Abstract
Interleukin-7 (IL-7) regulates T-cell homeostasis, and its availability is augmented in lymphopenic hosts. Naive CD8+ T cells transferred to lymphopenic mice acquire a memory-like phenotype, raising the possibility that IL-7 is the biological mediator of this effect. Here, we provide direct evidence that IL-7 induces the acquisition of memory-cell markers not only in CD8+ T cells but also in CD4+ T-cell subsets in immune-competent Indian rhesus macaques. The increase of these memory-like populations was dependent on the dose of the cytokine, and these cells were found in the blood as well as secondary lymphoid organs. Memory-like CD4+ and CD8+ T cells acquired the ability to secrete tumor necrosis factor alpha and, to a lesser extent, gamma interferon following stimulation with a cognate antigen. The phenotypic change observed in naive T cells was promptly reversed after discontinuation of IL-7. Importantly, IL-7 induced cycling of both CD4+ and CD8+ central memory and effector memory T cells, demonstrating its contribution to the maintenance of the entire T-cell pool. Thus, IL-7 may be of benefit in the treatment of iatrogenic or virus-induced T-cell depletion.
Interleukin-7 (IL-7) is a nonredundant cytokine produced by nonlymphoid cells that is essential for T-cell development in humans and mice and B-cell development in mice (13). IL-7 contributes to the maintenance of the size and subset composition of the peripheral T-cell pool by providing growth and survival signals through the IL-7 receptor (16, 22, 25). The IL-7 receptor has a private α-chain and shares the common γ-chain (γc) with other common cytokine receptor users, such as IL-2, IL-4, IL-15, and IL-21 (23). IL-7 together with these cytokines regulates the expansion and contraction of adaptive immune responses (23) and potentially the memory response to a given antigen. IL-7 modulates memory CD8+ T cells in response to a virus infection and, given in conjunction with adoptive transfer of T cells after herpes simplex virus type 1 infection, improves virus clearance (23, 30).
Overexpression of IL-7 maintains memory CD8+ T cells in an IL-15-independent fashion (15), albeit both IL-15 and IL-7 provide survival and proliferative signals to memory CD8+ T cells (26). Recent evidence has demonstrated the importance of IL-7 together with T-cell receptor (TCR) signals in the regulation of the homeostasis of memory CD4+ T cells as well (24).
Our investigators have previously demonstrated that IL-7 administration to immune-competent or modestly CD4+ T-cell-depleted macaques results in an expansion of naive CD4+ and CD8+ subsets and increases lymphocyte numbers in a dose-dependent manner, as also observed in murine models (6, 10). In macaques, a portion of the naive CD8+ T cells upregulated CD11a, thus acquiring a partial memory-like phenotype following IL-7 treatment (6). However, CD11a does not allow for the precise delineation of CD4+ and CD8+ naive T-cell (TN), central memory T-cell (TCM), and effector memory T-cell (TEM) populations in macaques, as demonstrated recently by the elegant study by Pitcher et al. (21), in which TN, TCM, and TEM in neonate and adult macaques were delineated by the use of antibodies that cross-reacted with the macaque CD28 and CD95 molecules. In this species, TN, TCM, and TEM are clearly distinguished within the CD4+ and CD8+ subsets by using these antibodies.
We therefore designed a study to directly quantitate the effects of different doses of IL-7 on the proliferation of CD4+ and CD8+ TN, TCM, and TEM by using these antibody combinations, and we found that IL-7 drives the proliferation of both naive CD4+ and CD8+ T cells, which acquire a memory-like phenotype. We also demonstrate that these memory-like T cells produce tumor necrosis factor alpha (TNF-α) and, to a lesser extent, gamma interferon (IFN-γ).
All together, these data demonstrate that IL-7 contributes not only to the maintenance of naive and memory CD8+ T cells but also, importantly, to that of naive and memory CD4+ T cells.
MATERIALS AND METHODS
Experimental design.
Juvenile rhesus macaques were injected subcutaneously with simian IL-7 as illustrated in Fig. 1. Two additional animals were inoculated by the same route with human IL-7 as previously described (6), and cryopreserved cells from peripheral blood were used. Animals were housed in accordance with the Guide for the Care and Use of Laboratory Animals (18a) and the U.S. Department of Agriculture through the Animal Welfare Act (Public Law 91-579). Simian IL-7 was expressed as an insoluble inclusion body. Following cell disruption, the inclusion bodies were washed extensively and solubilized in 6 M guanidine hydrochloride. Denatured, monomeric IL-7 was purified and refolded to an active form. The refolded IL-7 was extensively purified by a series of chromatographic purification steps. The final formulation was greater than 97% pure by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and reverse-phase high-performance liquid chromatography and contained less than 0.05 endotoxin units of residual endotoxin/mg. Biological activity was confirmed as previously described (6). All of the IL-7 used in this study was from the same bulk production lot. Prior to injection, IL-7 was stored in aliquots at minus 20°C. Blood samples were obtained from anesthetized monkeys prior to treatment as well as for several days during treatment. Axillary lymph nodes were obtained when indicated.
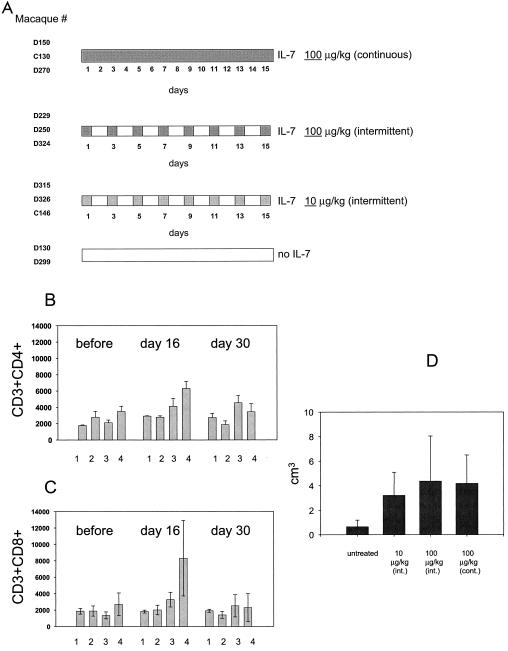
FIG. 1.
IL-7 therapy increases CD4+ and CD8+ T-cell numbers in peripheral blood and enlarges lymph node size. (A) Diagram of the study design. The total number of macaques (nine) treated with simian IL-7 in each group is indicated on the left. Two additional macaques treated with human IL-7 are not indicated in the figure (6). The regimens of IL-7 administration are summarized on the right, and the numbers represent days of treatment. (B and C) Reversible increases in the total number of CD3+ CD4+ and CD3+ CD8+ cells in IL-7-treated or untreated animals. Bars represent the mean lymphocyte (CD3+) number in groups of macaques receiving no IL-7 (lane 1), 10 μg of IL-7/kg intermittently (lane 2), 100 μg of IL-7/kg intermittently (lane 3), and 100 μg of IL-7/kg continuously (lane 4). The measurements were taken before IL-7 treatment and at days 16 and 30 of the experiment. (D) Axillary lymph node biopsies were collected 2 days after discontinuation of IL-7 administration, and lymph node size was measured and the volume was calculated using the formula R × π. Bars represent the mean values in each group.
Cell preparation and superantigen stimulation.
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood by density gradient sedimentation. Axillary lymph node cells were obtained by gentle mincing of tissues in complete medium (RPMI 1640 with l-glutamine; Invitrogen Corporation, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen Corporation) and 1% penicillin-streptomycin (Invitrogen Corporation). Staphylococcal enterotoxin B (SEB) stimulation for intracellular cytokine staining was performed as follows: 106 PBMCs or lymph node cells were placed in polypropylene tissue culture tubes (BD Biosciences, Franklin Lakes, N.J.) in 1 ml of complete medium with superantigen SEB (200 ng/ml; Toxin Technology, Sarasota, Fla.) or no antigen as a negative control with costimulatory monoclonal antibody CD49d (0.5 μg/ml; BD Immunocytometry Systems, San Jose, Calif.). The cultures were incubated at a 5° slant at 37°C in a humidified 5% CO2 atmosphere for 6 h, with the final 5 h including 10 μg of brefeldin A (Sigma-Aldrich, St. Louis, Mo.)/ml. After incubation, cells were washed in Dulbecco's phosphate-buffered saline (Life Technologies, Rockville, Md.) with 2% FBS, harvested, and kept at 4°C until processed for staining the next day.
Immunofluorescent staining and flow cytometric analysis: CD4+ and CD8+ TN and TCM.
Fresh or frozen PBMCs and/or lymph node cells were thawed and washed in RPMI 1640 with FBS. A total of 106 cells were washed and surface labeled with the following antibodies: CD4 PerCP (clone L200; BD Biosciences Pharmingen, San Diego, Calif.), CD8β PE or CD8β APC (clone 2ST8.5h7; Beckman Coulter, Miami, Fla.), CD28 APC or CD28 CyChrome (clone CD28.2; BD Biosciences Pharmingen), and CD95 PE or CD95 CyChrome (clone DX2; BD Biosciences Pharmingen). Surface-labeled cells were resuspended for 15 min in fixation-permeabilization solution (BD Biosciences Pharmingen), washed, and labeled with anti-Ki-67 fluorescein isothiocyanate (FITC) conjugate (clone B56; BD Biosciences Pharmingen) or isotype antibodies for 25 min at room temperature.
Intracellular cytokine staining.
Stimulated cells were first stained on the cell surface with the antibodies indicated above, fixed, and permeabilized as described above, and then they were stained with anti-TNF-α-FITC (clone 11; BD Biosciences Pharmingen) and anti-IFN-γ-FITC (clone B27; BD Biosciences Pharmingen). After staining, cells were resuspended in 1% paraformaldehyde in Dulbecco's phosphate-buffered saline and stored in the dark at 4°C. Flow cytometric analysis was performed on a dual-laser FACSCalibur apparatus (Becton Dickinson) and with CELLQuest software and Paint-a-Gate Plus software (BD Biosciences Pharmingen).
RESULTS
IL-7 expands T cells in blood and secondary lymphoid organs.
To determine the effects of IL-7 on different T-cell subsets, 11 Indian rhesus macaques were enrolled in this study and, of those, 9 were inoculated subcutaneously with simian IL-7. Two groups of three animals received 100 μg of the cytokine/kg of body weight for either 15 consecutive days or every other day, and another group of three animals received 10 μg/kg every other day. The control group consisted of two macaques that were not treated with IL-7 (Fig. 1A).
Total lymphocyte and CD4+ and CD8+ T-cell counts in blood were measured at the initiation of the study and at days 16 and 30 of treatment. The size of the axillary lymph node was measured 2 days after the discontinuation of IL-7 administration (day 17). An increase of the total CD4+ and CD8+ lymphocyte subsets by approximately twofold became evident by day 16 in macaques treated daily with IL-7 at 100 μg/kg (Fig. 1B and C, lanes 3 and 4), but this difference did not reach statistical significance. Similarly, a trend of enlargement was observed in lymph node size in animals treated with either 100-μg/kg dose regimen, but again, this increase only approached statistical significance (Fig. 1D). The IL-7 effect was reversed completely following discontinuation of the cytokine (Fig. 1B and C). Thus, IL-7, particularly at the highest daily dose, increased the number of T cells in the periphery as well as in the secondary lymphoid organs.
Effects of IL-7 on naive and memory CD4+ and CD8+ T cells.
Naive and memory T cells have been delineated in Indian rhesus macaques by using the CD28 and CD95 surface markers (3, 21). Naive CD4+ and CD8+ T cells are defined by CD95low and CD28mod/high expression and are found mostly in blood and lymph nodes but not at effector sites (lung and gut) and constitute the majority of fetal immune cells. Most TN are resting and produce low levels of cytokines, and their numbers decrease with age. In contrast, CD95high and CD28high expression on CD4+ and CD8+ T cells defines TCM that are functional and produce a cytokine. CD28low or CD28negative and CD95high T-cell subsets have been characterized as TEM (21). The combination of these antibodies conjugated with various fluorochromes was therefore used to delineate TN, TCM, and TEM populations before (day 0), during (day 16), and after (day 30) IL-7 treatment.
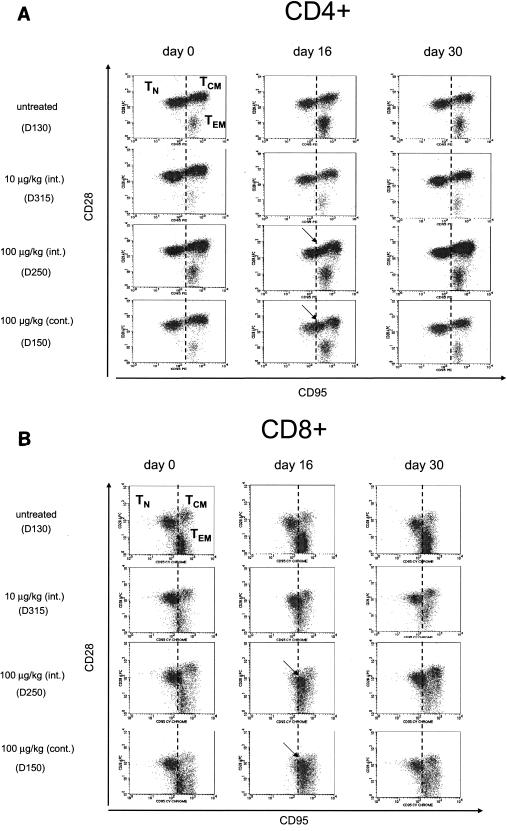
The three TN, TCM, and TEM populations were clearly distinguished by the use of these antibody combinations (Fig. 2, top left panels). Interestingly, by day 16 in both groups of macaques treated with 100 μg of IL-7/kg, upregulation of both CD28 and CD95 was observed in the CD4+ TN population, which resulted in a shift toward the TCM population (Fig. 2A). In CD8+ T cells, by day 16 CD95 was upregulated, whereas CD28 was downregulated and TN coalesced with both TEM and TCM (Fig. 2B). This effect of IL-7 was completely reversed after discontinuation of the cytokine, as demonstrated by the data obtained at day 30 (Fig. 2, right panels). The acquisition by TN of the memory-like phenotype occurred in all animals from the groups treated with 100 μg of IL-7/kg, whereas in macaques treated with a lower dose of IL-7 (10 μg/kg) the shift of TN was negligible in CD4+ TN and barely detectable in the CD8+ subsets (Fig. 2). As expected, no changes were observed in the untreated macaques (Fig. 2).
FIG. 2.
Shift of TN to memory-like CD4+ and CD8+ T cells following treatment with IL-7. CD4+ and CD8+ TN, TCM, and TEM were delineated with the use of CD28 and CD95 as described elsewhere (21). (A) Upregulation of both CD95 and CD28 in CD4+ TN that shifts toward CD4+ TCM (arrows). (B) Upregulation of CD95 and downregulation of CD28 in CD8+ TN and coalescence of the three cell populations, indicated by the arrows.
Kinetics of in vivo Ki-67 induction following IL-7 treatment.
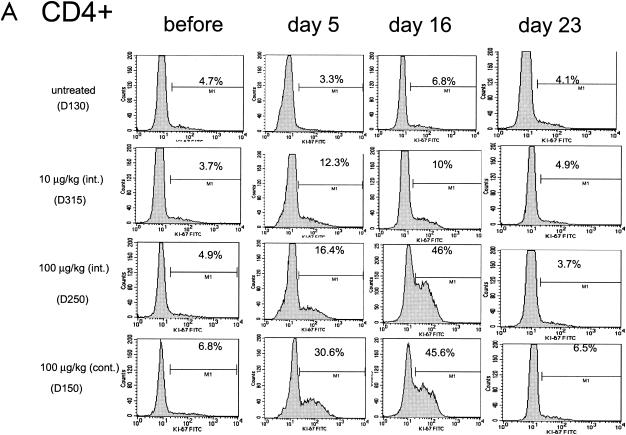
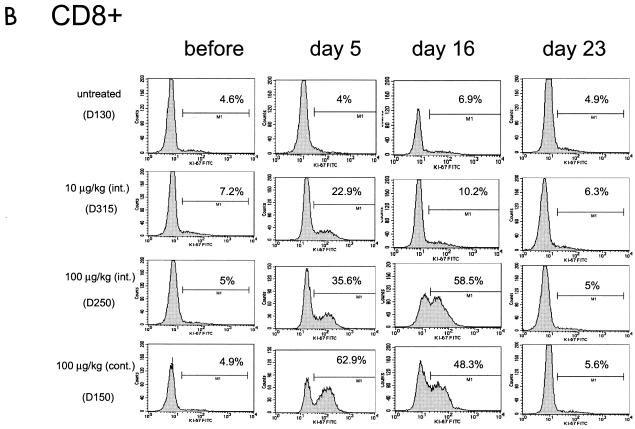
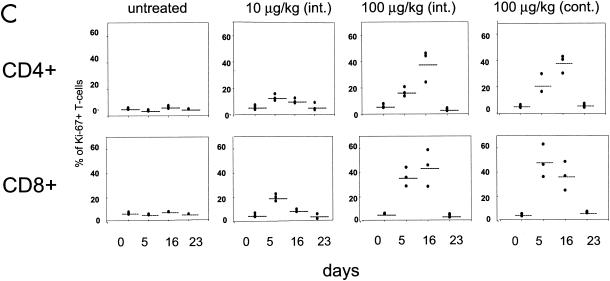
The ability of IL-7 to induce cycling of the CD4+ and CD8+ subsets was investigated by monitoring the expression of the Ki-67 nuclear antigen, a marker of T-cell proliferation, in total lymphocytes as well as in TN, TCM, and TEM. In the blood of untreated macaques, the percentage of Ki-67-positive CD4+ or CD8+ T cells was found to be consistently below 8% (Fig. 3, left panels). IL-7 treatment dramatically increased the number of both Ki-67-positive CD4+ and CD8+ T cells in animals treated with 100 μg of the cytokine/kg and, to a lesser extent, in those treated with 10 μg/kg, as demonstrated by the histogram for one representative animal in each group (Fig. 3A and B) and as an average in all animals (Fig. 3C). The percentage of cycling T cells was dose dependent, and approximately half of both CD4+ and CD8+ T cells became Ki-67 positive by day 16 following continuous treatment with 100 μg of the cytokine/kg. Interestingly, in macaques treated with 100 μg of IL-7/kg, the kinetics of Ki-67 appearance in the blood within CD4+ and CD8+ T cells differed. The highest level of Ki-67-positive CD8+ T cells was already reached by day 5 of cytokine treatment in most animals. In contrast, the number of Ki-67-positive CD4+ cells in these macaques was highest by day 16 of treatment. This cell cycle progression marker returned to pretreatment levels after the discontinuation of the cytokine in both the CD4+ and CD8+ T-cell subsets (see day 23 in Fig. 3).
FIG. 3.
Ki-67-positive cell cycle progression within CD4+ and CD8+ T cells. A transient increase in the percentage of Ki-67-positive cells within CD4+ (A) and CD8+ (B) T cells was seen in animals treated with simian IL-7. Data from one animal in each experimental group are presented in panels A and B, and the average value is summarized for all animals in panel C.
IL-7 induces cycling of TN and TCM CD4+ T cells.
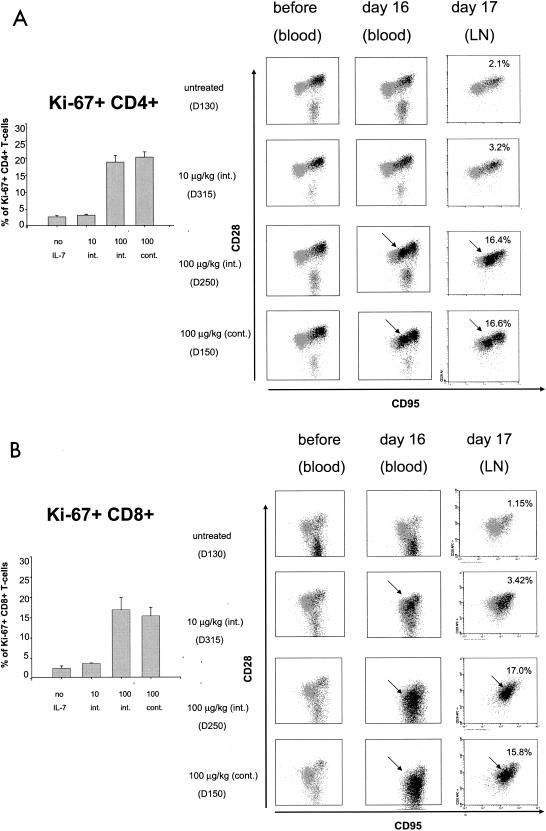
To assess the extent of cycling CD4+ T cells within TN and TCM, staining by the antibody to Ki-67 was overlapped by backgating on TN, TCM, and TEM. Before IL-7 treatment, cycling T cells were found mainly in TCM and TEM within the CD4+ T-cell population (Fig. 4A). However, following IL-7 treatment (day 16), a dose-dependent increase in the percentage of Ki-67-positive cycling T cells within the CD4+ T-cell subset (Fig. 4A) was observed and coincided with the memory-like cells defined as CD28high and CD95high (Fig. 2A and 4A, left panels). This memory-like population was almost negligible at 10 μg/kg and was highest with the 100-μg/kg continuous regimen of cytokine administration. In addition, following IL-7 treatment, 30 to 40% of CD4+ TEM were found to be Ki-67 positive (Fig. 4A and data not shown). To ascertain that this increase in Ki-67-positive cells was not due to trafficking rather than a total increase in the number of Ki-67-positive cells, we used the same approach on lymphocytes obtained from lymph node biopsies obtained 2 days (day 17) after IL-7 treatment suspension.
FIG. 4.
Cycling CD4+ and CD8+ T cells within TN, TCM, and TEM in blood and secondary lymphoid organs. (A) Backgating of Ki-67-positive cells (darker dots) on CD28+ and CD95+ T cells. Arrows indicate memory-like cells within the CD4+ T-cell compartment. Axillary lymph nodes were collected 2 days after discontinuation of IL-7 treatment from all macaques in the study, and the histograms (on the left) represent the mean percentages of Ki-67-positive CD4+ and CD8+ T cells in lymph nodes of animals in each group. (B) Percentages of Ki-67-positive CD8+ T cells are shown in the left panel for lymph nodes for all animals. Right panels show blood or lymph node samples of animals treated with each dose of IL-7.
Quantitation of the total number of cycling CD4+ T cells in the lymph nodes of all animals indicated that IL-7 induced an increase of severalfold in the number of Ki-67-positive T cells as observed in the blood (Fig. 4A, left panel). As expected in this locale, both TN and TCM were easily detected, whereas TEM were absent (Fig. 4A) (21). The increase in the number of Ki-67-positive CD4+ T cells in lymph nodes was dose dependent and mirrored that found in the blood. As in the blood, the shift of TN to the memory-like phenotype was dose dependent in CD4+ T cells. Thus, the changes induced by IL-7 in the peripheral blood extended to the secondary lymphoid tissues and, similar to blood, most of the naive CD4+ T cells were cycling at the highest dose of the cytokine.
Perturbation of TN, TCM, and TEM phenotypes in CD8+ T cells by IL-7 treatment.
As observed in Fig. 2A, IL-7 treatment had a dramatic dose-dependent effect on CD8+ T cells and, at the highest dose of IL-7, approximately half of the CD8+ T cells became Ki-67 positive within the first 5 days of treatment (Fig. 3C). The analysis of CD8+ T-cell subsets demonstrated that, at the higher dose of the cytokine, the TN, TCM, and TEM populations coalesced in blood and were not distinguishable (Fig. 4B). Similarly, in lymph nodes it was not possible to discern TN and TCM using the CD28 and CD95 antibodies (Fig. 4B, right panels). Thus, the effect of IL-7 appeared to be greater on CD8+ T cells and was so dramatic that the overlap in TN, TCM, and TEM did not allow for an accurate quantitation of the three subsets.
Cycling memory-like CD4+ and CD8+ TN, TCM, and TEM produce TNF-α.
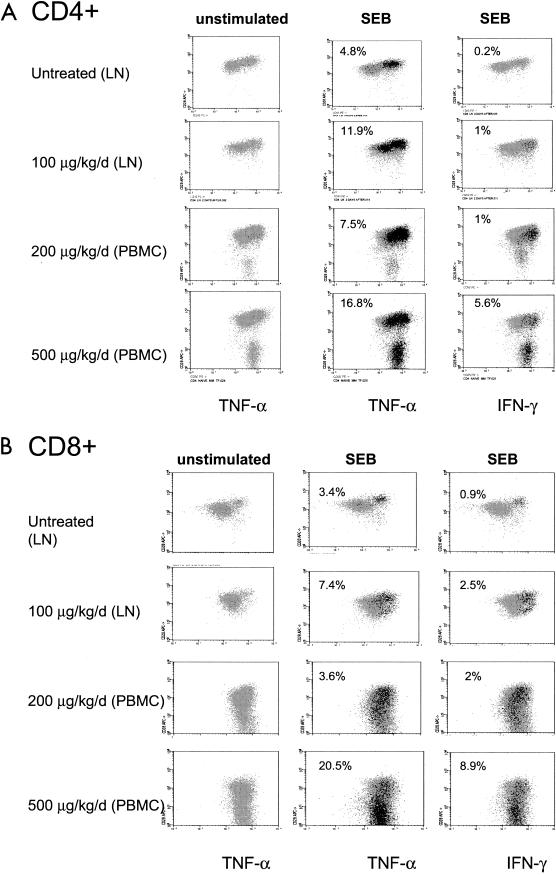
Since naive T cells in macaques do not produce TNF-α or IFN-γ (21), we investigated whether the memory-like cells induced by IL-7 acquired the ability to produce these cytokines. To that end, lymph node cells from IL-7-treated and control macaques were stimulated with SEB, and the abilities of T-cell subsets to produce either TNF-α or IFN-γ were measured independently. In parallel, cryopreserved PBMCs from macaques treated with continuous regimens of higher doses of IL-7 (200 and 500 μg/kg) were also tested in order to assess the ability of TEM, which are present in blood but not in lymph nodes, to produce cytokines (6).
Unstimulated cells did not produce TNF-α or IFN-γ, even in macaques treated with the high dose of the cytokine (Fig. 5). IL-7 treatment, however, dramatically increased the responsiveness of CD4+ T cells to SEB and, at the higher dose of the cytokine, up to 16% of CD4+ T cells produced TNF-α (Fig. 5A). IL-7 also increased IFN-γ production in TCM and, at the highest dose, in TEM (Fig. 5A). Interestingly, however, the memory-like CD4+ population induced by IL-7 appeared to produce almost exclusively TNF-α. In CD8+ T cells, both TNF-α and IFN-γ were produced following SEB stimulation and, as in the case of CD4+ T cells, TEM became strongly positive at the highest dose of IL-7 treatment (Fig. 5B).
FIG. 5.
IL-7 treatment increased the ability of CD4+ and CD8+ T cells to secrete TNF-α and IFN-γ. Cells from lymph nodes (LN) or blood (PBMCs) were stimulated ex vivo with SEB and analyzed for TNF-α and IFN-γ secretion. CD4+ (A) and CD8+ (B) T cells producing TNF-α or IFN-γ are shown.
Thus, in both lymph nodes and PBMCs, IL-7 induced a dose-dependent increase in the ability of CD4+ memory-like TCM and TEM to produce TNF-α. In the CD8+ T-cell subset, the memory-like population was less distinguishable, and TCM and TEM produced both cytokines.
DISCUSSION
Here we have demonstrated that IL-7 treatment induces a sizeable portion of CD28mod/high CD95low CD4+ TN to become CD28high CD95high and Ki-67 positive, phenotypically and functionally resembling CD4+ TCM. In addition, IL-7 at the highest dose also increased the level of CD95 positivity of TCM, which may promote apoptosis of these cells despite the ability of IL-7 to increase Bcl2. Importantly, IL-7 increased the ability of CD4+ TCM as well as CD4+ TEM to produce TNF-α and, to a lesser extent, IFN-γ.
In the case of CD8+ T cells, the effect of IL-7 was more dramatic and TN and TCM coalesced with TEM to form an indistinct Ki-67-positive cell population. Both TNF-α and IFN-γ were produced in Ki-67-positive CD8+ T-cell subsets. The effect of IL-7 was completely reversible and, by 1 week after IL-7 cessation, the total number of Ki-67-positive CD4+ and CD8+ T cells reverted to pretreatment levels. Whether these memory-like cells within the CD4+ and CD8+ T-cell populations regain the naive phenotype in the absence of exogenous IL-7, undergo apoptosis, or remain in the memory pool following cytokine withdrawal will require further investigation (12). In addition, the analysis of the Ki-67-positive populations as performed here has limitations, since more phenotypic and functional markers will be necessary to assess the differentiation status of the Ki-67-positive cells induced by IL-7 treatment.
Previously, our group had demonstrated that IL-7 treatment of cynomolgus monkeys resulted in an upregulation of CD11a on naive CD8+ T cells of a memory-like phenotype (6) and here, with the use of markers that more precisely delineate naive and memory subsets in macaques, we validated and extended our previous findings to CD8+ T cells and, importantly, we demonstrated that naive CD4+ T cells acquire a memory-like phenotype. These effects of IL-7 differ from in vitro observations, whereby IL-7 alone was able to induce proliferation of memory and, to a lesser extent, naive CD4+ and memory CD8+ T cells (7-9). Interestingly, however, the combination of IL-7, IL-15, and dendritic cell-derived cytokines in the culture had a strong additive effect and resulted in proliferation of naive and memory cells, suggesting that the combination of these factors is necessary and better mimics the in vivo situation.
Importantly, our data demonstrated that IL-7 treatment resulted in acquisition of a memory-like phenotype by a portion of naive CD4+ and CD8+ T cells and that this phenotypic change was accompanied by functional changes typically associated with effector cells, since a higher portion of the cells from the treated animals was able to produce TNF-α and IFN-γ in response to an antigen. Similarly, in murine models, a major component of the effect of IL-7 on naive T cells in lymphopenic hosts is its ability to induce cycling in response to low-affinity or self antigens (11, 25), which serves to expand and maintain the naive subset in states of T-cell deficiency. These data suggest that therapeutic administration of IL-7 in T-cell-replete hosts may accomplish the same physiologic response, thus potentially rendering IL-7 as a useful systemic adjuvant capable of rendering large numbers of the naive-cell repertoire transiently functional as effector cells.
The phenomenon of naive cells masquerading as memory-like cells was previously observed in lymphopenic mice following transfer of autologous naive cells (2, 12, 14, 17, 19, 27). Lymphopenia itself was shown to be closely associated with increased levels of endogenous IL-7 (1, 4, 5, 18). Our study suggests that the lymphopenia-induced conversion of naive cells to the memory-like phenotype observed in those experiments might be mainly due to IL-7.
Upregulation of CD95 (Fas) is not surprising, since Fas expression is induced by IL-2, IL-7, and IFN-γ alone or in combination (28, 31). In those previous experiments, cytokine-stimulated cell cycle progression correlated with upregulation of Fas and consequently led to activation-induced cell death. It is possible that this mechanism may be operating in our model system, as naive cells as well as memory cells upregulated their CD95 expression (together with up- or downregulation of CD28).
In this study we focused not only on the effects of exogenous IL-7 administration on peripheral lymphocytes but also on effects on the secondary lymphoid tissues. Thymic output of naive T cells was not studied, since measurement of levels of TCR excision circles is limited by the fast dilution of TCR excision circles in cycling cells, as previously observed (6). It is indeed unlikely that recent thymic output contributed to the increase in T-cell numbers, since treatment with IL-7 was short-term and likely insufficient to mobilize naive T cells from the thymus. In mice, prolonged treatment with IL-7 is necessary to mobilize naive T cells from the thymus (20).
All together, our data add to the concept that IL-7 is a potent cytokine that drives proliferation of naive T cells in both the blood and secondary lymphoid organs. These cells appear to have an increased ability to regulate antigens, and this finding may be exploited by the use of short-term treatment of IL-7 as a vaccine adjunct. Increased cycling within naive CD4+ and CD8+ T cells would increase the number of cells responsive to antigens.
In addition, IL-7 increased the ability of CD4+ and CD8+ TCM and TEM to produce cytokines. Given the importance of TCM in protection against pathogens (29), increasing the antigen-specific TCM may ease the generation of protective vaccines against pathogens such as human immunodeficiency virus type 1.
Acknowledgments
We are grateful to Louis Picker for helpful discussion. We thank Steven J. Snodgrass for editorial assistance.
REFERENCES
- 1.Bolotin, E., G. Annett, R. Parkman, and K. Weinberg. 1999. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 23:783-788. [DOI] [PubMed] [Google Scholar]
- 2.Cho, B. K., V. P. Rao, Q. Ge, H. N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 4.Fry, T. J., B. L. Christensen, K. L. Komschlies, R. E. Gress, and C. L. Mackall. 2001. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood 97:1525-1533. [DOI] [PubMed] [Google Scholar]
- 5.Fry, T. J., E. Connick, J. Falloon, M. M. Lederman, D. J. Liewehr, J. Spritzler, S. M. Steinberg, L. V. Wood, R. Yarchoan, J. Zuckerman, A. Landay, and C. L. Mackall. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 6.Fry, T. J., M. Moniuszko, S. Creekmore, S. J. Donohue, D. C. Douek, S. Giardina, T. T. Hecht, B. J. Hill, K. Komschlies, J. Tomaszewski, G. Franchini, and C. L. Mackall. 2003. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101:2294-2299. [DOI] [PubMed] [Google Scholar]
- 7.Geginat, J., S. Campagnaro, F. Sallusto, and A. Lanzavecchia. 2002. TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv. Exp. Med. Biol. 512:107-112. [DOI] [PubMed] [Google Scholar]
- 8.Geginat, J., A. Lanzavecchia, and F. Sallusto. 2003. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101:4260-4266. [DOI] [PubMed] [Google Scholar]
- 9.Geginat, J., F. Sallusto, and A. Lanzavecchia. 2001. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194:1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiselhart, L. A., C. A. Humphries, T. A. Gregorio, S. Mou, J. Subleski, and K. L. Komschlies. 2001. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J. Immunol. 166:3019-3027. [DOI] [PubMed] [Google Scholar]
- 11.Goldrath, A. W., and M. J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity 11:183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldrath, A. W., L. Y. Bogatzki, and M. J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaled, A. R., and S. K. Durum. 2002. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2:817-830. [DOI] [PubMed] [Google Scholar]
- 14.Kieper, W. C., and S. C. Jameson. 1999. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA 96:13306-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieper, W. C., J. T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C. D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lantz, O., I. Grandjean, P. Matzinger, and J. P. Di Santo. 2000. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat. Immunol. 1:54-58. [DOI] [PubMed] [Google Scholar]
- 17.Murali-Krishna, K., and R. Ahmed. 2000. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 165:1733-1737. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 18a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 19.Oehen, S., and K. Brduscha-Riem. 1999. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: a pitfall for T cell memory studies? Eur. J. Immunol. 29:608-614. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto, Y., D. C. Douek, R. D. McFarland, and R. A. Koup. 2002. Effects of exogenous interleukin-7 on human thymus function. Blood 99:2851-2858. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 22.Schluns, K. S., W. C. Kieper, S. C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426-432. [DOI] [PubMed] [Google Scholar]
- 23.Schluns, K. S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269-279. [DOI] [PubMed] [Google Scholar]
- 24.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680-686. [DOI] [PubMed] [Google Scholar]
- 25.Tan, J. T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K. I. Weinberg, and C. D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA 98:8732-8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan, J. T., B. Ernst, W. C. Kieper, E. LeRoy, J. Sprent, and C. D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanchot, C., A. Le Campion, B. Martin, S. Leaument, N. Dautigny, and B. Lucas. 2002. Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J. Immunol. 168:5042-5046. [DOI] [PubMed] [Google Scholar]
- 28.Wang, R., T. L. Ciardelli, and J. H. Russell. 1997. Partial signaling by cytokines: cytokine regulation of cell cycle and Fas-dependent, activation-induced death in CD4+ subsets. Cell. Immunol. 182:152-160. [DOI] [PubMed] [Google Scholar]
- 29.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 30.Wiryana, P., T. Bui, C. R. Faltynek, and R. J. Ho. 1997. Augmentation of cell-mediated immunotherapy against herpes simplex virus by interleukins: comparison of in vivo effects of IL-2 and IL-7 on adoptively transferred T cells. Vaccine 15:561-563. [DOI] [PubMed] [Google Scholar]
- 31.Zheng, L., C. L. Trageser, D. M. Willerford, and M. J. Lenardo. 1998. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J. Immunol. 160:763-769. [PubMed] [Google Scholar]