Abstract
Viral protein R (Vpr) of human immunodeficiency virus type 1 (HIV-1) is an accessory protein that plays an important role in viral pathogenesis. This pathogenic activity of Vpr is related in part to its capacity to induce cell cycle G2 arrest and apoptosis of target T cells. A screening for multicopy suppressors of these Vpr activities in fission yeast identified heat shock protein 70 (Hsp70) as a suppressor of Vpr-induced cell cycle arrest. Hsp70 is a member of a family of molecular chaperones involved in innate immunity and protection from environmental stress. In this report, we demonstrate that HIV-1 infection induces Hsp70 in target cells. Overexpression of Hsp70 reduced the Vpr-dependent G2 arrest and apoptosis and also reduced replication of the Vpr-positive, but not Vpr-deficient, HIV-1. Suppression of Hsp70 expression by RNA interference (RNAi) resulted in increased apoptosis of cells infected with a Vpr-positive, but not Vpr-defective, HIV-1. Replication of the Vpr-positive HIV-1 was also increased when Hsp70 expression was diminished. Vpr and Hsp70 coimmunoprecipitated from HIV-infected cells. Together, these results identify Hsp70 as a novel anti-HIV innate immunity factor that targets HIV-1 Vpr.
Heat shock proteins (HSPs) are produced in cells in response to a range of stress-related stimuli, including heat, UV radiation, and microbial/viral infections. In addition to previously recognized activity of HSPs as facilitators of protein folding and chaperones, recent studies revealed unique properties of HSPs in generating specific immune responses against cancers and infectious agents (reviewed in reference 3). Moreover, binding of HSPs to human immunodeficiency virus type 1 (HIV-1) proteins can enhance antiviral immunity, including natural killer (NK) cell, γδ T-cell and cytotoxic T-lymphocyte activities against HIV-1-infected cells (4). HSPs Hsp27 and Hsp70 are selectively expressed early after HIV-1 infection (50), suggesting that these proteins might be a part of the cellular innate antiviral immune responses. However, the specific targets of HSPs and their role in responses to HIV infection remain unclear.
HIV-1 viral protein R (Vpr) is highly conserved among HIV isolates, simian immunodeficiency virus, and other lentiviruses (47, 48). Accumulating evidence suggests that Vpr plays an important role in the viral life cycle and pathogenesis. For example, Vpr is required for efficient viral infection of macrophages, which serve as viral reservoirs throughout the course of infection (8, 15, 19, 20, 43). Chimpanzees and human subjects infected with the Vpr-defective viruses show slower disease progression, often accompanied by reversion of the mutated vpr gene back to the wild-type phenotype (27, 29, 40, 54). Rhesus monkeys infected with a pathogenic SIVmac239 strain, which carries two Vpr-related genes (vpr and vpx) believed to arise by duplication, did not get sick when both vpr and vpx genes were inactivated, although inactivation of either gene did not significantly affect disease progression (14). Interestingly, functionally defective Vpr mutations were found to be associated with long-term nonprogressive HIV infection and were shown to impair induction of apoptosis by Vpr (40, 54).
Vpr displays several distinct activities in the host cells. These include cytoplasmic-nuclear shuttling (19), induction of cell cycle G2 arrest (18), and cell killing (41). The cytoplasmic-nuclear shuttling of Vpr reflects its surmised role in nuclear transport of the viral preintegration complex, which is critical for efficient infection of nonproliferating cells, such as macrophages (7, 19, 38). The cell cycle G2 arrest induced by Vpr is thought to suppress human immune functions by preventing T-cell clonal expansion and to provide an optimized cellular environment for maximal levels of viral replication (15, 37). In addition, Vpr exerts a proapoptotic activity on an infected cell (6, 12, 13, 34). These Vpr-specific activities are functionally independent of each other and can be observed in a variety of eukaryotic cells (5). Consistently, Vpr behaves very similarly in fission yeast and mammalian cells, making fission yeast a particularly useful model to study the Vpr effects (reviewed in reference 55). Using this model, we searched for suppressors of Vpr activity and pulled out several HSPs as suppressors of G2 arrest in fission yeast. Analysis of the effects of one such protein, Hsp70, on Vpr activities in HIV-1-infected cells is presented in this report.
MATERIALS AND METHODS
Reagents.
The Hsp70 and the Hsp27 enzyme-linked immunosorbent assay (ELISA) kits were from Stressgen Biotechnologies (Victoria, British Columbia, Canada). The Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit II was purchased from BD Biosciences PharMingen (San Diego, Calif.). Mouse monoclonal antibody (MAb) against human Hsp70 was from Calbiochem (San Diego, Calif.), and goat polyclonal antibody against human Hsp70 (K-20) and mouse anti-c-Myc (9E10) MAb were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Rabbit polyclonal anti-Vpr antibody was a kind gift of Josephine Sire (INSERM, Marseille, France). Hsp70/Myc plasmid expressing Myc-tagged Hsp70 (45) was provided by Richard Vile (Mayo Clinic, Rochester, Minn.).
Cell cultures.
HEK 293T and HeLa cells were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Bio Whittaker), 100-U/ml penicillin, and 100-U/ml streptomycin. The 293T-632 cell line was derived from the 293T cells by introducing a heterodimer of the modified ecdysone receptor (VgEcR) and the retinoid X receptor (RXR) (57). The transfected 293T-632 cells expressing vpr and Hsp70 were selected with Geneticin (750 μg/ml; Mediatech) for vpr cloned on the pZY-1 plasmid and hygromycin (200 μg/ml) for Hsp70 cloned on the pZH-1 plasmid. Gene expressions in 293T-632 cells were induced by addition of 1 μM muristerone A (57). H9 cells (CD4+ T-cell line) stably expressing HSP70 were transfected with HSP70-expressing pcDNA3.1 plasmid and selected with zeocin (100 μg/ml).
Fission yeast cells were transformed using lithium acetate transformation procedure and cultured on a selective medium as described previously (53). Cells carrying the leu1- or ura4-selectable plasmids were maintained on agar plates of standard Edinburgh minimal medium (EMM) with appropriate amino acid supplements without leucine or uracil. Thiamine was added at 20 μM to repress gene expression from a strongly regulated nmt1 (no message in thiamine) promoter (53). All cells were normally grown at 30°C with constant shaking at 200 rpm. Cells were examined approximately 24 h after gene induction.
RNAi.
A 21-nucleotide-pair Hsp70 short interfering (si) DNA/RNA duplex (DNA-RNA hybrid) was chemically synthesized and purified by TriLink BioTechnologies, Inc. (San Diego, Calif.). The DNA strand of the duplex was homologous to the Hsp70 DNA sequence (GenBank accession no. M11717): 5′-AAGGCCAACAAGATCACCAT-3′. The antisense RNA strand was 5′-AUGGUGAUCUUGUUGGCCU(dTdT)-3′]. The control duplex carried three nucleotide substitutions (GCC to AAA in the DNA strand). Transfection of HeLa cells with these siDNA/RNA duplexes was performed in six-well plates with Oligofectamine (Invitrogen, Carlsbad, Calif.) according to the manufacturer's protocol. Five hours after transfection, culture medium containing transfection complexes was removed; cells were then washed with phosphate-buffered saline (PBS) twice and infected with recombinant HIV-1.
Preparation of HIV stocks and infection.
Phytohemagglutinin (PHA)-activated peripheral blood mononuclear cells (PBMCs) were infected with HIV-1 LAI, a T-cell line-adapted strain of the virus. Viral titers were normalized according to reverse transcriptase activity. After a 2 h of adsorption, cells were washed with RPMI 1640 without FBS, seeded at a density of 106 cells/ml, and cultured for 7 days in RPMI 1640 supplemented with 10% FBS (vol/vol).
Recombinant virus stocks for infection of HeLa CD4+ cells (MAGI) were generated by transfecting 293T cells with Vpr+ or Vpr− NLHXB infectious clones (38) with or without a vector encoding the Env protein of the amphotropic murine leukemia virus [pcDNA-Env(MLV); plasmid provided by N. Landau] by using Metafectene (Biontex). Seventy-two hours after transfection, recombinant virus particles were harvested and purified from the culture media by centrifugation through 30% sucrose cushion in PBS at 24,000 rpm in a Beckman SW-28 rotor for 2 h at 4°C. Viral titers were normalized for virus content by p24 ELISA (NEN Life Science, Inc., Boston, Mass.). Infection was performed in six-well plates by spinoculation according to a published protocol (36).
Analysis of G2 arrest.
For cell cycle analysis of human cells, approximately 1.5 × 106 cells were suspended in 500 μl of PBS and fixed by adding 5 ml of anhydrous ethanol. After an overnight incubation at 4°C, cell pellets were washed and incubated for 30 min in 500 μl of PBS containing 50-μg/ml propidium iodide (Sigma) and 1-mg/ml boiled RNase A (Sigma). The stained cells were analyzed for red fluorescence on a FACSCalibur (Becton Dickinson) fluorescence-activated cell sorter (FACS) by using Cell Quest software.
For analysis of Vpr-induced G2 arrest of fission yeast cells, a vpr-induced cell elongation (commonly known as the “cdc phenotype”) was used. The cdc phenotype is the result of a cell cycle G2/M delay or arrest (11, 33). Cell images were first captured on a Leica microscope, and the cell length was determined by using OpenLab software.
Flow cytometric analysis of apoptotic cells.
For quantification of apoptotic cells, FACS analysis of infected HeLa cells was performed with Annexin V-FITC Apoptosis Detection Kit II (BD PharMingen). Data were analyzed with the Cell Quest program.
Coimmunoprecipitation assays.
HeLa CD4+ (MAGI) cells transfected with Myc-tagged Hsp70-encoding plasmid and infected with HIV-1 were harvested, washed with PBS twice, and lysed in immunoprecipitation buffer containing 5.0 mM CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 50 mM NaCl, and 20 mM Tris (pH 7.5), according to reference 31. Equal amounts of the lysates (according to protein content) were immunoprecipitated with anti-Hsp70 or anti-Vpr polyclonal antibody and analyzed by Western blotting with polyclonal anti-Vpr or mouse anti-Hsp70 or anti-Myc MAbs.
RESULTS
Hsp70 suppresses the Vpr-induced cell cycle arrest.
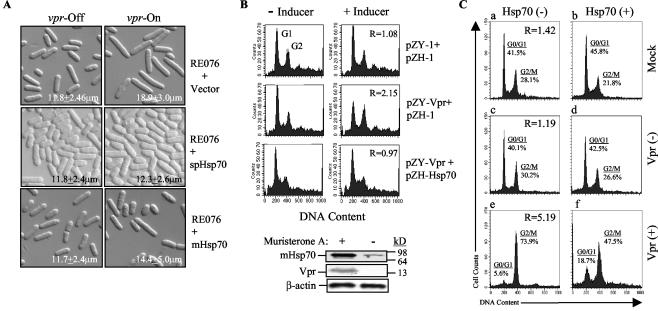
Because the yeast Hsp70 was pulled out in our search for suppressors of Vpr activity in Schizosaccharomyces pombe (unpublished result), we first tested the effect of Hsp70 on cell cycle in fission yeast. The expression vector for the fission yeast or mammalian Hsp70 was introduced into the RE076 S. pombe strain, which carries a single integrated copy of F34Ivpr under the control of an inducible nmt promoter (11). In the fission yeast, the Vpr protein with an F34I mutation is attenuated in the ability to induce cell death but fully retains the capacity to induce cell cycle G2 arrest (11). As shown in Fig. 1A (top right panel), expression of F34Ivpr-induced cell elongation typical of a cell cycle G2/M delay, which is commonly known as the “cdc phenotype” (11, 28, 33). Cell length measurements after 24 h of gene induction indicated that vpr-expressing cells had an average length of 18.9 ± 3.0 μm during log-phase growth, as compared to 11.8 ± 2.4 μm in vpr-repressing cells (top left panel). However, the Vpr-induced cell elongation was strongly suppressed when a cDNA clone encoding either a fission yeast (middle row panels) or the mammalian Hsp70 (bottom row panels) was expressed from the nmt1 promoter. Wild-type Vpr induces cell cycle G2 arrest and leads to cell death. A more heterogeneous size distribution in cells expressing vpr is likely explained by death of some cells due to residual cell-killing activity of F34Ivpr. A small proportion of elongated cells in cultures transfected with Hsp70 constructs likely represents cells that did not express the gene, as, despite culturing transformed cells on a selective medium, there is always a small percentage left with an untransformed phenotype (53). Importantly, Hsp70 did not affect the length of cells where Vpr had not been induced (left panels), indicating that Hsp70 specifically counteracted Vpr-induced G2 arrest in yeast cells. Thus, overexpression of S. pombe or mammalian Hsp70 in yeast inhibited Vpr-induced cell cycle G2 arrest.
FIG. 1.
Hsp70 suppresses Vpr-induced cell cycle G2 arrest. (A) Suppression of Vpr-induced G2 arrest in fission yeast. Vpr-induced cell elongation was used here as an indication of cell cycle G2 arrest (11). RE076 is a fission yeast strain that carries a single integrated copy of F34Ivpr (11, 56). RE076+Vector denotes the RE076 strain transformed with the pYZ1N vector control. RE076+spHsp70 and RE076+mHsp70 are RE076 strains transformed with fission yeast or mammalian Hsp70, respectively. vpr-Off, vpr gene expression suppressed; vpr-On, vpr gene expression induced. (B) Hsp70-mediated suppression of Vpr-induced G2 arrest in 293T-632 cells. Vpr-induced G2 arrest was measured by flow cytometric analysis after staining DNA with propidium iodide. The extent of G2 arrest was evaluated by the relative G2/G1 ratio (shown in the right-hand panels) between cells cultured in the presence and absence of the inducer (muristerone A): R = [G2/G1 (+ inducer)]/[G2/G1 (− inducer)] (57). A Western blot (bottom) shows coexpression of HIV-1 vpr and mammalian Hsp70 genes in muristerone A-induced 293T-632 cells. pZY-1 is a vector used for vpr expression, and pZH-1 was used for mHsp70 expression. Both vectors are induced by addition of 1 μM muristerone A (57). Results are shown for one representative experiment out of three performed. (C) Hsp70 suppresses Vpr-induced G2 arrest in HIV-1-infected cells. MAGI cells were transfected with an Hsp70-expressing vector and 24 h after transfection were infected with a Vpr-positive or Vpr-negative MLV-pseudotyped HIV-1. G2 arrest was analyzed 48 h after infection by flow cytometry as in panel B. The effect of Hsp70 was evaluated by relative G2/G1 ratio (shown in left panels) in cells transfected with Hsp70-expressing vector and control cells: R = [G2/G1 (Hsp70−)]/[G2/G1 (Hsp70+)]. Results are shown for one representative experiment out of two performed.
To extend this result to mammalian cells, we used an episomal system for ecdysone-inducible vpr expression described recently by Zhou and Ratner (57). In this system, vpr and a gene of choice (Hsp70 in our case) are maintained as episomes in 293T cells as part of pZY-1 or pZH-1 vectors, respectively, and can be induced by muristerone A. Results of the FACS analysis of such cells after propidium iodide staining of DNA are shown in Fig. 1B. Results are recalculated as a G2/G1 ratio of induced relative to cells cultured without the inducer: R = [G2/G1 (induced)]/[G2/G1 (uninduced)]. If this value is close to 1, the induced proteins do not affect the cell cycle distribution, while a value of >1 indicates induction of the G2 arrest. A G2/G1 ratio of 1.08 of cells carrying empty vectors in cultures grown with the inducer relative to cultures grown without the inducer (Fig. 1B, upper panels) indicates that muristerone A did not alter normal cell cycle distribution in cells (57). In contrast, a significant increase in this ratio, 2.15, observed in cells carrying vpr-expressing vector and grown with muristerone A indicated that vpr gene expression induced a G2 arrest of the cell cycle (Fig. 1B; middle row). Coexpression of mammalian Hsp70 with vpr blocked the Vpr-induced G2 arrest (G2/G1 ratio, 0.97; Fig. 1B, bottom row). Western blot analysis showed that both Vpr and Hsp70 were properly produced upon induction with muristerone A. The presence of small amounts of Hsp70 in uninduced cells is consistent with its role in normal cellular metabolism (25).
To analyze the effect of Hsp70 on Vpr-induced G2 arrest in the context of HIV-1 infection, we transfected HeLa cells with an Hsp70-expressing vector and infected them with an MLV-pseudotyped HIV-1. The efficiency of transfection of HeLa and derivative MAGI cells was routinely 60 to 70%, as measured by flow cytometry of samples in which a green fluorescent protein (GFP)-expressing vector was added to transfection mixtures. It should be noted that a high virus inoculum (1.1 × 107 cpm of reverse transcriptase activity per 106 cells) was used in this experiment to bring the percentage of infected cells close to 100%. Results presented in Fig. 1C demonstrate a dramatic decrease (5.19-fold) in the G2/G1 ratio in Hsp70-transfected culture infected with a Vpr-positive virus (panel f) as compared to culture transfected with an empty vector (panel e). Some decrease in G2 cells was observed in mock-infected cultures (panels a and b, relative G2/G1 ratio of 1.42) or cultures infected with a Vpr-deficient virus (panels c and d, relative G2/G1 ratio of 1.19), but it was substantially smaller.
Taken together, these results indicate that Hsp70, when overexpressed in an HIV-infected cell, partially reverses the Vpr-induced G2 arrest of the cell cycle progression.
Suppression of Hsp70 expression by RNAi increases the Vpr-dependent apoptosis.
To determine whether Hsp70 reduces Vpr effects during natural HIV-1 infection, we first analyzed the level of Hsp70 in cells infected with HIV-1. Consistent with the results of Brenner and coauthors (50), HIV-1 infection of both MAGI cells (modified HeLa cells expressing the CD4 receptor) and PBMCs resulted in a significant increase in the levels of intracellular Hsp70: from 0.3 ± 0.04 pg/cell to 1.5 ± 0.17 pg/cell (P = 0.009) 48 h after infection of MAGI cells and from 0.1 ± 0.026 pg/cell to 0.3 ± 0.021 pg/cell (P = 0.014) 96 h after infection of PBMCs.
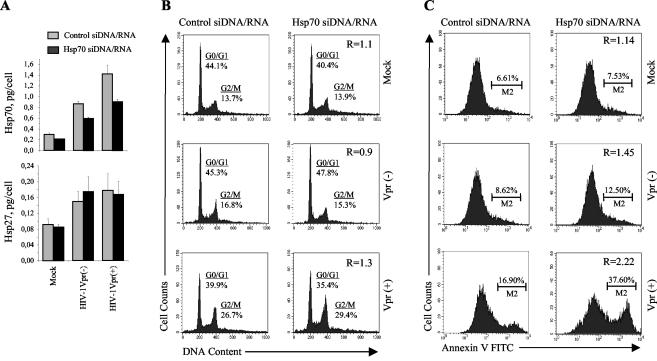
To inhibit Hsp70 expression, we employed the RNAi approach (9). Hybrid DNA/RNA molecules (siDNA/RNA) were used for silencing as such molecules have been shown to exert a much greater effect in both duration and degree of suppression than conventional siRNA (26). MAGI cells were transfected with siDNA/RNA against Hsp70 or control siDNA/RNA and infected with a Vpr-positive or Vpr-defective HIV-1 construct. The specificity of Hsp70 siDNA/RNA was confirmed by analysis of Hsp70 and Hsp27 expression with ELISA kits: while levels of Hsp70 were decreased, the level of Hsp27 was not affected (Fig. 2A). It should be noted here that the human Hsp70 family includes a number of isoforms that still differ in primary nucleotide sequence (44), and several of these isoforms are expressed upon HIV-1 infection (50). While we targeted the main cytosolic form of Hsp70, we did not expect to completely knock out expression of all inducible Hsp70 forms.
FIG. 2.
RNAi-mediated suppression of Hsp70 expression enhances Vpr-specific apoptosis of HIV-1-infected cells. MAGI cells were transfected with Hsp70-specific or control siDNA/RNA duplexes and then infected with Vpr-negative (Vpr−) or Vpr-positive (Vpr+) HIV-1 or mock infected. (A) Hsp70 and Hsp27 were quantified in cell lysates by ELISA 24 h after infection. Results are the mean ± standard error of triplicate wells. (B) Cell cycle distribution was analyzed by FACS 48 h after infection as in Fig. 1B. The effect of siDNA/RNA was evaluated by the ratio of G2/G1 in cultures transfected with control siDNA/RNA to G2/G1 in cultures transfected with Hsp70-specific siDNA/RNA (shown in the left column panels). Results are representative of two independent experiments. (C) Apoptotic cells were stained with Annexin V-FITC and analyzed by flow cytometry 24 h after infection. The effect of siDNA/RNA was evaluated by the ratio of apoptotic cells in cultures transfected with control and Hsp70-specific siDNA/RNA (shown in left column panels). Results are representative of two independent experiments.
The effect of siDNA/RNA on G2 arrest was calculated as a relative G2/G1 ratio between cells transfected with control and Hsp70 siDNA/RNA: R = [G2/G1(Hsp70 siDNA)]/[G2/G1 (control siDNA/RNA)]. In mock-infected cells, this ratio was 1.1, indicating that Hsp70 siDNA/RNA did not change the cell cycle distribution (Fig. 2B, upper panels). A close ratio (R = 0.9) was found in cells infected with a Vpr-deficient virus (middle row of panels). In cells infected with a Vpr-expressing HIV-1 construct, which induced substantial G2 arrest in control cells, Hsp70 siDNA/RNA slightly increased the number of G2-arrested cells, as evidenced by the relative G2/G1 ratio of 1.3 (bottom panels). While this effect was relatively small, it was reproduced in two independent experiments.
Following the Vpr-induced G2 arrest, cells go into apoptosis and die (41). The mechanism of Vpr-induced apoptosis is unclear (22, 42, 51) and may or may not be related to the mechanism of G2 arrest by Vpr (10, 35). Importantly, the apoptotic activity of Vpr is a recognized pathogenic factor in HIV-1 infection, contributing significantly to HIV-induced apoptosis of infected and bystander cells (2, 23, 29, 42). Given that Hsp70 reduces Vpr-induced G2 arrest, we investigated the effect of Hsp70 on Vpr-dependent apoptosis associated with HIV-1 infection. We used the same approach as for analysis of cell cycle progression (Fig. 2B), except that the percentage of apoptotic cells in cultures treated with Hsp70 siDNA/RNA relative to cultures treated with control siDNA/RNA was calculated: R = [M2(Hsp70 siDNA/RNA)]/[M2(control siDNA/RNA)]. Consistent with previous reports (34), infection of MAGI cells transfected with control siDNA/RNA with Vpr-expressing HIV-1 increased the percentage of apoptotic cells (M2 gate on the histogram) from 6.61% observed in mock-infected culture to 16.90% (left panels in Fig. 2C). The percentage of apoptotic cells was dramatically increased in cultures transfected with Hsp70 siDNA/RNA and infected with Vpr-expressing HIV-1 (R = 2.22; bottom panels). No such increase was observed in mock-infected cultures (R = 1.14), and only a minor increase was detected in cultures infected with Vpr-deficient virus (R = 1.45). These results suggest that the antiapoptotic activity of Hsp70 preferentially targets Vpr-specific apoptosis.
The effect of Hsp70 on HIV-1 replication.
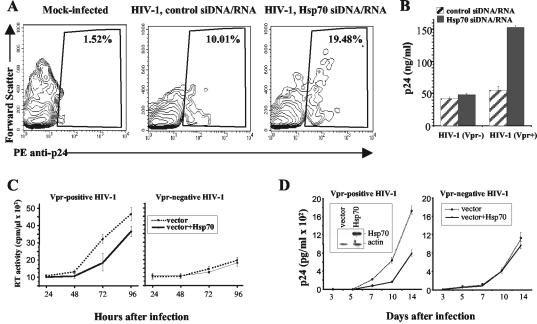
The ultimate purpose of HIV-1 accessory genes is to provide replication advantage to the virus. The capacity of Vpr to induce G2 growth arrest has been implicated as a positive factor for HIV-1 replication in vitro (16, 19). Our finding that Hsp70 counteracts this Vpr activity suggested that it may also reduce HIV-1 replication. We first tested this premise in MAGI cells transfected with Hsp70 siDNA/RNA. HIV-1 replication was analyzed 48 h after infection of MAGI cells by flow cytometry after staining the intracellular p24 (Fig. 3A) and by measuring extracellular p24 (Fig. 3B). It should be noted here that staining of intracellular p24 in this experiment does not detect all infected cells, as p24 expression in many cells at 48 h postinfection (this time point was chosen to correlate analysis of virus replication and cell cycle distribution) does not yet reach the levels that can be detected by this assay (32). A substantial increase in both the number of p24-expressing cells (from 10% to 19.5%) and the amount of extracellular p24 (from 45 ng/ml to 150 ng/ml) was observed in cultures transfected with Hsp70 siDNA/RNA and infected with a Vpr-positive HIV-1 as compared to cultures transfected with control siDNA/RNA. No such increase was seen with a Vpr-deficient virus (Fig. 3B), demonstrating that the observed effect was Vpr dependent.
FIG. 3.
Analysis of Hsp70 effects on HIV-1 replication. (A) MAGI cells were transfected with control or Hsp70 siDNA/RNA. Twenty-four hours after transfection, cells were infected with HIV-1 or mock infected. HIV-1 replication was analyzed 48 h after infection by staining intracellular p24 essentially as described previously (32). The percentage of p24-positive cells is shown. For mock-infected cells, only results with Hsp70 siDNA/RNA-transfected cells are presented. (B) The experiment was performed as in panel A, except that cells were infected with Vpr-positive (Vpr+) or Vpr-negative (Vpr−) HIV-1 and extracellular p24 was measured by ELISA. Results show mean ± standard deviation of three independent wells. (C) Triplicate cultures of MAGI cells, transfected with either an Hsp70-expressing (vector+Hsp70) or an empty vector, were infected with Vpr-positive or Vpr-negative HIV-1. Virus replication was measured at indicated times after infection by reverse transcriptase activity in culture supernatants. Results are presented as means ± standard deviation. (D) Triplicate cultures of H9 cells stably transfected with Hsp70-expressing (vector+Hsp70) or an empty vector were infected with Vpr-positive or Vpr-negative HIV-1. p24 in culture supernatants was measured at indicated time points after infection by ELISA. Results are presented as the mean ± standard deviation. The inset in the left panel shows Western blot analysis of Hsp70 and β-actin proteins in transfected H9 cultures.
Conversely, overexpression of Hsp70 reduced replication of the Vpr-positive HIV-1 in MAGI cells without affecting replication of the Vpr-negative virus (Fig. 3C). Of note, the MAGI cells were transiently transfected with the Hsp70-expressing vector; therefore, the experiment was not continued beyond the 96-h time point, when expression of Hsp70 started to subside (not shown). To determine the effects of Hsp70 on spreading HIV-1 infection, we analyzed replication of Vpr-positive and Vpr-negative viruses in H9 cells (a CD4+ T-cell line) stably transfected with Hsp70. Results presented in Fig. 3D demonstrate that replication of Vpr-positive, but not of a Vpr-deficient HIV-1, was reduced in Hsp70-transfected cells relative to cells transfected with an empty vector.
We conclude that Hsp70 exerts a certain degree of protection against HIV-1 infection.
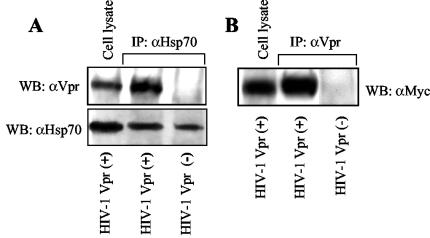
Hsp70 coimmunoprecipitates with Vpr.
The inhibitory effect of Hsp70 on such distinct activities of Vpr as G2 arrest and apoptosis suggested that Hsp70 might bind to Vpr, thus affecting its interaction with cellular partners. To test this possibility, we immunoprecipitated Hsp70 from HIV-1-infected MAGI cells and analyzed the immunoprecipitate by using Vpr- and Hsp70-specific antibodies. As shown in Fig. 4A, Vpr was detected in the immunoprecipitates from cells infected with a Vpr-positive, but not with Vpr-defective, HIV-1. No Vpr was detected when immunoprecipitation was performed with an antiactin antibody (not shown), demonstrating specificity of the observed interaction. In a reciprocal experiment, Vpr was immunoprecipitated from MAGI cells transfected with myc-tagged Hsp70 and infected with Vpr-positive HIV-1. Analysis using anti-Myc antibody revealed that Hsp70 was coimmunoprecipitated with Vpr (Fig. 4B). No actin was found in the immunoprecipitates (not shown), confirming the specificity of the observed interaction. These results demonstrate that Hsp70 interacts with Vpr within an HIV-infected cell. Therefore, Hsp70-Vpr interaction, which was initially observed in macrophages (21), can be extended now to other cell types.
FIG. 4.
Hsp70 coimmunoprecipitates with Vpr from HIV-1-infected cells. (A) MAGI cells were infected with Vpr-positive (Vpr+) or Vpr-negative (Vpr−) HIV-1. Forty-eight hours after infection, cells were lysed and immunoprecipitated with a polyclonal anti-Hsp70 antibody. The immunoprecipitates were analyzed for the presence of Hsp70 (using anti-Hsp70 MAb) and Vpr (using polyclonal anti-Vpr antibody) by Western blotting (WB). (B). MAGI cells were transfected with Myc-tagged Hsp70 and then infected with Vpr-positive or Vpr-negative HIV-1. Immunoprecipitation was performed with anti-Vpr polyclonal antibody and revealed by anti-Myc MAb.
DISCUSSION
In this report, we demonstrate that Hsp70 is induced in HIV-infected cells to target HIV-1 Vpr and reduce Vpr-dependent G2 arrest and apoptosis. Hsp70 also reduces HIV-1 replication in a Vpr-dependent fashion. Therefore, Hsp70 appears to function as a component of the innate cellular immunity against HIV-1. This new activity of Hsp70 is distinct from previously described mechanisms involving binding of Hsp70 to viral complexes to facilitate antigen presentation and enhance antiviral immunity, including antibody-dependent cellular cytotoxicity and cytotoxic T-lymphocyte-mediated killing of HIV-1-infected cells (4). While the latter activities of Hsp70 are related to the recognized ability of these proteins to stimulate antigen-presenting cells (3), the new role of Hsp70 identified in this report is to neutralize deleterious activities of a particular HIV-1 protein, Vpr. Interestingly, Vpr has been shown recently to be targeted by another intracellular chaperone molecule, cyclophilin A (52). However, in contrast to Hsp70, which inhibits Vpr activities in target cells, CypA actually assists Vpr expression in virus-producing cells and does not affect Vpr packaging. It remains to be determined whether Hsp70 bound to Vpr in a producing cell diminishes Vpr packaging. Another interesting question for future studies is whether Vpr contributes to Hsp70 incorporation into HIV-1 virions and what is the role (if any) of virion-associated Hsp70 in the new cycle of infection. Previous study (17) demonstrated that Gag is sufficient for Hsp70 incorporation, which, together with an established role of Vpr in the early steps of infection, argues that Vpr does not get in contact with Hsp70 within the virion.
The human Hsp70 family encompasses at least 11 genes that encode a group of highly related proteins, which include both cognate and highly inducible members located in various subcellular compartments (46). Hsp70, as well as its constitutively expressed and only slightly inducible form, Hsc70, is located in the cytoplasm, and therefore both forms have the opportunity to interact with Vpr. Results of subtractive immunoprecipitation suggest that both Hsp70 and Hsc70 can bind to Vpr (S. Iordanskiy, unpublished observation). Thus, it is likely that several Hsp70 isoforms contribute to the anti-Vpr activity in HIV-1-infected cells. Interestingly, suppression of Hsp70 by RNAi had little effect on Vpr-mediated G2 arrest but substantially increased Vpr-specific apoptosis in HIV-1-infected cell cultures (Fig. 2). This result suggests that the Hsp70 isoform targeted by siDNA/RNA used in our experiments has specificity for apoptosis-related factors activated by Vpr. Therefore, in addition to direct interaction with Vpr (Fig. 4), Hsp70 isoforms may reduce Vpr-specific effects by targeting Vpr-activated signaling pathways that lead to G2 arrest or apoptosis.
The anti-HIV activity of Hsp70 reported here seems at odds with our previous observation that Hsp70 can replace Vpr in HIV-1 nuclear import (1) and is therefore expected to stimulate HIV-1 nuclear translocation and replication. It should be noted that the current report deals with proliferating cells, where Vpr-dependent nuclear translocation is less critical for HIV-1 replication than in such nonproliferating cells as macrophages (49). Therefore, while active nuclear import seems to be important for HIV-1 infection of both proliferating and nonproliferating cells (24), it remains possible that Vpr does not contribute much to HIV-1 nuclear transport in dividing cells. In addition, the effect on viral replication was tested in this study by measuring intracellular or extracellular p24, thus reflecting the cumulative effect of pre- and postintegration steps of viral replication. Inhibition of postintegration steps could overcome any stimulatory effect of Hsp70 on HIV-1 nuclear import.
The results presented in this report suggest that high levels of Hsp70 could protect cells from HIV-1-specific cytotoxicity and reduce virus replication. One interesting possibility is that stressed cells may be less susceptible to HIV infection. Since Hsp70 is induced by a variety of physical and biochemical stressors, including bacterial and viral infection (39), it may be a factor that, in addition to cytokines (30), determines the outcome of HIV coinfection with another pathogen.
In summary, our results demonstrate that Hsp70 contributes to cellular innate anti-HIV immunity by targeting activity of Vpr. The balance between Vpr and Hsp70 may determine, at least in part, the level of HIV-1 replication and cytopathology. The Hsp70-mediated protection may be overwhelmed at late stages of infection, resulting in significant G2 arrest and high HIV-1 replication. Understanding the molecular details of Hsp70 interaction with Vpr could suggest new therapeutic approaches aimed at inactivating this pathogenic viral protein.
Acknowledgments
We would like to thank Josephine Sire (INSERM, Marseille, France) for anti-Vpr antibody, Lee Ratner (Washington University, St. Louis, Mo.) for providing the muristerone A-inducible gene expression vectors and 293T-632 cell lines, and Richard Vile (Mayo Clinic, Rochester, Minn.) for Hsp70/Myc plasmid. pcDNA-Env(MLV) plasmid encoding Env of the amphotropic MLV (from N. Landau) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported by NIH grants R01 AI33776 and R21 AI53806 to M.B. and AI40891 and GM63080 to Y.Z.
REFERENCES
- 1.Agostini, I., S. Popov, J. Li, L. Dubrovsky, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral pre-integration complex. Exp. Cell Res. 259:398-403. [DOI] [PubMed] [Google Scholar]
- 2.Azad, A. A. 2000. Could nef and vpr proteins contribute to disease progression by promoting depletion of bystander cells and prolonged survival of HIV-infected cells? Biochem. Biophys. Res. Commun. 267:677-685. [DOI] [PubMed] [Google Scholar]
- 3.Basu, S., and P. K. Srivastava. 2000. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones 5:443-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, B. G., and Z. Wainberg. 2001. Heat shock proteins: novel therapeutic tools for HIV-infection? Expert Opin. Biol. Ther. 1:67-77. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M., R. T. Elder, M. Yu, M. G. O'Gorman, L. Selig, R. Benarous, A. Yamamoto, and Y. Zhao. 1999. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J. Virol. 73:3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conti, L., P. Matarrese, B. Varano, M. C. Gauzzi, A. Sato, W. Malorni, F. Belardelli, and S. Gessani. 2000. Dual role of the HIV-1 vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 165:3293-3300. [DOI] [PubMed] [Google Scholar]
- 7.de Noronha, C. M. C., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzio, P., S. Choe, M. Ebright, R. Knoblauch, and N. R. Landau. 1995. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 69:7909-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley, N. R., and B. Goldstein. 2003. RNA interference: silencing in the cytoplasm and nucleus. Curr. Opin. Mol. Ther. 5:113-117. [PubMed] [Google Scholar]
- 10.Elder, R. T., M. Yu, M. Chen, S. Edelson, and Y. Zhao. 2000. Cell cycle G2 arrest induced by HIV-1 Vpr in fission yeast (Schizosaccharomyces pombe) is independent of cell death and early genes in the DNA damage checkpoint. Virus Res. 68:161-173. [DOI] [PubMed] [Google Scholar]
- 11.Elder, R. T., M. Yu, M. Chen, X. Zhu, M. Yanagida, and Y. Zhao. 2001. HIV-1 Vpr induces cell cycle G2 arrest in fission yeast (Schizosaccharomyces pombe) through a pathway involving regulatory and catalytic subunits of PP2A and acting on both Wee1 and Cdc25. Virology 287:359-370. [DOI] [PubMed] [Google Scholar]
- 12.Fukumori, T., H. Akari, S. Iida, S. Hata, S. Kagawa, Y. Aida, A. H. Koyama, and A. Adachi. 1998. The HIV-1 Vpr displays strong anti-apoptotic activity. FEBS Lett. 432:17-20. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor, E. M., and I. S. Chen. 2001. Analysis of apoptosis induced by HIV-1 Vpr and examination of the possible role of the hHR23A protein. Exp. Cell Res. 267:243-257. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 16.Gummuluru, S., and M. Emerman. 1999. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J. Virol. 73:5422-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurer, C., A. Cimarelli, and J. Luban. 2002. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol. 76:4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzinger, N. K., M. I. Bukrinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iordanskiy, S., Y. Zhao, P. DiMarzio, I. Agostini, L. Dubrovsky, and M. Bukrinsky. 27 May 2004. Heat-shock protein 70 exerts opposing effects on Vpr-dependent and Vpr-independent HIV-1 replication in macrophages. Blood 10.1182/blood-2004-01-0081. [DOI] [PubMed]
- 22.Jacotot, E., L. Ravagnan, M. Loeffler, K. F. Ferri, H. L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J. P. Briand, T. Irinopoulou, E. Daugas, S. A. Susin, D. Cointe, Z. H. Xie, J. C. Reed, B. P. Roques, and G. Kroemer. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacque, J. M., K. Triques, and M. Stevenson. 2002. Modulation of HIV-1 replication by RNA interference. Nature 418:435-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz, R. A., J. G. Greger, P. Boimel, and A. M. Skalka. 2003. Human immunodeficiency virus type 1 DNA nuclear import and integration are mitosis independent in cycling cells. J. Virol. 77:13412-13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiang, J. G., and G. C. Tsokos. 1998. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol. Ther. 80:183-201. [DOI] [PubMed] [Google Scholar]
- 26.Lamberton, J. S., and A. T. Christian. 2003. Varying the nucleic acid composition of siRNA molecules dramatically varies the duration and degree of gene silencing. Mol. Biotechnol. 24:111-120. [DOI] [PubMed] [Google Scholar]
- 27.Lang, S. M., M. Weeger, C. Stahl-Hennig, C. Coulibaly, G. Hunsmann, J. Müller, H. Müller-Hermelink, D. Fuchs, H. Wachter, M. M. Daniel, R. C. Desrosiers, and B. Fleckenstein. 1993. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 67:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, M., and P. Nurse. 1988. Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 4:287-290. [DOI] [PubMed] [Google Scholar]
- 29.Lum, J. J., O. J. Cohen, Z. Nie, J. G. Weaver, T. S. Gomez, X. J. Yao, D. Lynch, A. A. Pilon, N. Hawley, J. E. Kim, Z. Chen, M. Montpetit, J. Sanchez-Dardon, E. A. Cohen, and A. D. Badley. 2003. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Investig. 111:1547-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolis, L. 2003. Cytokines—strategic weapons in germ warfare? Nat. Biotechnol. 21:15-16. [DOI] [PubMed] [Google Scholar]
- 31.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 32.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda, M., Y. Nagai, N. Oshima, K. Tanaka, H. Murakami, H. Igarashi, and H. Okayama. 2000. Genetic studies with the fission yeast Schizosaccharomyces pombe suggest involvement of Wee1, Ppa2, and Rad24 in induction of cell cycle arrest by human immunodeficiency virus type 1 Vpr. J. Virol. 74:2636-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthumani, K., D. S. Hwang, B. M. Desai, D. Zhang, N. Dayes, D. R. Green, and D. B. Weiner. 2002. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J. Biol. Chem. 277:37820-37831. [DOI] [PubMed] [Google Scholar]
- 35.Nishizawa, M., M. Kamata, T. Mojin, Y. Nakai, and Y. Aida. 2000. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G2 arrest of the cell cycle. Virology 276:16-26. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Y. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 38.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santoro, M. G. 2000. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 59:55-63. [DOI] [PubMed] [Google Scholar]
- 40.Somasundaran, M., M. Sharkey, B. Brichacek, K. Luzuriaga, M. Emerman, J. L. Sullivan, and M. Stevenson. 2002. Evidence for a cytopathogenicity determinant in HIV-1 Vpr. Proc. Natl. Acad. Sci. USA 99:9503-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart, S. A., B. Poon, J. B. M. Jowett, and I. S. Y. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subbramanian, R. A., A. Kessous-Elbaz, R. Lodge, J. Forget, X. J. Yao, D. Bergeron, and E. A. Cohen. 1998. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med. 187:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tavaria, M., T. Gabriele, I. Kola, and R. L. Anderson. 1996. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones 1:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todryk, S., A. A. Melcher, N. Hardwick, E. Linardakis, A. Bateman, M. P. Colombo, A. Stoppacciaro, and R. G. Vile. 1999. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 163:1398-1408. [PubMed] [Google Scholar]
- 46.Todryk, S. M., M. J. Gough, and A. G. Pockley. 2003. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology 110:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tristem, M., C. Marshall, A. Karpas, and F. Hill. 1992. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11:3405-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tristem, M., A. Purvis, and D. L. Quicke. 1998. Complex evolutionary history of primate lentiviral vpr genes. Virology 240:232-237. [DOI] [PubMed] [Google Scholar]
- 49.Vodicka, M. A. 2001. Determinants for lentiviral infection of non-dividing cells. Somat. Cell Mol. Genet. 26:35-49. [DOI] [PubMed] [Google Scholar]
- 50.Wainberg, Z., M. Oliveira, S. Lerner, Y. Tao, and B. G. Brenner. 1997. Modulation of stress protein (hsp27 and hsp70) expression in CD4+ lymphocytic cells following acute infection with human immunodeficiency virus type-1. Virology 233:364-373. [DOI] [PubMed] [Google Scholar]
- 51.Yuan, H., Y.-M. Xie, and I. S. Y. Chen. 2003. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J. Virol. 77:2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zander, K., M. P. Sherman, U. Tessmer, K. Bruns, V. Wray, A. T. Prechtel, E. Schubert, P. Henklein, J. Luban, J. Neidleman, W. C. Greene, and U. Schubert. 2003. Cyclophilin A interacts with HIV-1 Vpr and is required for its functional expression. J. Biol. Chem. 278:43202-43213. [DOI] [PubMed] [Google Scholar]
- 53.Zhao, Y., J. Cao, M. R. G. O'Gorman, M. Yu, and R. Yogev. 1996. Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular function of fission yeast Schizosaccharomyces pombe. J. Virol. 70:5821-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, Y., M. Chen, B. Wang, J. Yang, R. T. Elder, X. Q. Song, M. Yu, and N. K. Saksena. 2002. Functional conservation of HIV-1 Vpr and variability in a mother-child pair of long-term non-progressors. Virus Res. 89:103-121. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, Y., and R. T. Elder. 2000. Yeast perspectives on HIV-1 VPR. Front. Biosci. 5:D905-D916. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, Y., R. T. Elder, M. Chen, and J. Cao. 1998. Fission yeast expression vectors adapted for positive identification of gene insertion and green fluorescent protein fusion. BioTechniques 25:438-440, 442, 444. [DOI] [PubMed] [Google Scholar]
- 57.Zhou, Y., and L. Ratner. 2001. A novel inducible expression system to study transdominant mutants of HIV-1 Vpr. Virology 287:133-142. [DOI] [PubMed] [Google Scholar]