Abstract
Pancreatic cancer is a devastating disease with poor prognosis. The association between vitamin A, retinol and carotenoid intake and the risk of pancreatic cancer occurrence remains controversial, and therefore it is necessary to make a meta-analysis to clarify the association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk. In the present study, PubMed and EMBASE databases were used to identify qualified studies. The association between dietary vitamin A, retinol and carotenoids was estimated by pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs). It was found that there was an inverse correlation between vitamin A, beta-carotene and lycopene intake and the risk of pancreatic cancer (for vitamin A, pooled OR = 0.85, 95%CI = 0.74–0.97, P = 0.015; for beta-carotene, pooled OR = 0.78, 95%CI = 0.66–0.92, P = 0.003; for lycopene, pooled OR = 0.84, 95%CI = 0.73–0.97, P = 0.020), which was more prominent in case-control study subgroup. In conclusion, dietary vitamin A, beta-carotene and lycopene might inversely correlate with pancreatic cancer.
Pancreatic cancer is a devastating disease with poor prognosis and the 5-year survival rate remains low at 8%1. It is the eighth and ninth leading cause of cancer-related death in men and women respectively throughout the world2. For patients with resectable pancreatic cancer, surgery is the mainstay of treatment. But the median overall survival time remains low in all pancreatic cancer stages3. There have been few therapeutic advances or effective treatments over the last few years4, highlighting the importance of identifying preventive factors for this malignancy. Risk factors such as smoking, obesity, diabetes mellitus, chronic pancreatitis and established genetic syndromes are known to be associated with pancreatic cancer5. A number of epidemiologic studies have been published in an attempt to explore the relationship between nutrient intake and the risk of pancreatic cancer occurrence. Various vitamins including vitamin B6, vitamin C7 and vitamin E8 have been implicated in the risk of pancreatic cancer occurrence according to previous studies.
Vitamin A (retinol) and its derivatives are a group of fat soluble compounds composed of a similar structure which are rich in cod liver oil and play important role in multiple biological processes9. Due to their ability to promote normal embryonic development and exert effects on cellular differentiation, they are essential for all stages of life from embryogenesis to adulthood10. However, they cannot be synthesized de novo by animals (including human) and must be obtained from the diets11. Recently, a myriad of epidemiological studies have demonstrated an inverse relationship between dietary vitamin A consumption and cancer development12. For instance, vitamin A has been proved to play a protective role in breast cancer13 and lung cancer14. However, the association between vitamin A (including retinol and carotenoid) and pancreatic cancer remains controversial15,16,17,18. Zablotska et al. conducted a case–control study to evaluate the association of dietary vitamin D, calcium and retinol and the risk of pancreatic cancer in USA, finding that there was no signification association between them18. Also, Kalapothaki et al. found that vitamin A intake was not related to pancreatic cancer risk when crude fiber intake was adjusted16. The results of clinical studies are not consistent with those of molecular researches. But other carotenoids, such as lycopene, alpha-and beta-carotene, are associated with pancreatic cancer risk17,19. Therefore, a meta-analysis is necessary to clarify the association between vitamin A, retinol and carotenoids intake and pancreatic cancer risk.
Results
Study characteristics and quality assessment
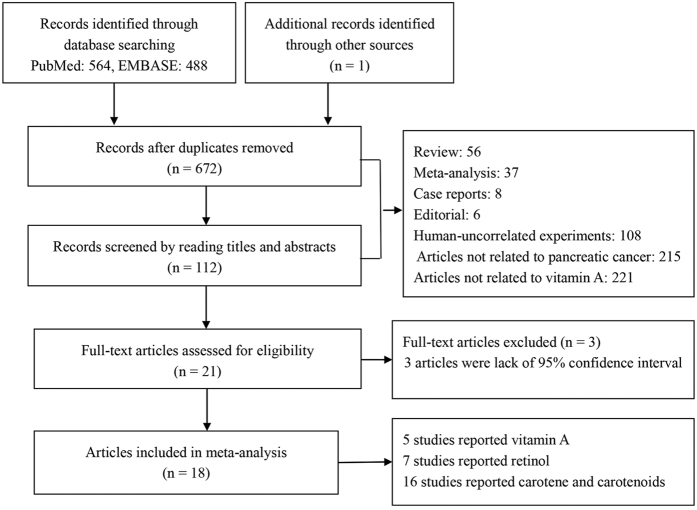
Initially, 672 articles were identified and 18 eligible studies were included in meta-analysis (Fig. 1). The characteristics of the studies and quality assessment results are shown in Table 1. The studies were published from 1990 to 2013. Since the subjects could be divided into males and females, the studies by Ji et al., Nkondjock et al. and Zablotska et al. were separated into two studies, respectively18,19,20. According to different designs of controls, the study conducted by Kalapothaki et al. was also divided into two studies16. Therefore, there were altogether 22 studies in our meta-analysis, among which 16 were performed in Caucasians, 3 in Asians and 3 in mixed population. Besides, 3 studies were conducted in males only, 3 studies were in females only, and the other 16 in both sexes As for the nutrient type, 6 studies focused on Vitamin A, 11 on retinol, and 17 on carotenoids including 6 on alpha-carotene, 14 on beta-carotene, 8 on lycopene, 6 on crytoxanthin and 7 on lutein and zeaxanthin. Quality assessment was conducted in all included studies, and the Newcastle-Ottawa-Scale (NOS) scores ranged from 6 to 9.
Figure 1. Flow chart of the study selection and inclusion process.
Table 1. Characteristics of the studies of vitamin A, retinol and carotenoids intake and pancreatic cancer risk included in this analysis.
| Author | Year | Ethnicity | Study design | Country | Sample size (cases/control) | Carotenoid types | OR(RR, HR)* | 95%CI | Adjustment for covariates | Newcastle- Ottawa Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Howe et al.15 | 1990 | Caucasian | Case-control | Canada | 249/505 | Vitamin A | RR 1.12 | 0.67–1.88 | None | 7 |
| Retinol | RR 1.25 | 0.79–1.99 | ||||||||
| Beta-carotene | RR 0.93 | 0.57–1.51 | ||||||||
| Bueno de Mesquita et al.39 | 1991 | Caucasian | Case-control | Netherlands | 164/480 | Beta-carotene | OR 0.61 | 0.38–0.97 | Adjusted for age, gender, response status, total smoking and dietary intake of energy | 7 |
| Olsen et al.32 | 1991 | Caucasian | Case-control | USA | 212/220 | Vitamin A | OR 1.10 | 0.60–1.80 | Adjusted for total energy, energy- adjusted total fat, age, cigarette usage, alcohol consumption, respondent- reported history of diabetes mellitus, and educational level | 8 |
| Retinol | OR 1.70 | 0.90–3.10 | ||||||||
| Beta-carotene | OR 0.60 | 0.30–1.00 | ||||||||
| Zatonski et al.34 | 1991 | Caucasian | Case-control | Poland | 110/195 | Retinol | RR 0.53 | 0.20–1.45 | Adjusted for cigarette lifetime consumption and calories | 8 |
| Ghadirian et al.33 | 1991 | Caucasian | Case-control | Canada | 179/239 | Retinol | OR 1.05 | 0.46–2.40 | Adjusted for age, sex, lifetime cigarette consumption, response status and energy | 8 |
| Beta-carotene | OR 0.69 | 0.32–1.48 | ||||||||
| Kalapothaki et al.16 | 1993 | Caucasian | Case-control (hospital control) | Greece | 181/181 | Vitamin A | OR 0.80 | 0.65–0.99 | Adjusted for age, gender, hospital, past residence, years of schooling, cigarette smoking, diabetes mellitus and energy intake | 6 |
| Kalapothaki et al.16 | 1993 | Caucasian | Case-control (visitor control) | Greece | 181/181 | Vitamin A | OR 0.74 | 0.59–0.93 | Adjusted for age, gender, hospital, past residence, years of schooling, cigarette smoking, diabetes mellitus and energy intake | 7 |
| Shibata et al.42 | 1993 | Caucasian | Prospective | USA | 63/13976 | Beta-carotene | RR 0.78 | 0.44–1.37 | Adjusted for sex, age and cigarette smoking | 7 |
| Ji et al.20 | 1995 | Asian (male) | Case-control | China | 261/847 | Carotene | OR 0.53 | 0.34–0.83 | Adjusted for age, income, smoking, response status and total calories | 8 |
| Retinol | OR 0.61 | 0.39–0.97 | ||||||||
| Ji et al.20 | 1995 | Asian (female) | Case-control | China | 184/680 | Carotene | OR 0.38 | 0.20–0.71 | Adjusted for age, income, smoking, green tea drinking, response status and total calories | 8 |
| Retinol | OR 0.48 | 0.26–0.88 | ||||||||
| Soler et al.40 | 1998 | Caucasian | Case-control | Italy | 362/1552 | Beta-carotene | OR 0.87 | 0.64–1.17 | Adjusted for age, sex, education, tobacco consumption and area of residence | 6 |
| Retinol | OR 1.37 | 1.01–1.85 | ||||||||
| Stolzenberg-Solomon et al.31 | 2002 | Caucasian | Prospective | Finland | 163/26948 | Vitamin A | HR 1.21 | 0.71–2.03 | Adjusted for energy intake, energy-adjusted folate intake | 8 |
| Carotenoids | HR 0.88 | 0.50–1.55 | ||||||||
| Beta-carotene | HR 0.97 | 0.56–1.68 | ||||||||
| Lycopene | HR 1.06 | 0.64–1.77 | ||||||||
| Nkondjock et al.19 | 2005 | Caucasian (male) | Case-control | Canada | 258/2331 | Alpha-carotene | OR 1.01 | 0.67–1.53 | Adjusted for age, province, smoking, educational attainment, BMI, folate, and total energy intake | 7 |
| Beta-carotene | OR 1.22 | 0.80–1.87 | ||||||||
| P-cryptoxanthin | OR 1.09 | 0.72–1.62 | ||||||||
| Lycopene | OR 0.69 | 0.46–0.96 | ||||||||
| Lutein + Zeaxanthin | OR 1.13 | 0.76–1.68 | ||||||||
| Total carotenoids | OR 1.22 | 0.80–1.86 | ||||||||
| Nkondjock et al.19 | 2005 | Caucasian (female) | Case-control | Canada | 204/2390 | Alpha-carotene | OR 1.56 | 0.98–2.42 | Adjusted for age, province, smoking, educational attainment, BMI, folate, and total energy intake | 7 |
| Beta-carotene | OR 0.96 | 0.60–1.51 | ||||||||
| P-cryptoxanthin | OR 1.23 | 0.80–1.87 | ||||||||
| Lycopene | OR 0.91 | 0.56–1.43 | ||||||||
| Lutein + Zeaxanthin | OR 1.25 | 0.79–1.98 | ||||||||
| Total carotenoids | OR 0.91 | 0.58–1.44 | ||||||||
| Lin et al.35 | 2005 | Asian | Case-control | Japan | 109/218 | Vitamin A | OR 1.09 | 0.62–1.92 | Adjusted for energy intake, age and pack-years of smoking | 7 |
| Retinol | OR 1.29 | 0.72–2.31 | ||||||||
| Carotene | OR 0.80 | 0.44–1.46 | ||||||||
| Bravi et al.17 | 2011 | Caucasian | Case-control | Italy | 326/652 | Retinol | OR 0.73 | 0.44–1.19 | Adjusted for year of interview, education, tobacco smoking, history of diabetes, body mass index, and total energy intake | 7 |
| Alpha-carotene | OR 0.69 | 0.43–1.12 | ||||||||
| Beta-carotene | OR 0.64 | 0.39–1.06 | ||||||||
| Beta-cryptoxanthin | OR 0.66 | 0.39–1.09 | ||||||||
| Lycopene | OR 0.76 | 0.46–1.25 | ||||||||
| Lutein + Zeaxanthin | OR 0.68 | 0.42–1.12 | ||||||||
| Total carotenoids | OR 0.64 | 0.40–1.03 | ||||||||
| Zhang et al.38 | 2011 | Mixed | Case-control | USA | 150/459 | Lutein + Zeaxanthin | OR 0.61 | 0.42–0.89 | Adjusted for energy intake, body mass index, race, education, smoking, history of diabetes, physical activity, alcohol consumption and energy intake by the residual method | 7 |
| Beta-cryptoxanthin | OR 0.89 | 0.61–1.28 | ||||||||
| Lycopene | OR 0.78 | 0.54–1.13 | ||||||||
| Alpha-carotene | OR 0.80 | 0.56–1.16 | ||||||||
| Beta-carotene | OR 0.65 | 0.44–0.94 | ||||||||
| Zablotska et al.18 | 2011 | Mixed (male) | Case-control | USA | 167/466 | Vitamin A | OR 0.68 | 0.27–1.70 | Adjusted for energy intake, body mass index, race, education, smoking, history of diabetes, physical activity, alcohol consumption and energy intake by the residual method | 8 |
| 171/490 | Retinol | OR 1.60 | 0.80–3.30 | |||||||
| Zablotska et al.18 | 2011 | Mixed (female) | Case-control | USA | 124/382 | Vitamin A | OR 1.20 | 0.44–3.50 | Adjusted for energy intake, body mass index, race, education, smoking, history of diabetes, physical activity, alcohol consumption and energy intake by the residual method | 8 |
| 131/407 | Retinol | OR 1.60 | 0.73–3.40 | |||||||
| Heinen et al.43 | 2011 | Caucasian | Prospective | Netherlands | 423/3868 | Alpha-carotene | HR 1.06 | 0.77–1.48 | Adjusted for age and sex. | 8 |
| Beta-carotene | HR 1.14 | 0.83–1.57 | ||||||||
| Lutein + Zeaxanthin | HR 1.03 | 0.76–1.41 | ||||||||
| Beta-cryptoxanthin | HR 0.77 | 0.56–1.07 | ||||||||
| Lycopene | HR 1.04 | 0.76–1.43 | ||||||||
| Han et al.44 | 2011 | Caucasian | Prospective | USA | 162/70332 | Beta-carotene | HR 0.65 | 0.42–0.99 | Adjusted for age, gender, ethnicity, education, body mass index, physical activity, cigarette smoking status, total alcohol consumption, family history of and total energy intake, pancreatic cancer, history of diabetes | 9 |
| Lutein + Zeaxanthin | HR 0.74 | 0.48–1.14 | ||||||||
| Lycopene | HR 0.82 | 0.53–1.26 | ||||||||
| Jansen et al.41 | 2013 | Caucasian | Case-control | USA | 384/983 | Alpha-carotene | OR 0.52 | 0.35–0.77 | Adjusted for energy, smoking, BMI, age, sex, and drinks of alcohol per week | 6 |
| Beta-carotene | OR 0.42 | 0.28–0.63 | ||||||||
| Beta-cryptoxanthin | OR 0.55 | 0.37–0.82 | ||||||||
| Lutein + Zeaxanthin | OR 0.46 | 0.31–0.70 | ||||||||
| Lycopene | OR 0.76 | 0.51–1.13 |
*OR: Odds ratio; RR: risk ratio; HR: hazard ratio.
Quantitative synthesis
Vitamin A and pancreatic cancer
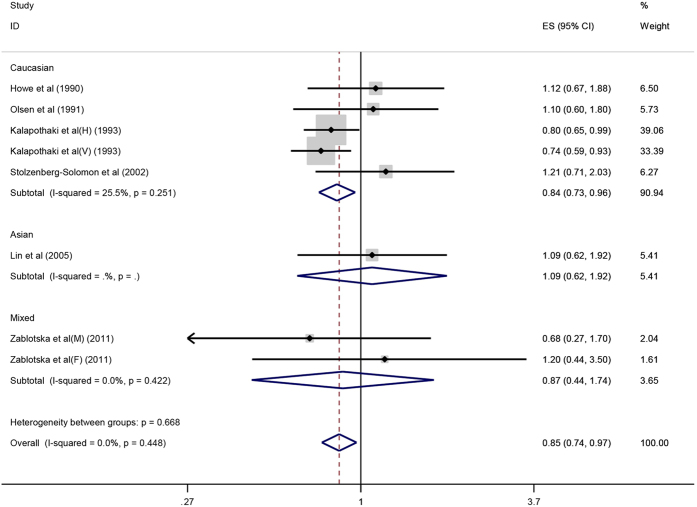
The results pooled by the fixed effect model indicated that there was an inverse association between vitamin A intake and pancreatic cancer risk (OR = 0.85, 95%CI = 0.74–0.97, P = 0.015) (Fig. 2). In addition, stratification analysis conducted by ethnicity and study design type revealed a significant association between vitamin A intake and pancreatic cancer risk in Caucasians subgroup (OR = 0.84, 95%CI = 0.73–0.96, P = 0.011) and case-control subgroup (OR = 0.83, 95%CI = 0.72–0.95, P = 0.007). Subsequently, publication bias was test by funnel plot and Egger’s test. The Egger’s test value suggested that significant publication bias was in the meta-analysis (P = 0.052). The results of metatrim suggested that the summary OR was 0.815 and corresponding 95%CI was 0.702 to 0.946. Besides, no single study could change the results in sensitive analyses, implying that the results of this meta-analysis were robust.
Figure 2. Forest plot of vitamin A intake and pancreatic cancer risk in different populations.
Retinol and pancreatic cancer
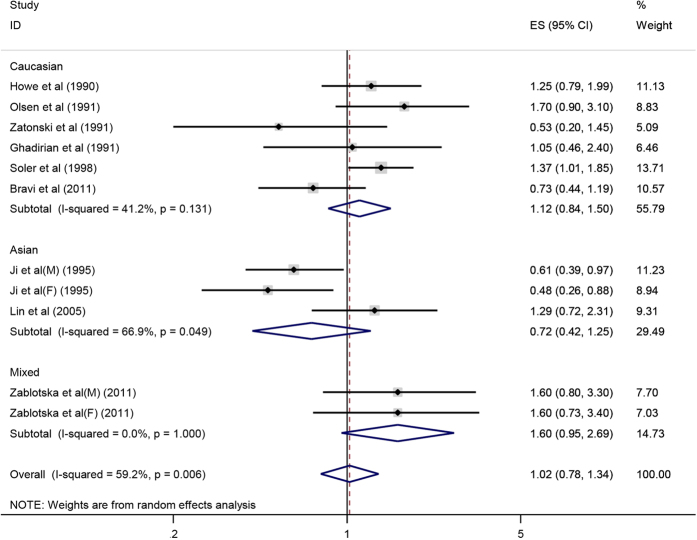
The meta-analysis based on 11 studies of 9 articles indicated that there was no significant correlation between retinol intake and pancreatic cancer risk (OR = 1.02, 95%CI = 0.78–1.34, P = 0.860). Subgroup analysis by ethnicity and the results showed no significant correlation between retinol intake and the risk of pancreatic cancer (Fig. 3). Additionally, the stability of the results was estimated by sensitive analysis, showing that a good stability and credibility. Publication bias was also tested by funnel plot and Egger’s test (P = 0.591), suggesting that there was no statistically significant publication bias in this meta-analysis.
Figure 3. Forest plot of retinol intake and pancreatic cancer risk in different populations.
Carotene, alpha-carotene, beta-carotene and pancreatic cancer
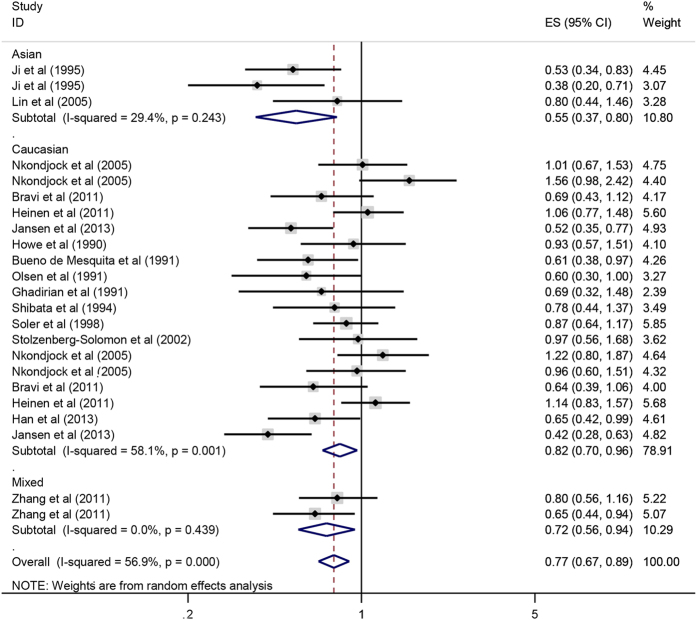
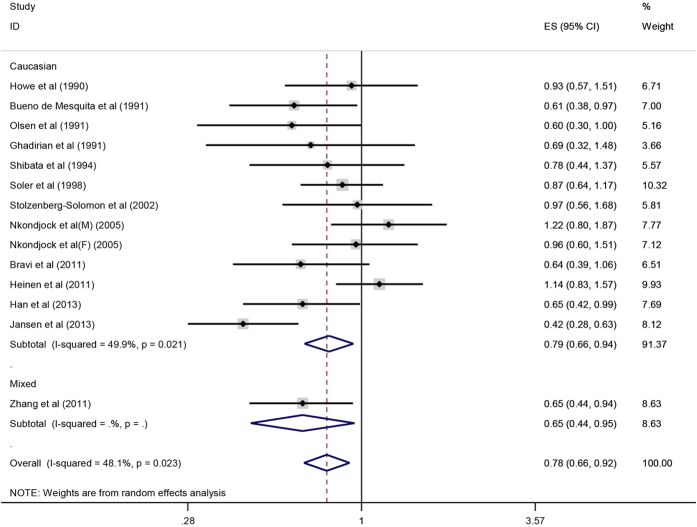
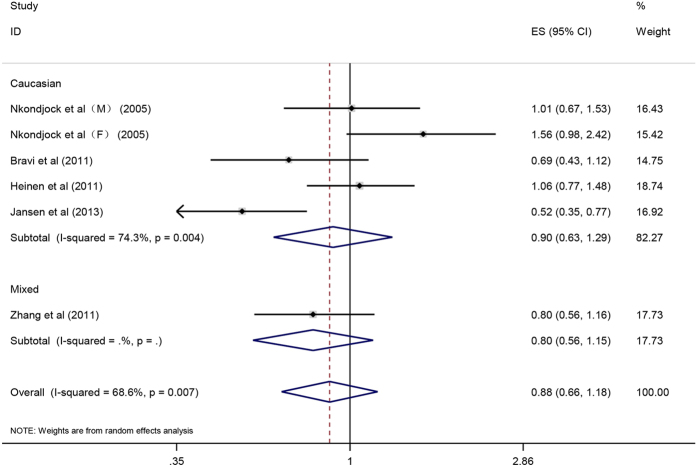
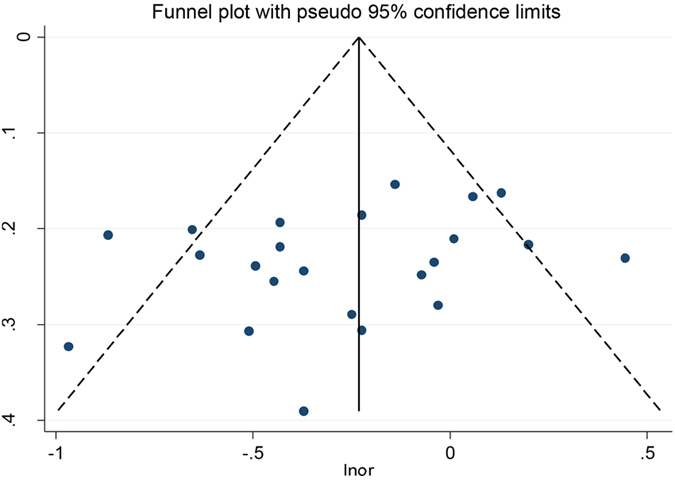
Overall, there was an inverse correlation between carotene intake and pancreatic cancer risk (OR = 0.77, 95%CI = 0.67–0.89, P < 0.001). After stratification by ethnicity, the association became stronger among Asian population (OR = 0.55, 95%CI = 0.37–0.80, P = 0.002) as compared with that in Caucasian (OR = 0.82, 95%CI = 0.70–0.96, P = 0.016) and the mixed population (OR = 0.72, 95%CI = 0.56–0.94, P = 0.016) (Fig. 4). The result of case-control subgroup showed an inverse association between carotene intake and pancreatic cancer risk (OR = 0.74, 95%CI = 0.62–0.87, P < 0.001), while there was no significant association between carotene intake and pancreatic cancer risk in prospective studies (OR = 0.94, 95%CI = 0.76–1.16, P = 0.577). As for nutrient types, the result showed that there was an inverse correlation between beta-carotene intake and pancreatic cancer risk (OR = 0.78, 95%CI = 0.66–0.92, P = 0.003) (Fig. 5), but no significant correlation was observed between alpha-carotene intake and pancreatic cancer risk (OR = 0.88, 95%CI = 0.66–1.18, P = 0.405) (Fig. 6). Sensitive analysis indicated that the results of this meta-analysis were stable. Publication bias was estimated by forest plot and Egger’s test (P = 0.170) and no significant publication bias was found in carotene meta-analysis (Fig. 7).
Figure 4. Forest plot of carotene intake and pancreatic cancer risk in different populations.
Figure 5. Forest plot of beta-carotene intake and pancreatic cancer risk in different populations.
Figure 6. Forest plot of alpha-carotene intake and pancreatic cancer risk in different populations.
Figure 7. Funnel plot for carotene intake and pancreatic cancer risk.
Lycopene and pancreatic cancer
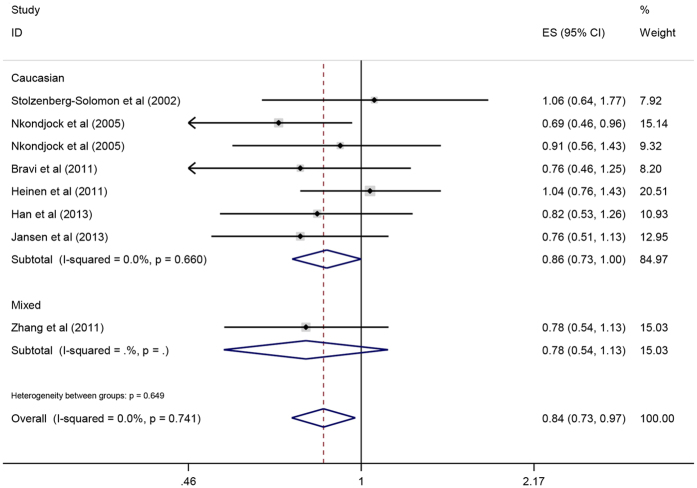
As shown in Fig. 8, there was an inverse correlation between lycopene intake and pancreatic cancer risk (OR = 0.84, 95%CI = 0.73–0.97, P = 0.020). When stratified by ethnicity, there was an inverse relationship between lycopene intake and pancreatic cancer risk in Caucasians (OR = 0.86, 95%CI = 0.73–1.00, P = 0.05), while this correlation was insignificant in the mixed population (OR = 0.78, 95%CI = 0.54–1.13, P = 0.187). With respect to the study design type, decreased the pancreatic cancer risk in case-control study (OR = 0.77, 95%CI = 0.64–0.92, P = 0.005), while prospective study showed no association between lycopene intake and pancreatic cancer (OR = 0.98, 95%CI = 0.78–1.23, P = 0.844). The sensitive analysis revealed that the results of this meta-analysis were credible and stable. Publication bias was evaluated by forest plot and Egger’s test (P = 0.857), and the results showed no significant publication bias in the meta-analysis.
Figure 8. Forest plot of lycopene intake and pancreatic cancer risk in different populations.
Cryptoxanthin and pancreatic cancer
The results showed no significant association between cryptoxanthin intake and pancreatic cancer risk (OR = 0.86, 95%CI = 0.67–1.12, P = 0.276). After stratification by ethnicity, there was no significant association in Caucasians and the mixed population. Similarly, there was no significant correlation in Caucasians and the mixed population. Subgroup analysis showed no significant correlation between cryptoxanthin intake and pancreatic cancer risk in case-control study and prospective study. The sensitive analysis implied that the result of the meta-analysis was robustness. The forest plot and Egger’s test (P = 0.522) suggested that no significant publication bias in this meta-analysis.
Lutein and zeaxanthin and pancreatic cancer
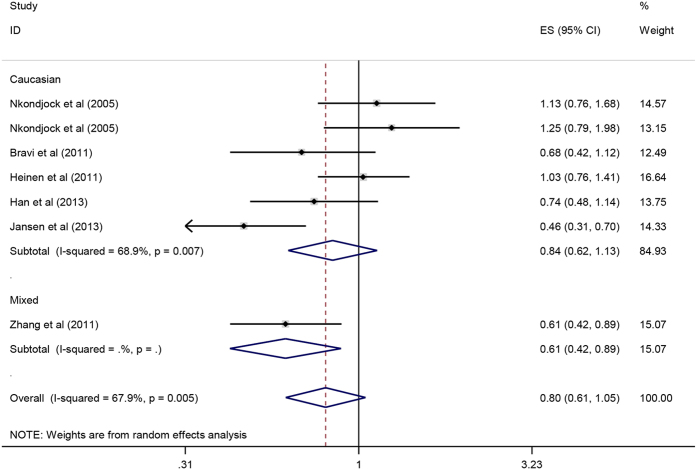
Pooled ORs and corresponding 95%CIs indicated that there was no significant correlation between lutein and zeaxanthin intake and pancreatic cancer risk (OR = 0.80, 95%CI = 0.61–1.05, P = 0.104). As it showed in Fig. 9, there was an inverse association between lutein and zeaxanthin intake and pancreatic cancer risk in the mixed population (OR = 0.61, 95%CI = 0.42–0.89, P = 0.010), but not in Caucasians population (OR = 0.84, 95%CI = 0.62–1.3, P = 0.251). Besides, subgroup analysis showed no significant association could be found in the subgroup of case-control study and prospective study. The sensitive analysis implied that this result was robust. Furthermore, the forest plot and Egger’s test (P = 0.664) showed no significant publication bias in our meta-analysis.
Figure 9. Forest plot of lutein and zeaxanthin intake and pancreatic cancer risk in different populations.
In summary, there was an inverse correlation between vitamin A (including some carotenoids) intake and pancreatic cancer risk, but no significant correlation was observed between retinol intake and pancreatic cancer risk. All the results were summarized in Table 2.
Table 2. The results of the association between vitamin A, retinol and carotenoids intake and the risk of pancreatic cancer in meta-analysis.
| Pooled OR | 95%CI | Pa | Model | I2 | Pb | The number of studies | |
|---|---|---|---|---|---|---|---|
| Vitamin A | |||||||
| Overall | 0.85 | 0.74–0.97 | 0.015 | Fixed | 0.0 | 0.448 | 8 |
| Subgroup | |||||||
| Caucasian | 0.84 | 0.73–0.96 | 0.011 | Fixed | 25.5 | 0.251 | 5 |
| Asian | 1.09 | 0.62–1.92 | 0.765 | Fixed | — | — | 1 |
| Mixed | 0.87 | 0.44–1.74 | 0.700 | Fixed | 0.0 | 0.422 | 2 |
| Case-control | 0.83 | 0.72–0.95 | 0.007 | Fixed | 0.0 | 0.549 | 7 |
| Prospective | 1.21 | 0.72–2.05 | 0.477 | Fixed | — | — | 1 |
| Retinol | |||||||
| Overall | 1.02 | 0.78–1.34 | 0.860 | Random | 59.2 | 0.006 | 11 |
| Subgroup | |||||||
| Caucasian | 1.12 | 0.84–1.50 | 0.437 | Random | 41.2 | 0.131 | 6 |
| Asian | 0.72 | 0.42–1.25 | 0.240 | Random | 66.9 | 0.049 | 3 |
| Mixed | 1.60 | 0.95–2.69 | 0.077 | Random | 0.0 | 1 | 2 |
| Carotenoids | |||||||
| Carotene | |||||||
| Overall | 0.77 | 0.67–0.89 | <0.001 | Random | 56.9 | <0.001 | 23 |
| Subgroup | |||||||
| Caucasian | 0.82 | 0.70–0.96 | 0.016 | Random | 58.1 | 0.001 | 18 |
| Asian | 0.55 | 0.37–0.80 | 0.002 | Random | 29.4 | 0.243 | 3 |
| Mixed | 0.72 | 0.56–0.94 | 0.016 | Random | 0.0 | 0.439 | 2 |
| Case-control | 0.74 | 0.62–0.87 | <0.001 | Random | 57.9 | 0.001 | 18 |
| Prospective | 0.94 | 0.76–1.16 | 0.577 | Random | 56.9 | <0.001 | 5 |
| Alpha-carotene | |||||||
| Overall | 0.88 | 0.66–1.18 | 0.405 | Random | 68.6 | 0.007 | 6 |
| Subgroup | |||||||
| Caucasian | 0.90 | 0.63–1.29 | 0.577 | Random | 74.3 | 0.004 | 5 |
| Mixed | 0.80 | 0.56–1.15 | 0.230 | Random | — | — | 1 |
| Case-control | 0.85 | 0.60–1.21 | 0.362 | Random | 72.2 | 0.006 | 5 |
| Prospective | 1.06 | 0.761.47 | 0.727 | Random | — | — | 1 |
| Beta-carotene | |||||||
| Overall | 0.78 | 0.66–0.92 | 0.003 | Random | 48.1 | 0.023 | 14 |
| Subgroup | |||||||
| Caucasian | 0.79 | 0.66–0.94 | 0.009 | Random | 49.9 | 0.021 | 13 |
| Mixed | 0.65 | 0.44–0.95 | 0.026 | Random | — | — | 1 |
| Case-control | 0.74 | 0.60–0.90 | 0.003 | Random | 49.8 | 0.036 | 10 |
| Prospective | 0.89 | 0.67–1.18 | 0.414 | Random | 35.0 | 0.202 | 4 |
| Lycopene | |||||||
| Overall | 0.84 | 0.73–0.97 | 0.020 | Fixed | 0.0 | 0.741 | 8 |
| Subgroup | |||||||
| Caucasian | 0.86 | 0.73–1.00 | 0.050 | Fixed | 0.0 | 0.660 | 7 |
| Mixed | 0.78 | 0.54–1.13 | 0.187 | Fixed | — | — | 1 |
| Case-control | 0.77 | 0.64–0.92 | 0.005 | Fixed | 0.0 | 0.933 | 5 |
| Prospective | 0.98 | 0.78–1.23 | 0.844 | Fixed | 0.0 | 0.645 | 3 |
| Cryptoxanthin | |||||||
| Overall | 0.86 | 0.67–1.12 | 0.276 | Random | 57.3 | 0.0507 | 5 |
| Subgroup | |||||||
| Caucasian | 0.86 | 0.61–1.21 | 0.387 | Random | 67.8 | 0.0811 | 4 |
| Mixed | 0.89 | 0.61–1.29 | 0.538 | Random | — | — | 1 |
| Case-control | 0.90 | 0.64–1.26 | 0.530 | Random | 66.0 | 0.0802 | 4 |
| Prospective | 0.77 | 0.56–1.07 | 0.114 | Random | — | — | 1 |
| Lutein + zeaxanthin | |||||||
| Overall | 0.80 | 0.61–1.05 | 0.104 | Random | 67.9 | 0.005 | 7 |
| Subgroup | |||||||
| Caucasian | 0.84 | 0.62–1.13 | 0.251 | Random | 68.9 | 0.007 | 6 |
| Mixed | 0.61 | 0.42–0.89 | 0.010 | Random | — | — | 1 |
| Case-control | 0.77 | 0.53–1.11 | 0.163 | Random | 74.5 | 0.003 | 5 |
| Prospective | 0.91 | 0.66–1.24 | 0.537 | Random | 32.7 | 0.223 | 2 |
Pa: P value for meta-analysis; Pb: P value for heterogeneity test.
Discussion
This meta-analysis included 18 articles focusing on the correlation between vitamin A, retinol and carotenoid intake and pancreatic cancer risk. The result showed that dietary vitamin A, carotene, beta-carotene and lycopene were inversely correlated with the risk of pancreatic cancer risk. However, retinol, alpha-carotene, cryptoxanthin, lutein and zeaxanthin intake had no relationship with pancreatic cancer risk.
Vitamin A is a necessity for cell growth and differentiation of epithelial tissues and must be obtained from diets in the human body. Provitamin A compounds, such as beta-carotene can transform into vitamin A, which is an essential molecule entailing multiple developmental pathways and influencing cell proliferation and differentiation in a variety of cell types21. Molecular studies had demonstrated that retinoids (vitamin A and its metabolites) could cause apoptosis in pancreatic cancer cells and thus suppress pancreatic cancer growth via activation of retinoic acid receptor-gamma, suggesting that vitamin A and its metabolites may play a protective role against pancreatic cancer22. Additionally, several preclinical studies showed that retinols play roles in many signaling pathways related with cell growth, adhesion and migration23,24,25. A recent study revealed that retinoic acid could inhibit pancreatic cancer cell migration and epithelial-mesenchymal transition by decreasing the expression of interleukin 6 (IL-6) in cancer-associated fibroblast (CAFs) cells25, suggesting that retinoids could be applied for prevention or therapy the recurrence and metastasis of pancreatic cancer. Actually, immunotherapy including 13-cis-retinoic acid and interleukin 2 had been used for treating locally advanced pancreatic cancer26. However, there is no clinical study focusing on vitamin A therapy in pancreatic cancer so far.
Other carotenoids such as lycopene and zeaxanthin that cannot convert into vitamin A may act as antioxidants against cancer initiation and progression. Lycopene has been proved to be a potent inhibitor for cell proliferation and growth in some cancer cells, such as endometrial cancer, breast cancer and lung cancer27,28. More recently, Assar and colleagues reported that lycopene could suppress the nuclear factor kappa B (NF-κB) signaling pathway through inhibiting phosphorylation of inhibitor of kappa B (IκB) in human prostate and breast cancer cells, probably due to the action of lycopene as an antioxidant to scavenge free radicals29. These data provide a potential strategy to prevent and treat pancreatic cancer by using lycopene.
Several meta-analyses or pooled analyses have investigated the association between the intake of other vitamins and pancreatic cancer risk. Fan et al. conducted a meta-analysis to assess the relationship between dietary vitamin C and pancreatic cancer risk and found that a higher vitamin C intake was inversely correlated with pancreatic cancer risk7. Similarly, dietary vitamin E was found to be a protective factor against pancreatic cancer8. However, a recent pooled analysis suggested that higher levels of vitamin D might increase pancreatic cancer risk30. To the best of our knowledge, this is the first meta-analysis about the relationship of dietary vitamin A, retinol, and carotenoids with pancreatic cancer risk. Overall, we found that dietary vitamin A had an inversely association with pancreatic cancer risk. On the contrary, several studies had investigated the relationship between vitamin A intake and the risk of pancreatic cancer and the results were negative. Partly, these conclusions may due to the small sample size of each study. Besides, the only prospective study showed no association between vitamin A intake and the risk of pancreatic cancer31. However, this prospective study was based on male smokers instead of the general population. No significant relationship was found between dietary retinol and pancreatic cancer risk, or in the subgroups of Caucasians, Asians and the mixed population. These results are consistent with previous case-control studies15,17,32,33,34,35.
The association between carotenoid intake and cancer risk had been investigated in many cancer types. Zhou et al. conducted a meta-analysis and found that beta-carotene and alpha-carotene were inversely correlated with risk of gastric cancer36. Another meta-analysis revealed that dietary alpha-carotene and lycopene could decrease the risk of prostate cancer37. In our meta-analysis, beta-carotene and lycopene intake were inversely associated with pancreatic cancer risk, while alpha-carotene and cryptoxanthin intake had no significant relationship with pancreatic cancer risk. Many previous observational studies including 10 case-control studies15,17,19,32,33,38,39,40,41 and 4 prospective studies31,42,43,44 on the relationship between beta-carotene intake and pancreatic cancer risk reported inconsistent results. These discrepant results from case-control studies might result from recall bias of self-reported dietary intake and different ethnicity. However, the results of 4 prospective studies did not suggest that beta-carotene acted as a protective factor against pancreatic cancer. Notably, a nested case–control study performed in Europe indicated that higher plasma concentrations of beta-carotene could decrease the risk of suffering from pancreatic cancer45. Besides, this article also suggested that higher plasma concentrations of zeaxanthin might be inversely related to pancreatic cancer risk, which is consistent with the result of our meta-analysis indicated that the relationship between the total lutein and zeaxanthin intake and pancreatic cancer risk in the subgroup of the mixed population. However, this subgroup result was based on only one case-control study38.
Between-study heterogeneity is common in meta-analysis46, and the heterogeneity test showed moderate between-study heterogeneity in most meta-analyses. The study characteristics of each study may lead to heterogeneity. In our meta-analysis, the study design, geographic location, publication year and sources of control and cases are various. To find the causes of heterogeneity for covariates, we conducted a meta-regression and subgroup analysis. As a result, meta-regression failed to determine any study characteristics including publication year, study type, study size and ethnicity as sources of heterogeneity. Subgroup analyses by study type and ethnicity were performed to explore the source of heterogeneity. However, between-study heterogeneity was permanent in some subgroups, suggesting that other unknown confounding factors may be present. In addition, the adjustments and the intake levels of nutrients are different between these studies.
Although the results obtained in our meta-analysis are statistically significant as a whole, several limitations should be noted in interpreting our study. First, most included studies in our meta-analysis were case-control studies, in which recall bias may be unavoidable. Both case-control and cohort study are observational study, they require fewer resources but provide less evidence compared with randomized controlled trial (RCT). However, given the extremely low morbidity of pancreatic cancer, there is too difficult to conduct RCT on the association between vitamin A, retinol and carotenoid intake and the risk of pancreatic cancer. Second, some confounding factors such as eating habits and residual confounding cannot be measured, which may affect the stability and credibility of our meta-analysis. Third, the individual sample sizes for each case in most studies included in this meta-analysis were relatively small and these studies were conducted in different populations whose heredity might be different. Finally, some pancreatic cancer cases may be familial heredity47, which may change the morbidity of pancreatic cancer and lead to an inaccurate results in epidemiological study.
In conclusion, the results of our meta-analysis indicate that high-level vitamin A, carotene, beta-carotene and lycopene intake might be the potential factors related to low pancreatic cancer risk. However, due to the limitations of the present meta-analysis mentioned above, it should be prudent to make recommendations based on the results of the present meta-analysis.
Materials and Methods
Literature search strategy
PubMed and EMBASE databases were used to identify observational studies that reported the association between vitamin A, retinol and carotenoid intake and the risk of PANCREATIC CANCER up to December 30th, 2015 by using the following key words “Vitamin A or Vitamin or diet or dietary or retinol or carotenoids or carotene or cryptoxanthin or lycopene or lutein or zeaxanthin” and “pancreatic” and “cancer or carcinoma or neoplasm or tumor or adenocarcinoma”. Additionally, some potential studies were identified via secondary searches which were conducted by searching reference lists of selected literatures.
Inclusion and exclusion criteria
The inclusion criteria were: (1) observational studies including case-control and cohort study design; (2) studies reporting the association between exposure factors including vitamin A, retinol and carotenoids and the risk of pancreatic cancer; (3) studies published in English or Chinese; (4) Providing the odds ratio (OR) (or relative risk [RR], hazard risk [HR]) data and the corresponding 95% corresponding interval (CI) for the highest vs. the lowest level of vitamin A intake or retinol intake or other carotenoid intake. The exclusion criteria were: (1) reviews, meta-analyses, case reports, editorials or human-uncorrelated experiments; (2) duplicated study (If duplicated studies were present, the study with the largest sample size was selected); (3) studies not reporting OR(or RR, HR) and 95%CI or lacking sufficient data to calculate OR(or RR and HR) and 95%CI.
Data extraction
The process of data extraction was conducted by two authors independently with a standardized form based on the inclusion and exclusion criteria mentioned above. Any divergence was resolved by rechecking until consensus was reached. The following information was collected: the last name of the first author, publication year, country, ethnicity, study design, number of cases and controls or total sample size, carotenoid types, OR(or RR, HR), the corresponding 95% CI from the most fully adjusted model for the highest vs. the lowest vitamin A intake, and the factors of adjustment for covariates.
Quality assessment
Newcastle-Ottawa-Scale (NOS) was applied in quality assessment48. During this process, the quality of the selected observational studies was evaluated independently by two authors. The NOS is a nine-point scale containing three parts: selection (four points), comparability (two points) and exposure/outcome assessment (three points). A study with a NOS score ≥6 was regarded as a high-quality study, and vice versa.
Statistical analysis
If the outcome under study is rare in all populations and subgroups under review, one can generally ignore the distinctions between the various measures of relative risk49. Given the low absolute risk of pancreatic cancer in the general populations, we interpreted all risk estimates as OR for simplicity. The relationship between vitamin A, retinol and other carotenoids and the risk of pancreatic cancer was assessed by calculating pooled OR and 95%CI respectively. Additionally, subgroup analysis was conducted by study design type and ethnicity if sufficient data was provided. All the statistical tests were two-sided and the results were considered as statistically significant if P ≤ 0.05. The heterogeneity test was performed by Q test and I2. When I2 > 50%, the random effect model was suggested to calculate the pooled OR and 95%CI. Otherwise, the fixed effect model was applied. Furthermore, sensitivity analysis was conducted by omitting each study once a time. We also assessed publication bias via funnel plots and Egger’s test50. This meta-analysis was performed by STATA12.0 (STATA Corporation, College Station, TX).
Additional Information
How to cite this article: Huang, X. et al. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 6, 38936; doi: 10.1038/srep38936 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (No. 81172077) and Shanghai Municipal Commission of Health and Family Planning, Key Developing Disciplines (No. 2015ZB0202).
Footnotes
Author Contributions The study designed by J.M.Z. Searched databases and collected full-text papers by X.Y.H. and Y.S.G. Extracted and analyzed the data by X.Y.H., Y.S.G. and X.S.Z. Statistical analyses by X.S.Z., H.J. and T.N. The main manuscript text wrote by X.Y.H., X.S.Z. and H.J. All authors reviewed the manuscript.
References
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics. CA: a cancer journal for clinicians 66, 7–30, doi: 10.3322/caac.21332 (2016). [DOI] [PubMed] [Google Scholar]
- Ryan D. P., Hong T. S. & Bardeesy N. Pancreatic adenocarcinoma. The New England journal of medicine 371, 1039–1049, doi: 10.1056/NEJMra1404198 (2014). [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. The New England journal of medicine 362, 1605–1617, doi: 10.1056/NEJMra0901557 (2010). [DOI] [PubMed] [Google Scholar]
- Sharma J., Duque M. & Saif M. W. Emerging therapies and latest development in the treatment of unresectable pancreatic neuroendocrine tumors: an update for clinicians. Therapeutic advances in gastroenterology 6, 474–490, doi: 10.1177/1756283x13498808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor A. A. & Gallinger S. Hereditary Pancreatic Cancer Syndromes. Surgical oncology clinics of North America 24, 733–764, doi: 10.1016/j.soc.2015.06.007 (2015). [DOI] [PubMed] [Google Scholar]
- Gong Z., Holly E. A. & Bracci P. M. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer causes & control: CCC 20, 1317–1325, doi: 10.1007/s10552-009-9352-9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H. et al. Association between vitamin C intake and the risk of pancreatic cancer: a meta-analysis of observational studies. Scientific reports 5, 13973, doi: 10.1038/srep13973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Liu X., Lu Q., Tang T. & Yang Z. Vitamin E intake and pancreatic cancer risk: a meta-analysis of observational studies. Medical science monitor: international medical journal of experimental and clinical research 21, 1249–1255, doi: 10.12659/msm.893792 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B. C. et al. Retinoic acid signaling pathways in development and diseases. Bioorganic & medicinal chemistry 22, 673–683, doi: 10.1016/j.bmc.2013.11.025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C. Retinoic acid signaling and mouse embryonic stem cell differentiation: Cross talk between genomic and non-genomic effects of RA. Biochimica et biophysica acta 1851, 66–75, doi: 10.1016/j.bbalip.2014.04.003 (2015). [DOI] [PubMed] [Google Scholar]
- Harrison E. H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochimica et biophysica acta 1821, 70–77, doi: 10.1016/j.bbalip.2011.06.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doldo E., Costanza G. & Agostinelli S. Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. 2015, 624627, doi: 10.1155/2015/624627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulan H. et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer causes & control: CCC 22, 1383–1396, doi: 10.1007/s10552-011-9811-y (2011). [DOI] [PubMed] [Google Scholar]
- Chen G., Wang J., Hong X., Chai Z. & Li Q. Dietary vitamin E intake could reduce the risk of lung cancer: evidence from a meta-analysis. International journal of clinical and experimental medicine 8, 6631–6637 (2015). [PMC free article] [PubMed] [Google Scholar]
- Howe G. R., Jain M. & Miller A. B. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. International journal of cancer. Journal international du cancer 45, 604–608 (1990). [DOI] [PubMed] [Google Scholar]
- Kalapothaki V. et al. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer causes & control: CCC 4, 383–389 (1993). [DOI] [PubMed] [Google Scholar]
- Bravi F. et al. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case-control study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 22, 202–206, doi: 10.1093/annonc/mdq302 (2011). [DOI] [PubMed] [Google Scholar]
- Zablotska L. B., Gong Z., Wang F., Holly E. A. & Bracci P. M. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer causes & control: CCC 22, 91–100, doi: 10.1007/s10552-010-9678-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkondjock A., Ghadirian P., Johnson K. C. & Krewski D. Dietary intake of lycopene is associated with reduced pancreatic cancer risk. The Journal of nutrition 135, 592–597 (2005). [DOI] [PubMed] [Google Scholar]
- Ji B. T. et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 4, 885–893 (1995). [PubMed] [Google Scholar]
- Mongan N. P. & Gudas L. J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation; research in biological diversity 75, 853–870, doi: 10.1111/j.1432-0436.2007.00206.x (2007). [DOI] [PubMed] [Google Scholar]
- Pettersson F., Dalgleish A. G., Bissonnette R. P. & Colston K. W. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. British journal of cancer 87, 555–561, doi: 10.1038/sj.bjc.6600496 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosewicz S., Brembeck F., Kaiser A., Marschall Z. V. & Riecken E. O. Differential growth regulation by all-trans retinoic acid is determined by protein kinase C alpha in human pancreatic carcinoma cells. Endocrinology 137, 3340–3347, doi: 10.1210/endo.137.8.8754760 (1996). [DOI] [PubMed] [Google Scholar]
- Rosewicz S. et al. Retinoids inhibit adhesion to laminin in human pancreatic carcinoma cells via the alpha 6 beta 1-integrin receptor. Gastroenterology 112, 532–542 (1997). [DOI] [PubMed] [Google Scholar]
- Guan J. et al. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer letters 345, 132–139, doi: 10.1016/j.canlet.2013.12.006 (2014). [DOI] [PubMed] [Google Scholar]
- Recchia F. et al. Chemoradioimmunotherapy in locally advanced pancreatic and biliary tree adenocarcinoma: a multicenter phase II study. Pancreas 38, e163–168, doi: 10.1097/MPA.0b013e3181abe222 (2009). [DOI] [PubMed] [Google Scholar]
- Levy J. et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutrition and cancer 24, 257–266, doi: 10.1080/01635589509514415 (1995). [DOI] [PubMed] [Google Scholar]
- Tang L., Jin T., Zeng X. & Wang J. S. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. The Journal of nutrition 135, 287–290 (2005). [DOI] [PubMed] [Google Scholar]
- Assar E. A., Vidalle M. C., Chopra M. & Hafizi S. Lycopene acts through inhibition of IkappaB kinase to suppress NF-kappaB signaling in human prostate and breast cancer cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, doi: 10.1007/s13277-016-4798-3 (2016). [DOI] [PubMed] [Google Scholar]
- Waterhouse M. et al. Vitamin D and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Case-Control Consortium. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 26, 1776–1783, doi: 10.1093/annonc/mdv236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg-Solomon R. Z., Pietinen P., Taylor P. R., Virtamo J. & Albanes D. Prospective study of diet and pancreatic cancer in male smokers. American journal of epidemiology 155, 783–792 (2002). [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Mandel J. S., Gibson R. W., Wattenberg L. W. & Schuman L. M. Nutrients and pancreatic cancer: a population-based case-control study. Cancer causes & control: CCC 2, 291–297 (1991). [DOI] [PubMed] [Google Scholar]
- Ghadirian P., Simard A., Baillargeon J., Maisonneuve P. & Boyle P. Nutritional factors and pancreatic cancer in the francophone community in Montreal, Canada. International journal of cancer. Journal international du cancer 47, 1–6 (1991). [DOI] [PubMed] [Google Scholar]
- Zatonski W. et al. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. International journal of cancer. Journal international du cancer 48, 390–394 (1991). [DOI] [PubMed] [Google Scholar]
- Lin Y. et al. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. Journal of gastroenterology 40, 297–301, doi: 10.1007/s00535-004-1537-0 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang T., Meng Q. & Zhai S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clinical nutrition (Edinburgh, Scotland), doi: 10.1016/j.clnu.2015.02.003 (2015). [DOI] [PubMed] [Google Scholar]
- Wang Y., Cui R., Xiao Y., Fang J. & Xu Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PloS one 10, e0137427, doi: 10.1371/journal.pone.0137427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Sequence variants in antioxidant defense and DNA repair genes, dietary antioxidants, and pancreatic cancer risk. International journal of molecular epidemiology and genetics 2, 236–244 (2011). [PMC free article] [PubMed] [Google Scholar]
- Bueno de Mesquita H. B., Maisonneuve P., Runia S. & Moerman C. J. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. International journal of cancer. Journal international du cancer 48, 540–549 (1991). [DOI] [PubMed] [Google Scholar]
- Soler M., Chatenoud L., La Vecchia C., Franceschi S. & Negri E. Diet, alcohol, coffee and pancreatic cancer: final results from an Italian study. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP) 7, 455–460 (1998). [DOI] [PubMed] [Google Scholar]
- Jansen R. J. et al. Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. Journal of gastrointestinal cancer 44, 152–161, doi: 10.1007/s12029-012-9441-y (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A., Mack T. M., Paganini-Hill A., Ross R. K. & Henderson B. E. A prospective study of pancreatic cancer in the elderly. International journal of cancer. Journal international du cancer 58, 46–49 (1994). [DOI] [PubMed] [Google Scholar]
- Heinen M. M., Verhage B. A., Goldbohm R. A. & van den Brandt P. A. Intake of vegetables, fruits, carotenoids and vitamins C and E and pancreatic cancer risk in The Netherlands Cohort Study. International journal of cancer. Journal international du cancer 130, 147–158, doi: 10.1002/ijc.25989 (2012). [DOI] [PubMed] [Google Scholar]
- Han X. et al. Antioxidant intake and pancreatic cancer risk: the Vitamins and Lifestyle (VITAL) Study. Cancer 119, 1314–1320, doi: 10.1002/cncr.27936 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurnink S. M. et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. International journal of cancer. Journal international du cancer 136, E665–676, doi: 10.1002/ijc.29175 (2015). [DOI] [PubMed] [Google Scholar]
- Munafo M. R. & Flint J. Meta-analysis of genetic association studies. Trends in genetics: TIG 20, 439–444, doi: 10.1016/j.tig.2004.06.014 (2004). [DOI] [PubMed] [Google Scholar]
- Roberts N. J. & Klein A. P. Genome-wide sequencing to identify the cause of hereditary cancer syndromes: with examples from familial pancreatic cancer. Cancer letters 340, 227–233, doi: 10.1016/j.canlet.2012.11.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. A., D O’Connell B. S., Peterson J., Welch V., Losos M. & Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2011).
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9, 1–30 (1987). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]