Abstract
During infection by herpes simplex virus type 1 (HSV-1), the virion protein VP16 activates the transcription of viral immediate-early (IE) genes. Genetic and biochemical assays have shown that the potent transcriptional activation domain of VP16 can associate with general transcription factors and with chromatin-modifying coactivator proteins of several types. The latter interactions are particularly intriguing because previous reports indicate that HSV-1 DNA does not become nucleosomal during lytic infection. In the present work, chemical cross-linking and immunoprecipitation assays were used to probe the presence of activators, general transcription factors, and chromatin-modifying coactivators at IE gene promoters during infection of HeLa cells by wild-type HSV-1 and by RP5, a viral strain lacking the VP16 transcriptional activation domain. The presence of VP16 and Oct-1 at IE promoters did not depend on the activation domain. In contrast, association of RNA polymerase II, TATA-binding protein, histone acetyltransferases (p300 and CBP), and ATP-dependent remodeling proteins (BRG1 and hBRM) with IE gene promoters was observed in wild-type infections but was absent or reduced in cells infected by RP5. In contrast to the previous evidence for nonnucleosomal HSV-1 DNA, histone H3 was found associated with viral DNA at early times of infection. Interestingly, histone H3 was underrepresented on IE promoters in a manner dependent on the VP16 activation domain. Thus, the VP16 activation domain is responsible for recruiting general transcription factors and coactivators to IE promoters and also for dramatically reducing the association of histones with those promoters.
The activation domain of VP16 (VP16 AD) from herpes simplex virus type I (HSV-1) has been widely used as a model for the study of transcriptional activation in eukaryotes. During infection, VP16 triggers the cascade of viral gene expression by activating transcription of the viral immediate-early (IE) genes (65). VP16 forms a DNA-binding complex with the cellular proteins Oct-1 and HCF-1 at specific cis elements present in the IE gene promoters (27, 40, 48, 68). The potent VP16 AD (10, 55), often artificially fused to a heterologous DNA-binding domain (47), can activate transcription in a wide range of organisms, including yeasts, insects, plants, and mammals (3, 47, 58, 66), indicating that mechanisms of transcriptional activation are broadly conserved through evolution.
Interactions of the VP16 AD with general transcription factors (GTFs) including transcription factor IIB (TFIIB), TFIIH, TATA-binding protein (TBP), and TBP-associated factors (TAFs) suggest that the VP16 AD might activate transcription by stimulating the assembly of an RNA polymerase II (RNA Pol II) preinitiation complex (13, 17, 24, 31, 56, 67). Consistent with this model, in vitro experiments have demonstrated the ability of the VP16 AD to promote the formation of the ternary complex formed by TFIIA, TFIID, and TATA box DNA (25). The VP16 AD might also recruit the RNA Pol II holoenzyme through interactions with components of the Mediator complex (7, 18, 33, 69). Other potential targets of the VP16 AD include chromatin-remodeling coactivator or adaptor proteins. The VP16 AD can interact physically or functionally with histone acetyltransferase (HAT) proteins including the yeast ADA/SAGA and NuA4 complexes and the human coactivators CBP, p300, and the hGCN5 complex (2, 3, 26, 28, 32, 57, 63). Acetylation of nucleosomal histones near gene promoters is generally correlated with increased transcription (5, 16, 49). In particular, CBP and p300 are very similar and ubiquitously expressed coactivators involved in cell cycle control, differentiation, and apoptosis, with HAT and factor acetyltransferase activities (60). Despite the sequence similarity of CBP and p300, gene deletion experiments suggest that the two proteins serve nonredundant but overlapping functions (23, 42, 54, 72). The VP16 AD can also interact with the ATP-dependent chromatin-remodeling complex SWI/SNF (14, 32, 38). BRM and BRG1 (BRM-related gene-1) are the mammalian homologs of the ATPase subunit of the yeast SWI/SNF complex. These ATPases have high sequence similarity but play different biological roles, as indicated by the phenotypes of mutant mice (6, 45) and by their differential recruitment to various gene promoters during cellular proliferation and differentiation (20).
The association of chromatin-modifying coactivators with the VP16 AD in heterologous systems is particularly intriguing, since previous evidence indicates that HSV-1 DNA is not packaged in nucleosomes during lytic infection. Nuclease assays have shown that little or none of the viral DNA delivered to infected cells is digested to fragment sizes consistent with nucleosomes (29, 30, 35). Electron microscopy studies have shown the accumulation of nonnucleosomal DNA in infected cells (36). Viral DNA is localized to an interchromosomal space that excludes cellular chromatin (41) and does not incorporate histone H2B (34). Collectively, these observations suggest that HSV-1 DNA is primarily nonnucleosomal during lytic infection. Thus, the purpose for the interaction of the VP16 AD with chromatin-remodeling coactivators on a presumably nonnucleosomal template remains enigmatic.
Given that the association of VP16 with such coactivators has arisen solely from artificial or heterologous experimental contexts, we tested whether the chromatin-modifying coactivator proteins were associated with viral IE gene promoters during infection. Using chemical cross-linking and immunoprecipitation (ChIP) assays, we found that HATs (p300 and CBP) and also ATP-dependent chromatin-remodeling enzymes (BRG1 and BRM) were present at viral IE promoters. Recruitment of the HATs and GTFs was fully dependent on the VP16 AD, whereas recruitment of the SWI/SNF components was only partially dependent on VP16. As predicted for a nonnucleosomal HSV-1 DNA, histone H3 was not detected on IE promoters at early times of infection. However, H3 was present at an IE gene coding region and at delayed-early (DE) and late (L) viral gene promoters, indicating that incoming viral DNA does associate with histones during lytic infection. Interestingly, during infections by a virus lacking the VP16 AD, histone H3 was clearly detected at IE promoters. Therefore, the VP16 AD is responsible either for preventing the deposition of histones or for the removal of histones from IE viral gene promoters.
MATERIALS AND METHODS
Cells and viruses.
HeLa and Vero cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Stocks of wild-type HSV-1 (strain KOS) and the VP16 truncation mutant RP5 (53) were prepared in Vero cells and titered by plaque assays. For gene expression and ChIP assays, HeLa cells (approximately 3 × 107) were infected with KOS at a multiplicity of infection of either 1 or 5 PFU/cell. Infections with RP5 were performed by using comparable virion numbers (approximately 100-fold lower PFU). In some experiments, cycloheximide (60 μg/ml) was added to the medium for 2 h prior to and during infection to inhibit protein translation.
ChIP assays.
To cross-link protein-DNA complexes, formaldehyde was added to the medium lying over infected cells to a final concentration of 1% for 15 min. The cross-linking reactions were quenched by adding glycine to a final concentration of 125 mM. Cells were collected, resuspended in a hypotonic buffer, and Dounce homogenized to release nuclei, which were collected by centrifugation. Nuclear pellets were sonicated to obtain DNA fragments with an average length of 300 to 400 bp. Aliquots corresponding to 10% of the input material were reserved.
Prior to immunoprecipitation (IP), samples were precleared by using protein G-agarose beads (Upstate, Charlottesville, Va.). IPs were performed using specific antibodies at concentrations ranging from 10 to 50 μg/ml at 4°C overnight in a solution containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% (wt/vol) bovine serum albumin, and 10 μg of salmon sperm DNA/ml. Antigen-antibody complexes were precipitated by using protein G-agarose beads. The beads were washed extensively before protein-DNA complexes were eluted by using 100 μl of 50 mM Tris-HCl (pH 8.0)-10 mM EDTA-1% sodium dodecyl sulfate for 20 min at 65°C. A second eluate, using 150 μl of 10 mM Tris-HCl (pH 8.0)-1 mM EDTA (TE) with 0.67% sodium dodecyl sulfate, was added to the first. The combined eluates are referred to as the pellet sample. Cross-links were reversed by adding NaCl to 200 mM and 10 μg of RNase A and incubating at 65°C overnight. After ethanol precipitation, samples were digested with proteinase K (Boehringer) at 42°C for 2 h and then extracted with phenol-chloroform. After another ethanol precipitation, DNA samples were resuspended in 75 μl of TE.
ChIP assays were performed using antibodies or antisera directed against VP16 (55), Oct-1 (a gift from W. Herr, Cold Spring Harbor Laboratory), TBP (a gift from R. W. Henry, Michigan State University), RNA Pol II (8WG16; Covance), CBP (A-22; Santa Cruz Biotechnology), p300 (N-15; Santa Cruz Biotechnology), hBRM (N-19; Santa Cruz Biotechnology), BRG1 (H-88; Santa Cruz Biotechnology), histone H3 acetylated at Lys9 and/or Lys14 (Upstate), and a C-terminal epitope of histone H3 (ab1791; Abcam). Control IPs using preimmune sera exhibited essentially the same results as mock IPs using no antibody (data not shown).
PCR analysis.
Semiquantitative PCRs were performed to detect specific viral or cellular gene fragments in the immunoprecipitated samples. These fragments included the promoters of the HSV IE genes (ICP0, ICP27, and ICP4), the coding region of the ICP27 gene, the promoters of viral DE (TK) and L (VP16 and glycoprotein C [gC]) genes, and the promoters of the cellular U3 snRNA and beta interferon (IFN-β) genes (Table 1). The dependence of these reactions on the viral DNA template and the specificity of each reaction for its intended product have been established (data not shown). Parallel PCRs were routinely performed on serial dilutions of input samples (typically corresponding to 0.5, 0.1, and 0.02% of the total material) to confirm that the signals observed were within the linear range of the assay and were comparable between different sets of primers. Signals from these input samples were not used to infer the absolute levels of occupancy of a given DNA fragment by a particular protein, since cross-linking and IP efficiencies cannot be accurately determined. Standard PCR conditions included 5 μl of immunoprecipitated DNA (6.7% of the pelleted material), 0.25 μM each primer, 2.5 U of Taq DNA polymerase (Invitrogen), 0.1 mM each deoxynucleoside triphosphate, 2 mM MgCl2, and 10% Enhancer solution (Invitrogen), with incubation at 95°C for 5 min followed by 30 to 35 cycles of 95°C for 30 s, 65°C for 30 s, and 72°C for 1 min, and ending with 5 min at 72°C. The Enhancer solution was omitted from reactions amplifying the ICP27 open reading frame (ORF), U3 snRNA promoter, and IFN-β promoter fragments. Annealing of primers for amplification of the IFN-β promoter was performed at 55°C. PCR products were electrophoresed in 1% agarose gels and stained with ethidium bromide. Figures represent negative images of the ethidium-stained gels.
TABLE 1.
Fragments amplified by PCR in ChIP and RT-PCR experiments
| Gene | Class | Location | Fragment endpoints relative to transcription start (+1) |
|---|---|---|---|
| ICP0 | IE | Promoter | −322 to −12 |
| ICP4 | IE | Promoter | −376 to −9 |
| ICP27 | IE | Promoter | −281 to −3 |
| ICP27 ORF | IE | Coding regiona | +1413 to +1614 |
| ICP27 ORF | IE | Coding regionb | +1328 to +1611 |
| TK | DE | Promoter | −297 to +1 |
| TK ORF | DE | Coding region | +113 to +644 |
| VP16 | L | Promoter | −270 to −45 |
| VP16 ORF | L | Coding region | +1290 to +1680 |
| gC | L | Promoter | −269 to −7 |
| U3 snRNA | Cellular | Promoter | −295 to −71 |
| IFN-β | Cellular | Promoter | −197 to +12 |
Used in ChIP assays.
Used in RT-PCR assays.
Gene expression assays.
Steady-state levels of viral mRNAs (ICP27, TK, and VP16) were determined by using reverse transcriptase PCR (RT-PCR) assays. Total RNA was isolated from infected cells by using the TRI REAGENT (Molecular Research Center, Cincinnati, Ohio), and cDNA was prepared by using 1 μg of total RNA and a randomly primed reverse transcription system (Promega). Viral gene fragments (Table 1) were amplified by using PCR conditions described previously (52).
RESULTS
VP16 associates with IE gene promoters with or without its transcriptional activation domain.
The VP16 protein comprises a core domain (encompassing amino acids 1 to 410) and a transcriptional activation domain (amino acids 413 to 490) (Fig. 1A). The core domain interacts with two cellular proteins, Oct-1 and HCF-1, to form a DNA-binding complex at specific cis-regulatory elements in the viral IE gene promoters (27, 40, 48, 68). The VP16 AD is required for efficient transcription of viral IE genes both in transfection experiments and during HSV-1 infection (53, 55, 62). Infection by RP5, a mutant viral strain lacking sequences encoding the VP16 AD (Fig. 1B), results in little or no IE gene expression (53, 70).
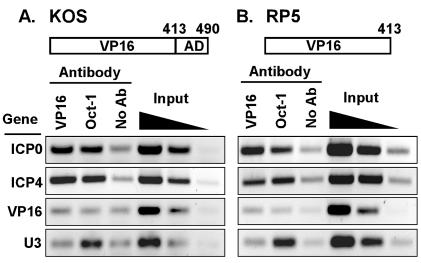
FIG. 1.
Detection of VP16 and Oct-1 at viral IE promoters during infection. ChIP assays were performed using antibodies against VP16 or Oct-1 in lysates of cells infected for 2 h by KOS (wild-type virus) (A) or RP5 (lacking sequences encoding the VP16 AD) (B). Controls include precipitations performed without specific antisera (No Ab) and aliquots of samples prior to precipitation (0.5, 0.1, and 0.02% input). Samples were analyzed by PCR detecting viral IE gene promoters (ICP0, ICP4), a viral L gene promoter (VP16), and the promoter of the cellular U3 snRNA gene. Negative images of ethidium bromide-stained gels are shown.
ChIP assays were adapted to detect the association of specific proteins with viral promoters during lytic infection by HSV-1. HeLa cells were fixed with formaldehyde at 2 h postinfection (hpi), when transcription of IE genes is robust. Sonicated nuclear lysates were immunoprecipitated with antisera directed against VP16 or Oct-1, or with protein G-agarose beads alone (no antibody). PCR products representing the promoters of the IE genes ICP0 and ICP4 were more abundant in reactions using antisera against VP16 and Oct-1 than in samples lacking primary antibodies (Fig. 1A), fulfilling the expectation that these proteins are associated with IE promoters during infection. Little or no PCR product corresponding to the VP16 promoter was detected in the VP16 and Oct-1 IPs, as expected for an L gene promoter. Anti-Oct-1, but not anti-VP16, immunoprecipitated the promoter region of the cellular U3 snRNA gene, which contains an Oct-1 binding site (50).
To test whether the VP16 AD was required for association with the IE promoters, parallel ChIP assays were performed using cells infected with the VP16 truncation mutant RP5. The binding of VP16 and of Oct-1 to the IE promoters was not altered by the absence of the VP16 AD (Fig. 1B). The comparable signals arising from the input samples of the two infections ensure that comparable amounts of viral DNA were present in the nuclear extracts (implying comparable infection efficiencies by the two viruses). The association of Oct-1 with the U3 snRNA promoter was likewise unaffected. Therefore, the reduced expression of IE genes in RP5 infections does not result from a failure of the activator to associate with its target genes but likely arises from a defect in transcriptional activation per se.
The VP16 AD is required to recruit GTFs to IE promoters during infection.
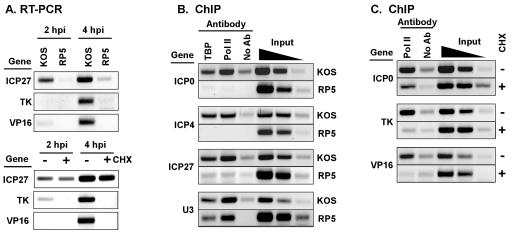
Transcription of HSV-1 genes depends on GTFs and RNA Pol II from the infected host cell (65). During infection of HeLa cells by wild-type virus (KOS), IE gene mRNAs were readily detected at 2 hpi, and DE and L mRNAs were detected at 4 hpi (Fig. 2A). In contrast, infection by RP5 (lacking the VP16 AD) resulted in a dramatic reduction in viral gene expression, in agreement with prior reports (53, 70). To test whether this decreased expression corresponded to a failure to recruit the general transcription machinery to IE gene promoters, ChIP assays were performed using antibodies directed against the GTF TBP and RNA Pol II. As shown in Fig. 2B, PCR products corresponding to the promoters of the IE genes ICP0, ICP4, and ICP27 were readily detected in the TBP and RNA Pol II IPs from cells infected with wild-type virus at 2 hpi. In contrast, these PCR products were not detected in parallel IPs from cells infected with RP5. This observation suggests that the association of GTFs with viral IE promoters does not arise nonspecifically during infection or sample preparation. The presence of the U3 snRNA promoter fragments in the IPs from both KOS- and RP5-infected cells demonstrates that the IP reactions were successful. We conclude that the recruitment of TBP and RNA Pol II (and also TFIIF [data not shown]) to IE gene promoters requires the VP16 AD and that the lack of IE gene transcription observed in RP5 infections correlates with the absence of GTFs at those promoters.
FIG. 2.
GTFs are recruited to IE promoters by the VP16 AD. (A) RT-PCR analysis of steady-state mRNA levels of viral IE (ICP27), DE (TK), and L (VP16) genes in cells infected with KOS or RP5 (top panels) or in the presence or absence of cycloheximide (CHX) (lower panels). (B) ChIP assays using antibodies against TBP and Pol II in lysates of cells infected for 2 h with KOS or RP5. Immunoprecipitated samples were analyzed by PCR detecting viral IE gene promoters ICP0, ICP27, and ICP4, and the cellular U3 snRNA promoter. Controls include precipitations performed without specific antisera (No Ab) and aliquots of samples prior to precipitation (0.5, 0.1, and 0.02% input). (C) ChIP assay performed as for panel B for KOS infections in the presence or absence of cycloheximide, using PCR to detect promoters of the viral genes ICP0, TK, and VP16.
In the course of these experiments, we noted that DE and L gene promoters were also associated with RNA Pol II at 2 hpi (Fig. 2C), even though transcription of these genes is not readily detected until 4 hpi (Fig. 2A). Because IE gene products themselves are transcriptional regulatory proteins that can further stimulate the expression of IE genes (as a positive feedback loop) as well as DE and L genes, the observation of TBP and RNA Pol II at the IE promoters may arise from the action of IE proteins rather than of VP16. To test this hypothesis, cells were infected by KOS in the presence of cycloheximide to inhibit IE protein synthesis. This treatment effectively blocked the cascade of viral gene expression; no DE or L gene mRNAs were detected at 2 or 4 hpi in the presence of cycloheximide (Fig. 2A). ChIP assays of cycloheximide-treated cells indicate that the presence of RNA Pol II at the ICP0 promoter was not affected (Fig. 2C), suggesting that recruitment of RNA Pol II to IE promoters arises directly from VP16 activity and not by the action of IE proteins themselves. In contrast, the association of RNA Pol II with DE and L genes (TK and VP16, respectively) was abolished in the presence of cycloheximide. Therefore, the presence of RNA Pol II at DE and L genes does indeed depend on IE protein synthesis, whereas its presence at IE gene promoters does not.
The VP16 AD recruits chromatin-modifying coactivators to IE gene promoters during infection.
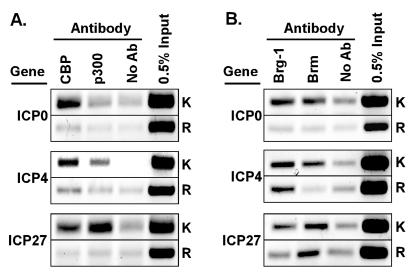
The association of chromatin-modifying coactivators with the VP16 AD in various heterologous systems led us to ask whether some of these factors are present on active IE gene promoters during HSV-1 infection. ChIP assays were performed on extracts of HeLa cells infected with KOS or RP5 by using antibodies specific to the closely related HATs CBP and p300. In extracts from KOS-infected cells, antibodies directed against CBP or against p300 immunoprecipitated the promoters of the ICP0, ICP4, and ICP27 genes (Fig. 3A), demonstrating that these HATs are recruited to IE viral promoters during HSV infection. Interestingly, CBP was preferentially associated with the ICP0 and ICP4 gene promoters whereas p300 was preferentially associated with the ICP27 promoter. Therefore, these two highly related HATs can be differentially recruited to IE gene promoters, despite the similarities in the cis-regulatory elements at those promoters.
FIG. 3.
Recruitment of chromatin-modifying coactivator proteins to IE promoters during HSV infection. (A) ChIP assays using antibodies specific for CBP or p300 in lysates of cells infected with KOS (K) or RP5 (R). (B) ChIP assays using antibodies specific for BRG1 or BRM in lysates of cells infected with KOS or RP5.
During infection of HeLa cells by RP5 (i.e., in the absence of the VP16 AD), recruitment of CBP and p300 to viral IE promoters was drastically reduced (Fig. 3A). No specific PCR signal was detected for the ICP0 and ICP27 promoters in samples immunoprecipitated with either anti-CBP or anti-p300, and only a weak signal was detected for the ICP4 promoter in the anti-CBP sample. These results reveal that the VP16 AD is required for the efficient recruitment of CBP and p300 to HSV-1 IE promoters during infection.
We also tested for the presence of another class of coactivator complex, namely, the ATP-dependent chromatin-remodeling complex SWI/SNF. BRM and BRG1 are the ATPase subunits of two distinct human SWI/SNF complexes (64). ChIP assays of nuclear extracts from KOS-infected cells using antibodies against BRG1 and BRM precipitated the promoters of the ICP0, ICP4, and ICP27 genes, indicating that the human SWI/SNF complexes are recruited to IE promoters during infection (Fig. 3B). As for the HAT complexes described above, BRG1 and BRM showed different preferences for interacting with various IE promoters. The ICP0 and ICP4 promoter fragments were preferentially detected in the BRG1 IP samples, whereas the ICP27 promoter fragment was preferentially detected in the BRM IP sample. In the absence of the VP16 AD (i.e., in RP5 infection), the association of BRG1 and BRM with the ICP0 promoter was lost. In contrast, BRG1 (but not BRM) was still associated with the ICP4 promoter, and BRM (but not BRG1) was still associated with the ICP27 promoter. Therefore, the ATP-dependent remodeling complexes are indeed recruited to viral DNA templates during lytic infection, and specific remodeling complexes preferentially associate with distinct IE gene promoters. Moreover, the VP16 AD is required or important for the association of these complexes with some promoters but not with others. The latter conclusion suggests that other activators present at IE promoters (for instance, Oct-1 or HCF-1) might contribute to the recruitment of BRG1 or BRM.
The recruitment of HATs and SWI/SNF complexes to viral IE promoters was apparently the direct result of the VP16 AD and not a consequence of IE proteins themselves. In ChIP experiments performed on KOS-infected cells in the presence of cycloheximide, the ICP0 and ICP27 promoter fragments were present in the IP pellets obtained by using antibodies recognizing CBP, p300, BRM, or BRG1 (data not shown), indicating that recruitment of these coactivators does not depend on IE protein synthesis.
Histone H3 is present on viral DNA but absent from IE gene promoters.
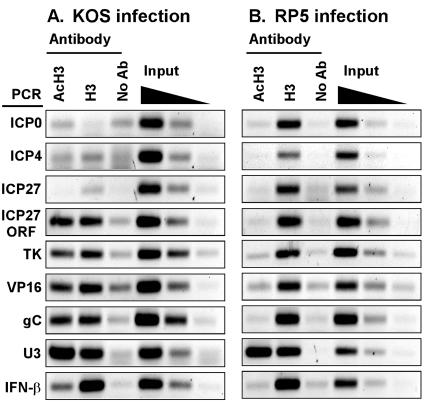
The presence of chromatin-modifying coactivators at viral promoters prompted us to test whether histones associate with viral DNA during early times of HSV-1 lytic infection. ChIP assays were performed on extracts of infected cells by using antisera that recognize either a C-terminal epitope of histone H3 or an acetylated N-terminal epitope of H3 (AcH3). As shown in Fig. 4A, the promoter regions of ICP27, ICP0, and ICP4 were not immunoprecipitated above control levels in either IP sample. The cellular U3 snRNA gene promoter was detected in both samples, and the IFN-β promoter was detected in the anti-H3 sample, confirming that the IP reactions were successful. This result indicates that little or no histone H3 (acetylated or nonacetylated) is associated with IE promoters early in infection. In contrast, the TK, VP16, and gC gene promoters were immunoprecipitated with anti-H3 and to some extent with anti-AcH3 antisera, indicating that some regions of the viral DNA do associate with histones. Moreover, the dearth of histones at IE genes was restricted to the promoter regions, since both anti-H3 and anti-AcH3 antisera immunoprecipitated the coding region of the ICP27 gene.
FIG. 4.
Histone H3 associates with HSV-1 DNA during infection but is underrepresented at transcriptionally active IE gene promoters. ChIP assays were performed using antibodies specific for histone H3 acetylated at lysines 9 and 14 (AcH3) or for a carboxyl-terminal epitope of histone H3 in lysates of cells infected for 2 h by KOS (A) or RP5 (B). Immunoprecipitated samples were analyzed by PCR detecting viral IE (ICP0, ICP4, and ICP27), DE (TK), or L (VP16 and gC) gene promoters, the cellular U3 snRNA or IFN-β promoter, or the coding region (ORF) of ICP27. Controls include precipitations performed without specific antisera (No Ab) and aliquots of samples prior to precipitation (0.5, 0.1, and 0.02% input).
These results suggest that transcription activity is correlated with the absence of histones at viral IE gene promoters. To test this hypothesis, we assayed the association of histone H3 at IE gene promoters during infection by the RP5 virus, when transcription of those genes is diminished. In marked contrast to the previous result, the anti-H3 antiserum efficiently immunoprecipitated the IE gene promoters in extracts from RP5-infected cells (Fig. 4B). These promoter fragments were not evident in IPs using the anti-AcH3 antiserum. This result strengthens the correlation between transcription and the absence of histones and indicates that the VP16 AD is responsible either for preventing the deposition of histones or for the removal of histones from viral IE gene promoters. Moreover, in RP5 infections, the TK, VP16, and gC gene promoters and the ICP27 ORF were also immunoprecipitated by the anti-H3 antiserum but not by the antiserum recognizing AcH3. The presence of the cellular gene promoters (U3 and IFN-β) in the IP pellets was the same for the two infections, confirming that the IP reactions were successful. We conclude that transcriptional activation of IE genes by VP16 has downstream effects on the acetylation status of histones associated with DE and L gene promoters.
DISCUSSION
Although much has been learned about mechanisms of transcriptional activation by using VP16 and other activation domains in heterologous or in vitro experimental systems, the validity of the models arising from such studies is best tested in an appropriate biological context. This report probes the physiological role of putative targets of VP16 by analyzing the recruitment of GTFs and transcriptional coactivator proteins to IE viral gene promoters by VP16 during HSV-1 infection of cultured mammalian cells.
We demonstrate that the recruitment of TBP and RNA Pol II (as representative GTFs) depends heavily on the VP16 AD, indicating that at least part of the in vivo mechanism of transcriptional activation by VP16 is the stimulation of preinitiation complex formation on target gene promoters. This conclusion is consistent with previous evidence that VP16 can interact directly in vitro with GTFs including TBP, TFIIB, TFIIA, and TFIIH (13, 17, 24, 31, 56, 67) and can stimulate in vitro assembly of a TFIID-TFIIA-DNA complex (25). Our results do not exclude the possibility that later stages of transcription, such as promoter escape or elongation, might also be stimulated by VP16 (71).
Our results also indicate that two different types of chromatin-modifying complexes, HATs and ATP-dependent chromatin-remodeling complexes, are recruited to viral IE gene promoters during HSV-1 infection. Recruitment of the HATs p300 and CBP was fully dependent on the VP16 AD, whereas recruitment of the SWI/SNF proteins BRM and BRG1 to some but not all IE promoters was affected by the VP16 truncation mutant. The ability of VP16 to recruit these HATs and remodeling enzymes during infection in vivo is consistent with results from assays using the Gal4-VP16 fusion protein in transfection or in vitro experiments (26, 32, 38, 63). The presence of BRM and BRG1 at some IE promoters during RP5 infection indicates that both VP16 AD-dependent and VP16 AD-independent mechanisms can recruit these proteins. Under other circumstances, recruitment of SWI/SNF complexes has been shown to be independent of a given activation domain and yet dependent on the DNA-binding domain of a regulatory protein (8, 21, 22). We cannot yet distinguish whether the partial recruitment of BRG1 and BRM to IE promoters depends on the core domain of VP16 or on other activators that bind these promoters. Although human SWI/SNF has been implicated as a component of the RNA Pol II holoenzyme (9, 39), our results are not fully consistent with that model. In the absence of the VP16 AD, we observed BRG1 and BRM present at certain IE promoters despite the absence of RNA Pol II, indicating that these ATPases can be recruited independently of the RNA Pol II holoenzyme, as seen in in vitro systems (73).
We find it intriguing that the coactivators were differentially recruited to the various IE promoters. For example, the ICP0 and ICP4 promoters seemed to favor the presence of CBP, whereas the ICP27 promoter was prominently associated with p300. Likewise, BRG1 was somewhat preferred at the ICP0 and ICP4 promoters, whereas BRM was slightly more prevalent at the ICP27 promoter. Together, these observations suggest that BRM and p300 may cooperate specifically at certain promoters, whereas BRG1 and CBP function together at other promoters. Curiously, these promoter preferences exist even though the important cis-regulatory elements, including TAATGARAT, GA-rich, and Sp1-binding sites, are common to all of the IE promoters. The differential recruitment may reflect additional, undefined promoter elements that might distinguish the various promoters. Alternatively, the specific arrangements of the binding sites within the various IE promoters (and thus the quaternary structure of the various regulatory proteins) might be responsible for preferential coactivator recruitment. Whether the promoter-specific differences in coactivator recruitment have functional consequences for gene expression remains to be determined. Collectively, our results reveal that assembly of the transcription machinery can be accomplished through multiple pathways and that subtle differences in promoters might have significant effects on the recruitment of particular factors.
Other transcriptional regulators, including Oct-1, HCF-1, GABP, and Sp1, also bind to the IE promoter regions (19, 40, 48, 65). We presume that the binding of these regulators to their cognate cis elements is not affected by the presence or absence of the VP16 AD, and in fact we show that Oct-1 is present at IE promoters during RP5 infection. However, the presence of these regulatory proteins is apparently not sufficient to recruit either the HATs or GTFs to the IE promoters, since the recruitment was ineffective during RP5 infection. This conclusion is somewhat surprising, given evidence that CBP and p300 can interact in vitro with GABP and Sp1 (1, 51, 61). This reinforces the value of testing such interactions in an appropriate in vivo context.
The recruitment of chromatin-modifying proteins to IE gene promoters might seem superfluous given previous evidence that HSV-1 DNA is not packaged in nucleosomes during lytic infection. In striking contrast to that model, our ChIP experiments detected a distinct association of HSV-1 DNA with histone H3, although this assay does not directly demonstrate that H3 is present in nucleosomal structures. ChIP assays using antibodies specific to other core histones (or variant histones) or other physical assays might be used to assess whether nucleosomes or some other histone-based structures are present on viral DNA. Interestingly, at 2 hpi in cells infected by wild-type virus, histone H3 was not detected at the IE gene promoters but was present at the ICP27 coding region and at DE and L gene promoters. Thus, histone H3 (perhaps in nucleosomes) was underrepresented at the promoters of actively transcribed genes. In contrast, H3 was present at IE promoters during RP5 infection, when those genes are transcriptionally silent. These results indicate that the VP16 AD is required either to exclude histones from IE promoters or to remove them, perhaps through the action of the chromatin-remodeling coactivators. In agreement with our observations, two recent reports demonstrated that nucleosomes are underrepresented in the fully remodeled PHO5 promoter in yeast upon transcription induction (4, 43). The effect of the VP16 AD on the acetylation of histones associated with viral DNA is also noteworthy. Although acetylated H3 was associated with the ICP27 ORF and the DE and L gene promoters during infection by wild-type virus, that acetylation was absent during RP5 infection. This result suggests that the cascade of viral gene expression requires histone modifications that are directly or indirectly dependent on the VP16 AD. Given that robust expression of DE and L genes is observed after 4 hpi, the H3 acetylation detected at 2 hpi at DE and L gene promoters might represent early events in the remodeling of these promoters.
Other virion proteins may also influence the association of histones with herpesvirus DNA. The tegument protein VP22 from bovine herpesvirus 1 can interact with histones in vitro (44). Moreover, VP22 from HSV-1 has been shown to interact in vitro with the template-activating factor 1 (TAF-1), a chromatin-remodeling protein and subunit of the INHAT (inhibitor of acetyltransferases) complex. The interaction of VP22 with TAF-1 disrupted the ability of TAF-1 to promote the loading of histones onto naked DNA in vitro (59). These observations raise the hypothesis that VP22 may interfere with nucleosomal deposition on the viral DNA during lytic infection. This hypothesis is further fueled by evidence that VP22 can interact with the VP16 AD (11), which our evidence indicates is involved in clearing histones from IE gene promoters. Whether during infection VP22 modulates the association of histones with IE promoters or with other regions of the HSV genome will be an interesting future question.
The presence of CBP, p300, BRG1, and BRM at IE promoters may reflect the mechanism by which histones are excluded from these promoters. These enzymes might also fulfill other functions for transcriptional activation in the absence of histones. For instance, CBP and p300 might be required for preinitiation complex formation or as a part of the RNA Pol II holoenzyme (37). Another possibility is that CBP and p300 may acetylate and thus regulate the activities of other proteins such as activators, high-mobility-group (HMG) proteins, coactivators, and GTFs (46, 49). Although SWI/SNF complexes are best known for their ability to modify nucleosomal structure, naked DNA also stimulates SWI/SNF ATPase activity, and conversely, SWI/SNF ATPase activity induces changes in DNA topology in nucleosomal and naked DNA templates (12, 15). Thus, SWI/SNF enzymatic activities on nonnucleosomal templates might be relevant for activating transcription on nucleosome-free HSV-1 viral DNA. Future experiments will address whether the enzymatic activities of these coactivators are explicitly required for the VP16-dependent activation of IE gene transcription.
Acknowledgments
We thank Bill Henry, Winship Herr, Bob Kingston, and Min-Hao Kuo for antibodies and antisera, and Dean Shooltz, Kanchan Champhekar, and Min-Hao Kuo for comments on the manuscript. We thank Shelley Berger and colleagues for communicating unpublished results.
This work was supported by NIH grant AI-27323 and by institutional funds from Michigan State University.
REFERENCES
- 1.Bannert, N., A. Avots, M. Baier, E. Serfling, and R. Kurth. 1999. GA-binding protein factors, in concert with the coactivator CREB binding protein/p300, control the induction of the interleukin 16 promoter in T lymphocytes. Proc. Natl. Acad. Sci. USA 96:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlev, N. A., R. Candau, L. Wang, P. Darpino, N. Silverman, and S. L. Berger. 1995. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J. Biol. Chem. 270:19337-19344. [DOI] [PubMed] [Google Scholar]
- 3.Berger, S. L., B. Pina, N. Silverman, G. A. Marcus, J. Agapite, J. L. Regier, S. J. Triezenberg, and L. Guarente. 1992. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell 70:251-265. [DOI] [PubMed] [Google Scholar]
- 4.Boeger, H., J. Griesenbeck, J. S. Strattan, and R. D. Kornberg. 2003. Nucleosomes unfold completely at a transcriptionally active promoter. Mol. Cell 11:1587-1598. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. E., T. Lechner, L. Howe, and J. L. Workman. 2000. The many HATs of transcription coactivators. Trends Biochem. Sci. 25:15-19. [DOI] [PubMed] [Google Scholar]
- 6.Bultman, S., T. Gebuhr, D. Yee, C. La Mantia, J. Nicholson, A. Gilliam, F. Randazzo, D. Metzger, P. Chambon, G. Crabtree, and T. Magnuson. 2000. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 6:1287-1295. [DOI] [PubMed] [Google Scholar]
- 7.Cantin, G. T., J. L. Stevens, and A. J. Berk. 2003. Activation domain-mediator interactions promote transcription preinitiation complex assembly on promoter DNA. Proc. Natl. Acad. Sci. USA 100:12003-12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, S. W., K. P. Davies, E. Yung, R. J. Beltran, J. Yu, and G. V. Kalpana. 1999. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat. Genet. 22:102-105. [DOI] [PubMed] [Google Scholar]
- 9.Cho, H., G. Orphanides, X. Sun, X. J. Yang, V. Ogryzko, E. Lees, Y. Nakatani, and D. Reinberg. 1998. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol. Cell. Biol. 18:5355-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousens, D. J., R. Greaves, C. R. Goding, and P. O'Hare. 1989. The C-terminal 79 amino acids of the herpes simplex virus regulatory protein, Vmw65, efficiently activate transcription in yeast and mammalian cells in chimeric DNA-binding proteins. EMBO J. 8:2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 69:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavin, I., P. J. Horn, and C. L. Peterson. 2001. SWI/SNF chromatin remodeling requires changes in DNA topology. Mol. Cell 7:97-104. [DOI] [PubMed] [Google Scholar]
- 13.Goodrich, J. A., T. Hoey, C. J. Thut, A. Admon, and R. Tjian. 1993. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell 75:519-530. [DOI] [PubMed] [Google Scholar]
- 14.Hassan, A. H., K. E. Neely, and J. L. Workman. 2001. Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104:817-827. [DOI] [PubMed] [Google Scholar]
- 15.Havas, K., A. Flaus, M. Phelan, R. Kingston, P. A. Wade, D. M. Lilley, and T. Owen-Hughes. 2000. Generation of superhelical torsion by ATP-dependent chromatin remodeling activities. Cell 103:1133-1142. [DOI] [PubMed] [Google Scholar]
- 16.Iizuka, M., and M. M. Smith. 2003. Functional consequences of histone modifications. Curr. Opin. Genet. Dev. 13:154-160. [DOI] [PubMed] [Google Scholar]
- 17.Ingles, C. J., M. Shales, W. D. Cress, S. J. Triezenberg, and J. Greenblatt. 1991. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature 351:588-590. [DOI] [PubMed] [Google Scholar]
- 18.Ito, M., C. X. Yuan, S. Malik, W. Gu, J. D. Fondell, S. Yamamura, Z. Y. Fu, X. Zhang, J. Qin, and R. G. Roeder. 1999. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators. Mol. Cell 3:361-370. [DOI] [PubMed] [Google Scholar]
- 19.Jones, K. A., and R. Tjian. 1985. Sp1 binds to promoter sequences and activates herpes simplex virus ′immediate-early' gene transcription in vitro. Nature 317:179-182. [DOI] [PubMed] [Google Scholar]
- 20.Kadam, S., and B. M. Emerson. 2003. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11:377-389. [DOI] [PubMed] [Google Scholar]
- 21.Kadam, S., G. S. McAlpine, M. L. Phelan, R. E. Kingston, K. A. Jones, and B. M. Emerson. 2000. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 14:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kal, A. J., T. Mahmoudi, N. B. Zak, and C. P. Verrijzer. 2000. The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev. 14:1058-1071. [PMC free article] [PubMed] [Google Scholar]
- 23.Kawasaki, H., R. Eckner, T. P. Yao, K. Taira, R. Chiu, D. M. Livingston, and K. K. Yokoyama. 1998. Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature 393:284-289. [DOI] [PubMed] [Google Scholar]
- 24.Klemm, R. D., J. A. Goodrich, S. Zhou, and R. Tjian. 1995. Molecular cloning and expression of the 32-kDa subunit of human TFIID reveals interactions with VP16 and TFIIB that mediate transcriptional activation. Proc. Natl. Acad. Sci. USA 92:5788-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, N., P. J. Horn, S. M. Sullivan, S. J. Triezenberg, T. G. Boyer, and A. J. Berk. 1998. DA-complex assembly activity required for VP16C transcriptional activation. Mol. Cell. Biol. 18:4023-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus, W. L., E. T. Manning, and J. T. Kadonaga. 1999. Biochemical analysis of distinct activation functions in p300 that enhance transcription initiation with chromatin templates. Mol. Cell. Biol. 19:8123-8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristie, T. M., J. H. LeBowitz, and P. A. Sharp. 1989. The octamer-binding proteins form multi-protein-DNA complexes with the HSV alpha TIF regulatory protein. EMBO J. 8:4229-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundu, T. K., V. B. Palhan, Z. Wang, W. An, P. A. Cole, and R. G. Roeder. 2000. Activator-dependent transcription from chromatin in vitro involving targeted histone acetylation by p300. Mol. Cell 6:551-561. [DOI] [PubMed] [Google Scholar]
- 29.Leinbach, S. S., and W. C. Summers. 1980. The structure of herpes simplex virus type 1 DNA as probed by micrococcal nuclease digestion. J. Gen. Virol. 51:45-59. [DOI] [PubMed] [Google Scholar]
- 30.Lentine, A. F., and S. L. Bachenheimer. 1990. Intracellular organization of herpes simplex virus type 1 DNA assayed by staphylococcal nuclease sensitivity. Virus Res. 16:275-292. [DOI] [PubMed] [Google Scholar]
- 31.Lin, Y. S., I. Ha, E. Maldonado, D. Reinberg, and M. R. Green. 1991. Binding of general transcription factor TFIIB to an acidic activating region. Nature 353:569-571. [DOI] [PubMed] [Google Scholar]
- 32.Memedula, S., and A. S. Belmont. 2003. Sequential recruitment of HAT and SWI/SNF components to condensed chromatin by VP16. Curr. Biol. 13:241-246. [DOI] [PubMed] [Google Scholar]
- 33.Mittler, G., T. Stuhler, L. Santolin, T. Uhlmann, E. Kremmer, F. Lottspeich, L. Berti, and M. Meisterernst. 2003. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 22:6494-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monier, K., J. C. Armas, S. Etteldorf, P. Ghazal, and K. F. Sullivan. 2000. Annexation of the interchromosomal space during viral infection. Nat. Cell Biol. 2:661-665. [DOI] [PubMed] [Google Scholar]
- 35.Muggeridge, M. I., and N. W. Fraser. 1986. Chromosomal organization of the herpes simplex virus genome during acute infection of the mouse central nervous system. J. Virol. 59:764-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller, U., C. H. Schroder, H. Zentgraf, and W. W. Franke. 1980. Coexistence of nucleosomal and various non-nucleosomal chromatin configurations in cells infected with herpes simplex virus. Eur. J. Cell Biol. 23:197-203. [PubMed] [Google Scholar]
- 37.Nakajima, T., C. Uchida, S. F. Anderson, J. D. Parvin, and M. Montminy. 1997. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 11:738-747. [DOI] [PubMed] [Google Scholar]
- 38.Neely, K. E., A. H. Hassan, A. E. Wallberg, D. J. Steger, B. R. Cairns, A. P. Wright, and J. L. Workman. 1999. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol. Cell 4:649-655. [DOI] [PubMed] [Google Scholar]
- 39.Neish, A. S., S. F. Anderson, B. P. Schlegel, W. Wei, and J. D. Parvin. 1998. Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Res. 26:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preston, C. M., M. C. Frame, and M. E. Campbell. 1988. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell 52:425-434. [DOI] [PubMed] [Google Scholar]
- 41.Puvion-Dutilleul, F., and S. Besse. 1994. Induction of complete segregation of cellular DNA and non-encapsidated viral genomes in herpes simplex virus type 1 infected HeLa cells as revealed by in situ hybridization. Chromosoma 103:104-110. [DOI] [PubMed] [Google Scholar]
- 42.Rebel, V. I., A. L. Kung, E. A. Tanner, H. Yang, R. T. Bronson, and D. M. Livingston. 2002. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc. Natl. Acad. Sci. USA 99:14789-14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinke, H., and W. Horz. 2003. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol. Cell 11:1599-1607. [DOI] [PubMed] [Google Scholar]
- 44.Ren, X., J. S. Harms, and G. A. Splitter. 2001. Bovine herpesvirus 1 tegument protein VP22 interacts with histones, and the carboxyl terminus of VP22 is required for nuclear localization. J. Virol. 75:8251-8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes, J. C., J. Barra, C. Muchardt, A. Camus, C. Babinet, and M. Yaniv. 1998. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2α). EMBO J. 17:6979-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth, S. Y., J. M. Denu, and C. D. Allis. 2001. Histone acetyltransferases. Annu. Rev. Biochem. 70:81-120. [DOI] [PubMed] [Google Scholar]
- 47.Sadowski, I., J. Ma, S. Triezenberg, and M. Ptashne. 1988. GAL4-VP16 is an unusually potent transcriptional activator. Nature 335:563-564. [DOI] [PubMed] [Google Scholar]
- 48.Stern, S., M. Tanaka, and W. Herr. 1989. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature 341:624-630. [DOI] [PubMed] [Google Scholar]
- 49.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh, D., H. Busch, and R. Reddy. 1986. Isolation and characterization of a human U3 small nucleolar RNA gene. Biochem. Biophys. Res. Commun. 137:1133-1140. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, T., A. Kimura, R. Nagai, and M. Horikoshi. 2000. Regulation of interaction of the acetyltransferase region of p300 and the DNA-binding domain of Sp1 on and through DNA binding. Genes Cells 5:29-41. [DOI] [PubMed] [Google Scholar]
- 52.Tal-Singer, R., T. M. Lasner, W. Podrzucki, A. Skokotas, J. J. Leary, S. L. Berger, and N. W. Fraser. 1997. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J. Virol. 71:5268-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tal-Singer, R., R. Pichyangkura, E. Chung, T. M. Lasner, B. P. Randazzo, J. Q. Trojanowski, N. W. Fraser, and S. J. Triezenberg. 1999. The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology 259:20-33. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, Y., I. Naruse, T. Hongo, M. Xu, T. Nakahata, T. Maekawa, and S. Ishii. 2000. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech. Dev. 95:133-145. [DOI] [PubMed] [Google Scholar]
- 55.Triezenberg, S. J., R. C. Kingsbury, and S. L. McKnight. 1988. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 2:718-729. [DOI] [PubMed] [Google Scholar]
- 56.Uesugi, M., O. Nyanguile, H. Lu, A. J. Levine, and G. L. Verdine. 1997. Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science 277:1310-1313. [DOI] [PubMed] [Google Scholar]
- 57.Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger, A. Eberharter, S. John, and J. L. Workman. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394:498-502. [DOI] [PubMed] [Google Scholar]
- 58.Van Doren, M., A. L. Williamson, and R. Lehmann. 1998. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8:243-246. [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen, H., M. Okuwaki, R. Hong, D. Chakravarti, K. Nagata, and P. O'Hare. 2003. Herpes simplex virus type 1 tegument protein VP22 interacts with TAF-I proteins and inhibits nucleosome assembly but not regulation of histone acetylation by INHAT. J. Gen. Virol. 84:2501-2510. [DOI] [PubMed] [Google Scholar]
- 60.Vo, N., and R. H. Goodman. 2001. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276:13505-13508. [DOI] [PubMed] [Google Scholar]
- 61.Vogel, J. L., and T. M. Kristie. 2000. The novel coactivator C1 (HCF) coordinates multiprotein enhancer formation and mediates transcription activation by GABP. EMBO J. 19:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker, S., R. Greaves, and P. O'Hare. 1993. Transcriptional activation by the acidic domain of Vmw65 requires the integrity of the domain and involves additional determinants distinct from those necessary for TFIIB binding. Mol. Cell. Biol 13:5233-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, L., S. R. Grossman, and E. Kieff. 2000. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc. Natl. Acad. Sci. USA 97:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, W., J. Cote, Y. Xue, S. Zhou, P. A. Khavari, S. R. Biggar, C. Muchardt, G. V. Kalpana, S. P. Goff, M. Yaniv, J. L. Workman, and G. R. Crabtree. 1996. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 15:5370-5382. [PMC free article] [PubMed] [Google Scholar]
- 65.Weir, J. P. 2001. Regulation of herpes simplex virus gene expression. Gene 271:117-130. [DOI] [PubMed] [Google Scholar]
- 66.Wilde, R. J., S. E. Cooke, W. J. Brammar, and W. Schuch. 1994. Control of gene expression in plant cells using a 434:VP16 chimeric protein. Plant Mol. Biol. 24:381-388. [DOI] [PubMed] [Google Scholar]
- 67.Xiao, H., A. Pearson, B. Coulombe, R. Truant, S. Zhang, J. L. Regier, S. J. Triezenberg, D. Reinberg, O. Flores, C. J. Ingles, et al. 1994. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol. Cell. Biol. 14:7013-7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao, P., and J. P. Capone. 1990. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol. Cell. Biol. 10:4974-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, F., R. DeBeaumont, S. Zhou, and A. M. Naar. 2004. The activator-recruited cofactor/Mediator coactivator subunit ARC92 is a functionally important target of the VP16 transcriptional activator. Proc. Natl. Acad. Sci. USA 101:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang, W. C., G. V. Devi-Rao, P. Ghazal, E. K. Wagner, and S. J. Triezenberg. 2002. General and specific alterations in programming of global viral gene expression during infection by VP16 activation-deficient mutants of herpes simplex virus type 1. J. Virol. 76:12758-12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yankulov, K., J. Blau, T. Purton, S. Roberts, and D. L. Bentley. 1994. Transcriptional elongation by RNA polymerase II is stimulated by transactivators. Cell 77:749-759. [DOI] [PubMed] [Google Scholar]
- 72.Yao, T. P., S. P. Oh, M. Fuchs, N. D. Zhou, L. E. Ch'ng, D. Newsome, R. T. Bronson, E. Li, D. M. Livingston, and R. Eckner. 1998. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93:361-372. [DOI] [PubMed] [Google Scholar]
- 73.Yudkovsky, N., C. Logie, S. Hahn, and C. L. Peterson. 1999. Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev. 13:2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]