM MgSO4, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride [PMSF]). A solution of 1% Nonidet P-40 in STM buffer was added to a final concentration of 0.025%, the cells were homogenized by 30 strokes in a tight glass-glass Dounce homogenizer (Wheaton) and examined immediately.
Nuclear envelope permeability assay.
Monolayers of permeabilized HeLa-3E cells were overlaid with appropriately diluted (see figure legends) lysates from virus-infected or plasmid-transfected cells. Samples were incubated for 1 h at 37°C and examined under an epifluorescence microscope. The effects of the following protease inhibitors were tested on the ability of cellular extracts to trigger protein redistribution: elastatinal, chymostatin, leupeptin, pepstatin A, antipain, PMSF, and N-(methoxysuccinyl)-l-alanyl-l-alanyl-l-prolyl-l-valine chloromethylketone (MPCMK) (all from Sigma) and benzyloxycarbonyl-Val-Ala-Asp-(OMe) fluoromethylketone (zVAD.fmk; Enzyme Systems Products; Dublin, Calif.). The extracts were preincubated with the inhibitors for 20 min at 4°C.
Immunofluorescence.
Cell fixation and staining was performed as described previously (7). Mouse monoclonal antibodies 6C5 (29) to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were kindly provided by V. Muronetz (Moscow State University). Secondary Cy3 anti-mouse antibodies were from Milan Analytica AG.
Fluorescence microscopy.
Cells were examined with a Leica DMLS fluorescence microscope equipped with green I3 (for the Timer protein and GFP derivatives), red N2.1 (also for the Timer protein), and blue A (for Hoechst-stained nuclei) filter cubes. The pictures were obtained with a Leica Digital Camera 100 (Leica DC100).
Electron microscopy.
Electron microscopy was performed as described previously (2). The cells were detached at 5 h postinfection (p.i.) and fixed with 2.5% glutaraldehyde and then with 1% OsO4 and embedded in EPON-812. Sections were stained with uranyl acetate and lead citrate before being photographed in a Philips CM100 electron microscope.
RESULTS
Discrimination between newly synthesized and “old” nuclear proteins in the cytoplasm of infected cells by using a fluorescent Timer protein.
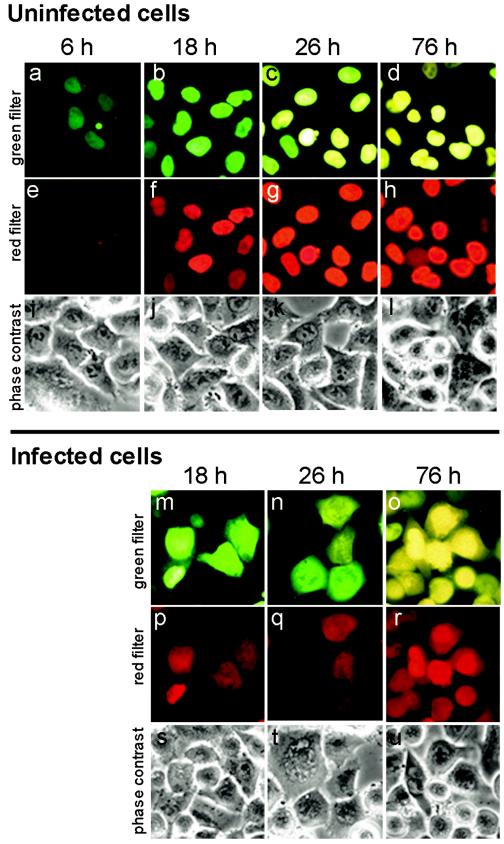
As mentioned in the introduction, the cytoplasmic accumulation of a nuclear protein may theoretically be explained by enhanced efflux of this protein from the nucleus and/or failure of the newly synthesized protein to be imported into the nucleus. To ascertain whether the former possibility is actually realized, we made use of a so-called fluorescent Timer protein (68). The color of fluorescence of nonaggregating Timer-NA (81), constructed on the basis of the original Timer (68), changes with time from green to yellow when inspected with a green filter, whereas the fluorescence of only the “old” Timer could be detected by using the red filter. Therefore, the color of fluorescence allows one to distinguish between “old” and recently synthesized “young” molecules. HeLa cells were transiently transfected with a plasmid expressing Timer-NA fused to the NLS of SV40 T antigen. As early as 6 h posttransfection, nuclear fluorescence in a proportion of cells was detected with the green filter (Fig. 1a). With time, upon protein “maturing”, fluorescence was detected in the overwhelming majority of the cells with both filters, and the color of fluorescence observed with the green filter gradually changed from green to yellow (Fig. 1a to d). The early steps of Timer “maturation” (i.e., between 6 and 18 h) could be more readily detected by using the red filter (compare panels a and b with panels e and f) because it did not transmit the fluorescence of the young protein at all (Fig. 1e). Due to incomplete synchrony of expression of the transfected gene, both green and yellow cells could be present in the same field at intermediate time intervals posttransfection (Fig. 1c). The change of the fluorescence color should be attributed to the “age” of the protein rather than to changes in its concentration because the addition of protein synthesis inhibitor relatively early after transfection did not prevent the color conversion (not shown).
FIG.1.
Cytoplasmic fluorescence in poliovirus-infected HeLa cells expressing the Timer-NA-NLS fusion was due to the efflux of the presynthesized nuclear protein. In control uninfected Timer-transfected cells (a to l), the newly synthesized protein was accumulated in the nuclei and emitted fluorescence, which shifted with time from green to yellow when inspected with the green filter (a to d) and gradually became visible and increased in intensity when inspected with the red filter (e to h). In poliovirus-infected cells at 4 h p.i. (m to u), fluorescence could be seen not only in the nuclei but also in the cytoplasm, and the colors of nuclear and cytoplasmic fluorescence in a given cell coincided. The exposures were 10, 5, and 2 s for the pictures taken at 6, 18 to 26, and 76 h posttransfection, respectively, due to a marked difference in the fluorescence levels.
Fluorescence was always confined entirely to the nuclei of uninfected cells (Fig. 1a to h). After poliovirus infection, however, significant fluorescence could be observed, with both filters, also located in the cytoplasm. The results obtained at 4 h p. i. in cells harboring the Timer protein of different “ages” are shown in Fig. 1m to r. Importantly, the color of cytoplasmic fluorescence in a given cell with the green filter always coincided with the color of its nuclear fluorescence. Were the cytoplasmic fluorescence in the infected cells due primarily to the newly synthesized Timer protein, it should have always been green. The cytoplasmic fluorescence observed in the infected cells with the red filter (Fig. 1p to r) obviously corresponded to the old molecules of the Timer protein because its young molecules were not detectable with this filter (cf., Fig. 1e).
We concluded that the fluorescence in the cytoplasm of infected cells was predominantly represented by the protein accumulated in the nucleus prior to infection rather than by the newly synthesized molecules.
Evidence for the increased leakiness of nuclear envelope of the infected cells.
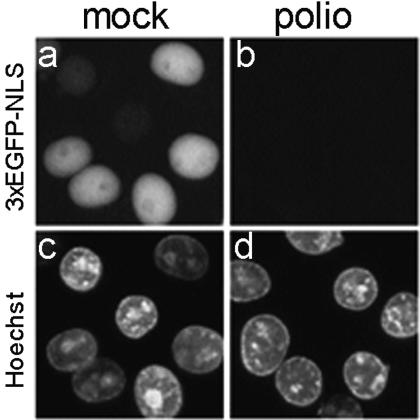
One of the simplest explanations for the facilitated protein traffic between the nucleus and cytoplasm in infected cells would be a general decrease in the barrier functions of the nuclear envelope. This explanation was supported by the following observations. Monolayers of mock-infected HeLa-3E cells stably expressing 3×GFP-NLS were treated with digitonin, known to destroy plasma membrane but to leave nuclear envelope intact (1). Nuclei of such cells, as expected, proved to be fluorescent (Fig. 2a). In contrast, the nuclei of similarly prepared permeabilized preparations of virus-infected cells at 2 h p.i. exhibited very low-level, if any, fluorescence (Fig. 2b), indicating that the fluorescent protein went out of the nuclei (and also out of the cytoplasm due to the digitonin-mediated increase in permeability of the plasma membrane). Similar results were obtained when isolated nuclei from mock-infected and poliovirus-infected HeLa-3E cells (rather than digitonin-permeabilized preparations of these cells) were inspected by fluorescence microscopy (not shown).
FIG. 2.
Loss of 3×GFP-NLS from nuclei of permeabilized poliovirus-infected but not mock-infected HeLa-3E cells. At 2 h p.i. (MOI of ∼100 PFU/cell), the monolayers of HeLa-3E cells were treated with digitonin, which by itself permeabilizes the plasma membrane but leaves the nuclear membrane intact, stained with Hoechst 33258, and inspected under fluorescence microscope by using the green (for 3×EGFP-NLS) and blue (for Hoechst) filter cubes. Note that this kind of Hoechst dye is not able to enter the cell through an intact plasma membrane, and therefore it stains only the permeabilized cells.
Thus, infection rendered the nuclear envelope readily permeable for at least some of nuclear proteins.
Facilitated entry of cytoplasmic proteins into nuclei of infected cells.
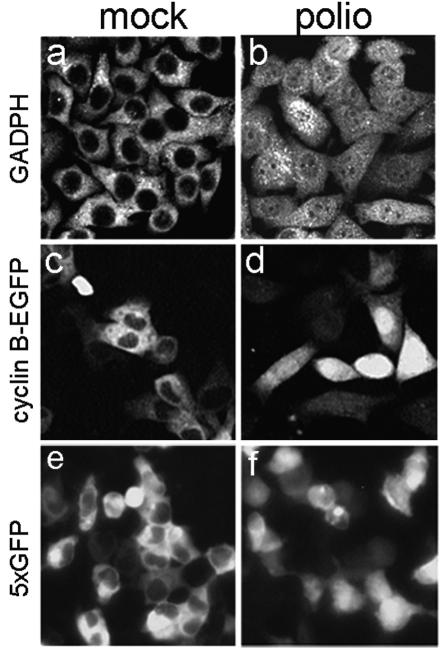
Next, we wanted to ascertain whether protein traffic in the opposite direction, i.e., from the cytoplasm to nucleus was similarly affected by the viral infection. An abundant glycolytic enzyme, GAPDH, and cyclin-B1 (in our case, a readily detectable fusion of this protein with EGFP), which are predominantly cytoplasmic in uninfected cells (Fig. 3a and c), became nearly evenly distributed throughout the infected cells already by 2 to 3 h p. i. (Fig. 3b and d). However, nuclear translocation of these two proteins may occur at certain physiological and pathological conditions (60, 67). Therefore, the ability of the cytoplasm-to-nucleus translocation was also tested with an artificial high-molecular-mass protein composed of five in-frame fused copies of EGFP. This protein, named 5×EGFP, lacked an NLS and was large enough (∼125 kDa) to be excluded from the nuclei in mock-infected cells (Fig. 3e), but it was distributed throughout the cell after poliovirus infection (Fig. 3f). The confocal microscope was unavailable for the experiments with cyclin B1 and 5×EGFP; furthermore, living cells were inspected in these experiments rather than fixed cells as in the GAPDH probes. Not surprisingly, the cells had a different appearance in different panels of Fig. 3, but the absence of nuclear exclusion was clear in all cases. The experiments with cyclin B1 and GAPDH were performed in HeLa and Hep-2 cells, respectively, to ascertain the generality of the phenomenon, whereas the experiments with 5×EGFP were carried out in 293 cells because this protein was more stable in these cells (uninfected or infected) than in HeLa cells, as evidenced by Western blotting (not shown). It should also be noted that the nucleus-to-cytoplasm relocation of EGFP-NLS and 3×EGFP-NLS was observed also in poliovirus-infected RD and L cells (not shown), demonstrating the generality of the phenomena described.
FIG. 3.
Entry of cytoplasmatic proteins in the nucleus after poliovirus infection. Mock-infected (a) and poliovirus-infected (b) Hep-2 cells were stained at 3 h p.i. with monoclonal anti-GAPDH antibodies. The secondary antibodies were Cy3 conjugated. The samples were analyzed with a Zeiss Axiovert 100 confocal microscope. HeLa cells were transfected with a plasmid expressing cyclin B-EGFP and 24 h posttransfection were mock infected (c) or poliovirus infected (d) and inspected under epifluorescence microscope at 2 h p.i. 293 cells were transiently transfected with a plasmid expressing five in-frame copies of EGFP and at 24 h posttransfection were infected with poliovirus. The mock-infected (e) and virus-infected (f) cells were investigated under a fluorescence microscope at 2 h p.i.
Thus, poliovirus infection facilitated protein translocation in both directions.
Electron microscopy of the nuclear envelope in infected cells.
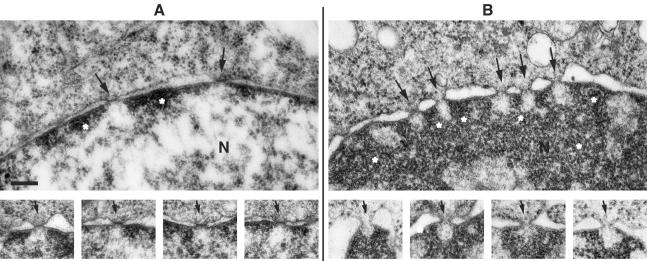
In an attempt to find morphological correlates of the increased leakiness of the nuclear envelope in the infected cells, electron microscopic examination of such cells in comparison with their uninfected counterparts was carried out. In uninfected HeLa cells, the central channel of the pores was found to be closed by an electron-dense, bar-like structure, seemingly anchored to the membranous nuclear envelope (Fig. 4a). Little condensed chromatin was lining the inner nuclear membrane, and the chromatin did not extend over the nuclear pore, thus leaving an electron translucent area at the site of the so-called “basket” component of the nuclear pore. In infected cells (Fig. 4b), the chromatin was more extensively condensed and also left electron translucent areas behind the nuclear pores. The space between inner and outer nuclear membrane was dilated. The diameter of the central channel of the pores was comparable to that in uninfected cells. Importantly, in practically all pores of the nuclear envelope of infected cells, the obstructing bar-like structure in the central channel was missing, giving the impression of a fully open pore. These alterations are fully compatible with the notion that poliovirus infection makes the pores more permissive for the free bidirectional diffusion of macromolecules.
FIG. 4.
Electron microscopy of nuclear envelope and nuclear pores of uninfected and infected HeLa cells. (A) Uninfected cells. The upper panel shows a segment from a nucleus (N) with nuclear pores (arrows) and small areas of condensed chromatin (asterisks) along the inner nuclear membrane. The sites of the nuclear pore baskets are devoid of condensed chromatin. The lower series of pictures represent examples of nuclear pores, all showing an obstructing bar-like structure in the central channel (arrows). (B) Poliovirus-infected cells at 5 h p.i. The upper panel shows a segment of a nucleus (N) with nuclear pores (arrows), dilated perinuclear space, and heavily condensed chromatin (asterisks). The lower series of pictures show examples of seemingly open nuclear pores (arrows) and the absence of the bar-like closures in the central channel. As in uninfected cells, the condensed chromatin is discontinuous behind the nuclear pores. All pictures in this figure are at an equal magnification. Bar, 200 nm.
Extracts of poliovirus-infected cells contain a proteolytic factor(s) increasing leakiness of the nuclear envelope.
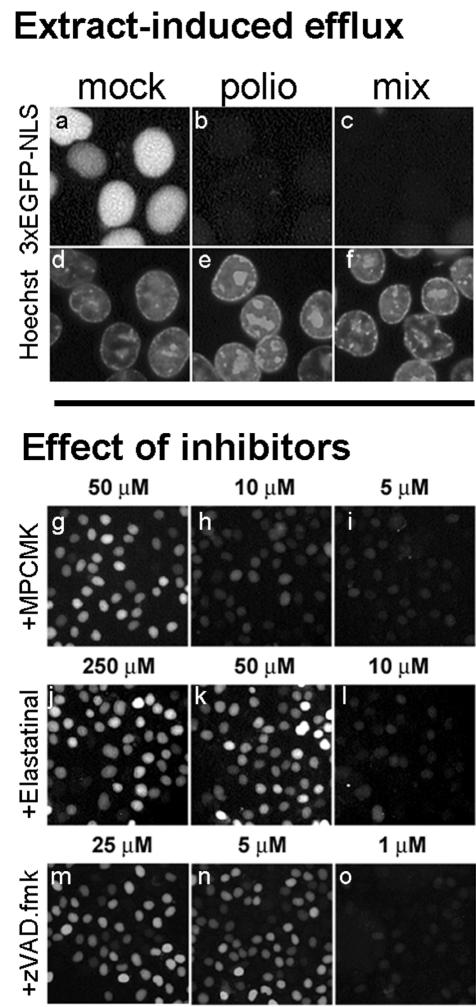
As a step toward elucidation of the mechanism of the nuclear envelope damage caused by poliovirus infection, we made use of monolayers of uninfected HeLa-3E cells permeabilized with digitonin. Such cells retained the fluorescent protein in the nuclei when incubated with 10-fold-diluted S100 cytoplasmic lysates from mock-infected cells (Fig. 5a) but completely lost it when treated with similarly diluted lysates from virus-infected cells (Fig. 5b). The difference between the effects of extracts from infected and mock-infected cells in their capacity to increase permeability of the nuclear envelope could have been ascribed to either the presence of some damaging activity in the former or a stabilizing activity in the latter. In an attempt to discriminate between these possibilities, the effect of a 10-fold diluted 1:9 mixture of lysates from infected and mock-infected cells was investigated. Even though the concentration of the extract from the infected cells was 10 times lower than in sample b, such a mixture efficiently triggered efflux of fluorescence from the nuclei of permeabilized uninfected cells (Fig. 5c), a finding consistent with the presence in the extracts from infected cells of a factor(s) decreasing the barrier properties of the nuclear envelope.
FIG. 5.
Stimulation of 3×GFP-NLS efflux from nuclei of permeabilized uninfected cells by extracts from poliovirus-infected cells. Monolayers of HeLa-3E cells were permeabilized with digitonin and incubated with 10-fold-diluted S100 extracts from mock-infected (a and d) or poliovirus-infected (b and e) HeLa cells for 1 h at 37°C. In panels c and f, the permeabilized cells were incubated with a 10-fold-diluted mixture of 1 and 9 μl of lysates from infected and mock-infected cells, respectively (the protein concentrations in all probes being equal). The effects of protease inhibitors (g to o) were assayed on permeabilized uninfected HeLa-3E cells by using the extract prepared as described for panels c and f. The extracts were preincubated with the inhibitors for 20 min at 4°C and then applied to the cells for 1 h at 37°C.
Since a likely candidate for the putative envelope-damaging factor in the extracts from infected cells could be a protease, the effects of several protease inhibitors on the traffic-enhancing activity of such extracts were investigated. The extracts from infected cells diluted as in the sample described in Fig. 5c were incubated with appropriate inhibitors for 20 min at 4°C and then added to monolayers of the permeabilized HeLa-3E cells. Elastase inhibitors, MPCMK and elastatinal, both known to suppress the proteolytic activity of poliovirus protease 2A (47), inhibited the capacity of the extracts to promote efflux of 3×GFP-NLS at a concentration of 50 μM (Fig. 5g and k; Table 1). A similar effect was exerted by some other known inhibitors of poliovirus (or rhinovirus) 2A, such as antipain (300 μM), chymostatin (20 μM), Zn2+ (5 mM), and Cd2+ (2.5 mM) but not by the compounds with no reported 2A-inhibitory activity (leupeptin, pepstatin A, and PMSF) (Table 1). A notable exception from this regularity was a strong inhibitory effect of a low concentration (5 μM) of a broad-spectrum caspase inhibitor, zVAD.fmk, on the envelope-permeabilizing activity of extracts from infected cell (Fig. 5n and Table 1). The protease activity of 2A was reported to be resistant to zVAD.fmk at concentrations at least 40 times higher than used here (83).
TABLE 1.
Effect of various protease inhibitors on the capacity of lysates from poliovirus-infected cells to promote efflux of 3×GFP-NLS from nuclei of permeabilized uninfected cellsa
| Inhibitor | Concn tested | Effectb | Remarks (known effects on poliovirus 2Apro)c | Reference |
|---|---|---|---|---|
| MPCMK | 50 μM | + | Inhibition with IC50 of 67 μM | 47 |
| Elastatinal | 50 μM | + | Inhibition at 250 μM | 47 |
| Antipain | 300 μM | + | Inhibition at 300 μM | 47 |
| Chymostatin | 20 μM | + | Inhibition of rhinovirus 2Apro with IC50 of 25 μM | 63 |
| zVAD.fmk | 5 μM | + | No inhibition at 200 μM | 83 |
| Leupeptin | 1 mM | − | Inhibition by 27% at 1 mM | 41 |
| Pepstatin A | 1 mM | − | No inhibition at 1 mM | 41 |
| PMSF | 1 mM | − | No inhibition at 1 mM | 41 |
| Zn2+ | 5 mM | + | Inhibition by 52% at 10 mM | 41 |
| Cd2+ | 2.5 mM | + | Inhibition by 56% at 10 mM | 41 |
| 65°C, 30 min | + |
The lysates were prepared and the experiments were carried out as indicated for Fig. 5 c and f.
+, Inhibition of the efflux-promoting activity of extracts from infected cells, i.e., nuclei of cells treated with such extracts exhibited fluorescence comparable to that of cells treated with extracts from mock-infected cells; −, no effect. Each assay was performed at least twice.
When data for poliovirus 2Apro were not available, relevant data for the rhinovirus 2Apro are given. IC50, 50% inhibitory concentration.
We concluded that a proteolytic activity or activities were involved in facilitating the exit of some proteins from the nuclei of permeabilized uninfected cells by extracts from the virus-infected cells. The results with inhibitors were consistent with the hypothesis that the viral 2A protease was a key component of the underlying mechanism, and they also suggested that a protease sensitive to zVAD.fmk played a role in this process.
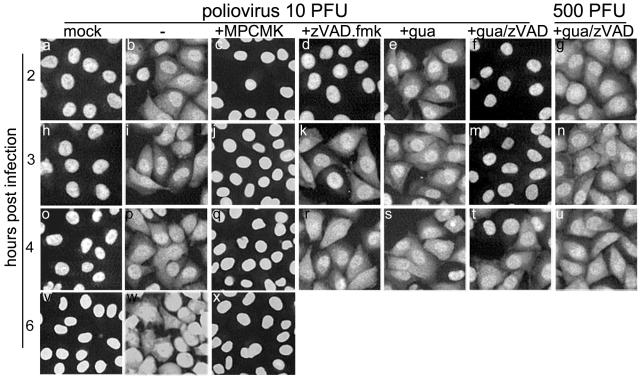
Effects of protease inhibitors on the efflux of nuclear protein from the nuclei of living infected cells.
One may wonder to what extent the results obtained above with permeabilized cells reflected the relocation of nuclear proteins observed in living infected cells. This question seemed the more pertinent because, in an apparent discrepancy with the results presented in Fig. 5, we have previously reported that zVAD.fmk failed to affect the infection-triggered relocation of nuclear proteins (6). It should be noted that the previous experiments were performed at an MOI of ∼500 PFU/cell. Under this condition, zVAD.fmk at 100 μM indeed did not prevent efflux of 3×GFP-NLS from the nuclei of infected cells, even if replication of the viral genome was inhibited by the addition of 2 mM guanidine-HCl (Fig. 6g). However, the inhibitory activity of zVAD.fmk could be detected in cells infected at a lower MOI, e.g., 10 PFU/cell. In this case, the appearance of cytoplasmic fluorescence in HeLa-3E cells could readily be detected at least as early as 2 h p.i., both in the absence (Fig. 6b) and in the presence (Fig. 6e) of guanidine-HCl. However, if the infection took place in the presence of zVAD.fmk, efflux of the fluorescent protein was delayed for 1 (Fig. 6d) or 2 (Fig. 6f and m) h, respectively. Thus, zVAD.fmk partially suppressed infection-triggered permeabilization of the nuclear envelope in the living cells as well.
FIG. 6.
Effects of inhibitors on the infection-triggered protein efflux from the nuclei. HeLa-3E cells were infected as described in Materials and Methods at MOIs of 10 or 500 PFU/cell. The inhibitors were used at the following concentrations: MPCMK, 1 mM; zVAD.fmk, 100 μM; and guanidine-HCl, 2 mM.
MPCMK at 1 mM fully prevented redistribution of the fluorescent protein in the infected cells (Fig. 6c, j, q, and x). The requirement for a significantly higher concentration of this inhibitor compared to the experiments with extracts could at least in part be due to its poor entry into the cells. Elastatinal at 0.5 mM failed to appreciably affect distribution of the fluorescent protein in the infected cells (not shown). This failure might also be due to the poor entry of the drug into the cell, as suggested by a relatively small (∼10-fold) decrease in the yield of infectious virus compared to an ∼1,000-fold decrease caused by 1 mM MPCMK (47). It is important to note that a complete inhibition of viral replication, e.g., by guanidine-HCl, did not prevent permeabilization of the nuclear envelope (see above).
We concluded that an MPCMK-sensitive protease activity was an essential component of the pathway leading to the alteration of the nuclear envelope in poliovirus-infected cells. This pathway may also involve a zVAD.fmk-sensitive event, perhaps a caspase-like activity (see, however, the Discussion).
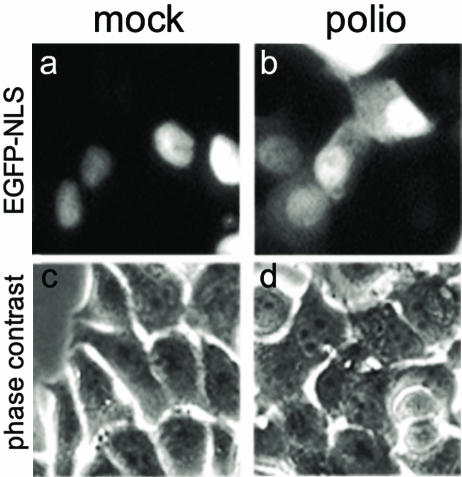
Are caspase-9 and caspase-3 involved in the alteration of nucleocytoplasmic traffic in infected cells?
It is known that apoptosis results in an increase in the permeability of the nuclear envelope, which involves the activity of caspase-9 (20). Poliovirus infection switches on an apoptotic program (2, 69), and caspase-9 is a major initiating caspase in this program (7). Therefore, we considered the possibility that poliovirus-triggered redistribution of intracellular proteins might be due to the infection-activated caspase-9. A relatively weak capacity of zVAD.fmk to prevent the redistribution (see above) is consistent with this hypothesis because caspase-9 is relatively insensitive to this drug (Y. Lazebnik, unpublished data). To ascertain whether this enzyme did play a significant role in the poliovirus-induced alterations of the permeability of nuclear envelope, cells selectively deficient in caspase-9 activity (due to the expression of a dominant-negative mutant of the enzyme) were used. Such cells, MCF7-Cas9DN were derived from MCF-7, which in addition lack active caspase-3 (38), and were shown previously to be deficient in developing the apoptotic response to poliovirus infection under conditions when cells with the full complement of caspases did so (7). MCF7-Cas9DN cells were transiently transfected with a plasmid expressing EGFP-NLS. As expected, the fluorescence in such cells was observed entirely in the nuclei (Fig. 7a). Poliovirus infection of these cells resulted in an early efflux of EGFP-NLS from the nuclei to the cytoplasm (Fig. 7b).
FIG. 7.
Efflux of EGFP-NLS from the nuclei of poliovirus-infected cells lacking caspase-3 and -9. MCF-Cas9DN cells were transiently transfected with pEGFP-NLS; at 48 h posttransfection they were mock infected (a) or infected with poliovirus at an input MOI of ∼500 PFU/cell (b) and inspected under a fluorescence microscope at 2 h p.i.
We concluded that the poliovirus-induced alterations of the nuclear structure could occur without the participation of caspase-3 and caspase-9.
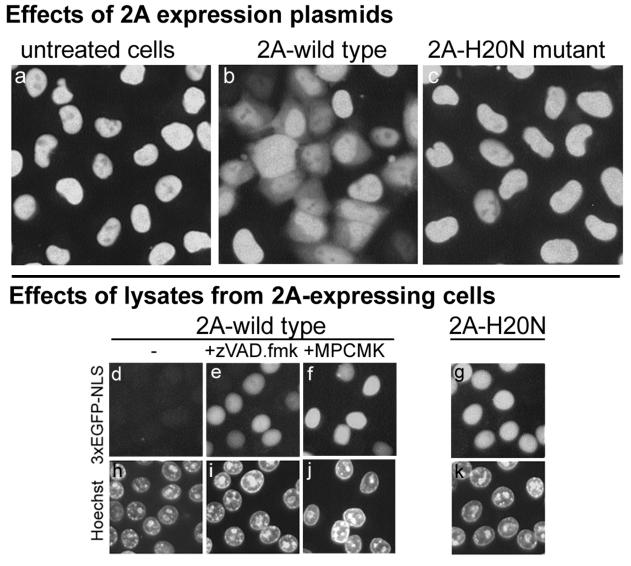
Effect of poliovirus 2A on nucleocytoplasmic traffic.
In order to ascertain which poliovirus proteins were responsible for the relocation of nuclear proteins, preliminary experiments with plasmids expressing different nonstructural viral proteins were carried out. Positive results were obtained with a 2A-expressing vector, and this effect was studied in more detail. The 2A-expressing plasmid, pUHC-2A, when transfected into HeLa-3E cells stably expressing 3×GFP-NLS, efficiently triggered the appearance of cytoplasmic fluorescence in a significant proportion of cells (Fig. 8b). As could be expected from a relatively inefficient transfection, the effect was not evident in all cells, and it developed markedly more slowly than in the virus-infected cells, probably due to the lack of RNA replication and the 2A-induced inhibition of cap-dependent translation (42). A similar plasmid, pUHC-2A(H20N), harboring the H20N mutation in the 2A-coding sequence known to inactivate the protease (82), failed to trigger efflux of the nuclear protein (Fig. 8c).
FIG. 8.
Effect of poliovirus 2A protease on the relocation of 3×GFP-NLS from the nuclei of uninfected cells. In the upper set of panels (a to c), HeLa-3E cells were untreated (a) or transfected with plasmids expressing either the wild-type poliovirus 2A (b) or 2A harboring a protease inactivating H20N mutation (c). The pictures were taken 12 h after the transfection. In the lower set of panels (d to k), twofold-diluted S15 extracts from HeLa cells expressing wild-type (d to f and h to j) and mutant (g and k) 2A were applied to the permeabilized uninfected HeLa-3E cells for 1 h at 37°C. When used, zVAD.fmk and MPCMK were at 5 and 50 μM, respectively, and preincubated with the extracts for 20 min at 4°C.
Cytoplasmic extracts from cells transfected with the 2A-expressing plasmid facilitated the efflux of fluorescence from permeabilized HeLa-EGFP cells (Fig. 8d), whereas extracts from cells expressing proteolytically inactive 2A mutant were devoid of such activity (Fig. 8g). The efflux-promoting activity of 2A-containing extracts was inhibited by zVAD.fmk (Fig. 8e) and MPCMK (Fig. 8f).
We concluded that the effect of 2A on nucleocytoplasmic traffic mimicked to a significant extent that of the intact virus. In line with the results obtained with the extracts from the infected cells, the activity of extracts from 2A-expressing cells was sensitive to zVAD.fmk.
DISCUSSION
The results described here, in particular those involving the Timer protein, as well as nuclei or permeabilized cells, clearly demonstrate that poliovirus infection facilitates efflux of some nuclear proteins into the cytoplasm. Although only artificial NLS-containing proteins were used in the present study to follow the efflux, a similar phenomenon was observed with a natural endogenous protein prothymosin-α (L. I. Romanova et al., unpublished data). As shown for three cytoplasmic proteins, GAPDH, cyclin B1-EGFP, and an NLS-lacking fusion of five copies of GFP, the traffic (perhaps free diffusion) of proteins is facilitated in the opposite direction as well. Gustin and Sarnow (32-34) presented evidence that poliovirus infection suppressed the active nuclear import of cytoplasmic proteins. Indeed, a drastic alteration of the nuclear pore structure and function appears to be accompanied by both an increased leakiness of the nuclear envelope and a suppression of the energy-dependent nucleocytoplasmic traffic. A combination of these two alterations fully explains all of the observed phenomena.
Viral intervention into the control of nucleocytoplasmic traffic appears to be a general phenomenon. It may serve a variety of purposes, e.g., import of viral components into, and export from, the nucleus (reviewed in references 19 and 75), contributing thereby to the efficiency of viral reproduction, as well as the development of host cell pathology. Different molecular mechanisms are exploited to accomplish these goals. Redistribution of nuclear proteins may be caused, for example, by the loss of an NLS due to infection-associated proteolytic activity (62). Stimulation of nuclear export of viral macromolecules, e.g., unspliced or incompletely spliced viral RNAs, may be exemplified by the existence of different retroviral trans-acting factors and RNA cis elements interacting with dedicated cellular shuttle carriers (76). Human immunodeficiency virus type 1-encoded protein Vpr can induce transient, localized “herniations” in the nuclear envelope, ensuring mixing of nuclear and cytoplasmic components (13). To permit the exit of large subviral components from the nucleus, a CMV protein phosphorylates proteins of the lamina, thereby promoting its dissolution (49). On the other hand, the matrix protein of vesicular stomatitis virus (53, 70) and related viruses (52) inhibits active nuclear export and import of proteins and RNAs by interacting with NPC, particularly Nup98. Inactivation of this nucleoporin is known to inhibit nucleocytoplasmic transport of a set of macromolecules (77). Virus-specific proteins of a number of viruses, e.g., influenza virus (11, 12), adenovirus (16), and herpesvirus (59), use a variety of mechanisms for control of differential nuclear export of host and viral mRNA species.
Although the features of the alteration of nucleocytoplasmic traffic in poliovirus-infected cells are only starting to emerge, it seems that the mechanism(s) involved differ from those already described. Electron microscopy provides clear evidence for a dramatic structural alteration of the nuclear pores, giving the impression that, in the infected cells, the gateway between the cytoplasmic and nuclear compartments becomes open. The destruction of the pores correlates well with the degradation (probably proteolysis) of certain nucleoporins (33, 34). In particular, Nup153 and p62 degraded upon poliovirus and rhinovirus infection (33, 34). Remarkably, p62, together with associated p58, p54, and p45, appear to form a doughnut-shaped “central granule” or “plug” with a diameter of ∼15 nm located within the channel of the nuclear pore (30, 31). It is tempting to correlate proteolysis of p62 with the loss of the electron-dense material separating nuclear and cytoplasmic compartments, but this hypothesis should await direct confirmation. Nup153 was mapped to the distal ring of the so-called basket at the nuclear side of the pore (51, 72). Its degradation might also contribute to the observed morphological alterations. Mixing of nuclear and cytoplasmic soluble content should occur as a consequence of these events. Of course, this does not necessarily imply redistribution of the proteins involved in immobile or extremely large complexes.
Phenomenologically, a similar increase in bidirectional leakiness of the nuclear envelope also takes place in apoptotic cells. The efflux of GFP-NLS from the nuclei of such cells could be observed, and immunofluorescence assays with antibodies to different NPC proteins have revealed significant alterations of the nuclear pore structure (20). Also, expression of poliovirus 2A may cause apoptotic reaction (26). Furthermore, caspase-dependent degradation of Nup153 was registered during apoptosis (9), although the sizes of the proteolytic products in apoptotic and rhinovirus-infected cells appeared to be different (34). The mechanisms of the alteration of nucleocytoplasmic traffic during the cellular response to poliovirus infection and apoptotic stimuli seem, however, to be different. As shown here, this alteration in the infected cells, in contrast to apoptotic cells (20), does not require active caspases-9 and caspases-3. Degradation of p62 is a characteristic of the virus-infected (33, 34) but not of the apoptotic (9, 20) cells.
The mechanism of structural and biochemical damage to the nuclear envelope caused by picornavirus infection has yet to be defined. Several lines of evidence suggest that proteolytic activity of the viral 2A protein may be involved. (i) Transient expression of wild-type 2A is accompanied by the nuclear efflux of a NLS-containing fluorescent protein, whereas 2A harboring a protease-inactivating mutation is devoid of such an activity. (ii) Cytoplasmic extracts from infected cells or cells expressing the wild-type 2A (but not the mutant 2A) trigger the loss of fluorescent protein from the nuclei of permeabilized uninfected cells. (iii) This capacity of the extracts is suppressed by MPCMK, elastatinal, and some other compounds known to inhibit the proteolytic activity of 2A. (iv) A similar effect was exerted by MPCMK on nuclear efflux in virus-infected cells, suggesting that the phenomena observed in permeabilized cells treated with extracts from infected or 2A-expressing cells mimic well those in virus-infected cells. It should be admitted that elastatinal was inefficient in this respect in the infected cells, but this may be explained by its weak anti-2A activity in vivo, as judged by relatively small effect on viral reproduction (47).
The capacity of extracts from poliovirus-infected or 2A-expressing cells to promote nuclear efflux in permeabilized uninfected cells to be efficiently inhibited by zVAD.fmk deserves special comments. This drug was known to not inhibit either the activity of 2A protease in vitro (83) or 2A-dependent poliovirus reproduction (2). In line with these data, it may be assumed that increased permeabilization of the nuclear pores during poliovirus infection involves an additional proteolytic activity or activities, which may work either in parallel or as a consequence of 2A activity. It should be noted that 2A was reported to activate host cell proteases, including a zVAD.fmk-sensitive one (83). The involvement in the degradation of p62 and Nup153 in poliovirus-infected cells (33, 34) of proteolytic activity(ies) other than 2A is suggested also by the fact that the 2A-specific cleavage site, Tyr-Gly, is absent from the former nucleoporin (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=protein&val=29791855), and the location of its two copies in the latter (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=protein&val=31418202) could not account for the pattern of the observed degradation products.
On the other hand, after the submission of the original version of the present study, it was reported that the O-methylated form of zVAD.fmk (the one used here) could inhibit the proteolytic activity of rhinovirus 2A (14). If this is true also of poliovirus 2A, then the involvement of another zVAD.fmk-sensitive enzyme in the 2A-mediated alteration of nucleocytoplasmic traffic should not necessarily be invoked. It may be noted that since the O-methylated form of zVAD.fmk undergoes intracellular demethylation and the demethylated form of the drug was not active against rhinovirus 2A protease (14), this may explain dependence of the zVAD.fmk effect on the MOI: there could be not enough active form of the inhibitor to suppress all 2A molecules formed at a high MOI.
The possibility of activation of a 2A-independent pathway targeting NPC cannot be ruled out as well. Regardless of the mechanism, the nuclear pore damage should exert a significant impact on the course of the infection, affecting both viral growth and host cell pathology. The obvious biological relevance of the facilitated bidirectional exchange of macromolecules between nuclei and cytoplasm was mentioned in the introduction. Here, we point to still another possible effect of the virus-triggered degradation of NPC. In addition to its role as a selective gateway in the nuclear envelope, NPC appears to be involved in the regulation of gene expression by controlling barriers that prevent the spread of heterochromatinization and silencing (23, 36). Although this involvement is only beginning to be appreciated, and the available data, especially with regard to higher eukaryota, remain rather scarce, it is tempting to speculate that NPC destruction in infected cells may contribute to chromatin heterochromatinization and hence to inhibition of host transcription. The validity of this speculation should be checked experimentally.
Acknowledgments
We thank Hermann Bujard, Dmitri Bulavin, Yuri Lazebnik, and Richard Lloyd for plasmids; Vladimir Muronetz for a preparation of GAPDH antibodies; and Bertrand Joseph for help with confocal microscopy. We are also grateful to Yuri Lazebnik for useful advice and critical reading of the manuscript.
This study was supported by grants from INTAS, the Ludwig Institute for Cancer Research, the Russian Foundation for Basic Research, the Scientific School Support Program, and the Russian Federal Program “Integration.”
REFERENCES
- 1.Adam, S. A., S. R. Marr, and L. Gerace. 1990. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell Biol. 111:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T., Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:342-353. [DOI] [PubMed] [Google Scholar]
- 3.Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis viral protein 2A localizes to nucleoli and inhibits cap-dependent mRNA translation. Virus Res. 95:45-57. [DOI] [PubMed] [Google Scholar]
- 4.Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59-73. [DOI] [PubMed] [Google Scholar]
- 5.Barton, D. J., and J. B. Flanegan. 1993. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J. Virol. 67:822-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belov, G. A., A. G. Evstafieva, O. Mikitas, A. B. Vartapetian, and V. I. Agol. 2000. Early alteration of nucleocytoplasmic traffic induced by some RNA viruses. Virology 275:244-248. [DOI] [PubMed] [Google Scholar]
- 7.Belov, G. A., L. I. Romanova, E. A. Tolskaya, M. S. Kolesnikova, Y. A. Lazebnik, and V. I. Agol. 2003. The major apoptotic pathway activated and suppressed by poliovirus. J. Virol. 77:45-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1982. Accumulation of poliovirus proteins in the host cell nucleus. Intervirology 18:189-196. [DOI] [PubMed] [Google Scholar]
- 9.Buendia, B., A. Santa-Maria, and J. C. Courvalin. 1999. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell Sci. 112:1743-1753. [DOI] [PubMed] [Google Scholar]
- 10.Burke, B., and J. Ellenberg. 2002. Remodeling the walls of the nucleus. Nat. Rev. Mol. Cell. Biol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z., and R. M. Krug. 2000. Selective nuclear export of viral mRNAs in influenza-virus-infected cells. Trends Microbiol. 8:376-383. [DOI] [PubMed] [Google Scholar]
- 12.Cros, J. F., and P. Palese. 2003. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res. 95:3-12. [DOI] [PubMed] [Google Scholar]
- 13.de Noronha, C. M. C., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity Induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 14.Deszcz, L., J. Seipelt, E. Vassilieva, A. Roetzer, and E. Kuechler. 2004. Antiviral activity of caspase inhibitors: effect on picornaviral 2A proteinase. FEBS Lett. 560:51-55. [DOI] [PubMed] [Google Scholar]
- 15.Detjen, B. M., J. Lucas, and E. Wimmer. 1978. Poliovirus single-stranded RNA and double-stranded RNA: differential infectivity in enucleate cells. J. Virol. 27:582-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobner, T., and J. Kzhyshkowska. 2001. Nuclear export of adenovirus RNA. Curr. Top. Microbiol. Immunol. 259:25-54. [DOI] [PubMed] [Google Scholar]
- 17.Emig, S., D. Schmalz, M. Shakibaei, and K. Buchner. 1995. The nuclear pore complex protein p62 is one of several sialic acid-containing proteins of the nuclear envelope. J. Biol. Chem. 270:13787-13793. [DOI] [PubMed] [Google Scholar]
- 18.Fahrenkrog, B., and U. Aebi. 2003. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat. Rev. Mol. Cell. Biol. 4:757-766. [DOI] [PubMed] [Google Scholar]
- 19.Fahrenkrog, B., D. Stoffler, and U. Aebi. 2001. Nuclear pore complex architecture and functional dynamics. Curr. Top. Microbiol. Immunol. 259:95-117. [DOI] [PubMed] [Google Scholar]
- 20.Faleiro, L., and Y. Lazebnik. 2000. Caspases disrupt the nucleo-cytoplasmic barrier. J. Cell Biol. 151:951-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falk, M. M., P. R. Grigera, I. E. Bergmann, A. Zibert, G. Multhaup, and E. Beck. 1990. Foot-and-mouth disease virus protease 3C induces specific proteolytic cleavage of host cell histone H3. J. Virol. 64:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Tomas, C. 1982. The presence of viral-induced proteins in nuclei from poliovirus-infected HeLa cells. Virology 116:629-634. [DOI] [PubMed] [Google Scholar]
- 23.Feuerbach, F., V. Galy, E. Trelles-Sticken, M. Fromont-Racine, A. Jacquier, E. Gilson, J. C. Olivo-Marin, H. Scherthan, and U. Nehrbass. 2002. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nat. Cell Biol. 4:214-221. [DOI] [PubMed] [Google Scholar]
- 24.Follett, E. A., C. R. Pringle, and T. H. Pennington. 1975. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J. Gen. Virol. 26:183-196. [DOI] [PubMed] [Google Scholar]
- 25.Fried, H., and U. Kutay. 2003. Nucleocytoplasmic transport: taking an inventory. Cell. Mol. Life Sci. 60:1659-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstaub, D., A. Gradi, Z. Bercovitch, Z. Grosmann, Y. Nophar, S. Luria, N. Sonenberg, and C. Kahana. 2000. Poliovirus 2A protease induces apoptotic cell death. Mol. Cell. Biol. 20:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Görlich, D., and U. Kutay. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607-660. [DOI] [PubMed] [Google Scholar]
- 28.Gosert, R., D. Egger, and K. Bienz. 2000. A cytopathic and a cell culture adapted hepatitis A virus strain differ in cell killing but not in intracellular membrane rearrangements. Virology 266:157-169. [DOI] [PubMed] [Google Scholar]
- 29.Grigorieva, J. A., M. B. Dainiak, A. G. Katrukha, and V. I. Muronetz. 1999. Antibodies to the nonnative forms of d-glyceraldehyde-3-phosphate dehydrogenase: identification, purification, and influence on the renaturation of the enzyme. Arch. Biochem. Biophys. 369:252-260. [DOI] [PubMed] [Google Scholar]
- 30.Grote, M., U. Kubitscheck, R. Reichelt, and R. Peters. 1995. Mapping of nucleoporins to the center of the nuclear pore complex by post-embedding immunogold electron microscopy. J. Cell Sci. 108:2963-2972. [DOI] [PubMed] [Google Scholar]
- 31.Guan, T., S. Muller, G. Klier, N. Pante, J. M. Blevitt, M. Haner, B. Paschal, U. Aebi, and L. Gerace. 1995. Structural analysis of the p62 complex, an assembly of O-linked glycoproteins that localizes near the central gated channel of the nuclear pore complex. Mol. Biol. Cell 6:1591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustin, K. E. 2003. Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex. Virus Res. 95:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustin, K. E., and P. Sarnow. 2001. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 20:240-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustin, K. E., and P. Sarnow. 2002. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 76:8787-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henke, A., M. Nestler, S. Strunze, H. P. Saluz, P. Hortschansky, B. Menzel, U. Martin, R. Zell, A. Stelzner, and T. Munder. 2001. The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology 289:15-22. [DOI] [PubMed] [Google Scholar]
- 36.Ishii, K., G. Arib, C. Lin, G. Van Houwe, and U. K. Laemmli. 2002. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell 109:551-562. [DOI] [PubMed] [Google Scholar]
- 37.Izumi, R. E., B. Valdez, R. Banerjee, M. Srivastava, and A. Dasgupta. 2001. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 76:17-29. [DOI] [PubMed] [Google Scholar]
- 38.Jänicke, R. U., M. L. Sprengart, M. R. Wati, and A. G. Porter. 1998. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273:9357-9360. [DOI] [PubMed] [Google Scholar]
- 39.Jans, D. A., C.-Y. Xiao, and M. H. C. Lam. 2000. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22:532-544. [DOI] [PubMed] [Google Scholar]
- 40.Jelachich, M. L., and H. L. Lipton. 2001. Theiler's murine encephalomyelitis virus induces apoptosis in gamma interferon-activated M1 differentiated myelomonocytic cells through a mechanism involving tumor necrosis factor alpha (TNF-α) and TNF-α-related apoptosis-inducing ligand. J. Virol. 75:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.König, H., and B. Rosenwirth. 1987. Purification and partial characterization of poliovirus protease 2A by means of a functional assay. J. Virol. 62:1243-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuechler, E., J. Seipelt, H.-D. Liebig, and W. Sommergruber. 2002. Picornavirus proteinase-mediated shutoff of host cell translation: direct cleavage of a cellular initiation factor, p. 301-311. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. American Society for Microbiology, Washington, D.C.
- 43.Lei, E. P., and P. A. Silver. 2002. Protein and RNA export from the nucleus. Dev. Cell 2:261-272. [DOI] [PubMed] [Google Scholar]
- 44.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McBride, A. E., A. Schlegel, and K. Kirkegaard. 1996. Human protein Sam 68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA 93:2296-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meerovitch, K., Y. V. Svitkin, H. S. Lee, F. Lejbkowicz, D. J. Kenan, E. K. Chan, V. I. Agol, J. D. Keene, and N. Sonenberg. 1993. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 67:3798-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molla, A., C. U. Hellen, and E. Wimmer. 1993. Inhibition of proteolytic activity of poliovirus and rhinovirus 2A proteinases by elastase-specific inhibitors. J. Virol. 67:4688-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molla, A., A. V. Paul, and E. Wimmer. 1991. Cell-free, de novo synthesis of poliovirus. Science 254:1647-1651. [DOI] [PubMed] [Google Scholar]
- 49.Muranyi, W., J. Haas, M. Wagner, G. Krohne, and U. H. Koszinowski. 2002. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 297:854-857. [DOI] [PubMed] [Google Scholar]
- 50.Neznanov, N., A. Kondratova, K. M. Chumakov, B. Angres, B. Zhumabayeva, V. I. Agol, and A. V. Gudkov. 2001. Poliovirus protein 3A inhibits tumor necrosis factor (TNF)-induced apoptosis by eliminating the TNF receptor from the cell surface. J. Virol. 75:10409-10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pante, N., R. Bastos, I. McMorrow, B. Burke, and U. Aebi. 1994. Interactions and three-dimensional localization of a group of nuclear pore complex proteins. J. Cell Biol. 126:603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen, J. M., L. S. Her, and J. E. Dahlberg. 2001. Multiple vesiculoviral matrix proteins inhibit both nuclear export and import. Proc. Natl. Acad. Sci. USA 98:8590-8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen, J. M., L. S. Her, V. Varvel, E. Lund, and J. E. Dahlberg. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollack, R., and R. Goldman. 1973. Synthesis of infective poliovirus in BSC-1 monkey cells enucleated with cytochalasin B. Science 179:915-916. [DOI] [PubMed] [Google Scholar]
- 55.Porter, A. G. 1999. Protein translocation in apoptosis. Trends Cell Biol. 9:394-401. [DOI] [PubMed] [Google Scholar]
- 56.Rubinstein, S. J., T. Hammerle, E. Wimmer, and A. Dasgupta. 1992. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J. Virol. 66:3062-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan, K. J., and S. R. Wente. 2000. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 12:361-371. [DOI] [PubMed] [Google Scholar]
- 58.Salina, D., K. Bodoor, P. Enarson, W. H. Raharjo, and B. Burke. 2001. Nuclear envelope dynamics. Biochem. Cell. Biol. 79:533-542. [PubMed] [Google Scholar]
- 59.Sandri-Goldin, R. M. 2001. Nuclear export of herpesvirus RNA. Curr. Top. Microbiol. Immunol. 259:2-23. [PubMed] [Google Scholar]
- 60.Schmitz, H.-D. 2001. Reversible nuclear translocation of glyceroaldehyde-3-phosphate dehydrogenase upon serum depletion. Eur. J. Cell Biol. 80:419-427. [DOI] [PubMed] [Google Scholar]
- 61.Semler, B. L., and E. Wimmer (ed.). 2002. Molecular biology of picornaviruses. American Society for Microbiology, Washington, D.C.
- 62.Shiroki, K., T. Isoyama, S. Kuge, T. Ishii, S. Ohmi, S. Hata, K. Suzuki, Y. Takasaki, and A. Nomoto. 1999. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol. 73:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sommergruber, W., H. Ahorn, A. Zöphel, I. Maurer-Fogy, F. Fessl, G. Schnorrenberg, H. Liebig, D. Blaas, E. Kuechler, and T. Skern. 1992. Cleavage specificity on synthetic peptide substrates of human rhinovirus 2 proteinase 2A. J. Biol. Chem. 267:22639-22644. [PubMed] [Google Scholar]
- 64.Steggerda, S. M., and B. M. Paschal. 2002. Regulation of nuclear import and export by the GTPase Ran. Int. Rev. Cytol. 217:41-91. [DOI] [PubMed] [Google Scholar]
- 65.Strom, A.-C., and K. Weis. 2001. Importin-β-like nuclear transport receptors. Genome Biol. 2:reviews3008.1-reviews3008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Svitkin, Y. V., and N. Sonenberg. 2003. Cell-free synthesis of encephalomyocarditis virus. J. Virol. 77:6551-6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takizawa, C. G., and D. O. Morgan. 2000. Control of mitosis by changes in the subcellular location of cyclin B1-Cdk1 and Cdc25C. Curr. Opin. Cell Biol. 12:658-665. [DOI] [PubMed] [Google Scholar]
- 68.Terskikh, A., A. Fradkov, G. Ermakova, A. Zaraisky, P. Tan, A. V. Kajava, X. Zhao, S. Lukyanov, M. Matz, S. Kim, I. Weissman, and P. Siebert. 2000. “Fluorescent timer”: protein that changes color with time. Science 290:1585-1588. [DOI] [PubMed] [Google Scholar]
- 69.Tolskaya, E. A., L. I. Romanova, M. S. Kolesnikova, T. A. Ivannikova, E. A. Smirnova, N. T. Raikhlin, and V. I. Agol,. 1995. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J. Virol. 69:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 71.Waggoner, S., and Sarnow, P. 1998. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 72:6699-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walther, T. C., M. Fornerod, H. Pickersgill, M. Goldberg, T. D. Allen, and I. W. Mattaj. 2001. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 20:5703-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weidman, M. K., R. Sharma, S. Raychaudhuri, P. Kundu, W. Tsai, and A. Dasgupta. 2003. The interaction of cytoplasmic RNA viruses with the nucleus. Virus Res. 95:75-85. [DOI] [PubMed] [Google Scholar]
- 74.Weis, K. 2003. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112:441-451. [DOI] [PubMed] [Google Scholar]
- 75.Whittaker, G. R., and A. Helenius. 1998. Nuclear import and export of viruses and virus genomes. Virology 246:1-23. [DOI] [PubMed] [Google Scholar]
- 76.Wodrich, H., and H. G. Krausslich. 2001. Nucleocytoplasmic RNA transport in retroviral replication. Results Probl. Cell Differ. 34:197-217. [DOI] [PubMed] [Google Scholar]
- 77.Wu, X., L. H. Kasper, R. T. Mantcheva, G. T. Mantchev, M. J. Springett, and J. M. van Deursen. 2001. Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc. Natl. Acad. Sci. USA 98:3191-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yalamanchili, P., R. Banerjee, and A. Dasgupta. 1997. Poliovirus-encoded protease 2Apro cleaves the TATA-binding protein but does not inhibit host cell RNA polymerase II transcription in vitro. J. Virol. 71:6881-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yalamanchili, P., U. Datta, and A. Dasgupta. 1997. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J. Virol. 71:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yalamanchili, P., K. Weidman, and A. Dasgupta. 1997. Cleavage of transcriptional activator Oct-1 by poliovirus encoded protease 3Cpro. Virology 239:176-185. [DOI] [PubMed] [Google Scholar]
- 81.Yanushevich, Y. G., D. B. Staroverov, A. P. Savitsky, A. F. Fradkov, N. G. Gurskaya, M. E. Bulina, K. A. Lukyanov, and S. A. Lukyanov. 2002. A strategy for the generation of non-aggregating mutants of Anthozoa fluorescent proteins. FEBS Lett. 511:11-14. [DOI] [PubMed] [Google Scholar]
- 82.Yu, S. F., and R. E. Lloyd. 1991. Identification of essential amino acid residues in the functional activity of poliovirus 2A protease. Virology 182:615-625. [DOI] [PubMed] [Google Scholar]
- 83.Zamora, M., W. E. Marissen, and R. E. Lloyd. 2002. Multiple eIF4GI-specific protease activities present in uninfected and poliovirus-infected cells. J. Virol. 76:165-177. [DOI] [PMC free article] [PubMed] [Google Scholar]