Abstract
Human immunodeficiency virus (HIV)-infected CD8 lymphocytes have been reported in vivo, but the mechanism of infection remains unclear. Experiments using the thy/hu mouse model support export of intrathymically infected CD8 precursors, while recent in vitro data suggest that mature CD8 lymphocytes upregulate CD4 upon activation (generating a CD8bright CD4dim phenotype) and are susceptible to HIV infection. To determine whether these mechanisms operate in vivo and to assess their relative importance in the generation of circulating HIV-infected CD8 lymphocytes, we quantified HIV long terminal repeat (LTR) DNA in CD8+ CD4− and CD8bright CD4dim lymphocytes isolated from HIV-infected individuals by fluorescence-activated cell sorting. HIV infection of CD8 lymphocytes was demonstrated in 17 of 19 subjects, with a significant inverse relationship between level of infection and CD4 lymphocyte count (R = −0.73; P < 0.001). The level of HIV infection of CD8bright CD4dim lymphocytes was significantly higher (median, 1,730 HIV LTR copies/106 cells; n = 9) than that of CD8+ CD4− lymphocytes (undetectable in seven of nine individuals; P < 0.01) and approached that of CD4 lymphocytes from the same individuals (median, 3,660 HIV LTR copies/106 cells). CD8bright CD4dim lymphocytes represented 0.8 to 3.3% of total CD8 lymphocytes and were most prevalent in the memory subset. Thus, HIV-infected CD8 lymphocytes commonly circulate in HIV-infected individuals and are generated through infection of activated CD8 lymphocytes rather than through export of intrathymically infected precursors. The high level of infection of CD8bright CD4dim lymphocytes could have a direct role in the decline in CD8 lymphocyte function that accompanies HIV disease progression.
Although the major cellular target of human immunodeficiency virus type 1 (HIV-1) is the CD4 lymphocyte, there is increasing in vitro (8, 17, 33, 35, 36) and in vivo (3, 8, 14, 20, 21, 29, 30) evidence that CD8 lymphocytes are also susceptible to HIV-1 infection. It is well established that in chronic HIV infection, deterioration in CD8 lymphocyte function accompanies progression to symptomatic disease (12, 19, 24). While this is generally ascribed to lack of CD4 lymphocyte help or to soluble viral factors, it is also possible that direct infection of CD8 lymphocytes by HIV may contribute to their functional decline. In addition to the potential immunosuppressive effect, infection of CD8 lymphocytes generates a reservoir of circulating HIV-infected cells, with implications for virus dissemination and antiviral escape (25).
Various mechanisms for HIV entry into CD8 lymphocytes have been proposed, including cell-to-cell transfer (5), selection of CD8-tropic HIV variants (34, 35), or entry through a conventional CD4-dependent pathway. Two opportunities for CD4-dependent HIV entry into CD8 lymphocytes have been identified. The first occurs during intrathymic CD8 lymphocyte development and the second upon activation of the mature CD8 lymphocyte. Intrathymic CD8 lymphocyte precursors express CD4 and are susceptible to HIV infection in vitro (7). In the thy/hu mouse model, maturation and export of HIV-infected lymphocyte precursors have been shown to generate circulating HIV-infected naïve lymphocytes (4). Such cells could survive in a resting state for years, acting as a reservoir of provirus unaffected by antiretroviral agents.
A number of investigators have shown that activation of mature CD8 lymphocytes in vitro leads to CD4 expression on the cell surface (8, 17, 31), and this mechanism is thought to generate the circulating CD8bright CD4dim lymphocytes observed in vivo (14). HIV infection of in vitro-activated CD8 lymphocytes has been demonstrated (8, 17, 35, 36). Infection of CD8 lymphocytes by this mechanism would target infection to cells responding to antigens, and thus, infection of a relatively small number of cells could have a disproportionately high impact on immune function.
To determine the relative importance of these different mechanisms of infection in the generation of circulating HIV-infected CD8 lymphocytes in vivo, we used PCR to quantify HIV long terminal repeat (LTR) DNA in CD8 lymphocyte subsets sorted on the basis of differentiation phenotype or CD4 expression. This method detects HIV provirus and HIV preintegration complexes, with each HIV DNA genome containing 2 LTR copies. It provides a measure of the level of HIV infection within a given cell population. Differentiation phenotype was defined in terms of CD45RA and CD27 expression, allowing cells to be divided into antigen-naïve, memory, and effector subsets. Intrathymic infection would be expected to generate HIV-infected naïve CD8 lymphocytes with no preference for CD8 lymphocytes expressing CD4. In contrast, infection during activation would be expected to generate HIV-infected, antigen-experienced CD8 lymphocytes, with higher levels of infection in CD8 lymphocytes expressing CD4.
We also used four-color flow cytometry to investigate the differentiation phenotype of CD4-expressing CD8 lymphocytes isolated from HIV-infected subjects. CD8 lymphocytes induced to express CD4 in vitro have been shown to have cytokine expression profiles in keeping with a significant role in immune function (18, 36), but little is known about the relationship between CD4 expression and progression from naïve to memory or effector status in vivo.
MATERIALS AND METHODS
Subjects.
Twenty 30- to 50-ml blood samples were drawn from 19 HIV-infected individuals attending health care services in Scotland (Table 1). Because each sample contained insufficient CD8 lymphocytes to assess HIV infection of all the cell subsets of interest, the samples were divided into two groups. CD8 lymphocytes in the first group were sorted on the basis of CD4 expression (n = 9), and those in the second were sorted on the basis of differentiation phenotype (n = 11). Subjects were selected in such a way as to ensure a range of disease stages, from asymptomatic to advanced AIDS, in each group. Three individuals were long-term nonprogressors (defined as persons who had maintained a CD4 count above 300 without antiretroviral therapy despite more than 10 years of infection), and 13 were receiving combination antiretroviral therapy at the time of sampling. The mean age of the subjects was 39 (range, 29 to 54).
TABLE 1.
Clinical profiles of HIV-infected subjectsa
| Study subjectb | CD4 count/μl | Plasma viral load/ml | Combination antiretroviral therapyc |
|---|---|---|---|
| 1 | 2 | 75,000 | No |
| 2 | 17 | 750,000 | Yes |
| 3 | 39 | 309 | Yes |
| 4 | 51 | 23,100 | No |
| 5 | 66 | 75,000 | Yes |
| 6 | 117 | 75,000 | Yes |
| 7 | 123 | 330,000 | Yes |
| 8 | 296 | 470 | Yes |
| 9 | 328 | <50 | Yes |
| 10 | 334 | <50 | No |
| 11 | 339 | 14,900 | Yes |
| 12 | 364 | 304 | No |
| 13 | 385 | 153 | Yes |
| 14 | 393 | 403 | Yes |
| 15 | 396 | 862 | No |
| 16 | 509 | <50 | Yes |
| 17 | 531 | <50 | Yes |
| 18 | 647 | 13,400 | No |
| 19A | 824 | 1,900 | Yes |
| 19B | 887 | <50 | Yes |
Ranked by CD4 count (from low to high).
Identification numbers of long-term nonprogressors are italicized. Blood samples were drawn from subject 19 on two occasions.
Yes, subject was prescribed at least three antiretroviral agents at the time when the sample was drawn.
Isolation of lymphocyte populations.
Each blood sample was taken into tubes containing EDTA. Peripheral blood mononuclear cells (PBMCs) were obtained by density centrifugation over Histopaque (Sigma Diagnostics, St. Louis, Mo.) and washed twice in phosphate-buffered saline (Invitrogen, Paisley, United Kingdom), and lymphocyte subsets were isolated. In order to achieve very high cell population purity, the populations of interest were first enriched by using immunomagnetic technology and then isolated by fluorescence-activated cell sorting (FACS). This two-stage process was performed for all subjects except subject 10, for whom cell sorting was performed directly on PBMCs.
Where CD8 lymphocytes were to be separated by differentiation phenotype, cell populations were enriched by the following steps: (i) γδ T lymphocytes were removed by negative selection using a magnetic cell sorting (MACS) γδ T-lymphocyte isolation kit (Miltenyi Biotec Ltd. [UK], Bisley, United Kingdom); (ii) from the γδ T-lymphocyte-negative population, CD8 lymphocytes were enriched by using MACS CD8 microbeads (Miltenyi Biotec); (iii) from the CD8-negative population, CD4 lymphocytes were enriched by using MACS CD4 microbeads (Miltenyi Biotec). Where CD8 lymphocytes were to be separated into populations on the basis of CD4 expression, steps ii and iii of this protocol were used.
Enriched populations were incubated for 30 min at 4°C with appropriate fluorescent dye-conjugated monoclonal antibodies (Table 2). Cells were then fixed in 0.75% paraformaldehyde (Sigma Diagnostics) at 4°C for 1 h, after which they were resuspended in phosphate-buffered saline and stored for a maximum of 4 days prior to flow sorting. High-purity CD4 lymphocytes and CD8 lymphocyte subpopulations were then isolated by using a FACSVantage (Becton Dickinson, Crawley, United Kingdom) flow sorter (Fig. 1). To ensure that the sorted CD8 lymphocytes were not contaminated by minor populations of other cell types expressing CD8 (such as CD4 lymphocytes and NK cells), the anti CD8 monoclonal antibody used was directed against the β chain of the CD8 molecule, which has been shown to define true CD8 lymphocytes (13). In sorting of the CD8bright CD4dim population, a tight lymphocyte gate and a low flow rate (approximately 2,000 events/s) were used in order to avoid cell aggregates and to minimize coincidence error.
TABLE 2.
Monoclonal antibodies used to stain cell populations for FACS and flow cytometry
| Cell population stained | Fluorescent dye-conjugated mouse anti-human mono- clonal antibodya | Clone | Company |
|---|---|---|---|
| CD8 lymphocytes to be separated by differentiation phenotype and PBMCs for assessment of differentiation phenotype of CD8 lymphocytes expressing CD4 | CD27 FITC | M-T271 | BD Biosciences |
| CD8β chain PE | 2ST8.5H7 | Coulter Immunotech | |
| CD45RA Cy-chrome | HI100 | Pharmingen | |
| CD4-APC | RPA-T4 | BD Biosciences | |
| CD8 lymphocytes to be separated by CD4 expression | CD8β chain PE | 2ST8.5H7 | Coulter Immunotech |
| CD4 Cy-chrome | RPA-T4 | BD Biosciences | |
| CD4 lymphocytes | CD3 PE | UCHT1 | BD Biosciences |
| CD4 Cy-chrome | RPA-T4 | BD Biosciences |
FITC, fluorescein isothiocyanate isomer 1; PE, phycoerythrin; APC, allophycocyanin.
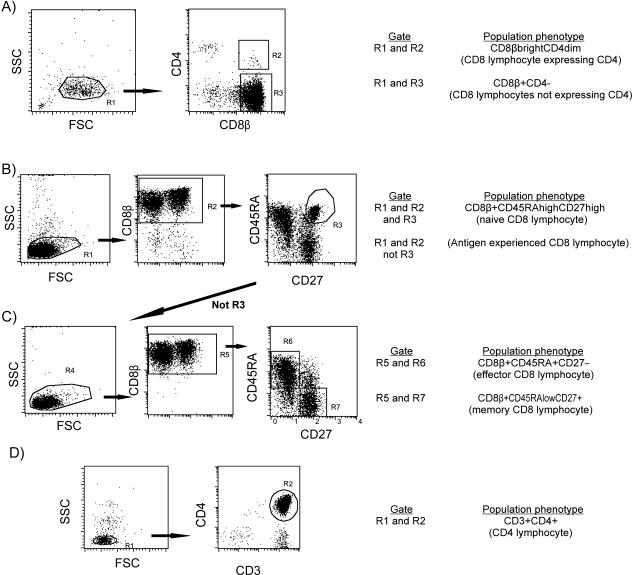
FIG. 1.
FACS of CD4 lymphocytes and CD8 lymphocyte subsets. (A) CD8 lymphocytes sorted on the basis of CD4 expression; (B and C) CD8 lymphocytes sorted on the basis of differentiation phenotype; (D) CD4 lymphocytes. Sorting on the basis of differentiation phenotype was performed in two stages: first, enriched CD8 lymphocytes were sorted into antigen-naïve and antigen experienced subsets (B); then a portion of the antigen-experienced subset was retained for HIV DNA analysis, and the remainder was further sorted into memory and effector subtypes (C). FSC, forward scatter; SSC, side scatter.
Nucleic acid extraction and HIV DNA quantification.
DNA was isolated from the cell subsets by phenol-chloroform extraction and ethanol precipitation, and the DNA concentration was measured by using a GeneQuant II spectrophotometer (Amersham Biosciences, Cambridge, United Kingdom). Real-time PCR was used to quantify HIV LTR DNA copies per microgram of DNA, and the copy number was then confirmed at limiting dilution. The real-time PCR was performed using a nested approach with a conventional primary reaction and a real-time secondary reaction. The primary reaction used primers GRAACCCACTGCTTAASSCTCAA (sense) and AAGCCGAGYCCTGCGTCGAGAG (antisense) (5′ base positions 506 and 686 of the HXB2 genome) with an annealing temperature of 55°C and ran for 18 cycles. The secondary reaction used primers CTCAATAAAGCTTGCCTTGAG (sense) and TGTTCGGGCGCCACTGCTAGAGA (antisense) (5′ base positions 524 and 626 of the HXB2 genome) (obtained from Oswell, Southampton, United Kingdom) and hybridization probes LCRed705-ACTCTGGTARCTAGAGATCCCTCAGA-phosphate and AAGTAGTGTGTGCCCGTCTGTTGT-fluorescein (Tib Molbiol, Berlin, Germany) and was performed on a Light Cycler (Roche, Mannheim, Germany) annealing at 55°C, with external standards. This method was sensitive to a single copy, as assessed against National Institute for Biological Standards and Control (NIBSC) HIV-1 DNA standards (provided by the European Union Programme EVA/MRC Centralised Facility for AIDS Reagents, NIBSC, Potters Bar, Hertfordshire, United Kingdom [grants QLK2-CT-1999-00609 and GP828102]; donated by J. Bootman), and was validated against serial dilutions of cloned full-length HIV-1.
Limiting-dilution PCR was then performed to confirm the estimated LTR copy number by using published Pan-LTR primers and thermocycling conditions (14). Ten replicate reactions were performed at the DNA concentration expected to contain 0.5 copy per reaction. Further dilutions were performed if necessary to produce replicates containing both positive and negative results. HIV DNA load and standard error were then estimated by using the QUALITY program (27) and were expressed as LTR DNA copies per 106 cells, assuming 6.6 μg of DNA per 106 cells. For samples where only a single positive replicate was generated due to limited availability of viral DNA, no standard error is provided. The correlation between the original real-time PCR estimate and the final result provided by limiting dilution was 0.79 (P < 0.001) (Spearman's rank correlation). This DNA extraction and LTR quantification protocol was found to have high reproducibility when tested on five replicate samples of paraformaldehyde-fixed PBMCs from an HIV-infected subject (mean, 60 LTR DNA copies/106 cells; range, 26 to 100).
Purity of cell subsets and calculation of attributable HIV DNA loads.
The level of CD4 lymphocyte contamination of CD8 lymphocyte subsets (defined as the percentage of cells that were CD4+ CD8β−) was assessed by flow cytometry and was found to be low (mean, 0.05% [Tables 3 and 4]). For subsets with adequate cell numbers (naïve and experienced CD8 lymphocytes and CD8β+ CD4− lymphocytes), at least 1,000 (mean, 4,880) ungated events were assessed. Within subjects, the purity of memory and effector populations was taken to be equivalent to that of antigen-experienced populations. For populations of rare cells (CD8bright CD4dim), sufficient cells were available to assess purity in four subjects. At least 500 (mean, 860) events were assessed, and due to the high ratio of background to cellular events, events falling outside the lymphocyte gate (defined on light scatter properties) were excluded. Where purity was not measured directly, the purity of CD8bright CD4dim lymphocytes was taken to be equivalent to that of CD8β+ CD4− lymphocytes. Where no purity data were available (subjects 9 and 17), a level of 0.2%, which represents the 75th percentile of the available CD4 contamination data, was used. Microscopic examination of sorted CD8bright CD4dim lymphocytes from one subject confirmed that they represented a single cell suspension free from aggregates.
TABLE 3.
Levels of CD4 lymphocyte contamination of CD8β+ CD4− and CD8βbright CD4dim lymphocytes
| Study subject | % CD4 lymphocyte contaminationa of:
|
|
|---|---|---|
| CD8β+ CD4− lymphocytes | CD8βbright CD4dim lymphocytes | |
| 1 | <5 × 10−3 | NA |
| 5 | <9 × 10−4 | NA |
| 6 | 0.1 | 0.56 |
| 7 | 0.01 | NA |
| 11 | 0.17 | 0.20 |
| 13 | 0.01 | NA |
| 14 | 0.13 | 2.44 |
| 15 | 0.3 | NA |
| 19B | <0.11 | <0.74 |
| Median | 0.1 | 0.65 |
NA, not available.
TABLE 4.
Levels of CD4 lymphocyte contamination of naïve and antigen-experienced CD8 lymphocytes
| Study subject | % CD4 lymphocyte contaminationa of:
|
|
|---|---|---|
| Naïve CD8 lymphocytes | Antigen-experienced CD8 lymphocytes | |
| 2 | 0.2 | <0.01 |
| 3 | <0.03 | NA |
| 4 | <0.03 | 0.1 |
| 8 | <0.03 | 0.03 |
| 9 | NA | NA |
| 10 | 0.41b | NA |
| 12 | <0.05 | <0.05 |
| 16 | 0.04 | 0.03 |
| 17 | NA | NA |
| 18 | 0.1 | 0.79 |
| 19A | 0.02 | 0.04 |
| Median | 0.04 | 0.03 |
NA, not available.
PBMCs from subject 10 were not enriched prior to FACS.
For each CD8 lymphocyte subset, the HIV DNA load attributable to CD4 lymphocyte contamination was calculated from the cell population purity data and the CD4 lymphocyte HIV DNA load. This figure was then subtracted from the observed HIV DNA load in the CD8 lymphocyte subset to give the HIV DNA load attributable to CD8 lymphocytes. Where CD8 lymphocytes were found to be infected, the HIV DNA load observed in the CD8 lymphocyte subset was >5 times that attributable to CD4 contamination in all but three cases. All HIV DNA loads given in Results for CD8 lymphocyte subsets are attributable to the CD8 lymphocytes.
Distribution of CD8 lymphocytes expressing CD4.
The differentiation phenotype, in terms of CD45RA and CD27 expression (Fig. 1), of CD8 lymphocytes expressing CD4 was assessed for 13 subjects by using a FACScalibur flow cytometer (Becton Dickinson). For seven subjects this assessment was performed on sorted naïve and experienced CD8 lymphocyte populations, while for the remainder appropriately stained PBMCs were used (Table 2). Populations were gated according to the differentiation phenotypes given (Fig. 1), and the percentage of CD8 lymphocytes expressing CD4 in each population was determined. A tight lymphocyte gate was used to exclude cell aggregates.
RESULTS
Extent of HIV infection of CD8 lymphocytes.
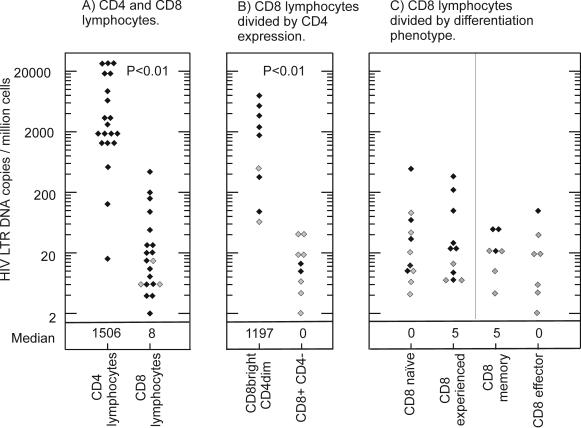
To assess the level of HIV infection of CD8 lymphocytes in subjects with a range of disease stages, HIV LTR copies were quantified in CD8 lymphocytes isolated from HIV-infected subjects. HIV infection of CD8 lymphocytes was demonstrated in 17 out of 19 subjects. The level of infection was low (median, 16 HIV LTR DNA copies per 106 CD8 lymphocytes; range, undetectable to 436 [Fig. 2A]), significantly lower than that of CD4 lymphocytes from the same subjects (median, 3,660 LTR DNA copies/106 cells; range, 16 to 25,512 [P < 0.001 by the Wilcoxon signed rank test] [Fig. 2A]). The possibility that the viral DNA found within the CD8 lymphocyte subsets represented contamination from CD4 lymphocytes was excluded by determining the purity of the CD8 lymphocyte populations and calculating attributable HIV DNA loads (see Materials and Methods). No significant correlation was found between HIV DNA loads in CD4 and CD8 lymphocytes (Spearman's correlation coefficient, 0.3; P > 0.1).
FIG. 2.
Levels of HIV infection in CD4 lymphocytes and CD8 lymphocyte subsets. (A) CD4 and CD8 lymphocytes; (B) CD8βbright CD4dim and CD8β+ CD4− lymphocytes; (C) CD8 lymphocytes divided by differentiation phenotype. Solid diamonds, attributable HIV DNA load. Shaded diamonds, samples where no virus was detected; the value given is half the lower limit of detection, which varies with the number of cells available for analysis. P values shown were calculated using the Wilcoxon signed rank test.
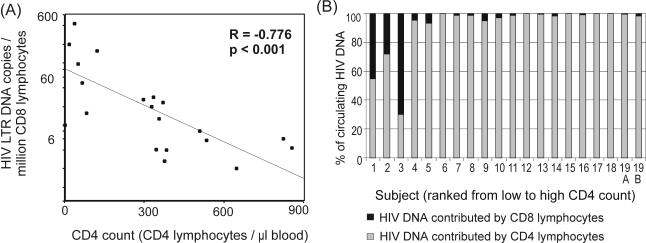
There was an inverse correlation between CD4 lymphocyte counts and CD8 lymphocyte HIV DNA loads (Spearman's correlation coefficient [R], −0.73; P < 0.001 [Fig. 3A]), indicating a progressive increase in the prevalence of infected CD8 lymphocytes with advancing disease. In individuals with advanced disease, the higher prevalence of infected CD8 lymphocytes, together with dwindling CD4 lymphocyte numbers, increased the contribution of CD8 lymphocytes to the total circulating HIV DNA load. Thus, for the individuals with the lowest CD4 lymphocyte counts, CD8 lymphocytes contributed more than 25% of the total HIV DNA load in circulating lymphocytes (Fig. 3B). In contrast, the CD8 lymphocytes of long-term nonprogressors appear relatively resistant to infection, with virtually no LTR copies detected.
FIG. 3.
Increased HIV infection of CD8 lymphocytes with disease progression. (A) Correlation between CD8 lymphocyte HIV DNA load and CD4 count (Spearman's rank correlation coefficient [R]). Cases with undetectable proviral loads were given a value of half the lower limit of detection. (B) Relative contributions of CD4 and CD8 lymphocytes to overall circulating HIV DNA loads were calculated from CD4 and CD8 lymphocyte HIV DNA loads and from CD4 and CD8 lymphocyte counts. Undetectable viral loads were given a value of zero.
Phenotype of HIV-infected CD8 lymphocytes.
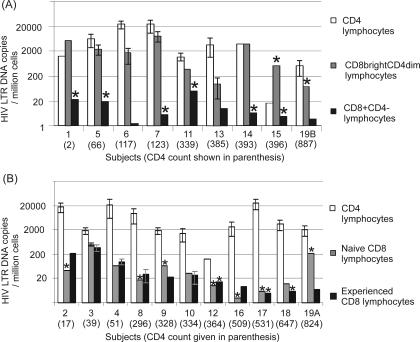
In order to determine the relative contributions of intrathymic infection versus infection of activated cells in the generation of circulating HIV-infected CD8 lymphocytes, HIV LTR DNA was quantified in subsets of CD8 lymphocytes. For the first nine subjects, CD8 lymphocyte subsets were divided on the basis of CD4 expression (Fig. 2B). CD8 lymphocytes expressing CD4 (CD8bright CD4dim) were found to be infected in seven out of nine subjects (78%) with relatively high levels of infection (median, 1,730 LTR DNA copies/106 cells; range, undetectable to 7,902), approaching those in the CD4 lymphocytes of the same nine subjects (Fig. 4A). In contrast, only two of nine subjects (22%) showed infection of CD8β+ CD4− lymphocytes, and this was at low levels (10 and 4 LTR DNA copies/106 cells). The increase in HIV DNA load in CD8bright CD4dim lymphocytes compared to that in CD8β+ CD4− lymphocytes was significant (P < 0.01 where undetectable values were assigned a value half the lower limit of detection, and P < 0.05 where undetectable values were assigned a value of zero, by the Wilcoxon signed rank test).
FIG. 4.
HIV DNA loads for each subject. HIV DNA loads for CD4 lymphocytes and CD8 lymphocyte subsets for each subject (ordered by ascending CD4 lymphocyte count) are given. (A) CD8 lymphocytes were divided on the basis of CD4 expression; (B) CD8 lymphocytes were divided by differentiation phenotype. Error bars indicate standard errors (see Materials and Methods); they are absent from samples where insufficient cells were available. Stars indicate samples where no virus was detected; the value given is half the lower limit of detection.
For the remaining 11 subjects, levels of HIV infection were compared in CD8 lymphocyte subsets divided on the basis of differentiation phenotype (Fig. 2C and 4B). Infection levels were generally quite low (range, <3 to 274 LTR DNA copies/106 cells), and although HIV DNA was more frequently detected in antigen-experienced than in antigen-naïve populations, there was no significant difference in HIV DNA loads (P = 0.7 by the Wilcoxon signed rank test). Further subdivision of the antigen-experienced cells into memory and effector subsets was performed for four of the subjects with detectable HIV DNA. Of these, three demonstrated infection in the memory subset and one demonstrated infection in the effector subset (Fig. 2C), again with no significant difference in viral DNA loads.
Prevalence and differentiation status of CD8 lymphocytes expressing CD4.
In order to investigate the relationship between CD8 lymphocyte differentiation and CD4 expression in vivo, we used four-color flow cytometry to assess CD4, CD8β, CD45RA, and CD27 expression in 13 HIV-infected subjects. Distinct CD8bright CD4dim populations were detected in all study subjects, and the density of CD4 expression on CD8bright CD4dim lymphocytes was approximately half (mean, 0.4; range, 0.3 to 0.6) that on true CD4 lymphocytes. The proportion of CD8 lymphocytes found to express CD4 was between 0.8 and 3.3% and was not found to correlate with CD4 or CD8 lymphocyte counts.
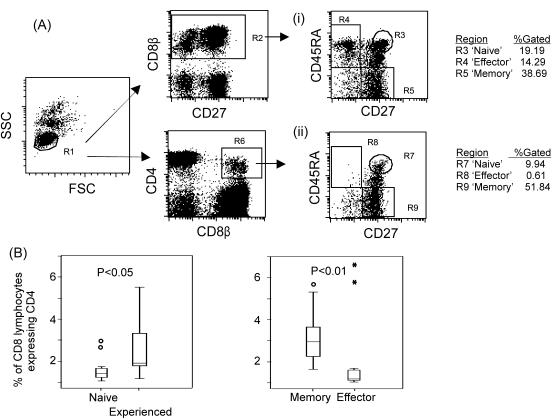
In keeping with the hypothesis of CD4 expression following activation, the vast majority of CD8bright CD4dim lymphocytes had an antigen-experienced phenotype (mean, 92%; range, 47 to 99%). Interestingly, most of the antigen-experienced CD8bright CD4dim lymphocytes displayed a memory (mean, 75%; range, 47 to 96%) as opposed to an effector (mean, 12%; range, 0 to 31%) phenotype. This preponderance of antigen-experienced and memory phenotypes is not simply a reflection of the distribution of phenotypes in the total CD8 lymphocyte population, because the proportion of CD8 lymphocytes expressing CD4 was significantly greater in the antigen-experienced population than in the antigen-naïve population (P < 0.05 by paired t test) and significantly greater in the memory subset than in the effector subset (P < 0.01 by paired t test [Fig. 5]).
FIG. 5.
Phenotype of CD8βbright CD4dim lymphocytes. (A) The differentiation phenotype of CD8βbright CD4dim lymphocytes was assessed in terms of CD45RA and CD27 expression. Dot plots from PBMCs of a representative subject (subject 10) are shown comparing the distribution of all CD8 lymphocytes (i) against that of CD8βbright CD4dim lymphocytes (ii). The lack of expression of CD4 on the CD45RA+ CD27− (effector) subset is clearly seen. (B) Percentages of CD8 lymphocytes found to express CD4 in antigen-naïve, antigen-experienced, memory, and effector subsets are shown for 13 subjects. Circles, outliers; stars, extreme outliers. P values were calculated by using the paired t test.
The differentiation phenotype of CD8bright CD4dim lymphocytes was also assessed in three healthy volunteers. All three demonstrated a differentiation pattern similar to that observed in the majority of HIV-infected subjects, with the greatest proportion of CD8 lymphocytes expressing CD4 observed in the memory subset (range, 3.8 to 6.3%) and markedly less found in the effector (range, 1.0 to 2.0%) and naïve (range, 1.1 to 2.8%) subsets.
DISCUSSION
This study confirms that CD8 lymphocytes are infected with HIV in vivo and demonstrates that CD8 lymphocytes expressing CD4 have a high frequency of infection. It is the first study to correct the level of infection of CD8 lymphocytes for directly measured CD4 lymphocyte contamination and therefore represents a methodological advance over previous studies (14, 21). The high infection levels found in CD8bright CD4dim lymphocytes support the hypothesis that infection of this subset could have a direct role in the AIDS-related decline in CD8 lymphocyte function.
Route of CD8 lymphocyte infection.
CD4-dependent HIV infection of CD8 lymphocytes has been demonstrated during intrathymic development (4, 7) and following activation of mature lymphocytes (8, 14, 17, 35). Given that CD8 lymphocyte precursors would be expected to lose CD4 before leaving the thymus, our observation of very low or undetectable viral DNA loads in CD8β+ CD4− lymphocytes implies that this route is contributing little to the circulating HIV-infected CD8 lymphocytes. In contrast, the higher viral DNA levels observed in the CD8bright CD4dim population suggests frequent infection of this subset of mature CD8 lymphocytes. This distribution of HIV DNA is in keeping with previous results from our group in which CD8+ CD4+ cells isolated by immunomagnetic methods were found to contribute a higher proportion to the overall proviral load than their CD4-negative counterparts (14). Similarly, in a recent paper, Brenchley et al. observed that there are 5- to 100-fold more HIV gag DNA copies in CD8+ CD4dull lymphocytes than in CD8 lymphocytes lacking CD4 expression (3).
The distribution of HIV DNA between CD8 lymphocytes at sequential stages of differentiation can also provide information regarding the likely route of infection. The use of phenotypic markers to define differentiation stages of CD8 lymphocytes has been a topic of much debate (11). CD27 and CD45RA have been demonstrated to define populations with distinct cytokine profiles, and a differentiation pathway from CD27+ CD45RA+ (antigen naïve) to CD27+ CD45RA− (memory) and then to CD27− CD45RA+ (effector) has been proposed (9, 10, 15). Further experiments indicated that >90% of CD27+ CD45RA+ CD8 lymphocytes were “true naïve” as defined by a panel of three further phenotypic markers (6), though early antigen-experienced cells may retain the CD27high CD45RA+ (naïve) phenotype (1). The relationship between differentiation markers and memory versus effector function remains unclear (1), and we therefore recognize that while we retain the labels “memory” and “effector” for the CD27+ CD45RA− and CD27− CD45RA+ subsets, respectively, a degree of functional overlap is likely.
Given the observed distribution of HIV DNA in CD8bright CD4dim versus CD8β+ CD4− lymphocytes, and the evidence that CD4 upregulation on CD8 lymphocytes follows antigen recognition (8, 17, 31), one would expect HIV DNA levels to be relatively high in antigen-experienced CD8 lymphocytes but very low in the antigen-naïve subset. Interestingly, although HIV DNA was more frequently detected in the antigen-experienced populations, it was also demonstrated in the naïve subset for 4 out of 11 subjects. These HIV-infected naïve CD8 lymphocytes are likely to be cells early in the activation process that have upregulated CD4 but have not yet lost their naïve markers. This interpretation is supported by our finding of CD4 expression on lymphocytes with the CD45RA+ CD27high (naïve) phenotype (Fig. 5) and suggests that, at least in some subjects, HIV infection of CD8 lymphocytes is occurring very early in the activation process. Recently published data showed negligible HIV DNA levels in naïve CD8 lymphocyte populations from which cells expressing CD4 had been removed (3), supporting our suggestion that CD4-expressing cells are responsible for the HIV observed in our naïve CD8 lymphocyte populations. Alternatively, the HIV DNA observed in our naïve cell populations may originate in contaminating nonnaïve cells which were excluded in the Brenchley et al. experiment through use of a more stringent definition of naïve cells (3).
The lack of correlation between the level of infection in CD4 and CD8 lymphocytes suggests that, although we propose that HIV enters both cells via the CD4 receptor, different factors influence the frequency of this event in the two cell types. The observation of a clear inverse correlation between CD8 lymphocyte HIV DNA load and CD4 count indicates that disease progression may be one factor that favors CD8 lymphocyte infection. Teasing out the relative importance of the many other host, viral, and therapeutic factors that are likely to influence CD8 lymphocyte infection would be of major value in future studies.
Phenotype of CD8bright CD4dim lymphocytes.
Given the finding that CD8bright CD4dim lymphocytes are a major target for HIV infection, a clear understanding of the natural history of these cells in HIV-infected subjects is a priority. In vitro, CD4 is markedly upregulated upon costimulation of naïve CD8 lymphocytes, with CD4 expression accompanying the appearance of activation markers and a change from a naïve to an antigen-experienced phenotype (17, 31). Our observation that the majority of CD8bright CD4dim lymphocytes circulating in vivo have an antigen-experienced phenotype is in keeping with this in vitro finding. CD4 expression was also observed in a small proportion of naïve CD8 lymphocytes, and, as discussed earlier, it is likely that these were recently activated, suggesting that CD4 expression is an early event in the activation process.
Within the antigen-experienced population, CD4 was expressed in a much higher proportion of CD27+ CD45RA− (memory) than terminally differentiated CD27− CD45RA+ (effector) CD8 lymphocytes. In the context of the proposed linear pathway of CD8 lymphocyte differentiation from naïve to memory and then to effector function (9, 10), these findings suggest that memory CD8 lymphocytes expressing CD4 either fail to differentiate to effector status or downregulate CD4 expression prior to further differentiation. Failure to differentiate could reflect deletion of CD4-expressing cells through HIV infection; however, the decreased frequency of CD4 expression in effector cells was also observed in three healthy volunteers, suggesting that non-HIV-related mechanisms are involved.
In 2 out of the 13 subjects assessed, an unusually high proportion of effector CD8 lymphocytes was found to express CD4 (indicated as outliers in Fig. 5B). These subjects had the highest overall proportion of CD8bright CD4dim lymphocytes (>4% of all CD8 lymphocytes), and, in contrast to the remaining 11 subjects, the proportion in the effector population was greater than that in the memory population. It is possible that the high levels of CD8bright CD4dim lymphocytes in these subjects reflected acute responses to intercurrent infection (23), and one of the subjects had clinical diagnoses of pneumonia and otitis media at the time of sampling.
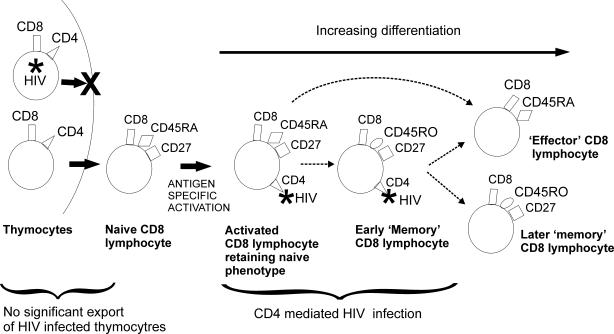
Taken together, the HIV DNA load and the CD4 expression data allow us to put forward a model for the route of HIV infection of circulating CD8 lymphocytes (Fig. 6). We propose that export of HIV-infected CD8 lymphocyte precursors from the thymus occurs rarely if at all and that the vast majority of infected CD8 lymphocytes acquire HIV through expression of CD4 during activation. CD4 expression can be induced early in the activation of naïve cells, and HIV infection can be rapid, in some cases occurring before the loss of naïve markers. The relationship between effector CD8 lymphocytes and both CD4 expression and HIV infection is interesting. Although we isolated effector cells from only four subjects, we have shown that in general, effector cells do not express CD4, and CD4-negative cells are rarely HIV infected. From this we can infer that effector cells are infrequent carriers of HIV. This suggests either that HIV infection of CD8 lymphocytes blocks progression to effector status (through, for example, cell death or interference with differentiation signals) or that CD4 expression occurs only on a subset of activated CD8 lymphocytes which have a different differentiation pathway. The picture is complicated by the two subjects with high levels of CD4 expression on effector lymphocytes and the one subject whose isolated effector CD8 lymphocytes were found to carry HIV DNA. Clearly, the dynamics of CD4 expression and HIV infection of this important subset of CD8 lymphocytes is an area where further study is a priority.
FIG. 6.
Proposed infection route of CD8 lymphocytes circulating in vivo. We propose that circulating HIV-infected CD8 lymphocytes are generated through CD4-mediated infection of activated CD8 lymphocytes and that export of HIV-infected CD8 precursors from the thymus occurs rarely if at all. Since only a minority of memory CD8 lymphocytes express CD4, we propose that expression is transient and therefore show a CD4-negative “later ” memory CD8 lymphocyte. It is also possible that CD4 is expressed on a stable minority of memory CD8 lymphocytes. Dotted arrows represent possible lineage associations between cell types.
Impact of HIV infection of CD8bright CD4dim lymphocytes.
The importance of CD8 lymphocytes in the control of HIV replication and the decline in CD8 lymphocyte function with progression to AIDS have been clearly demonstrated (16, 22, 32). The decline in CD8 lymphocyte function is commonly ascribed to lack of CD4 lymphocyte help and viral escape (reviewed in reference 22), but our group and others (8, 14, 17, 20, 21, 30, 35) have proposed that, in addition to the mechanisms referred to above, infection of CD8 lymphocytes with HIV may directly compromise CD8 lymphocyte function. The present finding of HIV infection in CD8bright CD4dim lymphocytes demonstrates targeted infection of cells responding to antigen. This process could clearly have a significant effect on the immune control of both HIV and opportunistic pathogens and may contribute to the observed correlation between immune activation and poor outcome (2).
Implications for therapeutic advances.
As the limitations of current antiviral therapies have become apparent, there has been increasing interest in therapeutic immune activation as a mechanism for achieving improved viral control. Strategies include the use of immune stimulants in conjunction with antiretroviral agents (26) and structured treatment interruptions where antiviral therapy is stopped to allow HIV to replicate and thus stimulate an anti-HIV immune response (28). In the light of our findings, it is clear that such therapies should be developed with caution, with evaluation of their effect on infection of CD8 lymphocytes. Structured treatment interruptions should be viewed as particularly hazardous, because they allow immune activation in the presence of actively replicating HIV.
In conclusion, we have shown that CD8 lymphocytes expressing CD4 contain high levels of HIV DNA in vivo, while CD8 lymphocytes lacking CD4 expression have low or undetectable levels. This finding supports the infection of activated mature CD8 lymphocytes over infection of thymic precursors as the major mechanism responsible for the generation of circulating HIV-infected CD8 lymphocytes. In the future it will be important to investigate factors influencing the expression of CD4 on CD8 lymphocytes and to formally assess the impact of infection of these cells on HIV immunopathogenesis.
Acknowledgments
This work was funded by Medical Research Council grant G84/5886.
We thank the medical and nursing staff who helped to obtain and process clinical specimens and the subjects, without whom this research would not have been possible.
REFERENCES
- 1.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., and S. L. Rowland-Jones. 2002. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 23:580-585. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley, J. M., B. J. Hill, D. R. Ambrozak, D. A. Price, F. G. Guenaga, J. P. Casazza, J. Kuruppu, J. Yazdani, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. [DOI] [PubMed] [Google Scholar]
- 5.De Maria, A., S. Colombini, S. Schnittman, and L. Moretta. 1994. CD8+ cytolytic T lymphocytes become infected in vitro in the process of killing HIV-1-infected target cells. Eur. J. Immunol. 24:531-536. [DOI] [PubMed] [Google Scholar]
- 6.De Rosa, S. C., L. A. Herzenberg, L. A. Herzenberg, and M. Roederer. 2001. Eleven-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat. Med. 7:245-248. [DOI] [PubMed] [Google Scholar]
- 7.De Rossi, A., M. L. Calabro, M. Panozzo, D. Bernardi, B. Caruso, G. Tridente, and L. Chieco-Bianchi. 1990. In vitro studies of HIV-1 infection in thymic lymphocytes: a putative role of the thymus in AIDS pathogenesis. AIDS Res. Hum. Retrovir. 6:287-298. [DOI] [PubMed] [Google Scholar]
- 8.Flamand, L., R. W. Crowley, P. Lusso, S. Colombini-Hatch, D. M. Margolis, and R. C. Gallo. 1998. Activation of CD8+ T lymphocytes through the T cell receptor turns on CD4 gene expression: implications for HIV pathogenesis. Proc. Natl. Acad. Sci. USA 95:3111-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamann, D., P. Baars, M. H. G. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. W. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamann, D., S. Kostense, K. C. Wolthers, S. A. Otto, P. A. Baars, F. Miedema, and R. A. van Lier. 1999. Evidence that human CD8+ CD45RA+ CD27− cells are induced by antigen and evolve through extensive rounds of division. Int. Immunol. 11:1027-1033. [DOI] [PubMed] [Google Scholar]
- 11.Hamann, D., M. T. Roos, and R. A. van Lier. 1999. Faces and phases of human CD8 T-cell development. Immunol. Today 20:177-180. [DOI] [PubMed] [Google Scholar]
- 12.Hoffenbach, A., P. Langlade-Demoyen, G. Dadaglio, E. Vilmer, F. Michel, C. Mayaud, B. Autran, and F. Plata. 1989. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J. Immunol. 142:452-462. [PubMed] [Google Scholar]
- 13.Hori, T., X. Paliard, R. de Waal Malefijt, M. Ranes, and H. Spits. 1991. Comparative analysis of CD8 expressed on mature CD4+ CD8+ T cell clones cultured with IL-4 and that on CD8+ T cell clones: implication for functional significance of CD8β. Int. Immunol. 3:737-741. [DOI] [PubMed] [Google Scholar]
- 14.Imlach, S., S. McBreen, T. Shirafuji, C. Leen, J. E. Bell, and P. Simmonds. 2001. Activated peripheral CD8 lymphocytes express CD4 in vivo and are targets for infection by human immunodeficiency virus type 1. J. Virol. 75:11555-11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern, F., E. Khatamzas, I. Surel, C. Frommel, P. Reinke, S. L. Waldrop, L. J. Picker, and H. D. Volk. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908-2915. [DOI] [PubMed] [Google Scholar]
- 16.Kersten, M. J., M. R. Klein, A. M. Holwerda, F. Miedema, and M. H. J. van Oers. 1997. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection: different kinetics in patients progressing to opportunistic infection or non-Hodgkin's lymphoma. J. Clin. Investig. 99:1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchen, S. G., Y. D. Korin, M. D. Roth, A. Landay, and J. A. Zack. 1998. Costimulation of naive CD8+ lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. J. Virol. 72:9054-9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitchen, S. G., S. LaForge, V. P. Patel, C. M. Kitchen, M. C. Miceli, and J. A. Zack. 2002. Activation of CD8 T cells induces expression of CD4, which functions as a chemotactic receptor. Blood 99:207-212. [DOI] [PubMed] [Google Scholar]
- 19.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. M. Keet, J. K. M. Eeftinck-Schattenkerk, A. D. M. E. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livingstone, W. J., M. Moore, D. Innes, J. E. Bell, P. Simmonds, J. Whitelaw, R. Wyld, J. R. Robertson, and R. P. Brettle. 1996. Frequent infection of peripheral blood CD8-positive T-lymphocytes with HIV-1. Lancet 348:649-654. [DOI] [PubMed] [Google Scholar]
- 21.McBreen, S., S. Imlach, G. R. Scott, C. Leen, J. E. Bell, and P. Simmonds. 2001. Preferential infection of the CD45RA+ (naive) subset of CD8+ lymphocytes by human immunodeficiency virus type 1 in vivo. J. Virol. 75:4091-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 23.Ortolani, C., E. Forti, E. Radin, R. Cibin, and A. Cossarizza. 1993. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem. Biophys. Res. Commun. 191:601-609. [DOI] [PubMed] [Google Scholar]
- 24.Pantaleo, G., A. De Maria, S. Koenig, L. Butini, B. Moss, M. Baseler, H. C. Lane, and A. S. Fauci. 1990. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc. Natl. Acad. Sci. USA 87:4818-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter, S. J., D. E. Dwyer, and N. K. Saksena. 2003. Differential cellular distribution of HIV-1 drug resistance in vivo: evidence for infection of CD8+ T cells during HAART. Virology 305:339-352. [DOI] [PubMed] [Google Scholar]
- 26.Robbins, G. K., M. M. Addo, H. Troung, A. Rathod, K. Habeeb, B. Davis, H. Heller, N. Basgoz, B. D. Walker, and E. S. Rosenberg. 2003. Augmentation of HIV-1-specific T helper cell responses in chronic HIV-1 infection by therapeutic immunization. AIDS 17:1121-1126. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigo, A. G., P. C. Goracke, K. Rowhanian, and J. I. Mullins. 1997. Quantitation of target molecules from polymerase chain reaction-based limiting dilution assays. AIDS Res. Hum. Retrovir. 13:737-742. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 29.Semenzato, G., C. Agostini, L. Chieco-Bianchi, and A. De Rossi. 1998. HIV load in highly purified CD8+ T cells retrieved from pulmonary and blood compartments. J. Leukoc. Biol. 64:298-301. [DOI] [PubMed] [Google Scholar]
- 30.Semenzato, G., C. Agostini, L. Ometto, R. Zambello, L. Trentin, L. Chieco-Bianchi, and A. De Rossi. 1995. CD8+ T lymphocytes in the lung of acquired immunodeficiency syndrome patients harbor human immunodeficiency virus type 1. Blood 85:2308-2314. [PubMed] [Google Scholar]
- 31.Sullivan, Y. B., A. L. Landay, J. A. Zack, S. G. Kitchen, and L. Al Harthi. 2001. Upregulation of CD4 on CD8+ T cells: CD4dim CD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology 103:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Baarle, D., S. Kostense, E. Hovenkamp, O. Ogg, N. Nanlohy, M. Callan, N. Dukers, A. McMichael, M. van Oers, and F. Miedema. 2002. Lack of Epstein-Barr virus (EBV)- and HIV-specific CD27− CD8+ T cells is associated with progression to viral disease in HIV infection. AIDS 16:2001-2011. [DOI] [PubMed] [Google Scholar]
- 33.Yang, L. P., J. L. Riley, R. G. Carroll, C. H. June, J. Hoxie, B. K. Patterson, Y. Ohshima, R. J. Hodes, and G. Delespesse. 1998. Productive infection of neonatal CD8+ T lymphocytes by HIV-1. J. Exp Med. 187:1139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerhouni, B., J. A. Nelson, and K. Saha. 2004. Isolation of CD4-independent primary human immunodeficiency virus type 1 isolates that are syncytium inducing and acutely cytopathic for CD8+ lymphocytes. J. Virol. 78:1243-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, J., A. Gupta, R. Dave, M. Yimen, B. Zerhouni, and K. Saha. 2001. Isolation of primary HIV-1 that target CD8+ T lymphocytes using CD8 as a receptor. Nat. Med. 7:65-72. [DOI] [PubMed] [Google Scholar]
- 36.Zloza, A., Y. B. Sullivan, E. Connick, A. L. Landay, and L. Al Harthi. 2003. CD8+ T cells that express CD4 on their surface (CD4dim CD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood 102:2156-2164. [DOI] [PubMed] [Google Scholar]