Abstract
A chimeric yellow fever-dengue 1 (ChimeriVax-DEN1) virus was produced by the transfection of Vero cells with chimeric in vitro RNA transcripts. The cell culture supernatant was subjected to plaque purification for the identification of a vaccine candidate without mutations. Of 10 plaque-purified clones, 1 containing no mutation (clone J) was selected for production of the vaccine virus. During subsequent cell culture passaging of this clone for vaccine production, a single amino acid substitution (K to R) occurred in the envelope (E) protein at residue 204 (E204) (F. Guirakhoo, K. Pugachev, Z. Zhang, G. Myers, I. Levenbook, K. Draper, J. Lang, S. Ocran, F. Mitchell, M. Parsons, N. Brown, S. Brandler, C. Fournier, B. Barrere, F. Rizvi, A. Travassos, R. Nichols, D. Trent, and T. Monath, J. Virol. 78:4761-4775, 2004). The same mutation was observed in another clone (clone E). This mutation attenuated the virus in 4-day-old suckling mice inoculated by the intracerebral (i.c.) route and led to reduced viremia in monkeys inoculated by the subcutaneous or i.c. route. The histopathology scores of lesions in the brain tissue of monkeys inoculated with either the E204K or E204R virus were reduced compared to those for monkeys inoculated with the reference virus, a commercial yellow fever 17D vaccine (YF-VAX). Both viruses grew to significantly lower titers than YF-VAX in HepG2, a human hepatoma cell line. After intrathoracic inoculation into mosquitoes, both viruses grew to a similar level as YF-VAX, which was significantly lower than that of their wild-type DEN1 parent virus. A comparison of the E-protein structures of nonmutant and mutant viruses suggested the appearance of new intramolecular bonds between residues 204R, 261H, and 257E in the mutant virus. These changes may be responsible for virus attenuation through a change in the pH threshold for virus envelope fusion with the host cell membrane.
Dengue, a mosquito-borne viral infection, has become a major global public health concern due to a dramatic growth in its prevalence. The disease is now endemic in more than 100 countries in the Americas, Southern Europe, Asia, and Australia. Two and a half billion people, approximately 40% of the world population, are now at risk of infection. Annually, over 50 million infections and 24,000 deaths are due to dengue (39).
Dengue virus (DEN virus), a member of the Flaviviridae family, has four distinct but closely related serotypes, serotypes 1 to 4. Infection with one serotype generally induces life-long immunity against that serotype, but it only confers a transient protection against the other three. Sequential infections increase the risk of dengue hemorrhagic fever and dengue shock syndrome, which are potentially lethal complications of the disease (13, 19, 32).
Flaviviruses are positive-strand RNA viruses that are primarily transmitted by infected mosquitoes or ticks. They include major human pathogens, such as the yellow fever (YF), DEN, Japanese encephalitis (JE), tick-borne encephalitis, and West Nile (WN) viruses. Mature virions contain three structural proteins, the capsid (C), small membrane (M), and major envelope (E) proteins. Seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) are synthesized in virus-infected cells. Both viral receptor binding and fusion domains reside within the E protein, and antibodies against this protein can neutralize virus infectivity and confer protection against disease (3).
Vaccine development for dengue has been difficult because simultaneous protection against all four serotypes is necessary. Protection against one or two serotypes may actually enhance the risk of subsequent infections with other serotypes, thereby putting the subjects at risk of acquiring dengue hemorrhagic fever or dengue shock syndrome. It is generally believed that a successful dengue vaccine must contain a tetravalent mixture of appropriate doses of each serotype. No vaccine is available at this time, although there are a few promising live attenuated vaccine candidates in the late stages of development (5, 6, 17, 18, 33-35).
We previously reported the construction and preclinical evaluation of four chimeric YF-DEN (ChimeriVax-DEN1 to -4) virus vaccine candidates in mice, monkeys (8-11), and mosquitoes (14, 16). Recently, a phase I clinical trial of the ChimeriVax-DEN2 vaccine virus was successfully completed as “proof of principle” (S. Kitchener, F. Guirakhoo, K. McCarthy, D. Morrison, S. Yoksan, and T. P. Monath, unpublished data). The premembrane (prM; precursor of M) and E genes used for construction of the ChimeriVax-DEN1 to -4 viruses were derived from wild-type (wt) DEN viruses isolated from humans, and the attenuation of DEN virus chimeras was mainly driven by the NS gene component of the YF 17D virus in the chimeric vaccine virus. The ChimeriVax-DEN1 and -DEN2 vaccines, but not the ChimeriVax-DEN3 and -DEN4 vaccines, accumulated mutations within their E genes when they were produced in Vero cells (10, 31). The effect of these mutations on the neurovirulence (NV) of the virus strains was determined in 4-day-old suckling mice. Mutations in the ChimeriVax-DEN2 vaccine did not affect its NV for suckling mice or its immunogenicity in monkeys (10). However, a single mutation (A to G at nucleotide [nt] 1590) resulting in an amino acid substitution from K to R at residue E204 in the E protein of the ChimeriVax-DEN1 vaccine was found to reduce NV in mice. Prior to including this mutant virus in a tetravalent vaccine formulation for human use, it was necessary to assess the safety of the virus with a reversion to the wt amino acid residue (K) at E204 in relevant models, including monkeys, human hepatoma cell lines, and mosquito vectors.
In this study, ChimeriVax-DEN1 strains with wt and mutant E-protein components were evaluated in suckling mice and monkeys inoculated by the subcutaneous route (s.c.; the intended route for human immunization) or the intracerebral route (i.c.; standard safety test used for release of the YF 17D vaccine). The growth kinetics of these viruses were compared to those of the YF 17D and wt DEN parent viruses in a human hepatoma cell line (HepG2) and in mosquito vectors (Aedes aegypti) inoculated by the intrathoracic (i.t.) route. A possible mechanism of attenuation is discussed based on the location of the E204 residue in the DEN1 E-protein structure that was modeled after the crystal structure of DEN2 virus (25).
MATERIALS AND METHODS
Cells and viruses.
Vero cells used for vaccine production were obtained from a qualified cell bank (Aventis Pasteur, Marcy l'Etoile, France). HepG2 cells were purchased from the American Type Culture Collection (Manassas, Va.). The production and biological activity of uncloned ChimeriVax-DEN1 (Vero passage 4 [P4] containing M39 H-to-R and E204 K-to-R substitutions; experiment 1) have been described previously (8). The plasmid-derived mutation in the M protein of the uncloned ChimeriVax-DEN1 P4 virus was found to have overattenuated the virus and was subsequently corrected in the ChimeriVax-DEN1 strain used for this study (10). Plaque-purified ChimeriVax-DEN1 viruses (clone B [E251 V→F], clone C [E311 E→D], clone E [E204 K→R], and clone J [no mutation]) were prepared from an uncloned Vero P2 virus which did not contain any mutations (10, 31). The ChimeriVax-DEN1 vaccine lot (VL) virus was produced at P10 from the premaster seed (PMS) virus (clone J, Vero P7, wt prME; experiment 2) by three passages performed according to current good manufacturing practices (cGMP) (10). A stock of the wt DEN1 parent virus (strain PUO359, which is the donor of prME genes for the ChimeriVax-DEN1 virus) was prepared in C6/36 cells. The YF 17D vaccine (YF-VAX) was purchased from Aventis Pasteur and used without dilution or further passage.
Animal studies.
All studies were performed according to an Institutional Animal Care and Use Committee-approved protocol in accordance with the USDA Animal Welfare Act (9 CFR parts 1 to 3) and the Guide for Care and Use of Laboratory Animals (30).
Mice.
The NV phenotype of DEN1 chimera clones was assessed in suckling mice. Pregnant ICR mice were purchased from Taconic Farm (Germantown, N.Y.). Suckling mice were pooled at the age of 2 to 3 days and fostered to dams (9 to 12 mice/dam). Mice were inoculated at the age of 3 to 4 days by the i.c. route with 0.02 ml of various dilutions of viruses. The mice were observed for 21 days, and mortality was recorded. The virus concentration administered to each group of animals was determined by back titration of inocula in a plaque assay on Vero cells.
Monkeys.
Two experiments were performed with macaques (at Sierra Division, Charles River Laboratories, Inc., Sparks, Nev.) to assess the viscerotropism (experiment 1) and NV (experiment 2) of ChimeriVax-DEN1 viruses with or without the E204 mutation. In the first experiment, rhesus monkeys (Macaca mulatta) were inoculated with chimeric DEN1 viruses by the s.c. route, whereas in the second experiment cynomolgus monkeys (Macaca fascicularis) were inoculated by the i.c. route. Because rhesus monkeys were not available, cynomolgus monkeys (a species approved under the World Health Organization [WHO] requirements for testing the YF vaccine [37]) were chosen for the second experiment. Prior to the second experiment, a pilot experiment with ChimeriVax-DEN1 to -4 viruses and YF-VAX was performed to assure the suitability of the cynomolgus monkeys as a replacement for rhesus monkeys.
Experiment 1.
A total of 12 (6 males and 6 females) experimentally naive, flavivirus-seronegative rhesus monkeys of 2.6 to 5.2 years of age, weighing 3.4 to 4.6 kg on the day prior to dosing, were assigned to three treatment groups, with each group containing 2 males and 2 females. Each animal received a single dose (∼5 log10 PFU/0.5 ml in minimal essential medium containing 50% fetal bovine serum [FBS]) of one of three viruses via s.c. injection as follows: group 1, ChimeriVax-DEN1 uncloned virus (Vero P4 [M39R, E204R]) (8); group 2, ChimeriVax-DEN1 clone E (Vero P6 [E204R]); group 3, ChimeriVax-DEN1 clone J (Vero P7, PMS [E204K]) (10). The uncloned virus and clone E contain the E204 K→R mutation, and clone J contains the parental genotype without mutations. The day of dosing was designated day 1. Blood samples were collected predose on day 1 and on days 2 through 11 for viremia analysis and on days 1 (predose) and 31 for neutralizing antibody analysis. Throughout the study, the animals were observed for changes in general appearance and behavior (at least twice daily), body weight (weekly), and food consumption (daily). After the last sample collection on day 31, all animals were returned to the animal colony.
Experiment 2.
ChimeriVax-DEN1 PMS (clone J, P7, E204K), ChimeriVax-DEN1 VL (clone J, P10, E204R) (each at 5 log10 PFU), and the YF-VAX reference vaccine (4.7 log10 PFU) were administered i.c. by injection (0.25 ml) into the left frontal lobe of 18 experimentally naive, flavivirus-seronegative cynomolgus monkeys (n = 6/group). The monkeys were observed for 30 days after inoculation and then were euthanized and necropsied. During the observation period, the monkeys were evaluated for changes in clinical signs (twice daily), body weight (weekly), and food consumption (daily). Clinical signs were assigned scores according to a clinical scoring system based on the WHO requirements for the YF vaccine (38). Blood samples were collected prestudy and preinoculation on day 1 and on days 3, 5, 7, 15, and 31 for clinical pathology analysis (serum chemistry and hematology parameters). Additional blood samples were collected preinoculation on day 1 and on days 2 to 11 for viremia analysis and on days 1 (predose) and 31 for measurement of the neutralizing antibody response. At necropsy, gross pathological findings were recorded, and a complete list of tissues (liver, spleen, heart, kidney, and adrenal glands) was collected and preserved. Slides were prepared from a selected subset of tissues and examined for histopathologic findings. Histopathology of the brain and spinal cord was performed by a neuropathologist according to WHO requirements for the YF vaccine (38). The histopathological evaluation was performed in a blinded manner. Lesions in the meninges and the brain or spinal cord matter were scored on a scale of 0 to 2, according to the following observations: grade 0, no visible lesions; grade 1 (minimal), one to three small focal, mostly perivascular infiltrates, consisting of several or more cells or very mild diffuse inflammatory infiltration; grade 2 (mild), more than three inflammatory infiltrates. The degree of NV was estimated for the target (known to reveal damage by flaviviruses) and discriminator (known to discriminate NV from attenuated strains) areas, as described previously (21, 28, 38). For cynomolgus monkeys, the substantia nigra and cervical and lumbar enlargements of the spinal cord represent the target areas, whereas basal ganglia and thalamic nuclei are considered discriminator areas. Individual and group mean lesion scores for the target and discriminator areas were calculated separately and as a combined score.
Viremia and antibody measurements.
Viremia was measured by a plaque assay on Vero cells (11, 12). Cells were inoculated with 0.1-ml samples of sera (undiluted or diluted 1:2 or 1:10) obtained from days 2 to 11 postinoculation. The minimal level of detection in viremia assays was 1 log10 PFU/ml. Viremia titers were expressed in PFU per milliliter. A plaque reduction method using Vero cells was used for measurement of the neutralizing antibody response to homologous viruses (chimeras or YF-VAX). In this test, a constant virus input (approximately 50 to 100 PFU) was neutralized by various dilutions of heat-inactivated test sera, and titers are expressed as the highest dilution of serum that inhibited plaque formation by 50% (plaque reduction neutralization titer [PRNT50]) (28).
Growth kinetics in HepG2 cells.
HepG2 cells were grown to confluence in T25 flasks with Eagle's minimal essential medium (Vitacell) supplemented with 8% FBS (HyClone) and 1% antibiotic-antimycotic mixture (Sigma). The cells were infected at a multiplicity of infection (MOI) of 0.001 with ChimeriVax-DEN1 PMS (clone J, P7, E204K), ChimeriVax-DEN1 VL (clone J, P10, E204R), or the parent viruses (YF-VAX and wt DEN1 strain PUO359). After adsorption at 37°C for 1 h, the inocula were removed, the cells were washed with phosphate-buffered saline three times to remove unbound viruses, and growth medium was added to the cultures. Samples were removed daily for 10 days, FBS was added to a final concentration of 50% to preserve virus infectivity, and samples were stored at −70°C. Virus titers were determined by plaque assays on Vero cells, using agarose double overlays and neutral red as previously described (28).
Mosquito infection.
F4 generations of a laboratory established colony of A. aegypti from Puerto Rico were inoculated with ChimeriVax-DEN1 PMS (clone J, P7, E204K), ChimeriVax-DEN1 VL (clone J, P10, E204R), or the reference parent viruses (YF 17D and wt DEN1 strain PUO359). To avoid potential infection barriers in the midgut that are associated with oral feeding, we anesthetized the mosquitoes in the cold and inoculated them i.t., using a microcapillary needle that had been pulled to a point with a Narishige (Tokyo) needle puller. Approximately 0.34 μl of virus standardized to 6.0 log10 PFU/ml was injected into each mosquito (2.5 log10 PFU/mosquito). Inoculated mosquitoes were maintained in cartons at 27°C with 80% humidity and 5% sugar water. Three mosquitoes per infection were removed at 48-h intervals for 10 days. Mosquitoes were frozen at −70°C until they were used for assays. Infectious virus titers were determined by real-time reverse transcription-PCR (TaqMan). Primers and probes were designed with the PrimerExpress software package (PE Applied Biosystems, Foster City, Calif.). The TaqMan probes were labeled at the 5′ end with the FAM reporter dye and at the 3′ end with a dark quencher dye. The ChimeriVax-DEN primers were serotype specific, whereas the YF 17D primers detected both ChimeriVax-DEN and YF 17D viruses (14).
Molecular modeling.
A molecular model of the E protein of DEN1 virus (strain PUO 359) was developed by homology modeling, with the structure of DEN2 virus as a starting point (25). The DS Modeling 1.1 (Accelrys, San Diego, Calif.) software package was used to develop the DEN1 virus homology model. The K→R substitution was built into the DEN1 virus model by allowing a 5-Å radius for movement of the amino acid side chains. For comparisons of the mutant and wt E proteins, both structures were superimposed by sequence alignment, and amino acids showing significant changes in position in the vicinity of the substitution were identified by inspection.
Statistical analyses.
Differences in the NV phenotypes of viruses inoculated into suckling mice were analyzed for significance by product-limit survival fit, and probability was calculated by a log-rank test. All other probability analyses between two groups or among groups of animals were performed by analysis of variance. Observed significance probabilities of 0.050 or less are often considered evidence that an analysis of variance model fits the data. All analyses were performed with JMP software, version 5.1.
RESULTS
NV properties of various clones of DEN1 chimeras in suckling mice.
During PMS production of the DEN1 chimera, 10 different plaque-purified clones (A to J) were sequenced (entire genome) to identify a clone with no amino acid substitutions. All but one clone (J) contained one or two substitutions within the envelope protein E (31). The uncloned DEN1 (wt prME, Vero P2) virus and representatives of cloned viruses were evaluated for their NV following i.c. inoculation of 3- to 4-day-old suckling mice (Table 1). The NV of all DEN1 viruses, inoculated at doses of 1.8 to 5.8 log10 PFU, was significantly lower than that of YF-VAX, inoculated at 2.5 log10 PFU (P = 0.001; log-rank test). Mice inoculated with chimeric DEN1 viruses at similar or higher doses than those of YF-VAX showed a longer average survival time (AST) (Table 1). Clone E, which contained two mutations (one nucleotide change at position 1590 [A→G] that resulted in a K→R substitution and one nucleotide change at position 3952 [A→T] that was silent), was significantly less virulent (P = 0.0001; log-rank test) than all other DEN1 clones, with an AST of 13 to 15 days. Interestingly, the only amino acid change identified in the E protein of the original, uncloned DEN1 chimera (Vero P4) was also the E204 K→R substitution. This virus was previously shown to induce a low level of viremia (mean peak titer, 0.7 log10 PFU/ml) for 1.3 days after s.c. inoculation into monkeys (8). Clone J, which contained no mutations and was shown to be significantly less NV than YF-VAX in 4-day-old mice (P = 0.001; log-rank test) (Table 1), was selected for production of the cGMP vaccine virus (10).
TABLE 1.
NV of various clones of ChimeriVax-DEN1 viruses in 4-day-old mice inoculated by the i.c. routea
| Group | ChimeriVax-DEN1 clone or other virus | Location of mutation (amino acid change) | Dilution | Dose (BT)b (log10 PFU) | No. of dead/total (% dead) | AST (days) |
|---|---|---|---|---|---|---|
| 1 | Uncloned (P2) | None | Neat | 5.0 | 11/11 (100) | 9.1 |
| Uncloned (P2) | None | 1:10 | 4.1 | 11/11 (100) | 10.2 | |
| 2 | B | E251 (V→F) | Neat | 5.8 | 10/11 (91) | 9.8 |
| B | E251 (V→F) | 1:10 | 5.0 | 11/11 (100) | 10.2 | |
| 3 | C | E311 (E→D) | Neat | 5.8 | 11/11 (100) | 8.5 |
| C | E351 (V→L) | 1:10 | 4.9 | 11/11 (100) | 9.5 | |
| 4 | E | E204 (K→R) | Neat | 5.9 | 3/11 (27) | 13 |
| E | E204 (K→R) | 1:10 | 4.8 | 1/11 (9) | 14 | |
| E | E204 (K→R) | 1:100 | 4.0 | 1/11 (9) | 15 | |
| 5 | J | None | Neat | 3.6 | 11/11 (100) | 10.8 |
| J | None | 1:10 | 3.0 | 11/11 (100) | 11.3 | |
| J | None | 1:100 | 1.8 | 9/11 (82) | 11.3 | |
| 6 | YF-VAX | NAc | 1:20 | 2.5 | 12/12 (100) | 8.3 |
For groups 1, 2, 3, and 5 versus group 6, P < 0.001. For groups 1, 2, 3, and 5 versus group 6, P < 0.0001. For group 5 versus group 6, P < 0.001. These are all statistically significant.
Back titration.
NA, not applicable.
Experiment 1. (i) Viremia-viscerotropism and immunogenicity of ChimeriVax-DEN1 viruses (with or without E204 mutation) in monkeys inoculated by the s.c. route.
To determine if the attenuation of clone E (E204R) for infant mice correlated with decreased viscerotropism-viremia and/or immunogenicity in monkeys, we inoculated the clone E and clone J (E204K, wt prME, PMS) viruses s.c. into monkeys. Twelve flavivirus-seronegative rhesus monkeys were divided into three groups (n = 4/group). Animals in each group received a single s.c. injection (approximately 5 log10 PFU virus/0.5 ml) of virus as shown in Table 2. During a 1-month observation period, there were no virus-related changes in clinical signs, food consumption, or body weight.
TABLE 2.
Viremia and neutralizing antibody responses in monkeys inoculated S.C. with 5 log10 PFU of each ChimeriVax-DEN1 virus/0.5 ml of buffera
| Group | Monkey | Virus (amino acid change) | Viremia (log10 PFU/ml) by postimmunization dayb
|
PRNT50 at day 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| 1 | R18265M | Uncloned, P4 (M39 H→R, E204 K→R) | —c | — | — | — | — | — | — | — | — | — | 640 |
| R175110F | — | — | — | 1.7 | — | — | — | — | — | — | 640 | ||
| F17572M | 1.3 | 1.0 | — | 1.0 | — | — | — | — | — | — | 320 | ||
| F171114F | — | — | — | — | — | — | — | — | — | — | 640 | ||
| GMT | 538 | ||||||||||||
| 2 | R182103M | Clone E, P6 (E204 K→R) | — | — | — | — | — | — | — | — | — | — | 5,120 |
| R17098F | — | 1.7 | — | — | — | — | — | — | — | — | 2,560 | ||
| R18261M | 1.7 | 2.5 | 1.3 | 2.0 | — | 2,560 | |||||||
| R175118F | — | — | 1.0 | — | — | — | — | — | — | — | 5,120 | ||
| GMT | 3,620 | ||||||||||||
| 3 | R182104M | Clone J, PMS, P7 (none) | 1.0 | 1.9 | 1.7 | 1.7 | 1.8 | 1.7 | 1.0 | 1.0 | 1.7 | — | 5,120 |
| R175108F | — | 1.7 | 2.8 | 2.2 | 1.0 | 2.0 | 1.7 | 2.0 | 2.2 | 1.7 | 10,240 | ||
| R182111M | 2.3 | 3.0 | 3.3 | 2.8 | 1.7 | 1.7 | — | — | — | — | 10,240 | ||
| R175104F | — | 2.4 | 1.3 | 2.0 | 2.3 | 1.7 | 1.7 | 2.2 | 3.0 | 3.1 | 10,240 | ||
| GMT | 8,611 | ||||||||||||
For group 1 versus group 2, P < 0.0045; for group 1 versus group 3, P < 0.0006; for group 2 versus group 3, P < 0.0130. These values are considered statistically significant.
Monkeys were immunized on day 1.
—, < 1.0 log10 PFU/ml.
(ii) Viremia and neutralizing antibody response.
As shown in Table 2, all four monkeys inoculated with the DEN1 PMS virus without the E204 K→R mutation (clone J, group 3) became viremic. Three of four monkeys inoculated with the clone E virus and two of four monkeys inoculated with uncloned DEN1 virus (both containing the E204 mutation) became viremic. Viremia was detected in all four animals in group 3 through day 11, whereas no animal in group 1 or 2 was viremic beyond day 5 (at a level of detection of 1 log10 PFU/ml). The mean peak virus titers were 0.75 (1.5 for viremic animals), 1.3 (1.7 for viremic animals), and 2.5 log10 PFU/ml for groups 1 to 3, respectively. The mean duration of viremia was 1 (2 for viremic animals), 1.5 (2 for viremic animals), and 8.5 days for groups 1 to 3, respectively. The magnitude and duration of viremia in group 3 (nonmutant, E204K) monkeys were significantly higher than the values observed for group 1 and 2 (mutant E204R) (Table 3) animals. The differences in peak viremia titers and duration of viremia between group 1 and 2 animals (both inoculated with mutant E204R viruses) were not statistically significant. Despite the lack of detectable viremia in some monkeys, all animals developed neutralizing antibody titers against homologous viruses (Table 2). The geometric mean neutralizing antibody titers (GMT PRNT50) were 538, 3,620, and 8,611 for groups 1, 2, and 3, respectively. Consistent with the level of viremia, the neutralizing antibody titers in monkeys immunized with the PMS virus (group 3, nonmutant, E204K) were significantly higher than the values for the other two groups (groups 1 and 2, with E204R mutations) (Table 2). The sera of group 1 monkeys (immunized with a DEN1 chimera with two amino acid substitutions in the envelope proteins [M39 H→R and E204 K→R]) had the lowest neutralizing antibody titers.
TABLE 3.
Summary of viremia shown in a
| Group | Virus (amino acid change) | No. of viremic animals/no. tested (%) | Mean value
|
|
|---|---|---|---|---|
| Peak titer (log10 PFU/ml) | Duration (days) | |||
| 1 | Uncloned (M39 H→R and E204 K→R) | 2/4 (50) | 0.75 (1.5)b | 1 (2)b |
| 2 | Clone E (E204 K→R) | 3/4 (75) | 1.3 (1.7)b | 1.5 (2)b |
| 3 | Clone J, PMS, P7 (none) | 4/4 (100) | 2.5 | 8.5 |
For group 1 versus group 2, P < 0.45 for mean titers and 0.67 for mean durations. for group 1 versus group 3, P < 0.009 for mean titers and 0.0004 for mean durations. for group 2 versus group 3, P < 0.053 for mean titers and 0.001 for mean durations. P values shown in bold are considered statistically significant.
Numbers in parentheses are for viremic animals only.
Experiment 2. (i) Safety and NV of ChimeriVax-DEN1 viruses (with or without the E204 mutation) in cynomolgus monkeys inoculated by the i.c. route.
Results from the inoculation of suckling mice with clone E indicated that the K→R substitution at the E204 residue affects the NV of the DEN1 chimera for infant mice. Subsequently, when clone E was inoculated into monkeys by the s.c. route, it induced a significantly lower magnitude and duration of viremia than treatment with a nonmutant virus (clone J PMS, P7). Interestingly, when clone J was passaged in Vero cells to produce the cGMP VL at P10, it acquired the same nucleotide change (nt 1590 A→G transition, resulting in K→R amino acid substitution) that was present in clone E. Similarly, the VL virus (P10) was less NV than the PMS (P7) virus in infant mice (10). Since attenuation of the DEN1 vaccine was dependent on a single amino acid substitution in the E protein (E204R), which theoretically could revert to the wt sequence (E204K) after vaccination, the safety profile of the nonmutant virus (E204K) was evaluated following i.c. inoculation of monkeys. Three groups of monkeys (n = 6/group) were inoculated i.c. with ChimeriVax-DEN1 PMS (P7, E204K), ChimeriVax-DEN1 VL (P10, E204R), or YF-VAX (as a reference). The animals were monitored for 30 days for clinical signs and then euthanized for pathological evaluations.
(ii) Viremia.
All six cynomolgus monkeys inoculated with the nonmutant PMS virus (E204K, group 1) became viremic. The duration of viremia was generally 4 to 5 days, with peak titers ranging from 1 to 3.3 log10 PFU/ml (Table 4). The mean duration of viremia was 4.2 days, with a mean peak viremia titer of 2.5 log10 PFU/ml (Table 5). Five of six monkeys inoculated with the mutant VL virus (E204R, group 2) became viremic. The duration of viremia was generally 1 to 4 days, with peak titers ranging from 1 to 2.1 log10 PFU/ml (Table 4). The mean duration was 2.5 days (3 days for viremic animals), with a mean peak viremia of 1.4 (1.6 for viremic animals) log10 PFU/ml (Table 5). All six monkeys inoculated with YF-VAX (group 3) became viremic. The duration of viremia was generally 2 to 4 days (with one exception, in which a viral titer of 1 log10 PFU/ml was observed at 9 days postinoculation following 4 days of undetectable titer), with peak titers ranging from 1 to 3 log10 PFU/ml (Table 4). The mean number of viremic days was 2.8 days, and the mean peak viremia was 2.2 log10 PFU/ml (Table 5). The peak duration and titer of viremia for group 1 were significantly higher than those for group 2. When group 1 (E204K) was compared with group 3 (YF-VAX), only the duration of viremia, but not the magnitude, was statistically different between the two groups. The duration and magnitude of viremia for the VL virus (E204R, group 2) were similar to those observed for YF-VAX (Table 5). For all groups, monkey viremia titers were below 500 and 100 mouse i.c. 50% lethal dose values (estimated to equal ∼20,000 and ∼4,000 PFU/0.03 ml [12], respectively, for YF-VAX), which are the maximum acceptable titers for individual monkey and group (i.e., present in no more than 10% of the monkeys) titers, respectively, as established under the WHO requirements for the YF 17D vaccine (38).
TABLE 4.
Viremia and neutralizing antibody responses in monkeys after i.c. inoculation with ChimeriVax-DEN1 PMS or ChimeriVax-DEN 1 VL virus (5 log10 PFU/0.25 ml) or with YF-VAX (4.7 log10 PFU/0.25 ml)a
| Group | Monkey | Virus (amino acid change) | Viremia (log10 PFU/ml) by postimmunization dayb
|
PRNT50 at day 31 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 11 | ||||
| 1 | F22220M | ChimeriVax-DEN1, PMS, P7 (none) | —c | 1 | 1 | 1.3 | 1.3 | 1 | — | — | — | — | 1,280 |
| F22236M | 3.3 | — | 2.2 | 2.3 | 1 | — | — | — | — | — | 5,120 | ||
| F22240M | 2.1 | 2.5 | 1.9 | 1.7 | — | — | — | — | — | — | 1,280 | ||
| F22276F | 2.2 | 1.8 | 1 | 1 | — | — | — | — | — | — | 1,280 | ||
| F22282F | 1.7 | 3.1 | 2.9 | 2.5 | — | — | — | — | — | — | 1,280 | ||
| F222106F | 1.6 | — | 2.7 | 2.8 | 2.6 | — | — | — | — | — | 1,280 | ||
| GMT50 | 1,613 | ||||||||||||
| 2 | F22203M | ChimeriVax-DEN1, VL, P10 (E204K→R) | 1 | — | 1.7 | 1.5 | 1.3 | — | — | — | — | — | 10,240 |
| F22205M | 2.1 | 1.8 | 1.3 | 1.3 | — | — | — | — | — | — | 2,560 | ||
| F22246M | 1.8 | 1.8 | 1.3 | 1 | — | — | — | — | 10,240 | ||||
| F22287F | — | — | 1.6 | — | 1 | — | — | — | — | — | 2,560 | ||
| F222112F | 1 | — | — | — | — | — | — | — | — | — | 10,240 | ||
| F222115F | — | — | — | — | — | — | — | — | — | — | 2,560 | ||
| GMT50 | 5,120 | ||||||||||||
| 3 | F22200M | YF-VAX | 1.9 | 2.3 | 2.6 | — | — | — | — | — | 1,280 | ||
| F22239M | 1 | 1.3 | 1.3 | 1.7 | — | — | — | — | 1 | — | 640 | ||
| F22256M | — | — | 1.8 | 2.2 | — | — | — | — | — | — | 2,560 | ||
| F22280F | — | 1 | 1 | — | — | — | — | — | — | 1,280 | |||
| F22291F | 1 | 1.7 | 2.8 | — | — | — | — | — | — | — | 1,280 | ||
| F22292F | 1.3 | 3 | — | — | — | — | — | — | — | — | 2,560 | ||
| GMT50 | 1,600 | ||||||||||||
For group 1 versus group 2, P < 0.016; for group 1 versus group 3, P < 0.664; for group 2 versus group 3, P < 0.021. P values in bold are considered statistically significant.
Monkeys were inoculated on day 1.
—, < 1.0 log10 PFU/ml.
TABLE 5.
Summary of viremia shown in a
| Group | Virus (amino acid change) | No. of viremic animals/no. tested (%) | Mean value
|
|
|---|---|---|---|---|
| Peak titer (log10 PFU/ml) | Duration (days) | |||
| 1 | ChimeriVax-DEN1, PMS, P7 (none) | 6/6 (100) | 2.5 | 4.2 |
| 2 | ChimeriVax-DEN1, VL, P10 (E204K→R) | 5/6 (83) | 1.4 (1.6)b | 2.5 (3)b |
| 3 | YF-VAX | 6/6 (100) | 2.2 | 2.8 |
For group 1 versus group 2, P < 0.021 for mean titers and 0.047 for mean durations; for group 1 versus group 3, P < 0.47 for mean titers and 0.025 for mean durations; for group 2 versus group 3, P < 0.081 for mean titers and 0.71 for mean durations. P values shown in bold are considered statistically significant.
Numbers in parentheses are for viremic animals only.
(iii) Immunogenicity.
All monkeys seroconverted after treatment with PMS or VL virus (Table 4). PRNT50 values ranged from 1,280 to 5,120 and from 2,560 to 10,240 in the PMS and VL virus-treated groups, respectively, and no monkey had cross-reacting antibodies to the YF 17D virus (data not shown). Antibody levels varied inversely with viremia levels for the two ChimeriVax-DEN1-treated groups (Table 4). All monkeys seroconverted after inoculation with YF-VAX. On day 31, PRNT50 values against YF virus ranged from 640 to 2,560. A day 31 serum from one of the YF-VAX-treated monkeys cross-reacted with heterologous DEN1 virus in a PRNT50 assay (data not shown). Such antibody cross-reactivity is not unexpected among flaviviruses. However, a remote exposure of this monkey to a heterologous flavivirus prior to prestudy antibody screening cannot be excluded.
Histopathology.
Vaccine-related histopathologic findings in non-central nervous system tissues were minimal to mild splenic lymphoid hyperplasia in four of six, three of six, and six of six animals treated with the ChimeriVax-DEN1 PMS (E204K, nonmutant), ChimeriVax-DEN1 VL (containing the E204R mutation), or YF-VAX virus, respectively (Table 6). Lymphoid hyperplasia was considered secondary to immunostimulation in this study.
TABLE 6.
Histopathological evaluation (lesion scores) of monkey brains and spinal cords after i.c. inoculation with ChimeriVax-DEN1 PMS or ChimeriVax-DEN 1 VL virus (5 log10 PFU/0.25 ml) or YF-VAX (4.7 log10 PFU/0.25 ml)a
| Group (virus) | Monkey | Lesion score
|
||
|---|---|---|---|---|
| Target areas | Discriminator area | Combined | ||
| 1 (ChimeriVax-DEN1, PMS, P7, no mutation) | F22220M | 0 | 0 | 0 |
| F22236M | 0 | 0 | 0 | |
| F22240M | 0 | 0 | 0 | |
| F22276F | 0 | 0 | 0 | |
| F22282F | 0.03 | 0.06 | 0.045 | |
| F222106F | 0 | 0 | 0 | |
| Mean (SD) | 0.01 (0.01) | 0.01 (0.02) | 0.01 (0.02) | |
| 2 (ChimeriVax-DEN1, VL, E204 K→R) | F22203M | 0 | 0.06 | 0.03 |
| F22205M | 0.08 | 0.31 | 0.195 | |
| F22246M | 0 | 0.06 | 0.03 | |
| F22287F | 0.17 | 0 | 0.085 | |
| F222112F | 0 | 0 | 0 | |
| F222115F | 0.20 | 0 | 0.10 | |
| Mean (SD) | 0.075 (0.091) | 0.072 (0.120) | 0.073 (0.070) | |
| 3 (YF-VAX) | F22200M | 0 | 0 | 0 |
| F22239M | 0.17 | 0.69 | 0.43 | |
| F22256M | 0.72 | 1.54 | 1.13 | |
| F22280F | 0.22 | 0.50 | 0.36 | |
| F22291F | 0.53 | 0.25 | 0.39 | |
| F22292F | 0.61 | 1.13 | 0.87 | |
| Mean (SD) | 0.38 (0.28) | 0.69 (0.57) | 0.53 (0.4) | |
For group 1 versus group 2, P values were 0.092, 0.25, and 0.055 for target area scores, discriminatory area scores, and combined scores, respectively for group 1 versus group 3, P values were 0.009, 0.016, and 0.010; for group 2 versus group 3, P values were 0.034, 0.027, and 0.021; for groups 1 and 2 versus group 3, P values were 0.0048, 0.0059, and 0.0032. P values shown in bold are considered statistically significant.
Central nervous system lesions were observed in one of six, five of six, and five of six monkeys inoculated with the ChimeriVax-DEN1 PMS, ChimeriVax-DEN1 VL, or YF-VAX virus, respectively. All of these lesions were inflammatory, with minimal and mild severity (grade 1 or 2). Scanty, mostly perivascular, infiltrates were noted in the brains and/or spinal cords of those monkeys with lesions. There was no involvement of neurons in any animal. Lesions in the ChimeriVax-DEN1-treated groups were generally minimal (grade 1), although one brain section of one monkey (F22205 M) that received the VL virus had a mild (grade 2) lesion. In the YF-VAX-treated group, grade 2 lesions were present in several sections of the brain in four monkeys. Target-area, discriminator-area, and combined lesion scores for ChimeriVax-DEN1-treated groups were much lower than those for the reference YF-VAX-treated group (Table 6). The differences in lesion scores in target and discriminator areas between the two ChimeriVax-DEN1-treated groups were not statistically significant (Table 6).
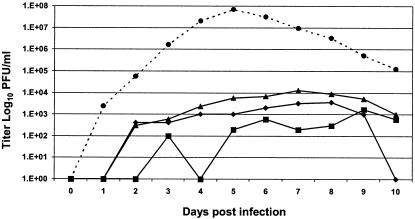
Growth kinetics of ChimeriVax-DEN1 virus (with or without the E204 mutation) in HepG2 hepatoma cells.
Both the ChimeriVax-DEN1 and parent wt DEN1 viruses grew slower and to significantly lower titers than the YF-VAX virus in HepG2 cells. Peak titers were noted on days 9, 8, 7, and 5 for the wt DEN1, nonmutant ChimeriVax-DEN1 PMS, ChimeriVax-DEN1 VL containing the E204 K→R mutation, and YF-VAX viruses, respectively. The virus concentrations at peak levels were ∼3.2, 3.6, 4.1, and 7.8 log10 PFU/ml for the wt DEN1, ChimeriVax-DEN1 PMS, ChimeriVax-DEN1 VL, and YF-VAX viruses, respectively (Fig. 1).
FIG. 1.
Growth kinetics of ChimeriVax-DEN1 PMS, ChimeriVax-DEN1 vaccine, wt DEN1 PUO359, and YF-VAX viruses in HepG2 cells. Squares, wt DEN1 (parent PUO359); diamonds, ChimeriVax-DEN1 P7; triangles, ChimeriVax-DEN1 P10; circles, YF-VAX.
Growth of ChimeriVax-DEN1 virus (with or without the E204 mutation) in mosquitoes.
The rationale for the next experiment was to ensure that the ChimeriVax-DEN1 mutant vaccine will remain safe in the human host and will not replicate in mosquitoes even if it reverts to the wt sequence in a vaccinated individual. The replication of ChimeriVax-DEN1 viruses was evaluated in mosquitoes. A. aegypti mosquitoes were inoculated by the i.t. route with nonmutant ChimeriVax-DEN1 PMS, ChimeriVax-DEN1 VL containing the E204 K→R mutation, wt DEN1 (strain PUO359), and YF 17D viruses. The growth of the viruses in the mosquitoes over 10 days was compared (Fig. 2). There were no significant differences in replication between the two chimeric viruses and YF 17D virus. The wt DEN1 virus titer was approximately 0.5 to 2.5 log higher than the titers of both the ChimeriVax-DEN1 viruses over the 10-day time period (Fig. 2).
FIG. 2.
Growth of chimeriVax-DEN1 PMS (P7), vaccine (P10), YF-VAX, and wt DEN1 (strain PUO359, donor of PrME genes for ChimeriVax-DEN1 virus) viruses in i.t. inoculated A. aegypti mosquitoes. Squares, wt DEN1 (parent PUO359); diamonds, ChimeriVax-DEN1 P7; triangles, ChimeriVax-DEN1 P10; circles, YF-VAX. Virus titers, calculated as mean (± standard deviation) log10 RNA transcripts/ml for three mosquitoes at each time point, were determined by real-time reverse transcription-PCR.
Effect of E204 mutation on structure of E protein.
The homology structure of 394 residues of the DEN1 E-protein ectodomain (strain PUO 359, representing the E protein of ChimeriVax-DEN1 [PMS, P7]) was modeled based on the known structure of the DEN2 virus (25) (Fig. 3A and B). The K residue at position 204 was changed to R, and the modeling was repeated for the mutant virus to represent the E-protein structure of the ChimeriVax-DEN1 (VL, P10) virus (Fig. 3C). Residue 204 is located within a short loop connecting the beta strands f and g of domain II (Fig. 3A) and is in proximity with the two alpha helices, alph-A and alph-B. Domain II also carries the conserved fusion peptide in its tip. This short loop is located within a hydrophobic pocket lined by residues that influence NV or the pH threshold for viral fusion (25). Figure 3B shows a close-up of the corresponding area in Fig. 3A with the amino acid 204K shown in stick representation. The side chain amino and imino groups of 204K and 261H make H bonds with the carbonyl oxygen (O) atoms of 252V (2.7 Å apart) and 253L (2.65 Å apart), respectively (Fig. 3B). In contrast, the mutation at 204 from K to R results in a local rearrangement, in which the distances of 204R and 261H to 252V and 253L increase to 5.10 and 8.11 Å, respectively. This movement results in the loss of intermolecular (i.e., between the two E monomers) H bonds between these residues. Instead, the guanidinium group of the 204R side chain in the mutant virus seems to make new intramolecular (i.e., within the same E monomer) bonds involving the imino group of 261H and the carboxylate O atoms of 257E (3.01 and ∼2.9 Å away from the guanidinium group, respectively) (Fig. 3C). The proximity of 204R and 257E suggests a salt bridge rather than H bonds, since both of them are charged at a neutral pH. Another interesting observation is that the side chain of 261H in the mutant virus is flipped compared to its position in the wt structure (compare the positions of 261H in Fig. 3B and C).
FIG. 3.
Structure of DEN1 E-protein dimer (aa 1 to 394) of ChimeriVax-DEN1 virus. (A) The position of the positively charged lysine (K) at residue 204 of the P7 (PMS, 204K) virus is shown by CPK (displays spheres sized to van der Waal [VDW] radii) representation. Three structural domains are shown in red (domain I), yellow (domain II), and blue (domain III). The structure was built based on the atomic coordinates (1OKE.pdb) of the DEN2 virus obtained from a protein data bank as deposited by Modis et al. (25), using homology modeling software (DS modeling 1.1) from Accelrys Inc. (B) Close-up of marked area in panel A. (C) The same area as in panel A from the E-protein model of the mutant DEN1 virus (P10, 204R shown in red). Selected amino acids in panels B and C are shown in stick representation. The distances between the nitrogen (N) of 204K or 204R and the N of 261H or oxygen (O) of 252V (the opposite strand) are shown in angstroms. Gray, carbon (C); blue, nitrogen (N); red, oxygen (O); yellow, sulfur (S).
DISCUSSION
Cloned vaccine viruses were produced by the transfection of Vero cells and subsequent plaque purification. This was an effective selection strategy to minimize undesired mutations because cloned viruses appeared to accumulate fewer mutations than uncloned viruses (31). Initially, two plaques (clones A and B) for each virus were subjected to direct plaque-to-plaque purification, which generally led to a mutation-free PMS stock virus at P7, with one exception; the first two plaque-purified clones of ChimeriVax-DEN1 virus contained mutations at E251 (V to F [clone B]) or E251 (V to F) and E435 (L to I) (clone A) (31). Several other clones needed to be plaque purified and sequenced to find a mutation-free PMS virus candidate. The 10th clone (clone J) was found to be mutation-free and was selected for further vaccine manufacturing. Representatives of different mutant clones were tested in a sensitive suckling mouse model that was shown to be capable of identifying a minor change in NV associated with a single amino acid substitution (29). All of the clones, except clone E, had NV phenotypes that were similar to that of the nonmutant clone (clone J) or uncloned (no mutations at Vero P2) virus. The NV of clone E was significantly reduced, as measured by survival analysis. Sequencing of clone E had revealed a single amino acid substitution (204K→R) in the E protein. Interestingly, an earlier uncloned version of this virus (ChimeriVax-DEN1, uncloned P4, 1999) contained the same amino acid substitution (in addition to another mutation [M39 H→R] in the M protein), was highly attenuated in mice, and induced a low level of viremia in monkeys (8, 9). The effect of this E-protein mutation on the viscerotropism (the induction of viremia) of the virus was assessed by s.c. inoculation of monkeys with ChimeriVax-DEN1 viruses containing (clone E, P6) or missing (clone J, P7) the E204 mutation. The uncloned ChimeriVax-DEN1 (P4, 1999) virus was selected as a reference strain, because its viremia and immunogenicity profiles had already been evaluated in monkeys, in both monovalent and tetravalent (combined with three other chimeras) vaccine preparations (8). The lowest level of viremia and the shortest duration of viremia were produced with the uncloned virus (with M39 and E204 substitutions). The highest peak viremia and the longest duration of viremia were induced by clone J (nonmutant). The levels of neutralizing antibody titers correlated with the magnitude of viremia; the nonmutant virus produced a significantly higher level of viremia and neutralizing antibody response than the two mutant viruses. All three viruses were well tolerated, and there were no test article-related changes in clinical signs, body weight, or food consumption. Upon further passages of clone J (PMS, P7) to produce master seed (P8), production seed (P9), and VL (P10) stocks, the same amino acid substitution (E240 K→R) was found in the VL virus. Sequencing revealed that this mutation first appeared at P8, was completely established at P10 (no virus could be found by consensus sequencing that did not have this mutation), was stable throughout multiple passages in Vero cells (up to P20), and resulted in an increase in plaque size (10).
The ChimeriVax-DEN1 virus containing the single amino acid substitution at E204 demonstrated a higher level of attenuation than the nonmutant virus with respect to NV and viscerotropism. This mutation arose spontaneously during passage of the nonmutant PMS (P7) virus during manufacture of the VL (P10) virus and appears to be an adaptation to replication in Vero cells. Since reversion to the wt sequence could occur during replication in the host, leading to an increase in virulence, we evaluated the safety profile of mutant and nonmutant viruses.
To assess the replication of these viruses in brain tissues, we inoculated monkeys i.c. with a mutant (VL) or nonmutant (PMS) virus and the reference YF-VAX virus. The only vaccine-related adverse effect was central nervous system inflammation that occurred less frequently and with a lower magnitude for the ChimeriVax-DEN1 PMS (E204K) and ChimeriVax-DEN1 VL (E204R) viruses than for the YF-VAX virus. The histopathological scores for the VL virus were slightly higher than those for the PMS virus, but these differences (target areas and discriminator areas) were not statistically significant and the scores for both ChimeriVax-DEN1 viruses were significantly lower than the scores for the licensed YF 17D vaccine, YF-VAX (Table 6). Since ChimeriVax-DEN variants both with and without the E204 mutation had such minimal lesion scores, it was not possible to demonstrate a lower NV in monkeys for the VL (E204R) variant, as was seen in a more sensitive suckling mouse model. As observed for monkeys inoculated by the s.c. route, viremia produced with the nonmutant virus (PMS) in i.c. inoculated monkeys was significantly higher than that observed in the VL virus (mutant) group. In all cases, viremia levels remained within the acceptable limits for individual monkey and group titers established under the WHO requirements for the YF 17D vaccine (38). All monkeys seroconverted after i.c. inoculation with either ChimeriVax-DEN1 virus. Antibody levels varied inversely with viremia levels for both DEN1 virus-treated groups. A similar inverse relationship between viremia level and neutralizing antibody response was observed when monkeys were inoculated with a tetravalent ChimeriVax-DEN1-4 vaccine formulation by the i.c. route (10). It is likely that the lower viremia reflects a robust innate immune response, which in turn enhances the adaptive (antibody) response to the virus. Previously, an inverse relationship between the level of viremia (viscerotropism) and histopathological brain scores was also observed with a mutant of a ChimeriVax-JE vaccine virus (E279 M→K) after i.c. inoculations (29). In that study, the level of viremia correlated with the level of the antibody response. It is possible that the tissue tropisms and/or antigen-presenting cells for the ChimeriVax-JE virus (an encephalitic virus) are different than those for ChimeriVax-DEN (nonencephalitic) viruses. The level of replication of the ChimeriVax-JE or ChimeriVax-WN (also an encephalitic virus) virus differed from that of the ChimeriVax-DEN1-4 vaccine after infections of blood-derived dendritic cells (S. Brandler, personal communication) or hepatoma cell lines at similar MOIs (data not shown).
The effect of the E204 mutation on the replication of ChimeriVax-DEN1 viruses was determined in HepG2 cell lines and in mosquitoes. Previous experiments with ChimeriVax-DEN1-4, wt DEN1-4, and YF 17D viruses in three human hepatic cell lines (Huh7, HepG2 [hepatocarcinoma cells], and THLE-3 [normal liver cells]) had shown that HepG2 and THLE-3, but not Huh7, cells can reveal differences in the replication efficiencies of these viruses (S. Brandler, N. Brown, T. Ermak, F. Mitchell, M. Parsons, Z. Zhang, J. Lang, T. P. Monath, and F. Guirakhoo, unpublished data). ChimeriVax-DEN1-4 replicated to significantly lower titers than YF-VAX in all HepG2 and THLE-3 cells, but not in Huh7 cells. However, when Huh7 cells were transplanted into SCID mice, attenuated and virulent DEN viruses could be differentiated (2). ChimeriVax-DEN1 strains with and without the K→R mutation at the E204 residue grew significantly less well (approximately 4 log10 PFU/ml) than YF-VAX. These data indicate that in the case of an R-to-K reversion at E204 of the ChimeriVax-DEN1 vaccine, the hepatotropism of the virus would remain significantly lower than that of YF-VAX. The high growth rates of YF-VAX in HepG2 and THLE-3 (S. Brandler et al., unpublished data) hepatic cell lines may correlate with severe and fatal hepatitis cases that have been reported for the YF 17D or YF 17DD strain in susceptible persons (27). Recent studies have described severe and fatal viscerotropic adverse events resembling wt YF virus infection associated with YF 17D vaccines (4, 23, 24, 26, 36). Sequencing evidence suggests that these adverse reactions might represent an aberrant host response to the YF 17D vaccine strain rather than a reversion of the vaccine virus to the wt (7, 24). The acquired and genetic host factors responsible for these adverse events (occurring at an estimated rate of 1:400,000) (4, 24, 26, 36) are unknown at present, although advanced age appears to play a role (23). In a healthy individual, the YF 17D vaccine induces a low level of viremia (∼2 log10 PFU/ml) with a short duration (3 to 4 days) and is quickly controlled and eliminated by the innate immune system before it can target liver parenchymal cells. Alternatively, it is possible that even if the virus reaches the liver, the infection can be blocked by Kupffer cells, which are macrophages residing in the liver, and result in aborted virus replication and the prevention of liver tissue injury. A wt DEN1 virus (strain Oster, isolated from a human case of DEN virus infection in 1989) was shown to penetrate human Kupffer cells (isolated from liver specimens) but did not produce viral progeny since it underwent apoptosis and was cleared by phagocytosis, even when infection was carried out at an extremely high MOI (100 PFU/cell) (22). The lack of ChimeriVax-DEN1-4 and wt DEN1-4 growth in hepatoma and normal hepatic cell lines (S. Brandler et al., unpublished data) demonstrated that the hepatotropism of ChimeriVax-DEN viruses is largely controlled by the prME genes, which are derived from DEN viruses. Whether the rate of rare viscerotropic adverse events in ChimeriVax-DEN-vaccinated individuals is lower than that for the YF-17D virus remains to be determined.
A further safety test included infections of A. aegypti mosquitoes, the principal vector for both YF and DEN viruses, by ChimeriVax-DEN1 viruses with or without the E204 mutation. Both chimeras and the YF 17D virus grew to similar levels, which were lower than that of the wt DEN1 virus parent. Similar to ChimeriVax-JE (1) and ChimeriVax-WN (15) viruses, the chimeric DEN1 vaccine virus replicated poorly in C6/36 mosquito-derived cells, did not infect susceptible mosquitoes (Aedes albopictus and A. Aegypti) by the oral route, and replicated to a level similar (which was lower than that of the wt DEN parent viruses) to that of YF-VAX by the i.t. route (14). These data indicate that the ChimeriVax-DEN1 virus remains safe (i.e., it does not become NV, is unlikely to become hepatotropic, and is unlikely to be transmitted by mosquitoes), even if it reverts to the wt sequence in a vaccinated individual.
The structure of the ChimeriVax-DEN1 (PMS, P7) E protein was modeled based on the atomic coordinates of 394 residues of the DEN2 E-protein ectodomain (S1 strain) determined in the presence of the detergent n-octyl-β-d-glucoside (25). The K residue at position 204 (204K) lines the interior of a ligand binding pocket. The mutations residing in the interior of this pocket are shown to affect the fusion or NV of flaviviruses (20, 29). The homology model of the E-homodimer structure of the vaccine virus (204R) was compared to that of the PMS (204K) virus. The side chains of 204K and 261H of one E monomer appeared to make H bonds with the backbone atoms of 252V and 253L of the opposite monomer. At position 204, the R in the E protein of the vaccine virus is predicted to reorient itself so that these H bonds are lost. Instead, the side chain of the mutant R is in proximity with 261H and 257E, resulting in the generation of new intramolecular H bonds between R and H and probably of a new salt bridge between R and E. Since the pK of histidine could be approximately 6.0, which is slightly below the fusion threshold (pH ∼6.4), we hypothesize that the predicted new H bonds between 204R and 261H and the salt bridge between R and E may affect the pH threshold of fusion. These changes might also have a significant impact on the dissociation of E monomers, the conformational changes in the finger-like domain II that are necessary for the transition to a trimer, and the subsequent fusion and infectivity of the virus. In a similar situation, a Vero cell-derived mutant of DEN3 virus with an E202 K-to-R substitution was shown to have a lower pH threshold for fusion than the wt parent virus (20). Because the DEN3 E protein is two amino acids shorter than those of the other three serotypes, the E202 residue in this virus is homologous to E204 in DEN1, -2, and -4 viruses. Interestingly, a mutant of the ChimeriVax-JE vaccine virus (29) with a reversion from M to K at residue 279 revealed a phenotype similar to that of the ChimeriVax-DEN1 virus; both mutant viruses induced lower viremia (viscerotropism) but slightly more brain lesions than the parent viruses in monkeys inoculated by the i.c. route, and both mutations mapped within the interior of the previously described ligand binding pocket (25).
In summary, the E204 mutation from K to R in ChimeriVax-DEN1 attenuated the virus for 4-day-old suckling mice and reduced viremia in monkeys inoculated by the s.c. or i.c. route. This mutation, however, did not affect the growth characteristics of the virus in human dendritic cells (data not shown) or hepatoma cells or in A. aegypti, a mosquito vector for both YF and DEN viruses. These studies indicate that in the hypothetical case of reversion of the mutation to the wt in a ChimeriVax-DEN1-vaccinated individual, the virus should remain highly attenuated and safe. Currently, the ChimeriVax-DEN1 mutant virus is being used as a component for a tetravalent vaccine formulation to be tested in upcoming clinical trials.
Acknowledgments
This work was supported by an Industry Challenge Grant (1 UC 1 AI49517-01) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Md., and by Aventis Pasteur, Marcy-L'Etoile, France. B. W. Johnson was supported by an American Society for Microbiology/National Center for Infectious Diseases (ASM/NCID) postdoctoral training fellowship.
We thank R. Tesh, the late R. Shope, and A. Travassos, all from the University of Texas Medical Branch, Galveston, Tex., for prescreening of monkey sera for antibodies against flaviviruses. We also thank N. Tobin for animal care and P. Papastathis for cell culture support (Acambis, Inc., Cambridge, Mass.).
REFERENCES
- 1.Bhatt, T. R., M. B. Crabtree, F. Guirakhoo, T. P. Monath, and B. R. Miller. 2000. Growth characteristics of the chimeric Japanese encephalitis virus vaccine candidate, ChimeriVax-JE (YF/JE SA14-14-2), in Culex tritaeniorhynchus, Aedes albopictus, and Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 62:480-484. [DOI] [PubMed] [Google Scholar]
- 2.Blaney, J. E., Jr., D. H. Johnson, G. G. Manipon, C. Y. Firestone, C. T. Hanson, B. R. Murphy, and S. S. Whitehead. 2002. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology 300:125-139. [DOI] [PubMed] [Google Scholar]
- 3.Burke, S., and T. P. Monath. 2001. Flaviviruses, p. 1049-1125. In D. M. Knipe et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 4.Centers for Disease Control and Prevention. 2002. Adverse events associated with 17D-derived yellow fever vaccination—United States, 2001-2002. Morb. Mortal. Wkly. Rep. 51:989-993. [PubMed] [Google Scholar]
- 5.Durbin, A. P., R. A. Karron, W. Sun, D. W. Vaughn, M. J. Reynolds, J. R. Perreault, B. Thumar, R. Men, C. J. Lai, W. R. Elkins, R. M. Chanock, B. R. Murphy, and S. S. Whitehead. 2001. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3′-untranslated region. Am. J. Trop. Med. Hyg. 65:405-413. [DOI] [PubMed] [Google Scholar]
- 6.Edleman, R., S. S. Wasserman, S. A. Bodison, R. J. Putnak, K. H. Eckels, D. Tang, N. Kanesa-Thasan, D. W. Vaughn, B. L. Innis, and W. Sun. 2003. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am. J. Trop. Med. Hyg. 69:48-60. [DOI] [PubMed] [Google Scholar]
- 7.Galler, R., K. V. Pugachev, C. L. Santos, S. W. Ocran, A. V. Jabor, S. G. Rodrigues, R. S. Marchevsky, M. S. Freire, L. F. Almeida, A. C. Cruz, A. M. Yamamura, I. M. Rocco, E. S. da Rosa, L. T. Souza, P. F. Vasconcelos, F. Guirakhoo, and T. P. Monath. 2001. Phenotypic and molecular analyses of yellow fever 17DD vaccine viruses associated with serious adverse events in Brazil. Virology 290:309-319. [DOI] [PubMed] [Google Scholar]
- 8.Guirakhoo, F., J. Arroyo, K. V. Pugachev, C. Miller, Z.-X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Soike, M. Ratterree, and T. P. Monath. 2001. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J. Virol. 75:7290-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guirakhoo, F., K. Pugachev, J. Arroyo, C. Miller, Z.-X. Zhang, R. Weltzin, K. Georgakopoulos, J. Catalan, S. Ocran, K. Draper, and T. P. Monath. 2002. Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 298:146-159. [DOI] [PubMed] [Google Scholar]
- 10.Guirakhoo, F., K. Pugachev, Z. Zhang, G. Myers, I. Levenbook, K. Draper, J. Lang, S. Ocran, F. Mitchell, M. Parsons, N. Brown, S. Brandler, C. Fournier, B. Barrere, F. Rizvi, A. Travassos, R. Nichols, D. Trent, and T. Monath. 2004. Safety and efficacy of chimeric yellow fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 78:4761-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guirakhoo, F., R. Weltzin, T. J. Chambers, Z.-X. Zhang, K. Soike, M. Ratterree, J. Arroyo, K. Georgakopoulos, J. Catalan, and T. P. Monath. 2000. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 74:5477-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guirakhoo, F., Z.-X. Zhang, T. J. Chambers, S. Delagrave, J. Arroyo, A. D. T. Barrett, and T. P. Monath. 1999. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology 257:363-372. [DOI] [PubMed] [Google Scholar]
- 13.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, B. W., T. V. Chambers, M. B. Crabtree, F. Guirakhoo, T. P. Monath, and B. R. Miller. 2004. Analysis of the replication kinetics of the ChimeriVax-DEN 1,2,3,4 tetravalent virus mixture in Aedes aegypti by real-time reverse transcriptase-polymerase chain reaction. Am. J. Trop. Med. Hyg. 70:89-97. [PubMed] [Google Scholar]
- 15.Johnson, B. W., T. V. Chambers, M. B. Crabtree, J. Arroyo, T. P. Monath, and B. R. Miller. 2003. Growth characteristics of the veterinary vaccine candidate ChimeriVax-West Nile (WN) virus in Aedes and Culex mosquitoes. Med. Vet. Entomol. 17:235-243. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, B. W., T. V. Chambers, M. B. Crabtree, T. R. Bhatt, F. Guirakhoo, T. P. Monath, and B. R. Miller. 2002. Growth characteristics of ChimeriVax-DEN2 vaccine virus in Aedes aegypti and Aedes albopictus mosquitoes. Am. J. Trop. Med. Hyg. 67:260-265. [DOI] [PubMed] [Google Scholar]
- 17.Kanesa-Thasan, N., R. Edelman, C. O. Tacket, S. S. Wasserman, D. W. Vaughn, T. S. Coster, G. J. Kim-Ahn, D. R. Dubois, J. R. Putnak, A. King, P. L. Summers, B. L. Innis, K. H. Eckels, and C. H. Hoke, Jr. 2003. Phase 1 studies of Walter Reed Army Institute of Research candidate attenuated dengue vaccines: selection of safe and immunogenic monovalent vaccines. Am. J. Trop. Med. Hyg. 69:17-23. [DOI] [PubMed] [Google Scholar]
- 18.Kanesa-Thasan, N., W. Sun, G. Kim-Ahn, S. Van Albert, J. R. Putnak, A. King, B. Raengsakulsrach, H. Christ-Schmidt, K. Gilson, J. M. Zahradnik, D. W. Vaughn, B. L. Innis, J.-F. Saluzzo, and C. H. Hoke. 2001. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine 19:179-188. [DOI] [PubMed] [Google Scholar]
- 19.Kliks, S. C., A. Nisalak, W. E. Brandt, L. Wahl, and D. S. Burke. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444-451. [DOI] [PubMed] [Google Scholar]
- 20.Lee, E., R. C. Weir, and L. Dalgarno. 1997. Changes in the dengue virus major envelope protein on passaging and their localization on the three-dimensional structure of the protein. Virology 232:281-290. [DOI] [PubMed] [Google Scholar]
- 21.Levenbook, I. S., L. J. Pelleu, and B. L. Elisberg. 1987. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and histological examination. J. Biol. Stand. 15:305-313. [DOI] [PubMed] [Google Scholar]
- 22.Marianneau, P., A. M. Steffan, C. Royer, M. T. Drouet, D. Jaeck, A. Kirn, and V. Deubel. 1999. Infection of primary cultures of human Kupffer cells by dengue virus: no viral progeny synthesis, but cytokine production is evident. J. Virol. 73:5201-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, M., L. H. Weld, T. F. Tsai, G. T. Mootrey, R. T. Chen, M. Niu, M. S. Cetron, and the GeoSentinel Yellow Fever Working Group. 2001. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg. Infect. Dis. 7:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, M., T. F. Tsai, B. Cropp, G.-J. J. Chang, D. A. Holmes, J. Tseng, W.-J. Shieh, S. R. Zaki, I. Al-Sanouri, A. F. Cutrona, G. Ray, L. H. Weld, and M. S. Cetron. 2001. Fever and multisystem organ failure associated with 17D-204 yellow fever: a report of four cases. Lancet 358:98-104. [DOI] [PubMed] [Google Scholar]
- 25.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monath, T. P., and A. D. Barrett. 2003. Pathogenesis and pathophysiology of yellow fever. Adv. Virus Res. 60:343-395. [DOI] [PubMed] [Google Scholar]
- 27.Monath, T. P. 2004. Yellow fever vaccine, p. 1095-1176. In S. Plotkin and W. Orenstein (ed.), Vaccine, 4th ed. Saunders, Philadelphia, Pa.
- 28.Monath, T. P., I. Levenbook, K. Soike, Z.-X. Zhang, M. Ratterree, K. Draper, A. D. T. Barrett, R. Nichols, R. Weltzin, J. Arroyo, and F. Guirakhoo. 2000. Chimeric yellow fever 17D- Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J. Virol. 74:1742-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monath, T. P., J. Arroyo, I. Levenbook, Z.-X. Zhang, J. Catalan, K. Draper, and F. Guirakhoo. 2002. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J. Virol. 76:1932-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institutes of Health. 1998. Guide for the care and use of laboratory animals, revised ed. Department of Health and Human Services publication no. (NIH) 85-23. National Institutes of Health, Bethesda, Md.
- 31.Pugachev, K. V., F. Guirakhoo, S. W. Ocran, F. Mitchell, M. Parsons, C. Penal, S. Girakhoo, S. O. Pougatcheva, J. Arroyo, D. W. Trent, and T. P. Monath. 2004. High fidelity of yellow fever virus RNA polymerase. J. Virol. 78:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigau-Pérez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 33.Sabchareon, A., J. Lang, P. Chanthavanich, S. Yoksan, R. Forrat, P. Attanath, C. Sirivichayakui, K. Pengsaa, C. Pojjaroen-Anat, W. Chokejindachai, A. Jagsudee, J.-F. Saluzzo, and N. Bhamarapravati. 2002. Safety and immunogenicity of tetravalent live-attenuated dengue vaccine in Thai adult volunteers: role of serotype concentration, ratio and multiple doses. Am. J. Trop. Med. Hyg. 66:264-272. [DOI] [PubMed] [Google Scholar]
- 34.Saluzzo, J. F. 2003. Empirically derived live-attenuated vaccines against dengue and Japanese encephalitis. Adv. Virus Res. 61:419-443. [DOI] [PubMed] [Google Scholar]
- 35.Sun, W., R. Edelman, N. Kanesa-Thasan, K. H. Eckels, J. R. Putnak, A. D. King, H. S. Houng, D. Tang, J. M. Scherer, C. H. Hoke, Jr., and B. L. Innis. 2003. Vaccination of human volunteers with monovalent and tetravalent live-attenuated dengue vaccine candidates. Am. J. Trop. Med. Hyg. 69:24-31. [DOI] [PubMed] [Google Scholar]
- 36.Vasconcelos, P. F. C., E. J. Luna, R. Galler, L. Silva, T. L. Coimbra, V. R. L. S. Barros, T. P. Monath, S. G. Rodrigues, C. Laval, Z. G. Cosata, M. F. G. Vilela, C. L. S. Santos, C. M. O. Papaiordanou, V. A. F. Alves, L. D. Andrade, H. K. Sato, E. S. T. Rosa, G. B. Froguas, E. Lacava, L. M. R. Almeida, A. C. R. Cruz, I. M. Rocco, R. T. M. Santos, and O. F. P. Oliva. 2001. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet 358:91-97. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 1988. Annex 9. Requirements for yellow fever vaccine. Addendum 1987. W.H.O. Tech. Rep. Ser. no. 771:208. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 38.World Health Organization. 1998. Requirements for yellow fever vaccine (requirements for biological substances no. 3.) W.H.O. Expert Committee on Biological Standardization, 46th report. W.H.O. Tech. Rep. Ser. 872. W.H.O. fact sheet no. 117, revised April 2002. World Health Organization, Geneva, Switzerland.
- 39.World Health Organization. 2002. W.H.O. fact sheet no. 117. World Health Organization, Geneva, Switzerland.