Abstract
The infection of human fetal thymus organ cultures (FTOC) with coxsackievirus B4 E2 (CVB4 E2) was investigated. Both positive- and negative-strand viral RNA were detected by real-time quantitative reverse transcription-PCR (RT-PCR) in CVB4 E2-infected FTOC, which supported high yields of virus production (∼106 50% tissue culture infective doses/ml), and in flow-sorted thymocyte populations for 7 days after inoculation. Cortical CD4+ CD8+ thymocytes were found to be the principal targets of infection. Inoculation of human FTOC with CVB4 E2 led to a marked and progressive depletion of immature thymocytes (CD4+ CD8+ cells) with no enhancement of Annexin V-positive cells. CVB4 E2 replication caused significant major histocompatibility complex (MHC) class I upregulation on these cells. MHC class I upregulation was correlated with positive- and negative-strand RNA quantitative detection and the release of infectious particles. In addition, chloroquine treatment of FTOC and single-thymocyte suspensions suggested that MHC class I upregulation on thymocytes was the result of direct infection rather than caused by production of soluble factors such as alpha interferon. Thus, CVB4 E2 can infect human fetal thymocytes, which subsequently results in quantitative and qualitative abnormalities of these cells.
Numerous clinical and epidemiological studies have linked type 1 diabetes with environmental factors such as enterovirus infection. Among enteroviruses, the group B coxsackieviruses, particularly coxsackievirus B4 (CVB4) have been commonly implicated as the responsible agent (9, 12, 18, 25, 47). CVB4 is a small, nonenveloped, positive-strand RNA enterovirus in the Picornaviridae family, which also includes poliovirus, echovirus, and coxsackievirus A and enterovirus 68-71. CVB4 has been reported as a possible etiological factor in promoting β-cell autoimmune destruction. The E2 strain of CVB4 was isolated from the pancreas of a child who died from diabetic ketoacidosis (48). CVB4 E2 induces diabetes with hyperglycemia and β-cell autoimmunity in some strains of mice (39) and can persistently infect human pancreas islets in vitro (8). The CVB genome has also been detected in peripheral blood of type 1 diabetic patients (9). Various mechanisms for virus-induced autoimmunity have been suggested, such as molecular mimicry (35), bystander activation, and direct lysis of infected target cells (17, 44).
In concert with individual genetic predisposition, type 1 diabetes is associated with a loss or absence of immune self-tolerance at central and peripheral levels (41). Self-tolerance is initiated in the thymus during T-cell ontogeny (13). During thymocyte maturation, interactions between peptide-displaying major histocompatibility complex (MHC) class I and II and randomly rearranged T-cell receptors lead to the development of MHC-restricted immune responses by positive selection and to the establishment of central T-cell tolerance by negative selection. MHC class I molecules are expressed at different levels on thymocyte subpopulations during T-cell development with higher expression on mature cells (3, 29). T-cell-positive and -negative selections depend on complex interactions between thymocytes and a variety of thymic stromal cells such as thymic epithelial cells (TEC). Thus, the thymic microenvironment is essential for generation of a functionally diverse T-cell repertoire that is nevertheless self-tolerant (23).
The association between CVB4 infection and its consequences on thymic microenvironment has not been extensively studied. Previous studies of CVB4 infection of lymphoid targets have been restricted to human T-cell lines and monocytes (16, 34) or to other type B coxsackieviruses, e.g., CVB3 (24, 38). Recently, we reported that CVB4 JVB (a laboratory reference strain) and CVB4 E2 persistently infect human TEC in vitro, and we hypothesized that this persistence could interfere with thymus function and contribute to the development of autoimmunity (5). Congenital rubella syndrome provides an example of in utero acquired infection causing type 1 diabetes (14); therefore, it has been suggested that fetal viral infections may be causally related to type 1 diabetes (10). Otonkoski et al. detected postnatally several types of diabetes-associated autoantibodies in an infant infected in utero by an enterovirus, which suggests that enterovirus infections in utero may induce β-cell autoimmunity (36). A vertical transmission of coxsackievirus B, associated with the infection of fetal thymus, has been observed (19, 33). Moreover, epidemiological studies showed that CVB4 infection during pregnancy was a risk factor for childhood-onset type 1 diabetes (11, 18). Here, we used explanted fetal thymus organ cultures (FTOC) to investigate CVB4 E2 infection in intact human thymus and to establish whether CVB4 E2 was able to infect the cellular components of the human thymus and what effects such an infection could have on those cells.
MATERIALS AND METHODS
Viruses.
CVB4 E2 (provided by J. W. Yoon, Julia McFurlane Diabetes Research Center, Calgary, Alberta, Canada) was generated as previously described (5). Briefly, CVB4 E2 was grown in Vero cells (American Type Culture Collection, Manassas, Va.) in Dulbecco Eagle medium (Cellgro, Herndon, Va.) supplemented with 10% fetal calf serum (FCS). Supernatants were collected 3 days after inoculation and clarified at 1,200 × g for 10 min. Virus titers were determined on Vero cells by limiting dilution assay for 50% tissue culture infection doses by the method of Reed and Muench. Human immunodeficiency virus type 1 (HIV-1) Ba-L (National Institutes of Health AIDS Research and Reference Reagent Program [contributed by S. Gartner, M. Popovic, and R. Gallo]) was generated in monocyte-derived macrophages as described previously (2).
FTOC inoculation.
Human fetal thymi (Advanced Bioscience Resources, Alameda, Calif.) were dissected into small pieces of ca. 6 × 106 cells (this number represents an average of cell counts for several FTOC pieces) with sterile scalpels, as described previously (20), and directly transferred into CVB4 E2 or HIV-1 Ba-L stocks at multiplicities of infection (MOIs) of 0.05 to 0.005 for 4 h at 37°C in a 5% CO2 incubator. Pieces were then transferred onto sterile filters (Millipore, Bedford, Mass.) placed on Gelfoam (Pharmacia-Upjohn, Kalamazoo, Calif.) rafts in 700 μl of Yssel's medium (Gemini Bio-Products, Calabasas, Calif.) in 24-well plates. CVB4-infected FTOC were incubated 7 days, and the medium was changed every 3 days. Cultures were treated with 10 μM chloroquine diphosphate salt (Sigma, St. Louis, Mo.), lamivudine (McKesson, Denver, Colo.) at 5 μM, and alpha-2b interferon (IFN-α2b; Intron A; Schering, Kenilworth, N.J.) were added at 1,000 IU/ml. At the termination of culture, individual thymus pieces were dispersed, washed, and stained for CD4, CD8, 7-AAD (BD Pharmingen, San Diego, Calif.), Annexin V, and MHC class I and then analyzed by fluorescence-activated cell sorting (FACS).
Neutralizing experiments.
To neutralize CVB4, virus stocks were treated for 90 min at 37°C with a neutralizing anti-CVB4 polyclonal rabbit antibody at a dilution of 1:10 (Eurobio, Les Ulis, France) before inoculation of the FTOC. Treatment of FTOC with IFN-α-neutralizing polyclonal rabbit and sheep antibodies (BioSource International, Camarillo, Calif.) was performed before, during, and after FTOC inoculation. FTOC were preincubated 2 h at 37°C with saturating amounts of neutralizing antibodies (1,000 neutralizing U/ml); inoculated with CVB4 E2, CVB4 JBV, or HIV-1 Ba-L in the presence of the antibodies; and incubated for 7 days. Every 3 days, medium containing fresh antibodies was replaced. Importantly, the neutralizing polyclonal sheep anti-IFN-α antibody binds with high avidity to IFN-αA, IFN-α2, and IFN-αG and with moderate avidity to IFN-αA/D, IFN-αF, IFN-αK, and IFN-αWA. The neutralizing polyclonal rabbit antibody binds strongly to IFN-αA/D, IFN-αA, and IFN-αK and moderately to IFN-αD (according to the manufacturer).
FACS staining and analysis.
Dispersed thymocytes from FTOC were washed, pelleted, and resuspended in 50 μl of monoclonal antibodies and incubated at 4°C for 30 min. After incubation, cells were rinsed, pelleted, and resuspended in 100 μl of phosphate-buffered saline (PBS)-2% FCS for FACS analysis. Intracellular staining for cytokeratin was performed as follows. After initial staining with antibodies to surface markers, cells were rinsed and pelleted. Pellets were resuspended in 200 μl of 1% paraformaldehyde, 1 mg of human gamma globulin/ml, and 0.1% Triton X-100 in PBS. Cells were incubated at room temperature for 10 min and then rinsed twice and pelleted. Pellets were then stained intracellularly with anti-cytokeratin antibody diluted in PBS-2% FCS for 20 min at 4°C. After incubation, cells were rinsed, pelleted, and resuspended in 100 μl of PBS-2% FCS for FACS analysis. Four-color FACS staining was performed with the indicated combinations of the following antibodies to CD4-allophycocyanin (APC) (Caltag, Burlingame, Calif.); CD8-peridinin chlorophyll protein (PerCP) and CD3-phycoerythrin (PE) (both from BD Bioscience, San Jose, Calif.); HLA-A, -B, and -C (pan-MHC class I, W6/32 clone)-PE (Dako, Carpinteria, Calif.); cytokeratin-fluorescein isothiocyanate (cytokeratin-FITC, clone J1B3; Immunotech, Marseille, France); CD11c-APC and CD8α-PerCP (both from BD Bioscience); and blood dendritic cell A-2-APC (BDCA-2; Miltenyi Biotech, Auburn, Calif.). Viability was assessed by staining with 7-AAD. Five-color FACS staining was performed with the indicated combinations of the antibodies to CD4-APC; CD8-Cascade Blue (a gift of Marty Bigos); Annexin V-FITC (Caltag); HLA-A-, HLA-B-, and HLA-C-PE (Dako); and 7-AAD. Samples were analyzed immediately after staining on a FACSCalibur or FACSDiVa (Becton Dickinson, San Jose, Calif.) by using FlowJo version 3.6.1 software (Tree Star, Inc., Stanford, Calif.).
Thymocyte sorting.
DP thymocytes were isolated with an anti-FITC Multisort kit (Miltenyi), CD4-FITC (BD Bioscience), and CD8 microbeads (Miltenyi). The purity was checked by FACS before CVB4 infection and was always ≥98%. For cell sorting, unfixed cells were stained as for FACS analysis with CD3-PE, CD4-APC, and CD8-FITC (Becton Dickinson) and then sorted into three populations by using a FACSVantage (Becton Dickinson): CD3+/− CD4+ CD8+ (DP), CD3+ CD4+ CD8− (SP4), and CD3+ CD4− CD8+ (SP8). Cells were directly sorted into lysis buffer and frozen at −80°C for RNA extraction. Gating for SP4 and SP8 was based on high expression of CD3.
ELISA.
Conditioned media from CVB4- and HIV-1-infected FTOC were collected at various times after inoculation and analyzed for IFN-α, IFN-β, IFN-γ, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor (GM-CSF) by enzyme-linked immunosorbent assay (ELISA) kits (BioSource). The IFN-α ELISA detects IFN-αA, IFN-α2, IFN-αA/D, IFN-αD, IFN-αK, and IFN-α4b.
Quantitative RT-PCR for CVB4 E2.
CVB4 E2 positive- and negative-strand RNA in FTOC were quantitated by two-step quantitative RT-PCR by using the TaqMan fluorogenic detection system with AmpliTaq Gold reagents (Applied Biosystems, Foster City, Calif.). Briefly, total RNA was extracted with RNeasy minikit (Qiagen, Valencia, Calif.) and resuspended in water according to the manufacturer's instructions. Total RNA was measured in a quantitative RT-PCR for rRNA expression with the TaqMan rRNA control reagent (Applied Biosystems). Positive- and negative-strand-specific RT was carried out with 10 ng of rRNA and either the reverse or the forward primer at 48°C for 30 min. PCR was performed with universal cycle conditions (10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C) on an ABI Prism 7700 sequence detector (Applied Biosystems). The following primers, used to detect CVB4 E2 RNA, were located within the enterovirus 5′-nontranslated region, which is highly conserved among enterovirus serotypes: CVB4 forward (5′-GTA GTC CTC CGG CCC CT) and CVB4 reverse (5′-AAT TGT CAC CAT AAG CAG CCA). The sequence of the CVB4 probe was 5′-VIC-ATG CGG CTA ATC CTA ACT GCG GAG-TAMRA (Applied Biosystems). The RNA copy number in each sample was determined by a standard curve generated from increasing copy number of a synthetic transcript corresponding to 435 nt of the CVB4 E2 genome. Briefly, 435 nt within the enterovirus 5′-nontranslated region were reverse transcribed and amplified with primers described previously (31). The PCR product was cloned in pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.), sequenced, and subcloned to two different T7 promoter-containing pBluescript II KS(+) vector to obtain a positive and a negative strain after in vitro transcription. Sense and antisense synthetic RNA generated with a T7 Megascript RNA transcription kit (Ambion, Austin, Tex.) were measured by spectrophotometry and diluted to obtain a standard curve. Accurate quantification of 101 to 106 RNA copies was achieved by use of a single amplification reaction, and CVB4 quantitative RT-PCR is more sensitive than previously reported enterovirus RT-PCR (31). The absence of contaminating DNA from plasmid in standards and genomic DNA in samples was checked by RT-PCR without the reverse transcriptase enzyme. Primers and probe pairs were designed with PrimerExpress software, and the data were analyzed with Sequence Detector version 1.6.3 (both from Perkin-Elmer, Boston, Mass.).
Statistical analyses.
Statistical analyses were performed by using an unpaired Student t test and the Spearman correlation test (StatView 5.0; Abacus Concepts, Berkeley, Calif.). The data are expressed as means ± the standard deviation (SD) for three to five independent experiments performed in triplicate.
RESULTS
CVB4 E2 replicates in human thymocytes.
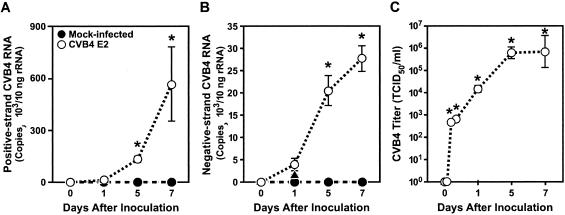
To determine the effects of CVB4 E2 on thymus function, we chose FTOC because both thymic architecture and cellular composition are maintained for at least 1 week (4). We also set up a CVB4-specific real-time RT-PCR that allowed us to detect as few as 10 copies of positive- and negative-strand CVB4 E2 RNA. Positive- and negative-strand CVB4 E2 RNA were detected by 24 h after inoculation, and virus production peaked between days 5 and 7 (Fig. 1A and B). Positive-strand RNA was three- to fivefold more abundant than negative-strand RNA, an expected finding since the negative-strand RNA represents unstable replicative intermediates. The infection was productive: infectious virus was released into the supernatant as assayed by titration on Vero cells (Fig. 1C), and virus progeny in supernatants correlated positively with the detection of positive- and negative-strand RNA (P < 0.002 and P < 0.0002, respectively).
FIG. 1.
CVB4 E2 replicates in human thymus. The production of positive-strand (A) and negative-strand (B) viral RNA and infectious virus (C) by CVB4-infected FTOC is shown.  , P < 0.001 (n = 3).
, P < 0.001 (n = 3).
To evaluate the cellular tropism of CVB4 E2 in human thymus, the distribution of viral RNA in the different thymocyte subpopulations was assessed by quantitative RT-PCR. Positive- and negative-strand RNAs were detected in both double-positive (DP) thymocyte and single positive 4 (SP4) subsets but were significantly more abundant in DP cells (P < 0.05) (Table 1). Viral RNA was detectable in single positive 8 (SP8) cells from infected cultures but was at the limit of the assay sensitivity or in any of the thymocyte subpopulations from mock-infected FTOC (data not shown).
TABLE 1.
Cellular tropism of CVB4 E2 in human thymocytesa
| Cell type | Mean no. of copies of RNA/10 ng of rRNA ± SD
|
|
|---|---|---|
| Positive-strand RNA | Negative-strand RNA | |
| DP | 5,900 ± 110* | 850 ± 190* |
| SP4 | 1,400 ± 570 | 60 ± 1 |
| SP8 | 12 ± 17 | <10 |
CVB4-infected FTOC were harvested 7 days after inoculation, and positive- and negative-strand CVB4 RNAs were assessed in sorted DP, SP4, and SP8 cells by quantitative RT-PCR. *, P < 0.05 DP versus SP4 and SP8 (n = 3).
CVB4 E2 induces thymocyte depletion.
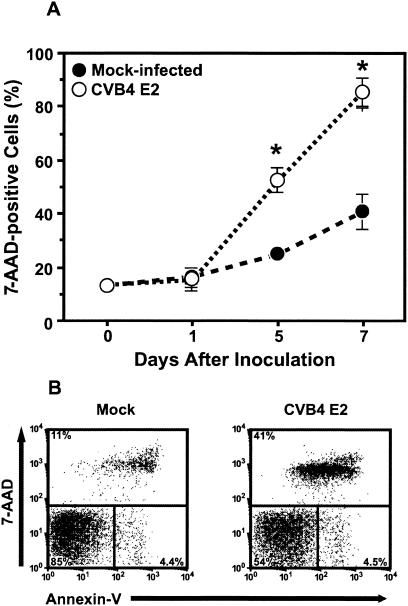
Given that CVB4 E2 actively replicates within thymocyte populations, we sought to determine the effects of infection on the major subpopulations. CVB4 E2 infection caused marked and progressive depletion of thymocytes (Fig. 2A). The number of total thymocytes was 40% lower in CVB4 E2-infected FTOC than in mock-infected FTOC (P < 0.001), and thymocyte depletion was highly correlated with the release of infectious virus (r = 0.968, P < 0.0001). To evaluate the effects of viral replication on major thymocyte subpopulations, we performed flow cytometry on thymocyte suspensions from CVB4 E2-infected FTOC 7 days after inoculation, and live thymocytes (as assessed by 7-AAD exclusion) were examined for expression of CD4 and CD8. The percentage of live thymocytes was reduced, and immature DP (P < 0.001) and CD4+CD8− cells were markedly depleted (P < 0.01) (Table 2). No significant change in the CD4− CD8+ live percentage was observed. In absolute cell numbers, SP8 numbers was reduced by 50%, whereas SP4 and DP numbers were reduced by 70%, suggesting that SP8 cells were more resistant to CVB4 E2 infection. Thymocyte subsets in CVB4 E2-infected FTOC were assessed for apoptosis by Annexin V staining in five-color time course experiments (42). Compared to mock-infected FTOC, no significant increase in the percentage of 7-AAD negative, Annexin V-positive DP cells was observed between 1 and 7 days after CVB4 E2 inoculation (Fig. 2B), suggesting that apoptosis was not involved in CVB4 E2-mediated thymocyte depletion.
FIG. 2.
CVB4 E2 infection is cytopathic in human thymus. (A and B) Staining of thymocytes with 7-AAD at 1, 5, and 7 days after CVB4 E2 inoculation of FTOC (A) and staining with 7-AAD and Annexin V 7 days after inoculation (B).  , P < 0.001 versus mock-infected FTOC (n = 3).
, P < 0.001 versus mock-infected FTOC (n = 3).
TABLE 2.
Effects of CVB4 E2 on thymocyte subpopulationsa
| Virus | % Live cellsb ± SD
|
|||
|---|---|---|---|---|
| Live thymocytes | DP cells | SP4 cells | SP8 cells | |
| Mock | 58 ± 14 | 79 ± 7 | 5 ± 2 | 3 ± 1 |
| CVB4 E2 | 26 ± 11 | 57 ± 12* | 3 ± 2† | 3 ± 1 |
Mock- and CVB4 E2-infected FTOC were harvested 7 days after inoculation, and the distributions of live cells and thymocyte subpopulations were assessed by flow cytometry.
Live thymocytes, the percentage of live cells determined by 7-AAD exclusion staining; DP, SP4, and SP8, thymocyte subpopulations were quantified as a percentage of total live cells. SP4 and SP8 cells were gated based on high expression of CD3. Other cells include CD4− CD8−, SP4 CD3low, and SP8 CD3low cells. *, P < 0.001 versus mock-infected thymocytes; †, P < 0.01 versus mock-infected thymocytes (n = 3).
CVB4 E2 induces MHC class I upregulation on thymocytes.
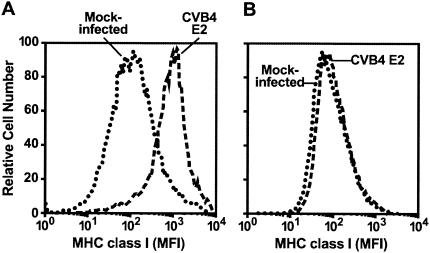
MHC class I expression on DP thymocytes was 4- to 10-fold higher in FTOC inoculated with CVB4 E2 than in mock-infected controls (P < 0.001) (Fig. 3A). The upregulation was observed as early as 3 days after inoculation (data not shown). Pretreatment of CVB4 E2 viral stocks with a neutralizing polyclonal anti-CVB4 antibody inhibited MHC class I upregulation (Fig. 3B).
FIG. 3.
CVB4 E2 infection upregulates MHC class I. (A and B) MHC class I mean fluorescence intensity (MFI) of DP thymocytes 7 days after inoculation with CVB4 E2 in the absence (A) or presence (B) of CVB4-neutralizing antiserum. Representative data from five experiments are shown.
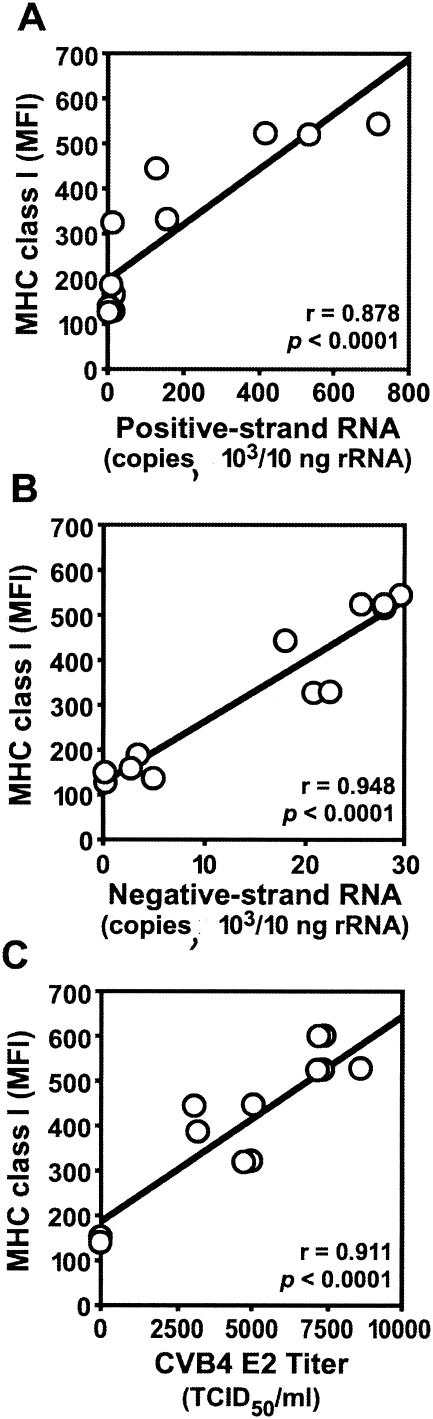
MHC class I upregulation on DP thymocytes correlated strongly with productive CVB4 E2 infection. After inoculation with CVB4 E2, MHC class I mean fluorescence intensity correlated with positive- and negative-strand RNA (Fig. 4A and B) and with the release of infectious particles in culture supernatants (Fig. 4C). Thus, MHC class I upregulation correlated with CVB4 E2 replication in the thymus.
FIG. 4.
Positive correlation between MHC class I upregulation and CVB4 E2 replication in human thymus. (A to C) MHC class I mean fluorescence intensity (MFI) on DP thymocytes versus production of positive-strand (A) and negative-strand (B) CVB4 E2 RNA and infectious virus (C) in FTOC 7 days after CVB4 inoculation.
CVB4 E2-mediated MHC class I upregulation is independent of IFN-α.
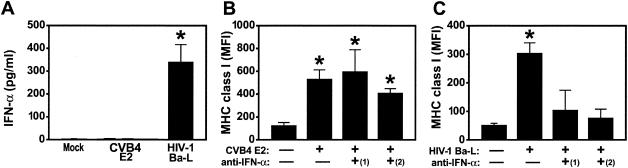
Since IFN-α modulates expression of MHC class I molecules in a variety of cell types (15, 21) and after HIV-1 infection of human thymus (20), we reasoned that MHC class I upregulation in CVB4-infected thymus was induced by this cytokine. To detect IFN-α, we harvested supernatants from CVB4 E2- and HIV-1 Ba-l-infected FTOC and performed ELISA for IFN-α. IFN-α was not detected in FTOC infected with CVB4 E2 (Fig. 5A) but was present at high levels in medium from HIV-1 Ba-L-infected FTOC (P < 0.001). No IFN-β or IFN-γ was detected in medium from either CVB4 E2- or HIV-1-infected FTOC (data not shown). Since IFN-α has numerous subtypes that could be undetected by ELISA, FTOC were treated with two neutralizing polyclonal antibodies against different IFN-α subtypes before, during, and after virus inoculation. Upregulation of MHC class I was unaffected by treatment of CVB4 E2-infected FTOC with sheep and rabbit antibodies (Fig. 5B), whereas treatment with neutralizing anti-IFN-α antibodies blocked MHC class I upregulation in HIV-1 Ba-l-infected FTOC (P < 0.001) (Fig. 5C). Importantly, treatment with antibodies did not affect virus replication since antibody-treated CVB4 E2- and HIV-1-infected FTOC were productively infected by each virus: CVB4 E2 titers in antibody-treated FTOC supernatants were similar to antibody-untreated CVB4 E2-infected FTOC supernatants [106.1 50% tissue culture infective doses per ml; data not shown]. These data suggest that IFNs do not mediate MHC class I upregulation on thymocytes in CVB4 E2-infected thymus.
FIG. 5.
(A) CVB4 E2-mediated MHC class I upregulation is independent of IFN-α. IFN-α was not detected in supernatants from CVB4 E2- and mock-infected FTOC but was detected after HIV-1 Ba-L infection. (B and C) MHC class I upregulation was not blocked by treatment of CVB4 E2-infected FTOC with rabbit [(1)] and sheep [(2)] polyclonal neutralizing anti-IFN-α antisera (B) but was completely blocked in HIV-1-Ba-l-infected FTOC (C). , P < 0.001 versus mock-infected cells (n = 5). MFI, mean fluorescence intensity.
CVB4 E2-induced MHC class I upregulation results from direct thymocyte infection.
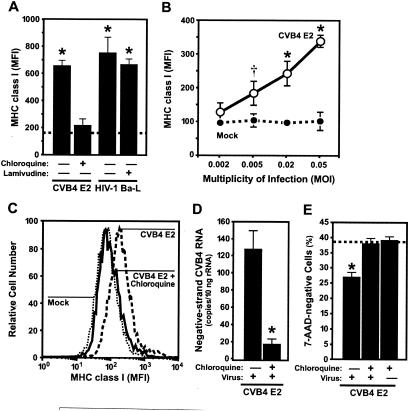
Next, we explored the possibility that other soluble factors are responsible for MHC class I upregulation on DP thymocytes. Supernatants from CVB4 E2-, HIV-1 Ba-l-, and mock-infected FTOC were transferred to freshly plated FTOC from a different donor in the presence of the lysosomotropic weak base, chloroquine, to prevent uncoating of CVB4 E2 (16) or the nucleoside analog lamivudine to inhibit infection by HIV-1 Ba-L (20). After 5 days, MHC class I expression on DP thymocytes from FTOC treated with CVB4 E2 supernatants in the presence of chloroquine was similar to that in mock-infected FTOC. In contrast, MHC class I on DP thymocytes was significantly upregulated after treatment with CVB4 E2 supernatants in the absence of chloroquine inhibition and with HIV-1 Ba-L supernatants both in the presence and in the absence of lamivudine (P < 0.001), implicating direct infection in CVB4 E2-induced MHC class I upregulation (Fig. 6A). Chloroquine and lamivudine had no effect on cell viability or MHC class I expression, and chloroquine-treated FTOC in which IFN-α was added showed significant MHC class I upregulation, indicating that chloroquine treatment by itself did not downregulate MHC class I expression (data not shown). In dose-response experiments, the magnitude of MHC class I upregulation was dependent on the MOI (Fig. 6B), further suggesting that CVB4 E2-induced MHC class I upregulation depends on direct infection.
FIG. 6.
CVB4 E2-mediated MHC class I upregulation is the result of direct infection of DP. (A) Chloroquine prevents MHC class I upregulation on DP thymocytes from FTOC inoculated with conditioned medium from CVB4 E2-infected FTOC, whereas lamivudine does not prevent MHC class I upregulation on DP from HIV-1 Ba-l-infected FTOC. The dashed line represents the mean MHC class I expression in mock-infected samples (n = 3). (B) MHC class I upregulation increases with MOI. , P < 0.001; †, P < 0.01 (versus mock-infected cells [n = 3]). (C) Significant MHC class I upregulation on purified DP thymocytes infected with CVB4 E2. The treatment of infected cells with chloroquine inhibited MHC class I upregulation. Representative stains for MHC class I are shown (n = 3). Chloroquine inhibits CVB4 E2 replication (D) and prevents virus-induced thymocyte depletion (E). The dashed line in panel E shows the viability of mock-infected cells. , P < 0.001 versus chloroquine-treated DP thymocytes (n = 3). MFI, mean fluorescence intensity.
To further explore the effects of direct CVB4 E2 infection of thymocytes on MHC class I upregulation, purified DP thymocytes from human thymus (98% purity, 91% viability with ≤0.07% pDC, and ≤0.04% immature dendritic cells) were inoculated with CVB4 E2 with or without chloroquine. DP thymocytes were harvested 3 days after inoculation and stained for CD4, CD8, MHC class I, and viability (7-AAD). Compared to mock-infected DP thymocytes, CVB4 E2-infected DP thymocytes showed increases in MHC class I expression (Fig. 6C) and in negative-strand RNA (Fig. 6D), a finding consistent with direct effects of infection. Infectious virus was detected in the supernatants (data not shown), and infected DP cells were depleted compared to the mock-infected cells (Fig. 6E) (P < 0.001). The phenotype of live cells did not vary during culture because mock- and CVB4 E2-infected FTOC had >96% DP thymocytes (data not shown). Chloroquine treatment reduced the levels of positive- and negative-strand RNA in purified DP thymocytes (Fig. 6D) (P < 0.001) and completely blocked CVB4 E2-induced MHC class I upregulation (Fig. 6C). Chloroquine did not affect cell viability but did prevent virus-induced depletion (Fig. 6E). These results suggest that MHC class I upregulation occurs through a direct infection of DP thymocytes.
DISCUSSION
This study shows that infection of human fetal thymus with CVB4 E2 leads to infection of immature thymocytes, followed by marked and progressive thymocyte depletion and upregulation of MHC class I expression on DP thymocytes. Positive- and negative-strand RNAs were present in FTOC up to 7 days after inoculation, indicating continuous CVB4 E2 replication. Virus replication in FTOC, as evidenced by quantitative negative-strand RNA detection, was undetectable at the beginning of the culture, increased with time, and was correlated with the production of infectious virus, indicating de novo viral production. Using thymocyte sorting, followed by quantitative RT-PCR, we were able to separate thymocyte subpopulations and detect CVB4 E2 RNA in DP and SP4 cells, meaning that both populations were infected by CVB4 E2, as shown by detection of positive and negative CVB4 E2 RNA. The higher viral RNA loads in sorted DP thymocytes indicate that CVB4 E2 has a tropism for these immature cells. In FTOC, DP thymocytes are an abundant subpopulation that represents an early step in thymic maturation and gives rise to mature SP progeny. No viral RNA was detected in SP8 cells, confirming their greater resistance to infection (46).
The release of infectious virus had obvious cytopathic effects. CVB4 E2, like most enteroviruses, causes cytolytic infections, but it can also establish nonlytic persistent infections (5, 8). Although other members of the enterovirus group, such as poliovirus 1, induce apoptosis under some conditions (1), CVB4 E2-induced apoptosis has been rarely reported. In our experiments, Annexin V staining showed no increase in apoptosis of infected thymocytes. Flow stainings showed that cellular depletion was most evident in DP and SP4 subpopulations. Thus, DP and SP4 thymocytes can support replication of CVB4 E2, and infection of these cells leads to obvious cytopathic effects. On the other side, SP8 cells were not depleted by CVB4 E2, which was in agreement with our inability to detect CVB4 E2 RNA in those cells. CVB4 E2 tropism has been reported in human T-cell lines where the virus replicates persistently without evidence of cytopathic effect (34). However, our study is different from those of others investigators because we report the first demonstration that human thymocyte subpopulations sustain CVB4 E2 replication and are depleted after CVB4 E2 infection. Indeed, the continuous increased virus progeny in supernatants of CVB4 E2-infected FTOC was correlated with positive- and negative-strand RNA detection and total thymocyte depletion. Together, these data indicate that CVB4 E2 replication in FTOC results in virus dissemination during the time course of the culture.
Herpesviruses, adenoviruses, and vaccinia virus decrease MHC expression (26, 37, 45), whereas MHC class I expression can be upregulated on vertebrate cells through indirect mechanisms by HIV-1 (27) and through direct mechanisms by West Nile virus (22, 27). In our FTOC cultures, MHC class I expression was upregulated on immature thymocytes and correlated strongly with markers of active viral replication, such as negative-strand viral RNA and the release of infectious virus. This effect was related to the virus since anti-virus neutralizing antibodies inhibited MHC class I. MHC class I upregulation was completely inhibited by treatment of CVB4 E2 supernatants with chloroquine, a lysosomotropic agent that increases the pH of acidic prelysosomal vacuoles and blocks viral penetration or uncoating (16). Thus, the ability of CVB4 E2 to modulate MHC class I expression on immature DP thymocytes involves at least entry or uncoating of the virus associated with replication and depletion of these cells. In single-cell suspensions of DP thymocytes, chloroquine blocked CVB4 E2-induced upregulation of MHC class I, reduced negative-strand viral RNA by 90%, and inhibited CVB4 E2-induced depletion. The low levels of negative-strand viral RNA (<20 copies/10 ng of rRNA) did not seem to influence MHC class I expression, suggesting that a threshold of replication was necessary for MHC class I upregulation. In addition, MHC class I upregulation was largely dependent on MOI. Moreover, CVB4 E2-induced MHC class I upregulation occurred even when IFN-α was blocked by neutralizing antibodies, and despite numerous attempts, we were unable to detect IFN-α, IFN-β, or IFN-γ in supernatants from CVB4-infected FTOC. Furthermore, chloroquine-treated supernatants of CVB4-infected FTOC did not upregulate MHC class I, which argues against the role of soluble factors released by CVB4-infected FTOC in the observed phenomenon. Together, these data showed that MHC class I upregulation on immature DP thymocytes was dependent on CVB4 E2 replication in this subpopulation, which strongly suggested a direct viral effect. Whether viral components and/or intracellular virus-induced factors are involved in this phenomenon remains to be determined.
Clearly, the mechanisms of upregulation of MHC class I obtained with CVB4E2 in thymic cells are distinct from those playing a role in the increased MHC expression obtained with HIV-1 in these cells. Indeed, HIV-1 infection induced high levels of IFN-α involved in indirect mechanisms of upregulation of MHC class I in our system. Our results of MHC class I upregulation on thymocytes obtained with HIV-1 are in agreement with those from another group, who reported that HIV-1 induced IFN-α secretion by pDC2 (20). Together, these data suggest that CVB4, in contrast to HIV-1, did not infect pDC2 or did not induce IFN-α production by these cells, which is in agreement with previous report of CVB4-induced production of IFN-α in monocytes, whereas pDC in peripheral blood did not secrete IFN-α in response to CVB4, in contrast to HSV-1, a well-known IFN-α inducer in these cells (7, 16). However, CVB4 E2 infection of FTOC altered pDC2 since we observed a significant depletion of pDC2 in CVB4 E2-infected FTOC compared to mock-infected cultures (0.1% versus 1.2% [data not shown]). Whether pDC2 were depleted by direct infection mechanisms was difficult to ascertain due to the low numbers of these cells in FTOC cultures. Alternatively, pDC2 can be depleted by bystander mechanisms since the viability of pDC is altered significantly when their cellular and culture environment changes (43). Further experiments are needed to clarify whether the lack of IFN-α responsiveness to CVB4 E2 in FTOC can be the consequence of pDC2 depletion.
The pattern of results obtained with CVB4 E2 compared to HIV-1 in our experiments, together with experiments showing that supernatants from CVB4 E2-infected FTOC, when treated with chloroquine and put on new FTOC, did not induce MHC I upregulation, demonstrated that the CVB4 E2-induced MHC-I upregulation was not due to virus-induced cytokine.
Whether TEC were infected in a similar manner to DP thymocytes was difficult to establish given the low abundance of TEC extracted from FTOC (5%). However, we observed that IL-6, and GM-CSF, cytokines produced by TEC in human thymus (30), were produced by CVB4 E2-infected FTOC (data not shown). Recently, we reported that CVB4 can infect productively primary cultures of human TEC that results in IL-6 and GM-CSF secretion by these cells (5). Altogether, these data suggest that CVB4 E2 infection of human FTOC can lead to TEC infection. Whether TEC from FTOC inoculated with CVB4 E2 were infected remains to be clarified; nevertheless, CVB4 E2 affected TEC in FTOC. Indeed, flow cytometry extracellular and intracellular stainings showed a two- to fourfold upregulation of MHC class I expression on FSChi SSChi CD4− CD8− cytokeratinintrac+ cells (TEC) isolated from CVB4 E2-infected FTOC compared to mock cultures (P < 0.001) (data not shown) by using appropriate isotype controls as previously described (32).
The rationale for choosing CVB4 E2 in our system was to examine the replication and the effects of a relevant CVB4 strain isolated from a patient with type 1 diabetes (48). Enterovirus RNA, with strong homology with CVB4 E2, has been detected in the peripheral blood of type 1 diabetes patients (47). In our system, CVB4 JBV strain was capable of infecting and replicating in human FTOC and induced effects similar to those of CVB4 E2 (data not shown). Thus, the viral replication and effects in human FTOC were not restricted to the CVB4 E2 strain.
Coxsackievirus B infection in midpregnancy (mice) has been associated with delayed thymic development of the fetus (28). Diabetogenic CVB4 can reach the thymus in the course of systemic infection of mice (6), which results in a significant increase of CD4− CD8− thymocytes, together with insulitis and hyperglycemia, which indicates that CVB4 infection may be associated with abnormal T-cell maturation in vivo. It has been suggested that a fetal infection with coxsackievirus B may initiate autoimmunity or cause persistent infection that may lead to progressive beta-cell destruction that could play a role in the pathogenesis of type 1 diabetes (11). Furthermore, massive thymic depletion has been linked to autoimmunity emergence in mice models (40). Together, these reports suggest that CVB4 E2 thymic infection results in morphological and functional anomalies of the thymus. Further studies are needed to elucidate whether CVB4 infection of the fetal thymus can play a role in the impairment of central T-cell self-tolerance, which may provide a better knowledge about the pathogenesis of CVB4-induced diabetes type 1.
Acknowledgments
F. Brilot was supported by Fondation Léon Frédéricq of Liège and by the Fonds pour la Recherche Industrielle et Agronomique (Fria, Belgium) and was awarded a travel grant from the Brussels-Wallonia Community of Belgium. This study was supported by the Vaugrenier Foundation for Tolerance Research (Geneva, Switzerland). D. Hober was supported by Université Lille 2 (MENRT) and CHRU Lille, France. Cheryl A. Stoddart was supported by the NIH (grant AI05418).
We thank Mike McCune for thoughtful discussions and ideas; Jean-Francois Vanbellinghen and Christophe Kreis for assistance with quantitative RT-PCR; Marty Bigos, Dax Darguello, and Valerie Stepps for flow cytometry assistance; and Mary Keir for assistance with cell culture.
REFERENCES
- 1.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T. Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:343-353. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz, R. D., S. Alexander, C. Bare, V. Linquist-Stepps, M. Bogan, M. E. Moreno, L. Gibson, E. D. Wieder, J. Kosek, C. A. Stoddart, and J. M. McCune. 1998. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J. Virol. 72:10108-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blue, M. L., H. Levine, J. F. Daley, K. R. Branton, and S. F. Schlossman. 1989. Expression of CD1 and class I MHC antigens by human thymocytes. J. Immunol. 142:2714-2720. [PubMed] [Google Scholar]
- 4.Bonyhadi, M. L., L. Su, J. Auten, J. M. McCune, and H. Kaneshima. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1073-1080. [DOI] [PubMed] [Google Scholar]
- 5.Brilot, F., W. Chehadeh, C. Charlet-Renard, H. Martens, V. Geenen, and D. Hober. 2002. Persistent infection of human thymic epithelial cells by coxsackievirus B4. J. Virol. 76:5260-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee, N. K., J. Hou, P. Dockstader, and T. Charbonneau. 1992. Coxsackievirus B4 infection alters thymic, splenic, and peripheral lymphocyte repertoire preceding onset of hyperglycemia in mice. J. Med. Virol. 38:124-131. [DOI] [PubMed] [Google Scholar]
- 7.Chehadeh, W., A. Bouzidi, G. Alm, P. Wattre, and D. Hober. 2001. Human antibodies isolated from plasma by affinity chromatography increase the coxsackievirus B4-induced synthesis of interferon-alpha by human peripheral blood mononuclear cells in vitro. J. Gen. Virol. 82:1899-1907. [DOI] [PubMed] [Google Scholar]
- 8.Chehadeh, W., J. Kerr-Conte, F. Pattou, G. Alm, J. Lefebvre, P. Wattre, and D. Hober. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J. Virol. 74:10153-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chehadeh, W., J. Weill, M. C. Vantyghem, G. Alm, J. Lefebvre, P. Wattre, and D. Hober. 2000. Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J. Infect. Dis. 181:1929-1939. [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist, G. 2000. Fetal virus infection a risk factor of diabetes mellitus type 1 in children. Lakartidningen 97:313-315. [PubMed] [Google Scholar]
- 11.Dahlquist, G., G. Frisk, S. A. Ivarsson, L. Svanberg, M. Forsgren, and H. Diderholm. 1995. Indications that maternal coxsackie B virus infection during pregnancy is a risk factor for childhood-onset IDDM. Diabetologia 38:1371-1373. [DOI] [PubMed] [Google Scholar]
- 12.Fohlman, J., and G. Friman. 1993. Is juvenile diabetes a viral disease? Ann. Med. 25:569-574. [PubMed] [Google Scholar]
- 13.Geenen, V., and G. Kroemer. 1993. Multiple ways to cellular immune tolerance. Immunol. Today 14:573-575. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg-Fellner, F., M. E. Witt, S. Yagihashi, M. J. Dobersen, F. Taub, B. Fedun, R. C. McEvoy, S. H. Roman, R. G. Davies, L. Z. Cooper, et al. 1984. Congenital rubella syndrome as a model for type 1 (insulin-dependent) diabetes mellitus: increased prevalence of islet cell surface antibodies. Diabetologia 27(Suppl.):87-89. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, H., K. Tanaka, F. Jay, G. Khoury, and G. Jay. 1985. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell 43:263-267. [DOI] [PubMed] [Google Scholar]
- 16.Hober, D., W. Chehadeh, A. Bouzidi, and P. Wattre. 2001. Antibody-dependent enhancement of coxsackievirus B4 infectivity of human peripheral blood mononuclear cells results in increased interferon-alpha synthesis. J. Infect. Dis. 184:1098-1108. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, M. S., and N. Sarvetnick. 1999. Viruses, host responses, and autoimmunity. Immunol. Rev. 169:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyoty, H., M. Hiltunen, M. Knip, M. Laakkonen, P. Vahasalo, J. Karjalainen, P. Koskela, M. Roivainen, P. Leinikki, T. Hovi, et al. 1995. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes 44:652-657. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki, T., N. Monma, R. Satodate, R. Kawana, and T. Kurata. 1985. An immunofluorescent study of generalized coxsackie virus B3 infection in a newborn infant. Acta Pathol. Jpn. 35:741-748. [DOI] [PubMed] [Google Scholar]
- 20.Keir, M. E., C. A. Stoddart, V. Linquist-Stepps, M. E. Moreno, and J. M. McCune. 2002. IFN-alpha secretion by type 2 predendritic cells up-regulates MHC class I in the HIV-1-infected thymus. J. Immunol. 168:325-331. [DOI] [PubMed] [Google Scholar]
- 21.Keskinen, P., T. Ronni, S. Matikainen, A. Lehtonen, and I. Julkunen. 1997. Regulation of HLA class I and II expression by interferons and influenza A virus in human peripheral blood mononuclear cells. Immunology 91:421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesson, A. M., Y. Cheng, and N. J. King. 2002. Regulation of immune recognition molecules by flavivirus, West Nile. Viral. Immunol. 15:273-283. [DOI] [PubMed] [Google Scholar]
- 23.Klein, L., and B. Kyewski. 2000. Self-antigen presentation by thymic stromal cells: a subtle division of labor. Curr. Opin. Immunol. 12:179-186. [DOI] [PubMed] [Google Scholar]
- 24.Klingel, K., S. Stephan, M. Sauter, R. Zell, B. M. McManus, B. Bultmann, and R. Kandolf. 1996. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J. Virol. 70:8888-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knip, M., and H. K. Akerblom. 1999. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107(Suppl. 3):S93-S100. [DOI] [PubMed] [Google Scholar]
- 26.Koszinowski, U., and H. Ertl. 1975. Altered serological and cellular reactivity to H-2 antigens after target cell infection with vaccinia virus. Nature 257:596-597. [DOI] [PubMed] [Google Scholar]
- 27.Kovalev, G., K. Duus, L. Wang, R. Lee, M. Bonyhadi, D. Ho, J. M. McCune, H. Kaneshima, and L. Su. 1999. Induction of MHC class I expression on immature thymocytes in HIV-1-infected SCID-hu Thy/Liv mice: evidence of indirect mechanisms. J. Immunol. 162:7555-7562. [PMC free article] [PubMed] [Google Scholar]
- 28.Lansdown, A. B. 1977. Histological observations on thymic development in fetal and newborn mammals subject to intrauterine growth retardation. Biol. Neonate 31:252-259. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor, D. A., J. Zemmour, P. D. Ennis, and P. Parham. 1990. Evolution of class-I MHC genes and proteins: from natural selection to thymic selection. Annu. Rev. Immunol. 8:23-63. [DOI] [PubMed] [Google Scholar]
- 30.Le, P. T., S. Lazorick, L. P. Whichard, Y. C. Yang, S. C. Clark, B. F. Haynes, and K. H. Singer. 1990. Human thymic epithelial cells produce IL-6, granulocyte-monocyte-CSF, and leukemia inhibitory factor. J. Immunol. 145:3310-3315. [PubMed] [Google Scholar]
- 31.Leparc, I., M. Aymard, and F. Fuchs. 1994. Acute, chronic, and persistent enterovirus and poliovirus infections: detection of viral genome by seminested PCR amplification in culture-negative samples. Mol. Cell. Probes 8:487-495. [DOI] [PubMed] [Google Scholar]
- 32.Liu-Wu, Y., A. Svenningsson, S. Stemme, J. Holm, and O. Wiklund. 1997. Identification and analysis of macrophage-derived foam cells from human atherosclerotic lesions by using a “mock” FL3 channel in flow cytometry. Cytometry 29:155-164. [PubMed] [Google Scholar]
- 33.Lozovskaia, L. S., S. M. Osipov, I. V. Zubkova, and V. D. Soboleva. 1997. Study of vertical transmission of coxsackie group enteroviruses in the etiology of congenital immunodeficiencies. Vopr. Virusol. 42:175-179. [PubMed] [Google Scholar]
- 34.Matteucci, D., M. Paglianti, A. M. Giangregorio, M. R. Capobianchi, F. Dianzani, and M. Bendinelli. 1985. Group B coxsackieviruses readily establish persistent infections in human lymphoid cell lines. J. Virol. 56:651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldstone, M. B. 1998. Molecular mimicry and immune-mediated diseases. FASEB J. 12:1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otonkoski, T., M. Roivainen, O. Vaarala, B. Dinesen, J. A. Leipala, T. Hovi, and M. Knip. 2000. Neonatal type I diabetes associated with maternal echovirus 6 infection: a case report. Diabetologia 43:1235-1238. [DOI] [PubMed] [Google Scholar]
- 37.Paabo, S., T. Nilsson, and P. A. Peterson. 1986. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc. Natl. Acad. Sci. USA 83:9665-9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidtke, M., B. Gluck, I. Merkle, P. Hofmann, A. Stelzner, and D. Gemsa. 2000. Cytokine profiles in heart, spleen, and thymus during the acute stage of experimental coxsackievirus B3-induced chronic myocarditis. J. Med. Virol. 61:518-526. [PubMed] [Google Scholar]
- 39.See, D. M., and J. G. Tilles. 1995. Pathogenesis of virus-induced diabetes in mice. J. Infect. Dis. 171:1131-1138. [DOI] [PubMed] [Google Scholar]
- 40.Shih, F. F., L. Mandik-Nayak, B. T. Wipke, and P. M. Allen. 2004. Massive thymic deletion results in systemic autoimmunity through elimination of CD4+ CD25+ T regulatory cells. J. Exp. Med. 199:323-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha, A. A., M. T. Lopez, and H. O. McDevitt. 1990. Autoimmune diseases: the failure of self tolerance. Science 248:1380-1388. [DOI] [PubMed] [Google Scholar]
- 42.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labeled Annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 43.Weijer, K., C. H. Uittenbogaart, A. Voordouw, F. Couwenberg, J. Seppen, B. Blom, F. A. Vyth-Dreese, and H. Spits. 2002. Intrathymic and extrathymic development of human plasmacytoid dendritic cell precursors in vivo. Blood 99:2752-2759. [DOI] [PubMed] [Google Scholar]
- 44.Whitton, J. L., and R. S. Fujinami. 1999. Viruses as triggers of autoimmunity: facts and fantasies. Curr. Opin. Microbiol. 2:392-397. [DOI] [PubMed] [Google Scholar]
- 45.Yewdell, J. W., and J. R. Bennink. 1999. Mechanisms of viral interference with MHC class I antigen processing and presentation. Annu. Rev. Cell. Dev. Biol. 15:579-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 47.Yin, H., A. K. Berg, T. Tuvemo, and G. Frisk. 2002. Enterovirus RNA is found in peripheral blood mononuclear cells in a majority of type 1 diabetic children at onset. Diabetes 51:1964-1971. [DOI] [PubMed] [Google Scholar]
- 48.Yoon, J. W., M. Austin, T. Onodera, and A. L. Notkins. 1979. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]