Abstract
It is often stated that individuals of a species can differ significantly in their innate resistance to infection with herpes simplex virus type 1 (HSV-1). Three decades ago Lopez reported that C57BL/6 mice could survive a 5,000-fold-higher inoculum of HSV-1 given intraperitoneally than mice of the A or BALB/c strain (Nature 258:152-153, 1975). Susceptible strains of mice died of encephalitis-like symptoms, suggesting that viral spread to the central nervous system was the cause of death. Although Lopez's study documented that C57BL/6 mice were resistant to the development of HSV-1 encephalitis and mortality, the resistance of C57BL/6 mice to other steps of the HSV-1 infection process was not assessed. The results of the present study extend these observations to clarify the difference between resistance to (i) HSV-1 pathogenesis, (ii) HSV-1 replication, (iii) HSV-1 spread, and (iv) the establishment of latent HSV-1 infection. Although C57BL/6 mice are more resistant to HSV-1 pathogenesis than BALB/c mice, the results of the present study establish that HSV-1 enters, replicates, spreads, and establishes latent infections with virtually identical efficiencies in C57BL/6 and BALB/c mice. These observations raise questions about the validity of the inference that differences in natural resistance are relevant in explaining what differentiates humans with recurrent herpetic disease from the vast majority of asymptomatic carriers of HSV-1 and HSV-2.
C57BL/6 mice are more resistant than other inbred strains of mice to the pathogenesis caused by inoculation with murine cytomegalovirus (CMV) (27, 49), poxviruses (23, 50), polyomavirus (10), Trypanosoma congolense (61), Leishmania major (46), Angiostrongylus costaricensis (16), and Brucella abortus (4). In many of these studies, microbes are injected directly into the peritoneal cavity, and little to no data are presented on the kinetics of microbial replication and spread in the host. Nonetheless, it is often implied that the reduced pathogenesis observed in C57BL/6 mice is a consequence of the inefficient replication of the infectious agent.
A 1975 publication entitled “Genetics of natural resistance to herpesvirus infections in mice” stimulated considerable interest in the hypothesis that animal hosts can differ significantly in their natural susceptibility to infection with herpes simplex virus type 1 (HSV-1) (30). The intriguing observation was made that C57BL/6 mice survive intraperitoneal (i.p.) inoculation with 106 PFU of HSV-1, while the A and BALB/c mouse strains die after i.p. inoculation with 106, 105, or 104 PFU of HSV-1 (30). These observations revealed what appeared to be a previously unrecognized principle of the host-virus interaction: animal hosts can differ tremendously in their susceptibility to HSV-1 pathogenesis. An extensive literature developed out of this observation, and by 1982 it was found that a rapid innate alpha/beta interferon (IFN-α/β) response was integral to the increased resistance of C57BL/6 mice to HSV-1 pathogenesis following i.p. challenge with 106 PFU of HSV-1 (26, 65-67).
Physiologically relevant combinations of IFN-α/β (0.075 nM) and IFN-γ (1.5 nM) can synergize to inhibit HSV-1 replication by ∼1,000-fold in vitro (47). Likewise, treatment of mouse eyes with combinations of recombinant IFN-β and IFN-γ prior to ocular challenge with 105 PFU of HSV-1 causes (i) a >1,000-fold reduction in viral titers in the tear film 24 h after inoculation and (ii) a >200-fold reduction in the HSV-1 genome load in latently infected trigeminal ganglia (TG) (47). In light of these observations, we were intrigued by the possibility that the natural resistance of C57BL/6 mice might be due to the combined activities of a rapid IFN-α response (65) and a rapid NK cell-mediated response that could rapidly deliver IFN-γ to sites of viral infection (41). However, upon reviewing the literature, we were struck by the lack of quantitative methods used to measure the magnitude of natural resistance to HSV-1 replication and spread.
Reduced viral pathogenesis and increased survival are the most prevalent outcomes that have been used to measure the natural resistance of C57BL/6 mice to HSV-1 challenge (2, 30, 38, 40, 55). Measurements of pathogenesis and survival frequency are qualitative measurements and can be compared only by nonparametric statistics. More importantly, pathogenesis and death are end points that occur 5 to 10 days after HSV-1 inoculation and are the cumulative results of the interaction of the hundreds of variables that dictate the efficiency of viral replication, viral spread, innate immune responses, local inflammatory responses, and the development of an acquired immune response. Therefore, although an extensive literature establishes that C57BL/6 mice are more resistant to HSV-1 pathogenesis than susceptible mouse strains, such measurements do not address the quantitative question, “How much more resistant are C57BL/6 mice to HSV-1 infection than susceptible mouse strains?”
Many studies have addressed facets of this question, but a clear answer has never emerged. Initial comparisons of the 50% lethal doses of HSV-1 in C57BL/6 and BALB/c mice suggested that C57BL/6 mice were >1,000 times more resistant to HSV-1 challenge than BALB/c mice. The experimental basis for this conclusion was that C57BL/6 mice survived i.p. challenge with 106 PFU of HSV-1, whereas 50% of BALB/c mice died after i.p. challenge with just 200 PFU of HSV-1 (30). The difference in survival is striking. However, the ratio of these 50% lethal doses does not provide a valid basis for concluding that C57BL/6 mice are >1,000 times more resistant to HSV-1 than BALB/c mice (40, 51, 52). Kirchner and colleagues established this point when they demonstrated that although most C57BL/6 mice survive i.p. challenge with 106 PFU of HSV-1, a 100-fold reduction in the viral inoculum dramatically alters the outcome; C57BL/6 mice do not usually survive i.p. challenge with 104 PFU of HSV-1 (65). This observation was explained by the finding that 104 PFU of HSV-1 is sufficient to establish a viral infection in C57BL/6 mice but is not sufficiently immunogenic to trigger a rapid IFN-α/β response (65).
Kirchner's unexpected results brought into sharp focus the importance of the question, “How much more resistant are C57BL/6 mice to HSV-1 infection than susceptible mice?” A clear consensus that resolves this question never emerged in the literature. Some reports indicated that C57BL/6 mouse cells are far less permissive for HSV-1 replication than BALB/c mouse cells (1, 3, 59). Others concluded that C57BL/6 mouse cells and BALB/c mouse cells are equally permissive for HSV-1 replication (13, 30). Some suggested that the in vivo replication and spread of HSV-1 are significantly different in C57BL/6 and BALB/c mice (24, 40, 58). The 1989 report of Simmons and La Vista indicated that HSV-1 replicates to equivalent titers in the skin of C57BL/10 and BALB/c mice but that the spread of viral infection to the spinal ganglia is restricted in C57BL/10 mice (52). More recently, the results of Ellison et al. indicated that there is no difference between the numbers of HSV-1 genomes found in the latently infected TG of C57BL/6 and BALB/c mice (14).
Although an extensive literature developed out of Lopez's landmark observation (30), a satisfactory answer has never emerged to one fundamentally important question: “Should the inherited resistance of C57BL/6 mice to pathogenesis be considered (i) a broadly applicable paradigm of inherited resistance to HSV-1 or (ii) an isolated experimental phenomenon?” Like all scientific principles, the answer to this question lies in the predictive value of the paradigm. The predictive value of the paradigm that C57BL/6 mice are inherently resistant to HSV-1 can be objectively assessed by determining how much more resistant C57BL/6 mice are to the processes of HSV-1 infection, replication, spread, and pathogenesis than susceptible mouse strains, such as BALB/c mice. Given the ambiguity in the published literature on these points, we initiated the present study to address the following questions: “Relative to susceptible BALB/c mice, to what extent does the increased resistance of C57BL/6 mice cause (i) a reduction in HSV-1 replication and spread from the site of inoculation, (ii) a reduction in the number of latent HSV-1 genomes established in the TG, (iii) a reduction in the pathogenesis of HSV-1 infections, and (iv) an increase in the innate resistance of C57BL/6 scid mice to HSV-1 infection relative to the innate resistance of BALB/c scid mice?”
Experimental results that address these questions are presented herein. Additionally, the effect of natural resistance was compared to two other variables that are potentially relevant in dictating the outcomes of HSV-1 infection, namely, (i) differences in viral virulence and (ii) the extent of damage to the keratinized epithelial barrier at the site of viral inoculation. In light of our results, we conclude that the resistance of C57BL/6 mice to the processes of HSV-1 infection, replication, spread, and pathogenesis is generally overstated in the literature.
MATERIALS AND METHODS
Cells, viruses, and mice.
Vero cells (American Type Culture Collection, Manassas, Va.) were propagated in Dulbecco's modified Eagle medium (DMEM) containing 0.15% HCO3− supplemented with 10% fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 mg/ml) (referred to below as complete DMEM). Wild-type HSV-1 strains KOS (53) and McKrae (25) were propagated in Vero cells. A recombinant strain of KOS that expresses green fluorescent protein (GFP), KOS-GFP, was constructed by the insertion of a CMV immediate-early promoter-GFP gene cassette into the intergenic region between the UL26 and UL27 genes of HSV-1 strain KOS.
Female BALB/c, C57BL/6, BALB/c scid, and C57BL/6 scid mice (5 to 6 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the guidelines of the Institutional Animal Care and Use Committee where the work was performed. Prior to HSV-1 infection, mice were anesthetized by i.p. administration of xylazine (6.6 mg/kg of body weight) and ketamine (100 mg/kg). Mice were inoculated by scarifying the cornea with a 26-gauge needle, blotting tear film from the eyes, and placing 105 PFU of virus (in a volume of 4 μl) on each eye. In experiments in which a light scarification procedure was used, mouse eyes were scratched twice from front to back and twice from top to bottom. When heavy scarification was used, mouse eyes were scratched ∼15 times from front to back and ∼15 times from top to bottom.
Analysis of HSV-1 replication in mice.
Viral titers in the ocular tear films of mice were determined by swabbing the ocular surfaces of both eyes at the indicated times after inoculation with a cotton-tipped applicator. Following collection of a tear film sample, the tip of the applicator was placed in 0.4 ml of complete DMEM and viral titers were determined by a microtiter plate plaque assay. Fluorescent photographs of the faces of mice infected with HSV-1 strain KOS-GFP were taken at an original magnification of ×2 on a Nikon TE300 inverted fluorescent microscope (Nikon Instruments, Lewisville, Tex.). Mice were anesthetized by i.p. administration of xylazine (6.6 mg/kg) and ketamine (100 mg/kg), and a DXC-970MD charge-coupled device camera (Sony Corporation, New York, N.Y.) and Metavue software (Universal Imaging Corporation, Downingtown, Pa.) were used to capture 8 to 10 photographs that covered the right side of each mouse's face. Digital images were stitched together by using Paint Shop Pro (Jasc Software, Eden Prairie, Minn.).
Measurement of HSV-1 DNA load in latently infected mouse TG.
DNA was isolated from the combined left and right TG of mice 32 days after inoculation by a phenol-chloroform extraction procedure (60), and the number of HSV-1 genomes per TG was determined by competitive PCR as described previously (18, 19). A solution containing 1× Taq buffer, 50 μM each deoxynucleoside triphosphate, 0.25 μM each VP16 primer (17), 5% glycerol, and 230 fg of a VP16 competitor template per ml (∼2,000 competitors per 50-μl reaction mixture) was prepared. Forty-two microliters of this master mix was placed in 0.65-ml tubes and overlaid with mineral oil, and 100 ng of TG DNA (3 μl) was added to each tube. The tubes were heated to 90°C in a thermal cycler, and 2.5 U of Taq polymerase diluted in 5 μl of Taq buffer was added to each sample. PCR samples were incubated for 35 cycles of 94°C for 1 min, 15 s, 59.5°C for 1 min, 15 s, and 72°C for 40 s. VP16 and competitor PCR product yields were measured on a Cyclone PhosphorImager (Perkin-Elmer Life Sciences, Boston, Mass.) as previously described (19, 22).
Statistics.
Analysis of numerical data and statistical analyses were performed with the Microsoft Excel, Modstat (Modern Microcomputers, Mechanicsville, Va.), and CoStat (Cohort Software, Monterey, Calif.) software packages. All data are presented as means ± standard errors of the means (SEM). All viral titers were transformed by adding a value of 1 such that all data could be plotted and analyzed on a logarithmic scale.
RESULTS
KOS replicates efficiently in the eyes of C57BL/6 mice.
The replication of HSV-1 strain KOS was compared in the eyes of C57BL/6 and BALB/c mice. Mouse eyes received light scarification (4 scratches per eye) and were inoculated with 105 PFU of KOS per eye (n = 8 mice per group). Between days 1 and 5 postinoculation (p.i.), KOS replicated to high and equivalent titers in the eyes of C57BL/6 and BALB/c mice (data not shown). Shedding of KOS ceased in the eyes of both C57BL/6 and BALB/c mice by day 7 p.i. (data not shown). Despite extensive replication and viral shedding at the site of inoculation, 100% of the mice survived infection with KOS, and little to no overt pathogenesis was observed in C57BL/6 and BALB/c mice. Specifically, neither C57BL/6 nor BALB/c mice infected with KOS developed any symptoms of herpes encephalitis (i.e., hyperexcitability, tremors, hindlimb paralysis, etc.), and mice of both strains displayed little to no fur loss in the periocular skin and developed little scarring of the corneal tissues beyond that caused by the process of corneal scarification. In short, the majority of KOS-infected C57BL/6 and BALB/c mice were not readily distinguishable from uninfected mice. Thus, HSV-1 strain KOS replicates efficiently in the eyes of C57BL/6 and BALB/c mice for the first 5 days after inoculation but causes little to no overt pathogenesis in these animals.
KOS-GFP replicates efficiently in the eyes of C57BL/6 mice.
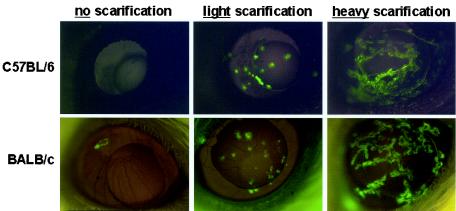
The expression of GFP from a recombinant virus, HSV-1 strain KOS-GFP, was compared in the eyes of C57BL/6 and BALB/c mice that received either no, light, or heavy scarification. In mice that received no scarification, only a single focus of GFP expression was visible in 1 of 10 inoculated BALB/c mouse eyes by 24 h p.i. (Fig. 1). GFP expression was not visible at 24 h p.i. in any eyes of C57BL/6 mice that received no scarification (Fig. 1). In C57BL/6 and BALB/c mice that received light or heavy scarification (4 or ∼30 scratches per eye, respectively), high levels of GFP expression were observed at 24 h p.i. in the corneal lesions produced by scarification but not in the remainder of the eye (Fig. 1). GFP expression in mouse eyes was directly proportional to the amount of corneal scarification, but the extent of green fluorescent viral infection did not appreciably differ between C57BL/6 and BALB/c mice (Fig. 1).
FIG. 1.
Foci of KOS-GFP infection in the eyes of C57BL/6 and BALB/c mice. C57BL/6 and BALB/c mice that received either 0, 4, or ∼30 scratches per eye (no, light, or heavy scarification, respectively) were inoculated with 105 PFU of HSV-1 strain KOS-GFP/eye (n = 5 mice per group). Twenty-four hours after inoculation, mouse eyes were photographed under illumination with the 360- to 400-nm spectrum of light that excites GFP fluorescence (magnification, ×4). Representative photomicrographs from each treatment group are shown except for the BALB/c no-scarification group. Among BALB/c mice that received no scarification, GFP fluorescence was not visible in 9 of 10 mouse eyes 24 h after inoculation with KOS-GFP.
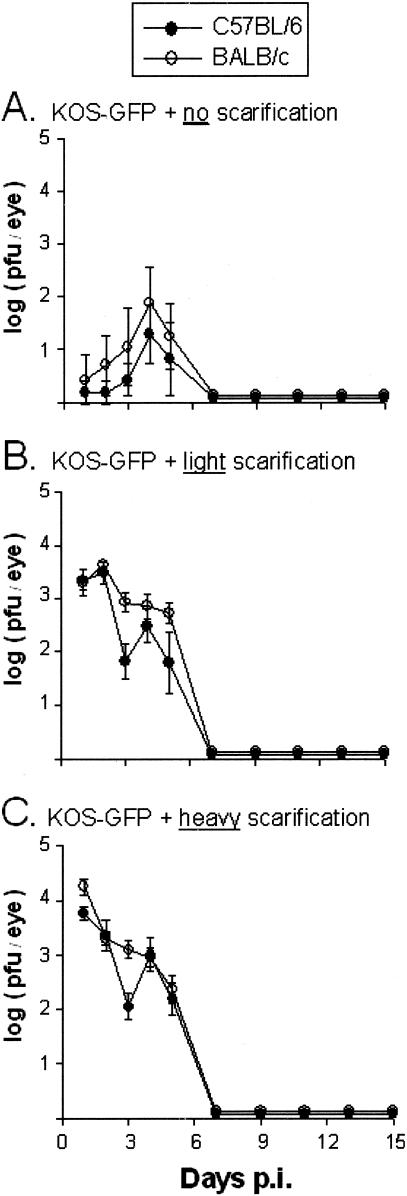
The replication of HSV-1 strain KOS-GFP in the eyes of C57BL/6 and BALB/c mice that received either no, light, or heavy scarification was compared. At 24 h p.i., infectious KOS was detected in the eyes of only 1 of 5 C57BL/6 mice and 1 of 5 BALB/c mice that received no scarification (Fig. 2A). By day 4 p.i., however, 3 of 5 C57BL/6 mice and 4 of 5 BALB/c mice that received no scarification shed low titers of infectious KOS-GFP (Fig. 2A). In contrast, 100% of C57BL/6 and BALB/c mice that received light or heavy scarification shed high titers of infectious KOS-GFP between days 1 and 5 p.i. (Fig. 2B and C). Shedding of KOS-GFP ceased in the eyes of all mice by day 7 p.i. Despite extensive replication and viral shedding at the site of inoculation, 100% of the C57BL/6 and BALB/c mice survived infection with KOS-GFP, and no overt pathogenesis was observed. Thus, HSV-1 strain KOS-GFP replicates with the same efficiency in the eyes of C57BL/6 and BALB/c mice but causes no overt pathogenesis in these animals.
FIG. 2.
Viral replication in C57BL/6 and BALB/c mice infected with KOS-GFP. C57BL/6 and BALB/c mice that received either 0, 4, or ∼30 scratches per eye (no, light, or heavy scarification, respectively) were inoculated with 105 PFU of HSV-1 strain KOS-GFP/eye (n = 5 mice per group). For mice that received no (A), light (B), or heavy (C) scarification, mean viral titers recovered from C57BL/6 and BALB/c mouse eyes (PFU per eye) are plotted as a function of the day after inoculation on which samples were collected. Error bars, SEM.
C57BL/6 mice are conditionally resistant to ocular infection with McKrae.
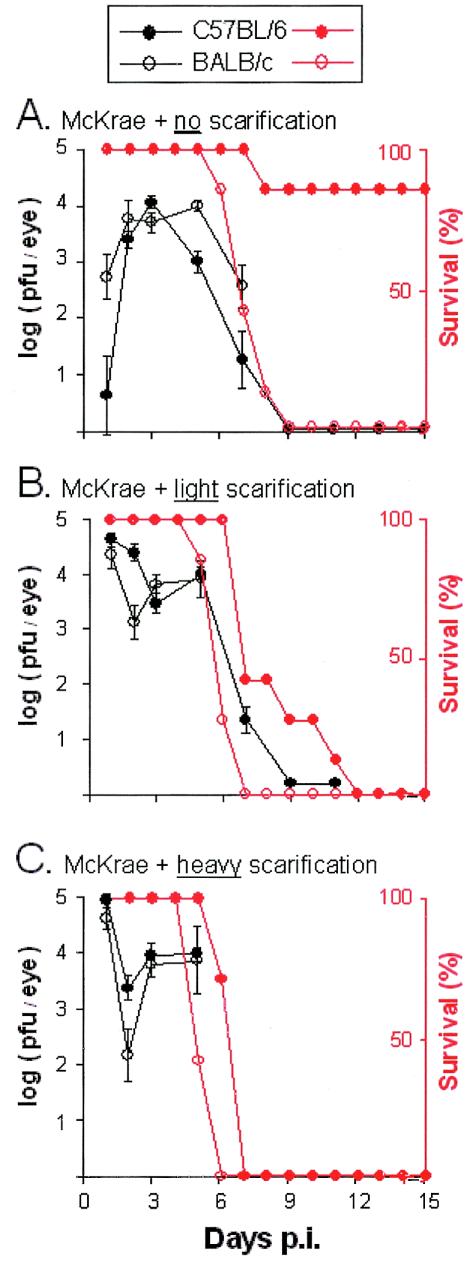
The replication of HSV-1 strain McKrae in C57BL/6 and BALB/c mice that received either no, light, or heavy scarification was compared. At 24 h p.i., only 1 of 7 C57BL/6 mice that received no scarification shed detectable levels of infectious McKrae (Fig. 3A). In contrast, BALB/c mice that received no scarification shed significantly higher titers of infectious virus at 24 h p.i. (Fig. 3A) (P < 0.05 by paired t test). Between days 2 and 3 p.i., shedding of McKrae increased to equivalent levels in the eyes of C57BL/6 and BALB/c mice that received no scarification (Fig. 3A). All C57BL/6 and BALB/c mice that received either light or heavy scarification shed high titers of infectious McKrae between days 1 and 5 p.i. (Fig. 3B and C). Therefore, only in the absence of corneal scarification was the “resistant” phenotype of C57BL/6 mice made apparent.
FIG. 3.
Survival and viral replication in C57BL/6 and BALB/c mice infected with McKrae. C57BL/6 and BALB/c mice that received either 0, 4, or ∼30 scratches per eye (no, light, or heavy scarification, respectively) were inoculated with 105 PFU of HSV-1 strain McKrae/eye (n = 7 mice per group). For mice that received no (A), light (B), or heavy (C) scarification, mean viral titers recovered from C57BL/6 and BALB/c mouse eyes (PFU per eye) are plotted in black as a function of the day after inoculation on which samples were collected. Error bars, SEM. For each scarification group, percent survival of McKrae-infected C57BL/6 and BALB/c mice is plotted in red as a function of the day after inoculation.
The survival of C57BL/6 and BALB/c mice infected with HSV-1 strain McKrae was compared over a 28-day observation period. When mice received no scarification, 6 of 7 C57BL/6 mice survived ocular infection with McKrae whereas none of 7 BALB/c mice survived the infection (Fig. 3A) (P = 0.002 by Fisher's exact test). When the efficiency of inoculation was increased by light or heavy scarification, infection with HSV-1 strain McKrae was uniformly lethal in 100% of C57BL/6 and BALB/c mice (Fig. 3B and C). Although the final outcome was the same, C57BL/6 mice survived an average 2.6 or 1.4 days longer than their BALB/c counterparts that received light or heavy corneal scarification, respectively. Therefore, C57BL/6 mice consistently survived ocular challenge with HSV-1 strain McKrae for a longer period than BALB/c mice (P < 0.001 by paired t test). However, only in the absence of corneal scarification was the “resistant” phenotype of C57BL/6 mice sufficient to prevent McKrae infection from progressing to fatal encephalitis.
C57BL/6 scid mice are not inherently more resistant to HSV-1 infection than BALB/c scid mice.
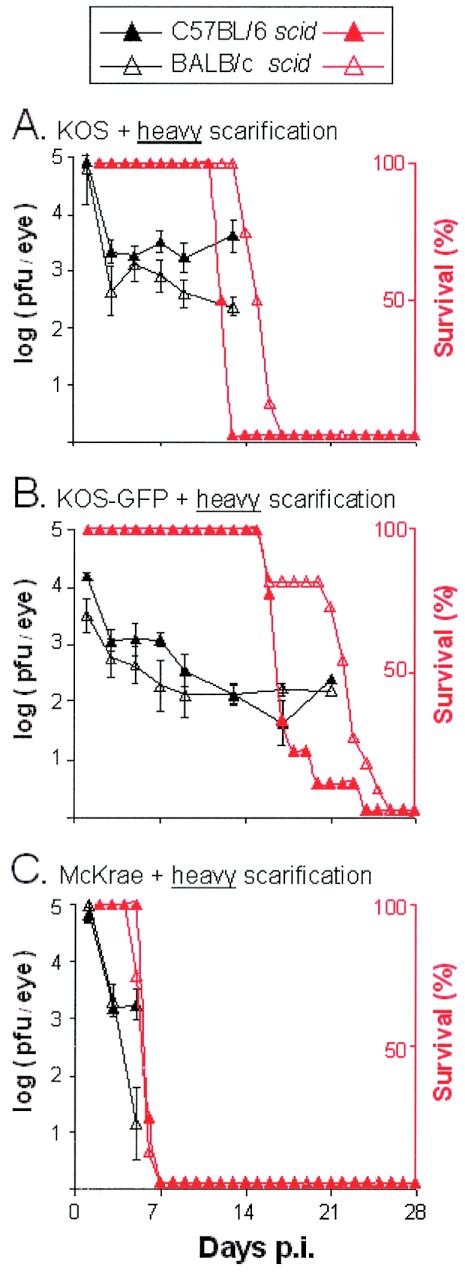
An experiment was conducted to test the hypothesis that the resistant C57BL/6 genetic background would prolong the survival of C57BL/6 scid mice relative to that of BALB/c scid mice. The replication of HSV-1 strains KOS, KOS-GFP, and McKrae was compared in C57BL/6, BALB/c, C57BL/6 scid, and BALB/c scid mice that received heavy scarification (n = 8 mice per group). Each of the three viruses replicated to equivalent titers in C57BL/6, BALB/c, C57BL/6 scid, and BALB/c scid mice between days 1 and 5 p.i. (P > 0.05 by two-way analysis of variance). In agreement with previous results, ocular shedding of KOS, KOS-GFP, and McKrae ceased in immunocompetent C57BL/6 and BALB/c mice by day 7 p.i. In contrast, immunodeficient C57BL/6 scid and BALB/c scid mice shed infectious virus at all times after inoculation (Fig. 4).
FIG. 4.
Viral replication in C57BL/6 scid and BALB/c scid mice infected with HSV-1 strain KOS (A), KOS-GFP (B), or McKrae (C). Mice that received ∼30 scratches per eye (heavy scarification) were inoculated with 105 PFU of the indicated HSV-1 strain/eye (n = 8 mice per group). For each group of mice, mean viral titers recovered from eyes (PFU per eye) are plotted in black as a function of the day after inoculation on which samples were collected. Error bars, SEM. Percent survival of HSV-1-infected C57BL/6 scid and BALB/c scid mice is plotted in red as a function of the day after inoculation.
The frequency and duration of survival of immunocompetent C57BL/6 and BALB/c mice were compared. All C57BL/6 and BALB/c mice infected with HSV-1 strain KOS or KOS-GFP survived ocular infection (Table 1). In contrast, only 1 of 8 C57BL/6 mice and none of 8 BALB/c mice survived ocular infection with HSV-1 strain McKrae. Considering those mice that died, C57BL/6 mice survived for 7.2 ± 0.6 days after inoculation with McKrae and BALB/c mice survived for 5.3 ± 0.2 days (Table 1). Thus, C57BL/6 mice survived McKrae infection for an average 1.9 days longer than BALB/c mice (P = 0.002 by paired t test).
TABLE 1.
Effect of host genetic background on survival of HSV-1-infected mice
| Virusa and mouse strain | Survival of:
|
|||
|---|---|---|---|---|
| Wild-type miceb
|
scid micec
|
|||
| Frequency (%)d | Duration (days)e | Frequency (%) | Duration (days) | |
| KOS | ||||
| C57BL/6 | 100 | 32 | 0 | 12.5 ± 0.2* |
| BALB/c | 100 | 32 | 0 | 15.4 ± 0.4 |
| KOS-GFP | ||||
| C57BL/6 | 100 | 32 | 0 | 18.0 ± 0.9* |
| BALB/c | 100 | 32 | 0 | 21.9 ± 1.0 |
| McKrae | ||||
| C57BL/6 | 12.5 | 7.2 ± 0.6* | 0 | 5.8 ± 0.1* |
| BALB/c | 0 | 5.3 ± 0.2 | 0 | 5.3 ± 0.2 |
Mice received heavy corneal scarification and were inoculated with 105 PFU of the indicated HSV-1 strain/eye.
Immunocompetent C57BL/6 and BALB/c mice (n = 8 per group).
Immunodeficient C57BL/6 scid and BALB/c scid mice (n = 8 per group).
Percentage of mice that survived until 32 days p.i.
Mean duration of survival ± standard error for those mice that survived for less than 30 days after inoculation with HSV-1. Mice that did not die of HSV-1 infection were sacrificed 32 days p.i. *, P < 0.05 by a paired t test comparing the duration of survival of matched pairs of HSV-1-infected C57BL/6 versus BALB/c wild-type mice or that of matched pairs of HSV-1-infected C57BL/6 scid versus BALB/c scid mice.
The innate resistance of C57BL/6 scid and BALB/c scid mice to HSV-1 infection was compared. HSV-1 strain McKrae was rapidly lethal in C57BL/6 scid and BALB/c scid mice, and all McKrae-infected mice died between days 5 and 6 p.i. (Table 1; Fig. 4). In contrast, C57BL/6 scid and BALB/c scid mice infected with HSV-1 strain KOS or KOS-GFP died between days 12 and 24 p.i. (Table 1; Fig. 4). Following inoculation with KOS or KOS-GFP, BALB/c scid mice survived an average of 3 to 4 days longer than C57BL/6 scid mice (Table 1; Fig. 4) (P = 0.002 by paired t test). Therefore, it is not evident that the C57BL/6 genetic background is functionally superior to the BALB/c genetic background in its capacity to confer innate resistance to HSV-1 infection on scid mice.
KOS-GFP spreads with equivalent efficiency in C57BL/6 scid and BALB/c scid mice.
The circuits and synapses of the peripheral nervous system are the primary conduit by which HSV spreads in vivo. Thus, the pattern by which ocular HSV-1 infection spreads in mice is a round trip from (i) the eye to (ii) the ophthalmic branch of the TG, to (iii) the brainstem, to multiple nerve centers including (iv) the maxillary and mandibular branches of the TG, to (v) the skin of the nose and the periocular skin (57).
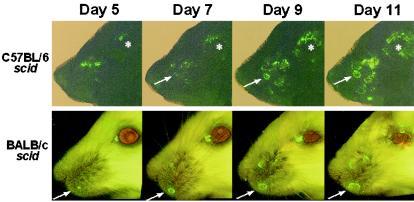
The round-trip spread of HSV-1 strain KOS-GFP from the eyes to the facial skin of C57BL/6, BALB/c, C57BL/6 scid, and BALB/c scid mice (n = 3 per group) was recorded by photographing the right side of each mouse's face between days 1 and 13 p.i. Between days 1 and 2 p.i., green fluorescent virus-infected cells were visible in the corneas of all KOS-GFP-infected mice but on no other part of the face (data not shown). Between days 4 and 5 p.i., GFP expression was absent from the cornea but began to reappear on the periocular skin and nose as small foci of green florescent viral infection in the facial epithelium. In immunocompetent C57BL/6 and BALB/c mice, limited spread of KOS-GFP infection through the facial epithelium was observed before viral replication was completely suppressed by day 8 p.i. (data not shown). In C57BL/6 scid and BALB/c scid mice, small foci of green fluorescent viral infection appeared in the skin by day 5 p.i. Thereafter, KOS-GFP infection spread centripetally through the epithelium, as is demonstrated by the enlargement of individual sites of green fluorescence between days 5 and 11 p.i. (Fig. 5). These results indicate that the green fluorescent virus, KOS-GFP, spreads through the peripheral nervous system and facial skin of C57BL/6 scid mice with an efficiency similar to that observed in BALB/c scid mice.
FIG. 5.
Spread of a green fluorescent virus on the skin of C57BL/6 scid and BALB/c scid mice between days 5 and 11 p.i. Composite images of a representative C57BL/6 scid mouse and a representative BALB/c scid mouse were taken 5, 7, 9, and 11 days after inoculation with KOS-GFP. Mice received ∼30 scratches per eye and were inoculated with 105 PFU of KOS-GFP/eye. Composite images were created by stitching together overlapping digital photographs that were taken at the indicated times p.i. on a Nikon TE300 inverted fluorescent microscope (magnification, ×2). Arrows point to a single focus of KOS-GFP infection spreading on the face of each mouse over time. Asterisks mark the location of the eye of the C57BL/6 scid mouse.
KOS and KOS-GFP establish equivalent numbers of latent viral genomes in TG of C57BL/6 and BALB/c mice.
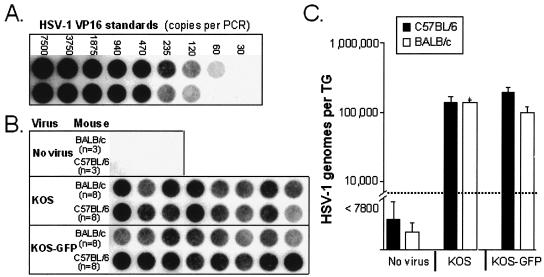
The efficiencies with which HSV-1 establishes latent infections in the TG of C57BL/6 and BALB/c mice were compared. DNA was extracted from the TG of uninfected, latently KOS-infected, and latently KOS-GFP-infected C57BL/6 and BALB/c mice. HSV-1 VP16 and competitor PCR products were coamplified from TG DNA samples isolated from C57BL/6 and BALB/c mice (Fig. 6B). VP16 PCR products were not amplified from uninfected TG DNA but were consistently amplified from latently HSV-1-infected TG DNA (Fig. 6B). In parallel reactions, VP16 and competitor PCR products were coamplified from viral DNA standards in order to define the relationship between the VP16 PCR product yield and the number of HSV-1 genomes per PCR (Fig. 6A) (r2 = 0.995). The number of HSV-1 genomes per TG in individual C57BL/6 and BALB/c mice was calculated to be the VP16 gene copy number per PCR multiplied by 260 (i.e., ∼1/260th of the DNA from a single TG was added to each PCR). C57BL/6 and BALB/c mice latently infected with KOS each contained an average of 1.0 × 105 viral genomes per TG (Fig. 6C). Likewise, C57BL/6 and BALB/c mice latently infected with KOS-GFP contained an average of 2.0 × 105 and 1.0 × 105 viral genomes per TG, respectively (Fig. 6C). Therefore, HSV-1 strains KOS and KOS-GFP establish latent infections with comparable efficiencies in the TG of C57BL/6 and BALB/c mice.
FIG. 6.
Measurement of KOS and KOS-GFP viral genome loads in the TG of latently HSV-1 infected mice. (A and B) Dot blots of VP16 PCR products hybridized to a 32P-labeled probe. HSV-1 VP16 PCR products were amplified from a standard curve of plasmid DNA containing the VP16 sequence (A) and from DNA isolated from the TG of C57BL/6 and BALB/c mice (B). TG DNA samples were obtained from uninfected C57BL/6 and BALB/c mice (n = 3 each), C57BL/6 and BALB/c mice latently infected with KOS (n = 8 each), and C57BL/6 and BALB/c mice latently infected with KOS-GFP (n = 8 each). Latently HSV-1 infected mice were sacrificed 32 days p.i. (C) Numbers of HSV-1 genomes per TG in latently infected C57BL/6 (solid bars) and BALB/c (open bars) mice sacrificed 32 days after inoculation with KOS or KOS-GFP. Dashed line indicates the lower limit of detection of the PCR assay.
DISCUSSION
Productive HSV-1 replication in C57BL/6 versus BALB/c mice.
It is often stated that C57BL/6 mice are resistant to HSV or that C57BL/6 mice are resistant to HSV infection (7, 9, 12, 21, 40, 48, 66). These statements range from ambiguous to inaccurate and have led many scientists to the conclusion that C57BL/6 mice are resistant to the processes of HSV-1 infection, replication, and/or viral spread. In the present study, we attempt to clarify this misconception.
There are reports that HSV-1 replicates inefficiently in primary cells derived from C57BL/6 mice, which would suggest that HSV-1 replicates inefficiently in C57BL/6 mice (1, 3). However, these findings contradict the original (1975) conclusions of Lopez, who wrote, “Preliminary evidence indicates that resistance is not a property of structural cells and is probably mediated immunologically. Embryo fibroblast cultures were prepared from A/J, BALB/c and C57BL/6 mice and inoculated with HSV-1. Virus replicated to the same titer in each monolayer, indicating that resistance was not mediated by the inability of HSV-1 to replicate in structural cells of resistant mice” (30).
The findings of the present study are consistent with the conclusion of Lopez (30). Throughout the course of this study, viral titers were measured in 336 matched pairs of swabs collected between days 1 and 5 p.i. from the HSV-1-infected eyes of C57BL/6 and BALB/c mice. Regardless of whether the mice were infected with strain KOS, KOS-GFP, or McKrae, equivalent titers of HSV-1 were recovered from matched pairs of C57BL/6 and BALB/c mice (P = 0.35, paired t test; hypothesis H0: titerC57BL/6 − titerBALB/c = 0). Therefore, the results of the present study indicate that host genetic background has no measurable effect on the efficiency with which HSV-1 replicates in the eyes of C57BL/6 and BALB/c mice. Of course, one caveat of the present study is that only the corneal route of infection was studied. Although the study of Simmons and La Vista (52) suggests that HSV-1 also replicates with identical efficiencies in the flank epithelia of C57BL/6 and BALB/c mice, it remains to be determined if C57BL/6 mice and BALB/c mice differ in their resistance to HSV-1 infection when challenged at other anatomic sites such as the footpad or ear pinna.
Establishment of latent HSV-1 infection in C57BL/6 versus BALB/c mice.
The results of the present study indicate that equivalent numbers of latent HSV-1 strain KOS genomes are established in the TG of C57BL/6 and BALB/c mice. Likewise, equivalent numbers of latent KOS-GFP genomes are established in the TG of C57BL/6 and BALB/c mice. Our results corroborate the earlier findings of Ellison et al., who observed that equivalent numbers of latent HSV-1 strain KOS genomes were established in the TG of C57BL/6, beige, Swiss Webster, BALB/c, and CBA mice (14). Despite equivalent total numbers of HSV-1 genomes per TG, it is possible that large differences could exist in (i) the number of latently HSV-1 infected neurons and (ii) the copy number of viral genomes per neuron in the TG of C57BL/6 and BALB/c mice. The study of Ellison et al. suggests that this is not the case and demonstrates that the number of latently HSV-1 infected neurons per TG does not differ more than twofold between C57BL/6 and BALB/c mice (14).
Although the lack of difference in the latent HSV-1 genome load between the TG of C57BL/6 and BALB/c mice could be due to the insensitivity of the PCR assay, prior results argue against this possibility. A similar PCR procedure has demonstrated that HSV-1 ICP0− mutants establish 3- to 15-fold fewer latent viral genomes per TG than wild-type virus in immunocompetent mice (19). Likewise, a similar PCR assay documented that treatment of mouse eyes with combinations of recombinant IFN-β and IFN-γ prior to ocular challenge with 105 PFU of wild-type HSV-1 caused a > 200-fold reduction in the latent HSV-1 genome load per TG (47). Therefore, taken together with the findings of Ellison et al. (14), the results of the present study clearly demonstrate that host genetic background has no significant effect on the efficiency with which HSV-1 establishes a latent infection in the TG of C57BL/6 and BALB/c mice.
The innate resistance of C57BL/6 scid versus BALB/c scid mice.
Despite the absence of mature lymphocytes, scid mice possess all of the normal components of the innate immune system (44). Therefore, like scid mice provide a tool for studying the capacity of the innate immune system to combat the spread of microbial infections without the masking (i.e., dominant) effect of the adaptive immune response which begins to predominate 1 week after inoculation (5, 62).
The genetic background of the C57BL/6 mouse is believed to contain allelic variants of several genes that confer superior innate resistance to herpesvirus infection relative to that of susceptible mouse strains (8, 32, 35, 39, 41, 45, 49, 51). In the present study, the predictive value of this hypothesis was tested by comparing the innate resistance of C57BL/6 scid mice and BALB/c scid mice to the processes of HSV-1 replication, spread, and pathogenesis. No significant differences were observed in HSV-1 titers recovered from the eyes of C57BL/6 scid mice and BALB/c scid mice. Likewise, the zosteriform spread of HSV-1 strain KOS-GFP in the facial epithelia of C57BL/6 scid mice and BALB/c scid mice was qualitatively similar at all times after inoculation. Although no differences were noted in the spread of KOS-GFP across the faces of C57BL/6 scid and BALB/c scid mice, it should be noted that differences in spread within the peripheral nervous system may be detectable by more sensitive methods (e.g., the method of Summers et al. [57] or Luker et al. [34]). Finally, comparison of pathogenesis and death confirmed our expectation that C57BL/6 scid mice would survive infection with HSV-1 strain McKrae for slightly longer (0.5 days) than BALB/c scid mice. However, daily inspection of scid mice infected with one of the less virulent strains, KOS or KOS-GFP, revealed an unexpected pattern. KOS and KOS-GFP infections progressed to fatal encephalitis 3 to 4 days sooner in C57BL/6 scid mice than in BALB/c scid mice (Table 1). Based on these observations, it is not evident that the allelic differences between the C57BL/6 and BALB/c genetic backgrounds have an appreciable effect on the innate resistance of scid mice to HSV-1 infection, replication, spread, or pathogenesis. This corroborates earlier findings that the resistance of C57BL/6 mice to HSV-1 pathogenesis is absolutely dependent on both (i) the development of a rapid IFN-α/β response (64, 65) and (ii) T-lymphocyte-mediated suppression of HSV-1 replication in infected tissues between days 5 and 7 after inoculation (11, 54).
Analysis of variables that may affect the pathogenic outcome of HSV-1 infection.
Three variables that can potentially impact the pathogenic outcome of HSV-1 infections were compared in the present study: (i) the relative virulence of viral strains, (ii) the degree of epithelial scarification at the time of viral inoculation, and (iii) host genetic background. Without question, the genetic differences between the viral strains McKrae, KOS, and KOS-GFP had a tremendous impact on the pathogenesis that developed in HSV-1-infected mice. In contrast, the genetic differences between C57BL/6 and BALB/c mice had a relatively minor impact on the course of HSV-1 infection, and a difference in the resistance of C57BL/6 and BALB/c mice to HSV-1 pathogenesis was evident only in a subset of the experiments. The relevant conclusions from these comparisons are summarized, as follows.
(i) Relative virulence of HSV-1 strains.
In the present study, 100% of C57BL/6 mice (n = 47) and BALB/c mice (n = 47) survived infection with HSV-1 strain KOS or KOS-GFP. Despite the fact that KOS and KOS-GFP infections caused little to no visible pathogenesis in C57BL/6 and BALB/c mice (i.e., little to no loss of fur on the periocular skin), these viruses replicated to high titers in the eyes of mice. In contrast, only 7 of 29 (24%) C57BL/6 mice and none of 29 BALB/c mice survived infection with HSV-1 strain McKrae. Despite the obvious differences in pathogenesis, similar titers of McKrae and KOS were recovered from the eyes of infected mice during the productive phase of infection. An important question that remains to be definitively resolved is which genetic locus or loci in the HSV-1 genome account(s) for the tremendous difference in virulence between virulent and avirulent strains of HSV-1. Given that the increased virulence of HSV-1 strains such as McKrae correlates with an increased resistance to IFN-α/β (56), it would be of interest to determine if the virulence of HSV-1 strain McKrae maps to the interferon resistance locus of HSV-1 that is formed by the adjacent ICP0 and ICP34.5 genes (20, 36, 37). Although there are some data to support this hypothesis (42), a rigorous analysis remains to be done.
(ii) Degree of epithelial scarification.
An outer layer of keratinized epithelial cells covers the body and serves as a physical barrier to HSV-1 infection. During inoculation, the experimental procedure of scarification breaks this barrier and allows HSV-1 to directly infect the underlying cells that are permissive for HSV-1 replication. In the absence of corneal scarification, KOS-GFP was slow to establish a productive infection in C57BL/6 and BALB/c mice relative to mice that received light or heavy corneal scarification (Fig. 1 and 2). In agreement with previous reports (43), HSV-1 strain McKrae was far more efficient at establishing a productive infection in C57BL/6 and BALB/c mouse eyes that received no scarification (Fig. 3). In particular, the ∼1,000-fold-higher titers of McKrae recovered 2 days after inoculation of unscarified corneas demonstrates that HSV-1 strain McKrae is far more efficient than KOS-GFP in its capacity to overcome the innate immune response of the host (compare Fig. 2A and 3A). This observation may be relevant to the hypothesis that the increased virulence of HSV-1 McKrae is due to an increased resistance to the innate IFNs, IFN-α/β (56). Moreover, this hypothesis is consistent with the reciprocal observation of Leib et al. (28) that IFN-α/β is a critical determinant of the resistance of the unscarified mouse cornea to infection with HSV-1 strain KOS.
(iii) Host genetic background.
The results of the present study corroborate earlier observations that demonstrate that the resistance of C57BL/6 mice to HSV-1 pathogenesis and death is observed only under a subset of experimental conditions. For example, Zawatsky et al. showed that C57BL/6 mice survive i.p. challenge with 106 PFU of HSV-1 but do not survive i.p. challenge when the HSV-1 inoculum is diluted to 104 PFU (64). Likewise, Zawatsky et al. showed that 6-week-old C57BL/6 mice survive i.p. challenge with 106 PFU of HSV-1 but that the same HSV-1 inoculum is uniformly lethal in 3-week-old C57BL/6 mice (64). In the present study, we present a similar finding. Most C57BL/6 mice survive ocular inoculation with 105 PFU of HSV-1 strain McKrae provided that the viral inoculum is applied to the unscarified cornea. However, when the site of corneal inoculation is scarified, HSV-1 strain McKrae is uniformly fatal in both C57BL/6 and BALB/c mice (Fig. 3). Thus, the capacity of C57BL/6 mice to resist HSV-1 pathogenesis and death is a conditional phenotype.
Conclusions.
Adult C57BL/6 mice mount an unusually rapid IFN-α/β response when infected with HSV-1 (65-67). Plasmacytoid dendritic cells appear to be a major source of this IFN-α response in vivo (6), and their relative absence in juvenile mice appears to account for the age dependence of the resistant phenotype observed in C57BL/6 mice (63). Based on our studies and review of the literature, it appears to us that most of the manifestations of the “resistant ” phenotype ascribed to C57BL/6 mice can be explained in terms of a transient IFN-α/β-mediated delay in HSV-1 spread from the site of viral inoculation into the peripheral nervous system. For example, we reproducibly observed a 1- to 2-day delay in the onset of McKrae-induced pathogenesis in C57BL/6 mice relative to that in BALB/c mice. Under experimental conditions where either (i) the efficiency of HSV-1 inoculation is low (Fig. 3A) or (ii) the induction of IFN-α/β is rapid (65), a transient delay in HSV-1 replication at the inoculation site may be sufficient to explain the increased survival of C57BL/6 mice relative to that of other inbred strains of mice.
Such experimental details and inferences still do not address a fundamentally important question: “Should the resistance of C57BL/6 mice to pathogenesis be considered a broadly applicable paradigm of inherited resistance to HSV-1?” Based on the fact that HSV-1 enters, replicates, spreads, and establishes latent infections with virtually identical efficiencies in C57BL/6 and BALB/c mice, individuals of the species Mus musculus do not appear to differ fundamentally in their innate resistance to HSV-1 infection. Likewise, although it has been implied that differences in host resistance are relevant in explaining what differentiates humans with recurrent herpetic disease from the vast majority of asymptomatic carriers of HSV-1 and HSV-2 (15, 29, 31, 33), there is little epidemiological evidence to support this hypothesis.
We conclude that it is quite possible that other explanations may account for this epidemiological observation. For example, the highly variable conditions under which viruses are transmitted in nature (e.g., size of inoculum, duration of sexual contact, abrasion of the epithelium) may be sufficient, in and of themselves, to explain the disparate clinical outcomes of HSV-1 and HSV-2 infection that are observed in human beings. Alternatively, given the tremendous range of virulence observed in HSV-1 laboratory strains, genetic variation in viral strains (e.g., differences in viral resistance to IFN-α/β) may be an important factor in the progression of HSV-1 and/or HSV-2 infections to recurrent herpetic disease. Further investigation will be required to determine if, in fact, differences in (i) viral strain and (ii) conditions of viral transmission have a significant impact on the progression of HSV-1 and HSV-2 infections in humans.
Acknowledgments
This work was supported by grants from the Louisiana Board of Regents Support Foundation (LEQSF-2001-2004-RD-A-33), the W. M. Keck Foundation of Los Angeles, the National Institute of Allergy and Infectious Diseases (R01 AI54104), the National Eye Institute (R01 EY02672 and P30 EY002377), and by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, N.Y.
REFERENCES
- 1.Abghari, S. Z., R. D. Stulting, S. M. Nigida, D. N. Downer, and A. J. Nahmias. 1986. Comparative replication of HSV-1 in BALB/c and C57BL/6 mouse embryo fibroblasts in vitro. Investig. Ophthalmol. Vis. Sci. 27:909-914. [PubMed] [Google Scholar]
- 2.Abghari, S. Z., R. D. Stulting, S. M. Nigida, Jr., D. N. Downer, and A. J. Nahmias. 1986. Spread of HSV and establishment of latency after corneal infection in inbred mice. Investig. Ophthalmol. Vis. Sci. 27:77-82. [PubMed] [Google Scholar]
- 3.Abghari, S. Z., R. D. Stulting, Z. Zhu, R. F. Schinazi, and H. E. Kaufman. 1994. Effect of genetically determined host factors on the efficacy of vidarabine, acyclovir and 5-trifluorothymidine in herpes simplex virus type 1 infection. Ophthal. Res. 26:95-104. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, C. L., and M. Parent. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90:367-382. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft, G. J., and J. P. Kelly. 1994. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology 191:424-431. [DOI] [PubMed] [Google Scholar]
- 6.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98:3520-3526. [DOI] [PubMed] [Google Scholar]
- 7.Brenner, G. J., N. Cohen, and J. A. Moynihan. 1994. Similar immune response to nonlethal infection with herpes simplex virus-1 in sensitive (BALB/c) and resistant (C57BL/6) strains of mice. Cell. Immunol. 157:510-524. [DOI] [PubMed] [Google Scholar]
- 8.Brown, M. G., A. O. Dokun, J. W. Heusel, H. R. Smith, D. L. Beckman, E. A. Blattenberger, C. E. Dubbelde, L. R. Stone, A. A. Scalzo, and W. M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934-937. [DOI] [PubMed] [Google Scholar]
- 9.Brucher, J., I. Domke, C. H. Schroder, and H. Kirchner. 1984. Experimental-infection of inbred mice with herpes simplex virus. 6. Effect of interferon on in vitro virus replication in macrophages. Arch. Virol. 82:83-93. [DOI] [PubMed] [Google Scholar]
- 10.Chang, S. S., and W. H. Hildemann. 1964. Inheritance of susceptibility to polyoma virus in mice. J. Natl. Cancer Inst. 33:303-313. [PubMed] [Google Scholar]
- 11.Chmielarczyk, W., H. Engler, R. Ernst, U. Opitz, and H. Kirchner. 1985. Injection of anti-thy-1.2 serum breaks genetic resistance of mice against herpes simplex virus. J. Gen. Virol. 66:1087-1094. [DOI] [PubMed] [Google Scholar]
- 12.Domkeopitz, I., P. Poberschin, S. Mittnacht, and H. Kirchner. 1987. Role of interferon in persistent infection of macrophages with herpes simplex virus. Virology 159:306-311. [DOI] [PubMed] [Google Scholar]
- 13.Ellermann-Eriksen, S., J. Justesen, and S. C. Mogensen. 1986. Genetically determined difference in the antiviral action of alpha/beta interferon in cells from mice resistant or susceptible to herpes simplex virus type 2. J. Gen. Virol. 67:1859-1866. [DOI] [PubMed] [Google Scholar]
- 14.Ellison, A. R., L. Yang, C. Voytek, and T. P. Margolis. 2000. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 268:17-28. [DOI] [PubMed] [Google Scholar]
- 15.Gallina, G., V. Cumbo, P. Messina, V. Caprera, D. Lio, and C. Caruso. 1989. Lack of correlation between HLA-B35 resistance against herpes labialis and antibody titers to HSV-1. Oral Surg. Oral Med. Oral Pathol. 68:167-170. [DOI] [PubMed] [Google Scholar]
- 16.Geiger, S. M., E. Abrahams-Sandi, P. T. Soboslay, W. H. Hoffmann, A. W. Pfaff, C. Graeff-Teixeira, and H. Schulz-Key. 2001. Cellular immune responses and cytokine production in BALB/c and C57BL/6 mice during the acute phase of Angiostrongylus costaricensis infection. Acta Trop. 80:59-68. [DOI] [PubMed] [Google Scholar]
- 17.Halford, W. P., V. C. Falco, B. M. Gebhardt, and D. J. J. Carr. 1999. The inherent quantitative capacity of the reverse transcription-polymerase chain reaction. Anal. Biochem. 266:181-191. [DOI] [PubMed] [Google Scholar]
- 18.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harle, P., B. Sainz, D. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-α/β. Virology 293:295-304. [DOI] [PubMed] [Google Scholar]
- 21.He, J. C., H. Ichimura, T. Iida, M. Minami, K. Kobayashi, M. Kita, C. Sotozono, Y. I. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J. Interferon Cytokine Res. 19:609-615. [DOI] [PubMed] [Google Scholar]
- 22.Hill, J. M., W. P. Halford, R. Wen, L. S. Engel, L. C. Green, and B. M. Gebhardt. 1996. Quantitative analysis of polymerase chain reaction products by dot blot. Anal. Biochem. 235:44-48. [DOI] [PubMed] [Google Scholar]
- 23.Jacoby, R. O., and P. N. Bhatt. 1987. Mousepox in inbred mice innately resistant or susceptible to lethal infection with ectromelia virus. II. Pathogenesis. Lab. Anim. Sci. 37:16-22. [PubMed] [Google Scholar]
- 24.Kastrukoff, L. F., A. S. Lau, and M. L. Puterman. 1986. Genetics of natural resistance to herpes simplex virus type 1 latent infection of the peripheral nervous-system in mice. J. Gen. Virol. 67:613-621. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, H. E., A. B. Nesburn, and E. D. Maloney. 1962. IDU therapy of herpes simplex. Arch. Ophthalmol. 67:583-591. [DOI] [PubMed] [Google Scholar]
- 26.Kirchner, H., R. Zawatzky, E. Storch, I. Domke, and W. Chmielarczyk. 1985. On the role of endogenous interferon in natural and induced antiviral resistance. Antivir. Res. 5(Suppl. 1):155-159. [DOI] [PubMed] [Google Scholar]
- 27.Lee, S. H., A. Zafer, Y. de Repentigny, R. Kothary, M. L. Tremblay, P. Gros, P. Duplay, J. R. Webb, and S. M. Vidal. 2003. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J. Exp. Med. 197:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lekstrom-Himes, J. A., P. Hohman, T. Warren, A. Wald, J. M. Nam, T. Simonis, L. Corey, and S. E. Straus. 1999. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J. Infect. Dis. 179:1077-1085. [DOI] [PubMed] [Google Scholar]
- 30.Lopez, C. 1975. Genetics of natural resistance to herpesvirus infections in mice. Nature 258:152-153. [DOI] [PubMed] [Google Scholar]
- 31.Lopez, C. 1981. Resistance to herpes simplex virus type 1 (HSV-1). Curr. Top. Microbiol. Immunol. 92:15-24. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, C. 1980. Resistance to HSV-1 in the mouse is governed by 2 major, independently segregating, non-H-2 loci. Immunogenetics 11:87-92. [DOI] [PubMed] [Google Scholar]
- 33.Lopez, C., D. Kirkpatrick, S. E. Read, P. A. Fitzgerald, J. Pitt, S. Pahwa, C. Y. Ching, and E. M. Smithwick. 1983. Correlation between low natural killing of fibroblasts infected with herpes simplex virus type 1 and susceptibility to herpesvirus infections. J. Infect. Dis. 147:1030-1035. [DOI] [PubMed] [Google Scholar]
- 34.Luker, G. D., J. L. Prior, J. Song, C. M. Pica, and D. A. Leib. 2003. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 77:11082-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg, P., P. Welander, H. Openshaw, C. Nalbandian, C. Edwards, L. Moldawer, and E. Cantin. 2003. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J. Virol. 77:11661-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norose, K., A. Yano, X. M. Zhang, E. Blankenhorn, and E. Heber-Katz. 2002. Mapping of genes involved in murine herpes simplex virus keratitis: identification of genes and their modifiers. J. Virol. 76:3502-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedersen, E. B., S. Haahr, and S. C. Mogensen. 1983. X-linked resistance of mice to high doses of herpes simplex virus type 2 correlates with early interferon production. Infect. Immun. 42:740-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pepose, J. S., and J. A. Whittum-Hudson. 1987. An immunogenetic analysis of resistance to herpes simplex virus retinitis in inbred strains of mice. Investig. Ophthalmol. Vis. Sci. 28:1549-1552. [PubMed] [Google Scholar]
- 41.Pereira, R. A., A. Scalzo, and A. Simmons. 2001. A NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J. Immunol. 166:5869-5873. [DOI] [PubMed] [Google Scholar]
- 42.Perng, G. C., K. R. Mott, N. Osorio, A. Yukht, S. Salina, Q. H. Nguyen, A. B. Nesburn, and S. L. Wechsler. 2002. Herpes simplex virus type 1 mutants containing the KOS strain ICP34.5 gene in place of the McKrae ICP34.5 gene have McKrae-like spontaneous reactivation but non-McKrae-like virulence. J. Gen. Virol. 83:2933-2942. [DOI] [PubMed] [Google Scholar]
- 43.Perng, G. C., R. L. Thompson, N. M. Sawtell, W. E. Taylor, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1995. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J. Virol. 69:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson, S. R., A. Kurimasa, M. Oshimura, W. S. Dynan, E. M. Bradbury, and D. J. Chen. 1995. Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc. Natl. Acad. Sci. USA 92:3171-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstreich, D. L., A. C. Weinblatt, and A. D. O'Brien. 1982. Genetic-control of resistance to infection in mice. Crit. Rev. Immunol. 3:263-330. [PubMed] [Google Scholar]
- 46.Sadick, M. D., R. M. Locksley, C. Tubbs, and H. V. Raff. 1986. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon gamma in response to Leishmania antigens in vitro. J. Immunol. 136:655-661. [PubMed] [Google Scholar]
- 47.Sainz, B., Jr., and W. P. Halford. 2002. Alpha/beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76:11541-11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarmiento, M. 1988. Intrinsic resistance to viral infection: mouse macrophage restriction of herpes simplex virus replication. J. Immunol. 141:2740-2748. [PubMed] [Google Scholar]
- 49.Scalzo, A. A., N. A. Fitzgerald, A. Simmons, A. B. La Vista, and G. R. Shellam. 1990. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J. Exp. Med. 171:1469-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schell, K. 1960. Studies on the innate resistance of mice to infection with mousepox. II. Route of inoculation and resistance; and some observations on the inheritance of resistance. Aust. J. Exp. Biol. Med. Sci. 38:289-299. [DOI] [PubMed] [Google Scholar]
- 51.Simmons, A. 1989. H-2-linked genes influence the severity of herpes simplex virus infection of the peripheral nervous-system. J. Exp. Med. 169:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmons, A., and A. B. La Vista. 1989. Neural infection in mice after cutaneous inoculation with HSV-1 is under complex host genetic-control. Virus Res. 13:263-270. [DOI] [PubMed] [Google Scholar]
- 53.Smith, K. O. 1964. Relationship between the envelope and the infectivity of herpes simplex virus. Proc. Soc. Exp. Biol. Med. 115:814-816. [DOI] [PubMed] [Google Scholar]
- 54.Speck, P., and A. Simmons. 1998. Precipitous clearance of herpes simplex virus antigens from the peripheral nervous systems of experimentally infected C57BL/10 mice. J. Gen. Virol. 79:561-564. [DOI] [PubMed] [Google Scholar]
- 55.Sprecher, E., and Y. Becker. 1987. Herpes simplex virus type 1 pathogenicity in footpad and ear skin of mice depends on Langerhans cell density, mouse genetics, and virus strain. J. Virol. 61:2515-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su, Y. H., J. E. Oakes, and R. N. Lausch. 1993. Mapping the genetic region coding for herpes simplex virus resistance to mouse interferon alpha/beta. J. Gen. Virol. 74:2325-2332. [DOI] [PubMed] [Google Scholar]
- 57.Summers, B. C., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 75:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor, J. L., and W. J. O'Brien. 1987. Interferon production in inbred mice during herpetic eye disease. Curr. Eye Res. 6:259-264. [DOI] [PubMed] [Google Scholar]
- 59.Thomas, E. E., A. S. Lau, S. U. Kim, D. Osborne, and L. F. Kastrukoff. 1991. Variation in resistance to herpes simplex virus type 1 of oligodendrocytes derived from inbred strains of mice. J. Gen. Virol. 72:2051-2057. [DOI] [PubMed] [Google Scholar]
- 60.Treco, D. A. 1990. Preparation and analysis of DNA, p. 2.0.3-2.2.3. In F. M. Ausubel (ed.), Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 61.Uzonna, J. E., R. S. Kaushik, J. R. Gordon, and H. Tabel. 1998. Experimental murine Trypanosoma congolense infections. I. Administration of anti-IFN-γ antibodies alters trypanosome-susceptible mice to a resistant-like phenotype. J. Immunol. 161:5507-5515. [PubMed] [Google Scholar]
- 62.Vollstedt, S., S. Arnold, C. Schwerdel, M. Franchini, G. Alber, J. P. Di Santo, M. Ackermann, and M. Suter. 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol. 78:3846-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vollstedt, S., M. Franchini, H. P. Hefti, B. Odermatt, M. O'Keeffe, G. Alber, B. Glanzmann, M. Riesen, M. Ackermann, and M. Suter. 2003. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 197:575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zawatzky, R., H. Engler, and H. Kirchner. 1982. Experimental infection of inbred mice with herpes simplex virus. 3. Comparison between newborn and adult C57BL/6 mice. J. Gen. Virol. 60:25-29. [DOI] [PubMed] [Google Scholar]
- 65.Zawatzky, R., I. Gresser, E. DeMaeyer, and H. Kirchner. 1982. The role of interferon in the resistance of C57BL/6 mice to various doses of herpes simplex virus type 1. J. Infect. Dis. 146:405-410. [DOI] [PubMed] [Google Scholar]
- 66.Zawatzky, R., J. Hilfenhaus, F. Marcucci, and H. Kirchner. 1981. Experimental infection of inbred mice with herpes simplex virus type 1. 1. Investigation of humoral and cellular immunity and of interferon induction. J. Gen. Virol. 53:31-38. [DOI] [PubMed] [Google Scholar]
- 67.Zawatzky, R., H. Kirchner, J. DeMaeyer-Guignard, and E. DeMaeyer. 1982. An X-linked locus influences the amount of circulating interferon induced in the mouse by herpes simplex virus type 1. J. Gen. Virol. 63:325-332. [DOI] [PubMed] [Google Scholar]